Indissolvable drug oral sustained-release composition and preparation method thereof
A technology for sustained-release compositions and insoluble drugs, applied in the field of medicine, can solve the problems of inability to obtain satisfactory sustained and controlled-release formulation development prescription process, incomplete dissolution, too fast dissolution, etc., and achieve stable and effective localized release effect and complete release. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
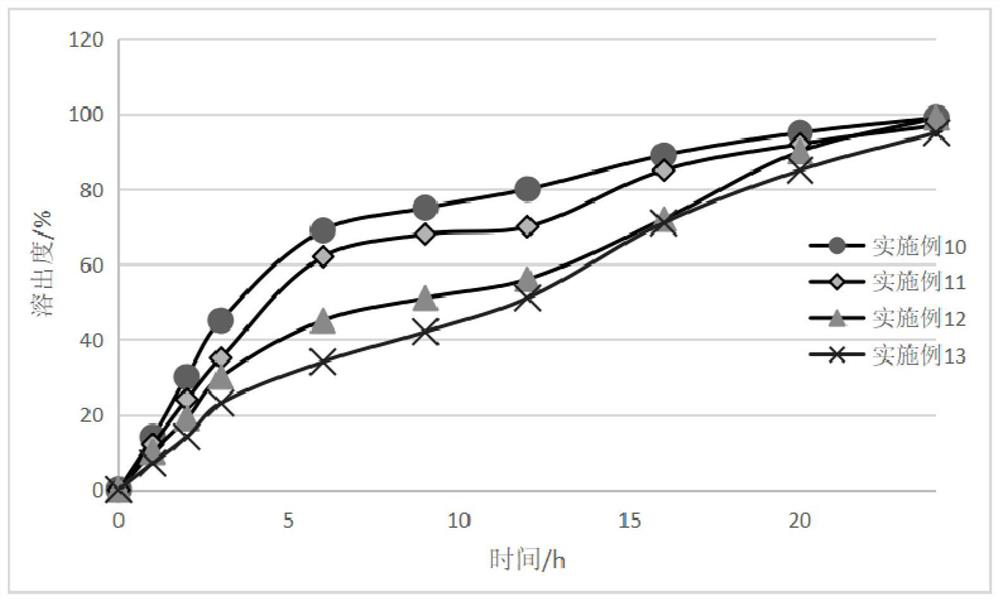
Embodiment 1~4
[0057] The used prescription of embodiment 1~4 and consumption ratio are as shown in table 1 below, and the material total amount of every batch is 500g (excluding solvent weight):
[0058] Table 1
[0059]
[0060] *Solvents will be removed by drying during processing.
[0061] Embodiment 1~4 preparation process steps:
[0062] 1) Dissolve Eudragit S 100 in an appropriate amount of ethanol (95%); add Apremilast, talcum powder and triethyl citrate into an appropriate amount of ethanol (95%), and homogenize with a homogenizer for about 10 minutes. Then pour it into the Eudragit S 100 solution, and use a stirrer to continuously stir the suspension during the granulation process;
[0063] 2) Spray the suspension in step 1) on the microcrystalline cellulose through WBF-2G fluidized bed top spray granulation, and control the atomization pressure at 0.5-2.0bar during the process (adjust 0.5bar every 10min to the maximum value) ) and spray speed 5 ~ 15g / min (up to 5g / min every ...
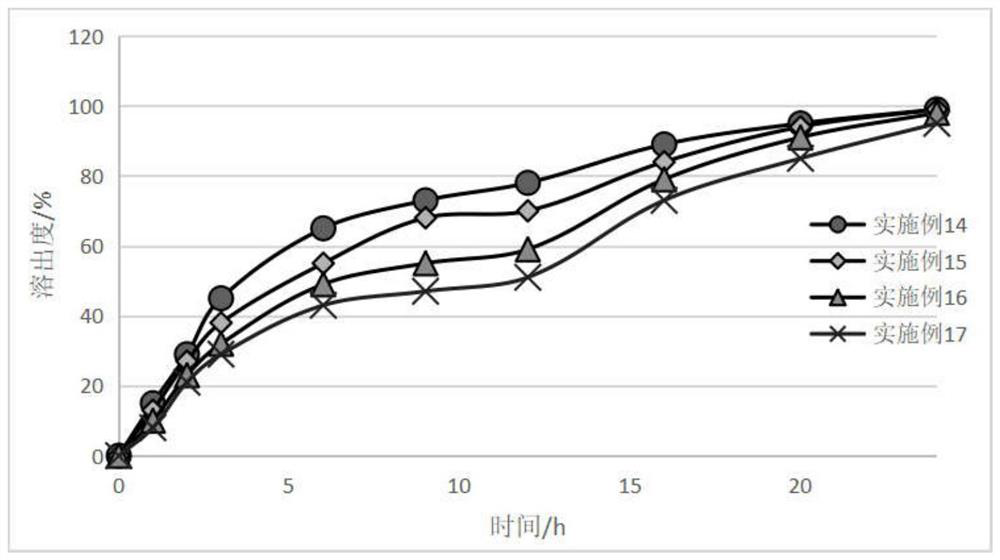
Embodiment 5~8
[0067] The prescriptions and dosage ratios used in Examples 5 to 8 are shown in Table 2 below, wherein the particle size distribution d90 of the bulk drug used in Apremilast is 7.4 microns, and the total amount of materials in each batch is 500g (excluding solvent weight).
[0068] Table 2
[0069]
[0070]
[0071] *Based on weight of solid matter in Eudragit aqueous dispersion.
[0072] **The amount of purified water includes the amount of water in the Eudragit water dispersion and the amount of added water. The purified water will be dried and removed during processing. In the prescription, other components except the liquid strong adsorption carrier and the binder are prepared into a suspension with the solvent, and the amount of the solvent used is that the solid content of the suspension is about 20% to 25%.
[0073] Embodiment 5~8 preparation process steps:
[0074] 1) Add anti-sticking agent, plasticizer, surfactant, and main ingredient to a certain amount of s...
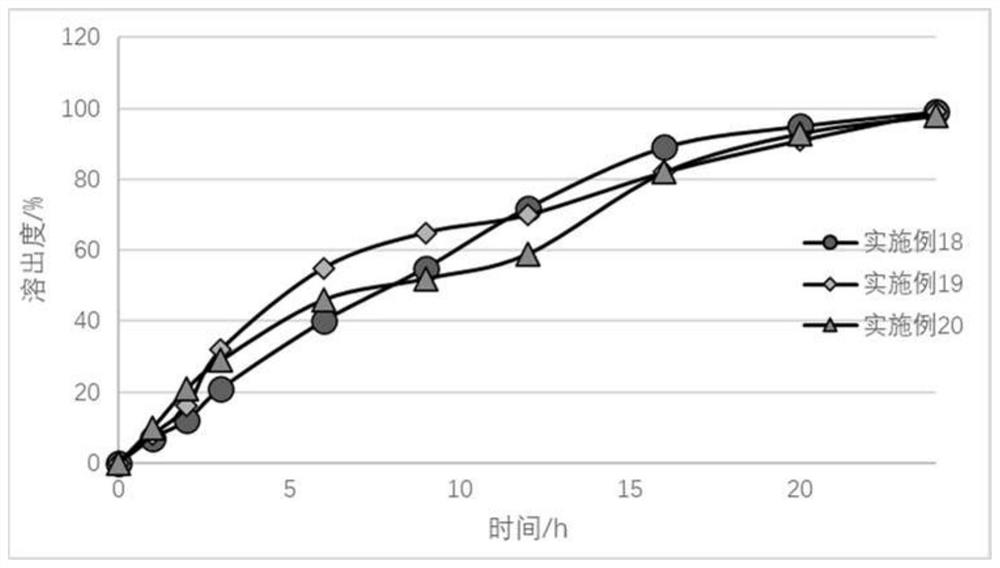
Embodiment 9
[0079] The prescription and dosage ratio used in embodiment 9 are shown in the following table 3, wherein the particle size distribution d90 of the crude drug used in apremilast is 15 microns, and the total amount of material in each batch is 500g (excluding solvent weight).
[0080] table 3
[0081]
[0082] *In the prescription, other components except liquid strong adsorption carrier and adhesive are prepared into a suspension with the solvent. The amount of solvent used is that the solid content of the suspension is 25.4%, and the purified water will be dried during processing remove.
[0083] Embodiment 9 preparation process steps:
[0084] 1) Slowly pour the enteric-coated material Eudragit L100 into water and stir for 5-10 minutes. After the enteric-coated material is completely wetted, slowly add sodium hydroxide powder or solution and keep stirring, and finally continue stirring for 30 minutes. Then add raw material drug, plasticizer and anti-adhesive agent, con...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com