Crystal form A of Bictegravir sodium salt, and preparation method and use of crystal form A of Bictegravir sodium salt
A crystal form and sodium salt technology, applied in organic chemical methods, medical preparations containing active ingredients, pharmaceutical formulations, etc., can solve the problems of reducing tablet and filling production efficiency, poor physical properties of crystal form powder, and bioavailability Reduce problems such as improving product appearance, excellent compressibility, and reducing adsorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] The preparation of embodiment 1 Bictegravir sodium salt
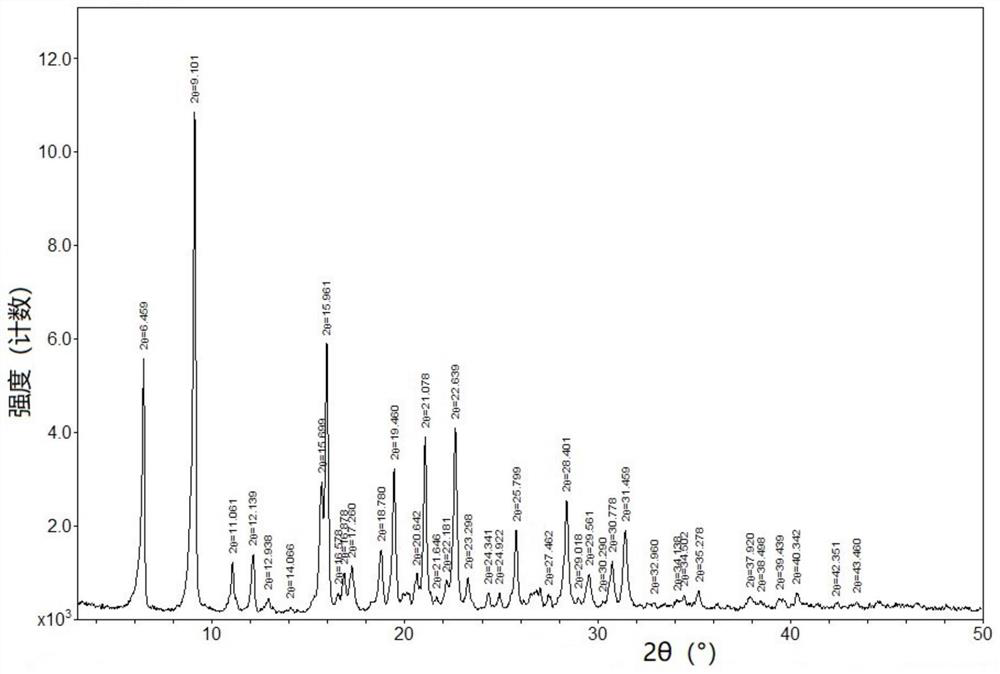
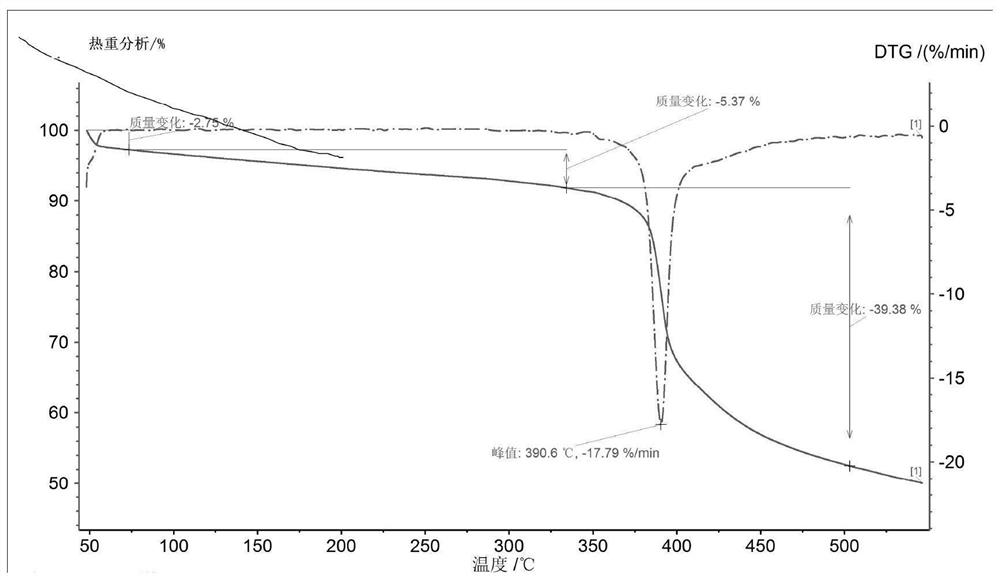
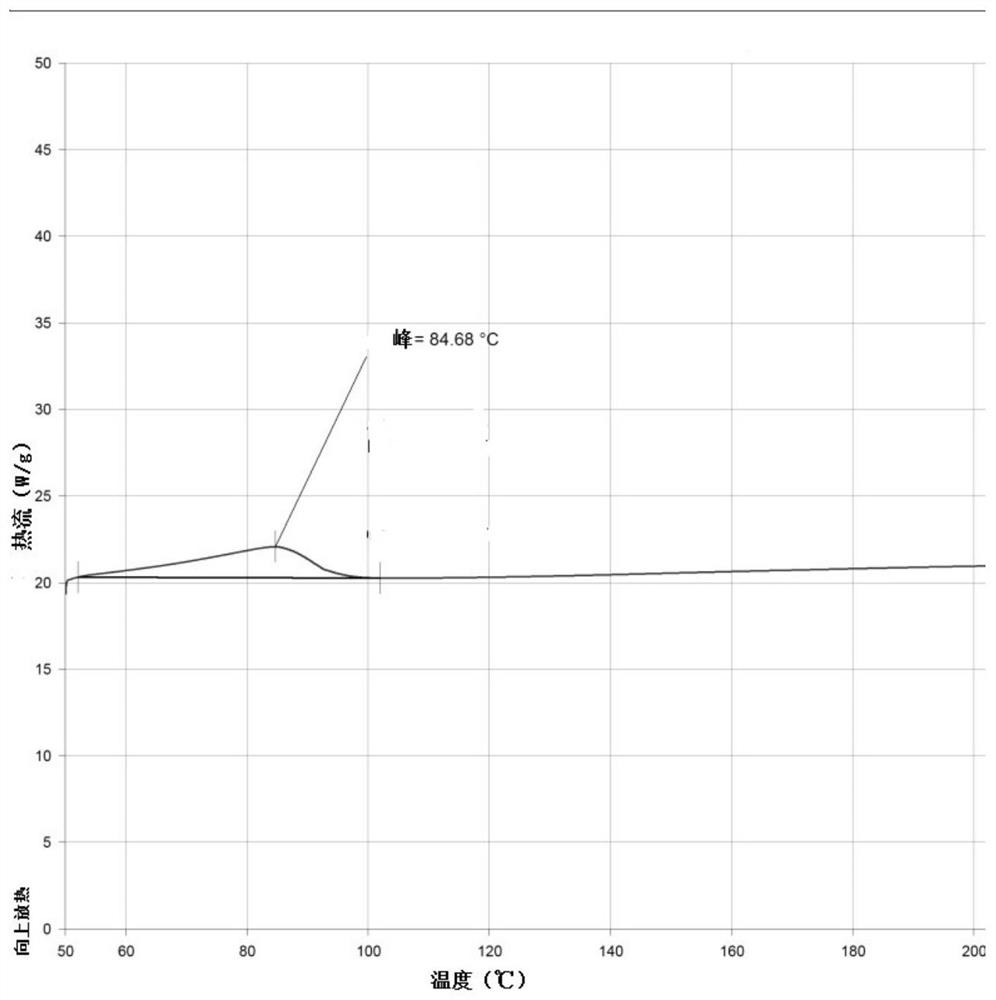
[0034] Add 10g of Bictegravir into 100mL of dichloromethane, stir to dissolve, cool down to 0-10°C; add 1.26g of sodium methoxide to 12mL of methanol, stir to dissolve, cool down to 0-10°C, slowly add the above methylene chloride solution dropwise After 20 minutes of dripping, the dripping was completed, stirring and crystallizing at 0-10°C for 3h, suction filtration, rinsing the filter cake with an appropriate amount of dichloromethane, and drying at 50°C for 16h to obtain 9.8g of off-white solid, which is the crystal form of Bictegravir sodium salt a.
[0035] The XRPD peak data of the crystal form A of Bictegravir sodium salt are shown in Table 1:
[0036] Table 1
[0037]
[0038]
[0039] Embodiment 2 fluidity test
[0040] According to the "United States Pharmacopoeia", the fluidity of the crystal form A prepared by the present invention is studied through the compressibility coefficient, and the b...
Embodiment 3
[0045] Embodiment 3 compressibility test
[0046] Adopt manual tablet press to carry out tabletting, when tabletting, select to be able to be pressed into the circular parallel punching of cylindrical tablet (guarantee the isotropy of tablet). Take a certain amount of original research crystal form and crystal form A respectively, use a certain pressure to press into round tablets, place them in a desiccator for 24 hours, and use a tablet hardness tester to test their radial crushing force (hardness , N). Measure the diameter (D) and thickness (L) of the tablet with a vernier caliper, and use the formula T=2N / πDL to calculate the tensile strength of the powder at different hardnesses. Under a certain pressure, the greater the tensile strength, the better its compressibility. When the amount of sample in the screening stage is small, the recommended parameters in the table below can be used for testing. The recommended parameters for the tensile strength test are as follows:...
Embodiment 4
[0051] Embodiment 4 adhesion test
[0052] Add an appropriate amount of crystal form A and the original research crystal form into a suitable mold, press a certain pressure for tableting, stay for about half a minute after tableting, and weigh the amount of powder absorbed by the punch. After using this method for several times of continuous pressing, record the cumulative final adsorption amount of the punch, the highest adsorption amount and the average adsorption amount during the pressing process. The experimental data are shown in Table 4:
[0053] Table 4
[0054] crystal form Cumulative final adsorption amount (mg) Maximum adsorption capacity (mg) Average adsorption capacity (mg) Form A 0.14 0.10 0.028 Original crystal form 0.21 0.17 0.042
[0055] The results show that compared with the original crystal form, the average adsorption amount of the crystal form A of the present invention is only 0.028 mg, which is far lower than that ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| tensile strength | aaaaa | aaaaa |
| tensile strength | aaaaa | aaaaa |
| adsorption capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com