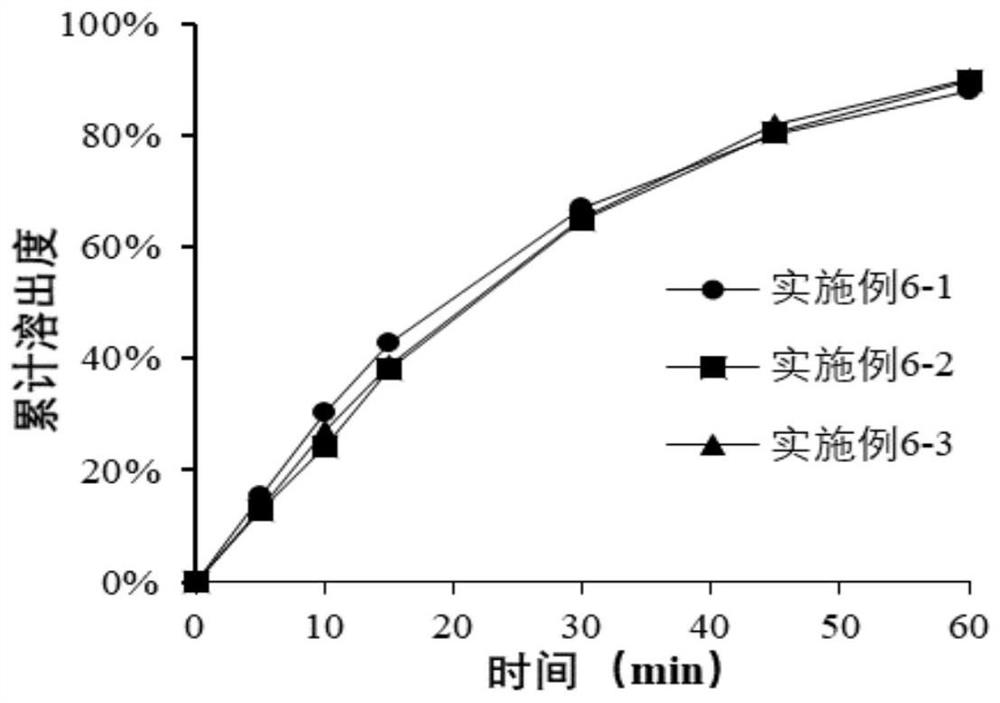
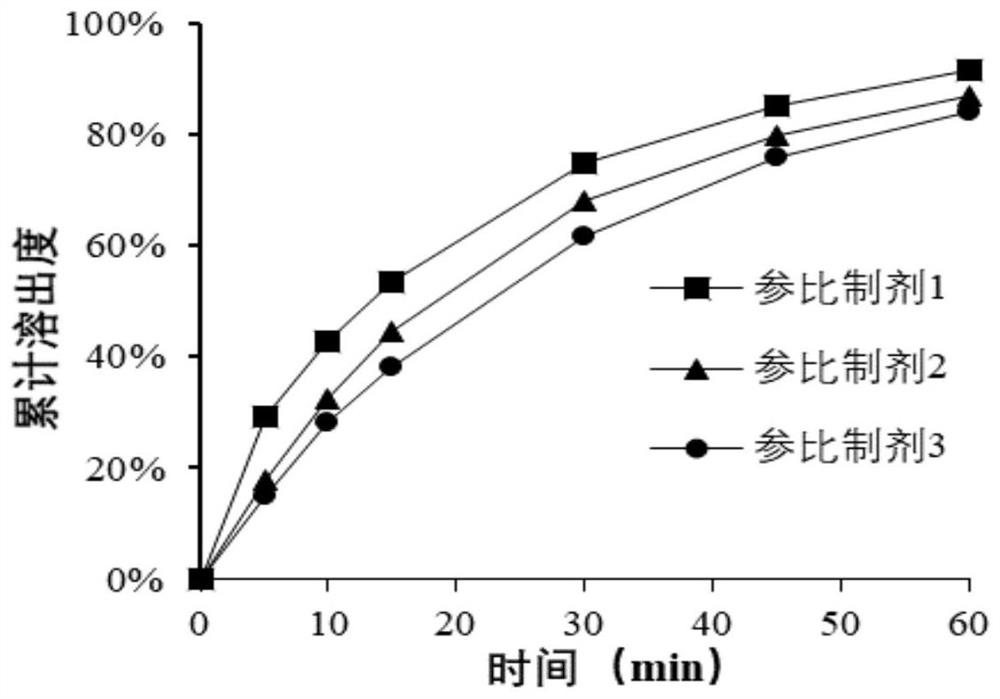
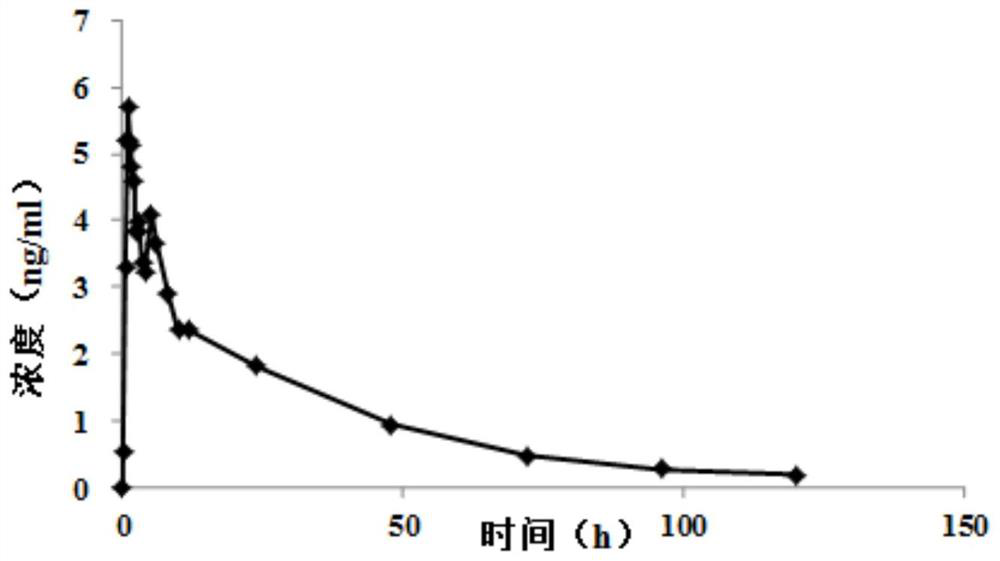
Pharmaceutical composition of bazedoxifene acetate tablets and preparation method thereof
A technology of bazedoxifene acetate and its composition, which is applied in the field of pharmaceutical preparations, can solve the problems of clinical treatment effect and potential safety hazards, large inter-batch differences, and poor reproducibility, so as to improve inter-batch quality differences and ensure uniform content degree of effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0074] Embodiment 1 Bazedoxifene acetate particle size investigation test
[0075] Get the bazedoxifene acetate bulk drug of different particle diameters, prepare bazedoxifene acetate tablet according to following prescription and preparation process, carry out dissolution rate measurement to gained tablet under the condition of pH4.5 and 0.25%SDS, to investigate The effect of the particle size of the bulk drug on the dissolution of the tablet, the results are shown in Table 1.
[0076] prescription:
[0077] Material name Prescription amount (mg) bazedoxifene acetate 22.60 lactose 127.94 Vitamin C 6.00 precrossified starch 15.50 Low-substituted hydroxypropyl cellulose 54.25 Colloidal silica 4.65 microcrystalline cellulose 62.00 Sodium dodecyl sulfate 4.65 Glyceryl behenate 6.20
[0078] Preparation Process:
[0079] Mix bazedoxifene acetate, vitamin C, colloidal silicon dioxide and 1 / 2 prescription amount...
Embodiment 2
[0083] Embodiment 2 wet granulation time research
[0084] According to the prescription of embodiment 1, adopt different wet granulation time to prepare bazedoxifene acetate tablet respectively, all the other preparation processes are identical with embodiment 1. The prepared tablets were tested for dissolution under the conditions of pH 4.5 and 0.25% SDS to investigate the influence of different granulation times on the dissolution of the tablets. The results are shown in Table 2.
[0085] Table 2 Wet granulation time investigation trial sample dissolution curve detection results
[0086]
[0087]
[0088] It can be seen from the above table that when the granulation time is in the range of 2min to 6min, the dissolution rate of the samples in Examples 2-1 to 2-7 is above 80% in 60 minutes, especially when the granulation time is in the range of 2min to 6min. At 3 minutes, the dissolution rate can reach more than 90%, indicating that the granulation time can meet the p...
Embodiment 3
[0089] Example 3 Wetting agent solution addition time investigation
[0090] According to the prescription of Example 1, Bazedoxifene Acetate Tablets were prepared at different adding times of the wetting agent solution, and the rest of the preparation process was the same as in Example 1. The prepared tablets were tested for dissolution under the conditions of pH 4.5 and 0.25% SDS to investigate the effect of adding time of different wetting agent solutions on the dissolution of the tablets. The results are shown in Table 3.
[0091] Table 3 Wetting agent solution addition time investigation test results of dissolution curve of trial samples
[0092]
[0093] It can be seen from the above table that when the adding time of the wetting agent is in the range of 90s to 120s, the dissolution rates of the samples in Examples 3-1 to 3-4 are all above 75% within 60 minutes, especially when the wetting agent solution is added When the time is in the range of 100s to 120s, the dis...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com