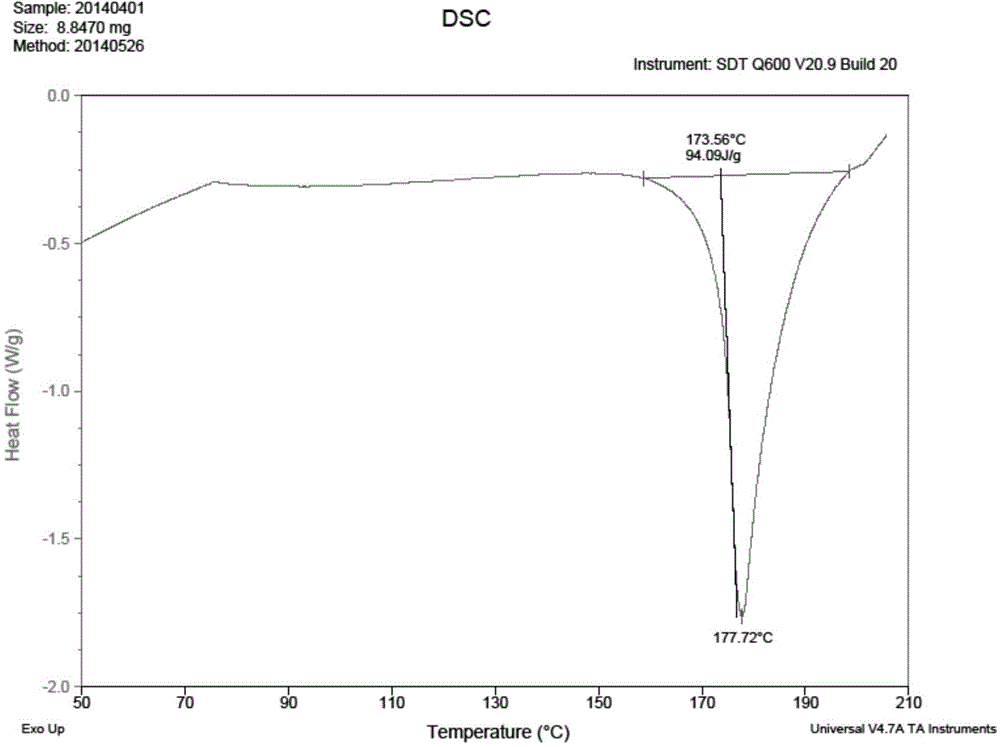
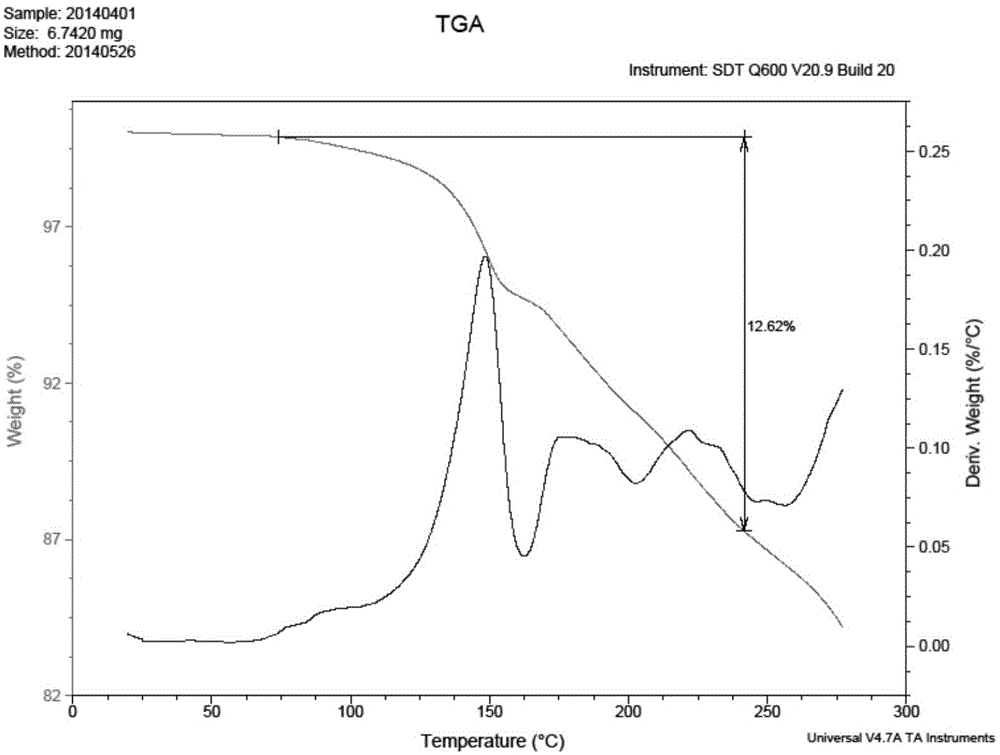
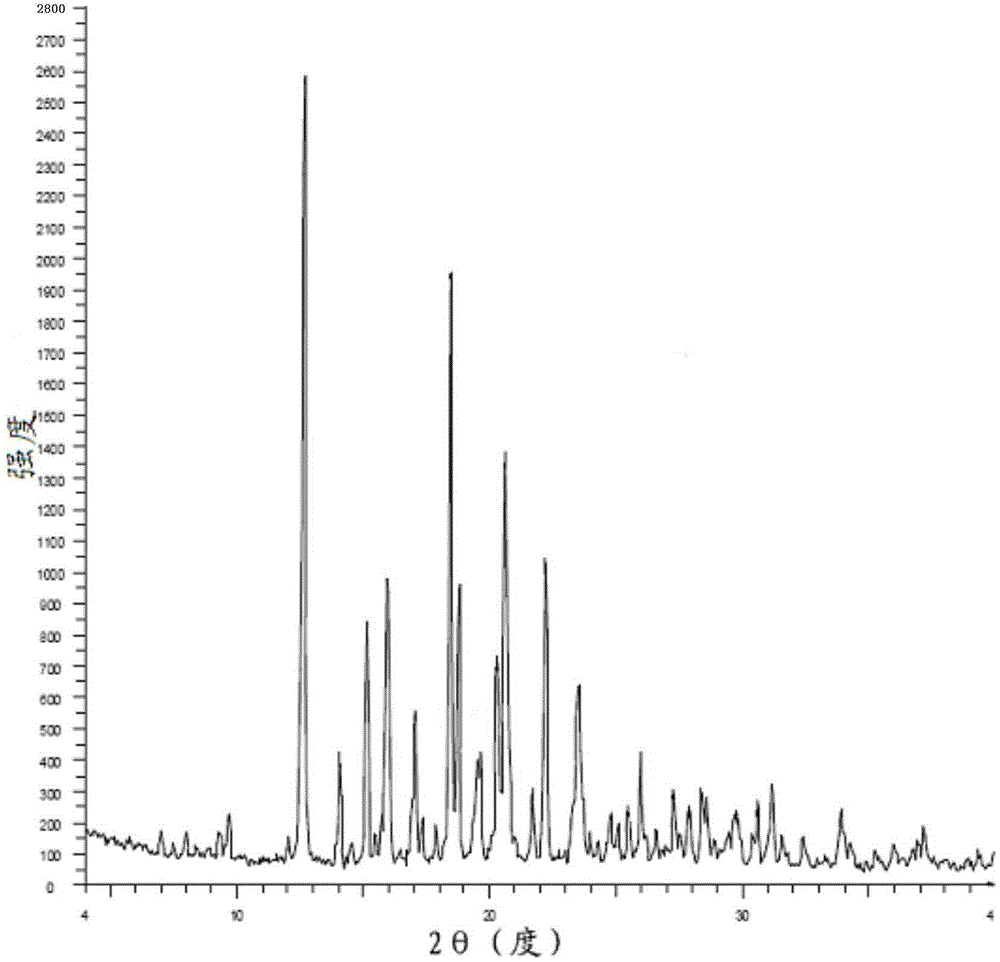
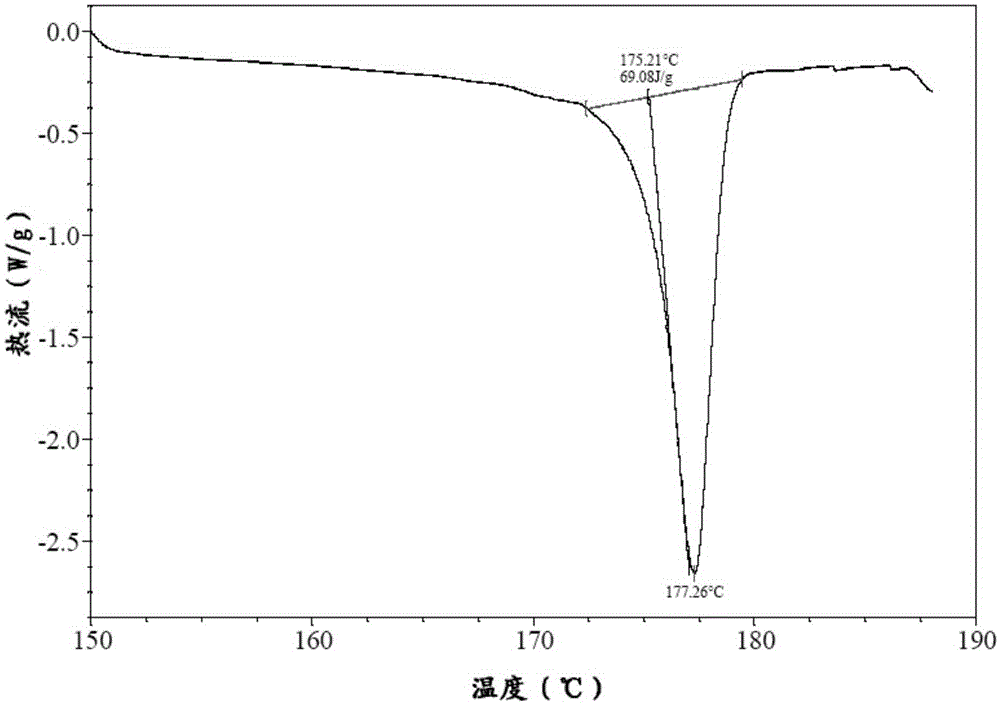
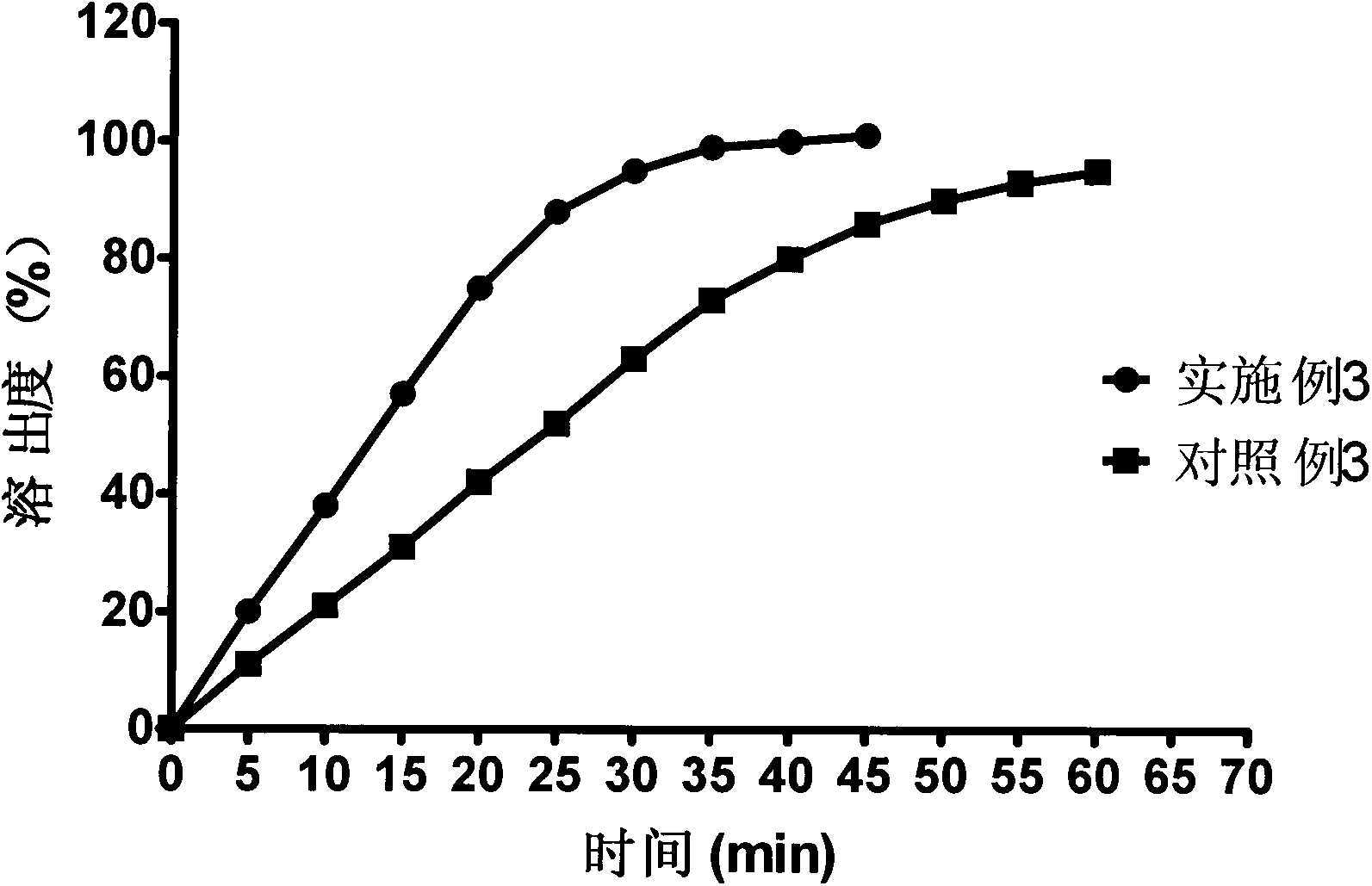
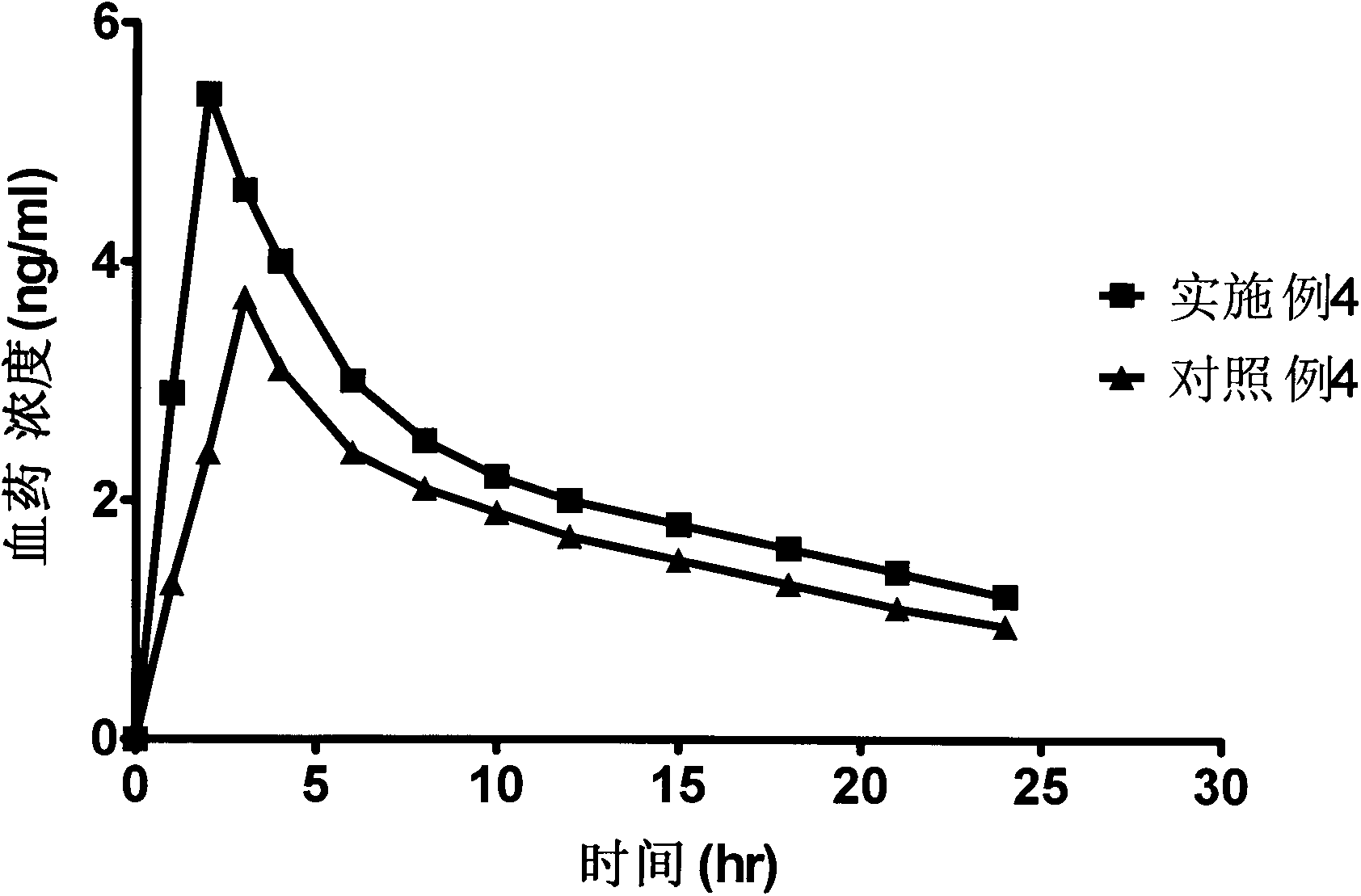
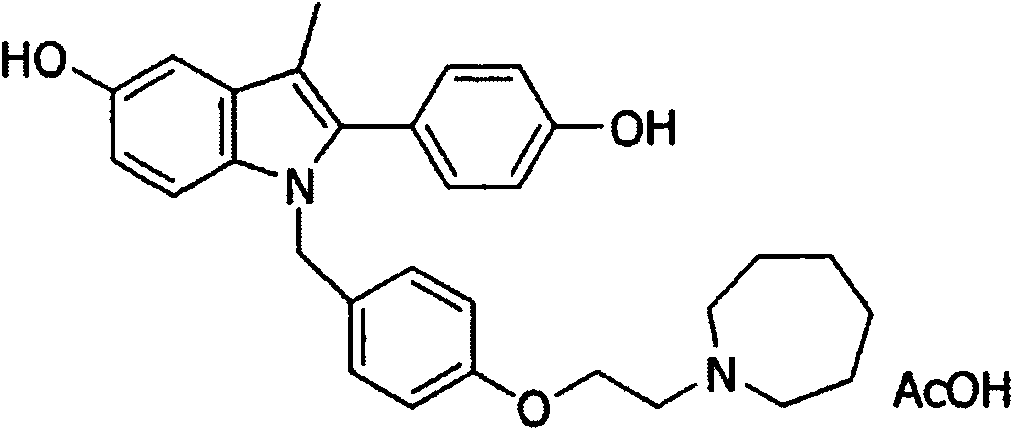
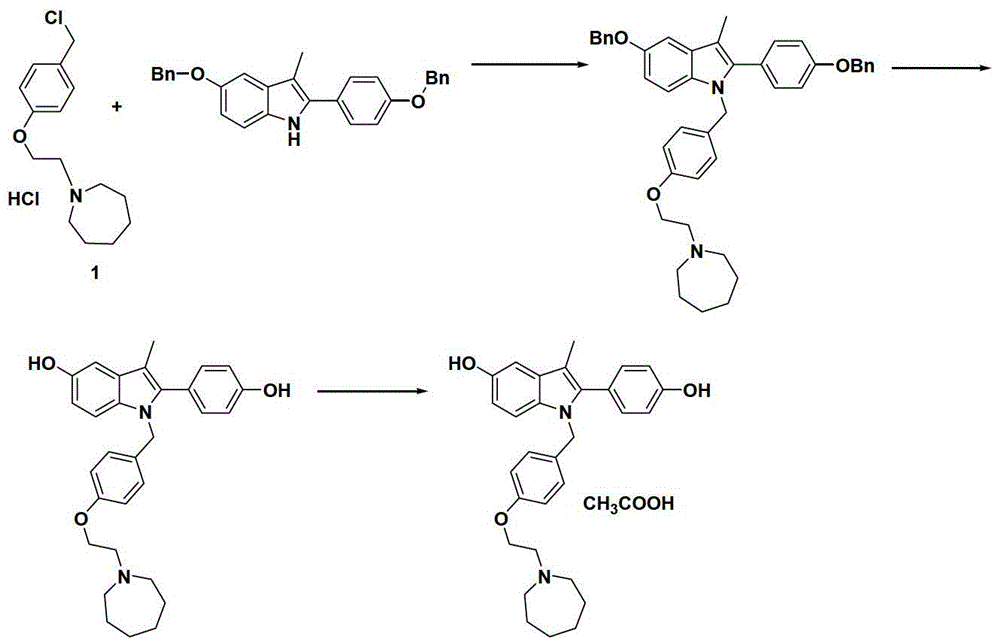
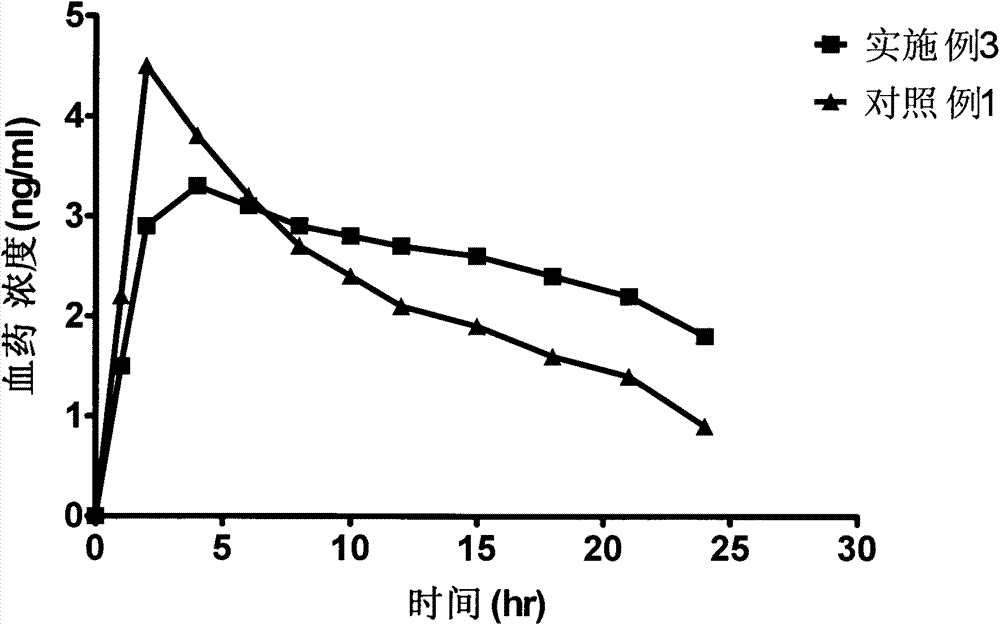
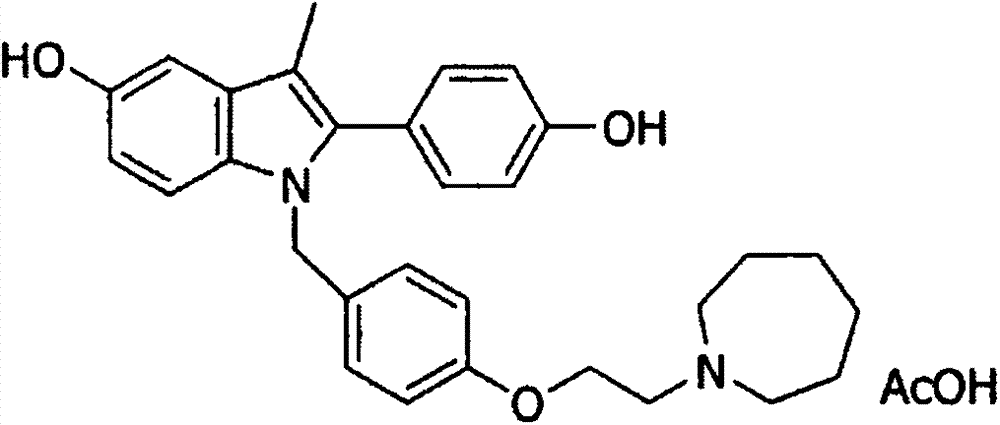
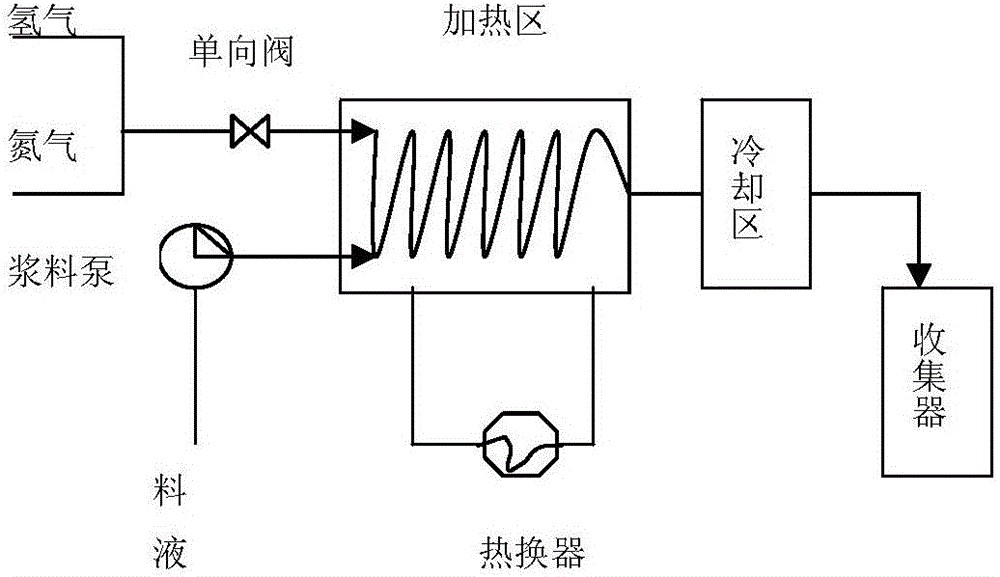
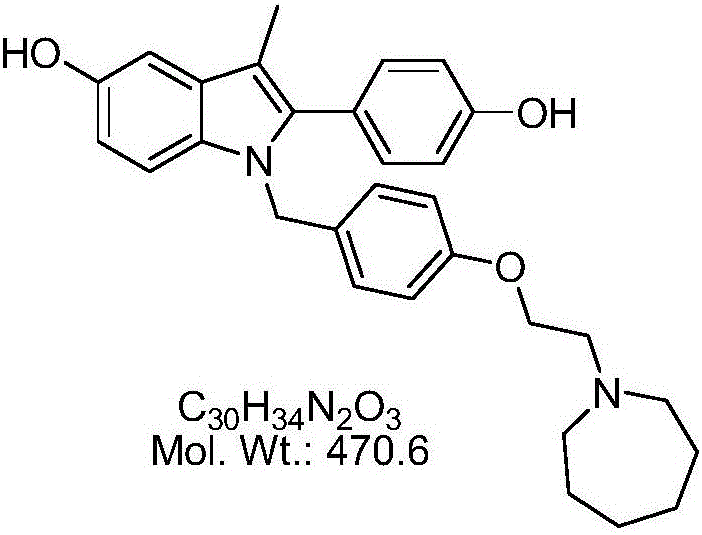
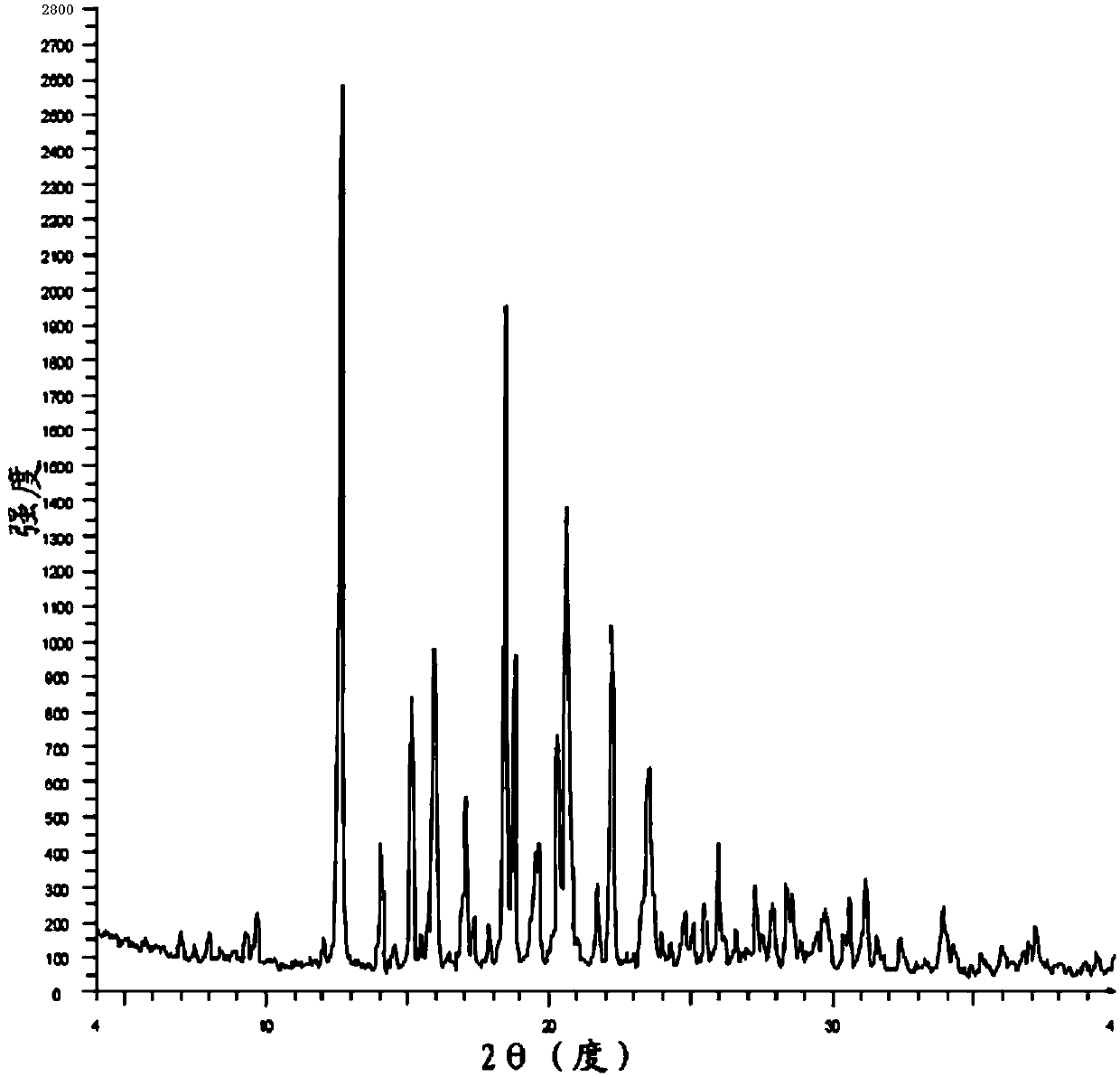
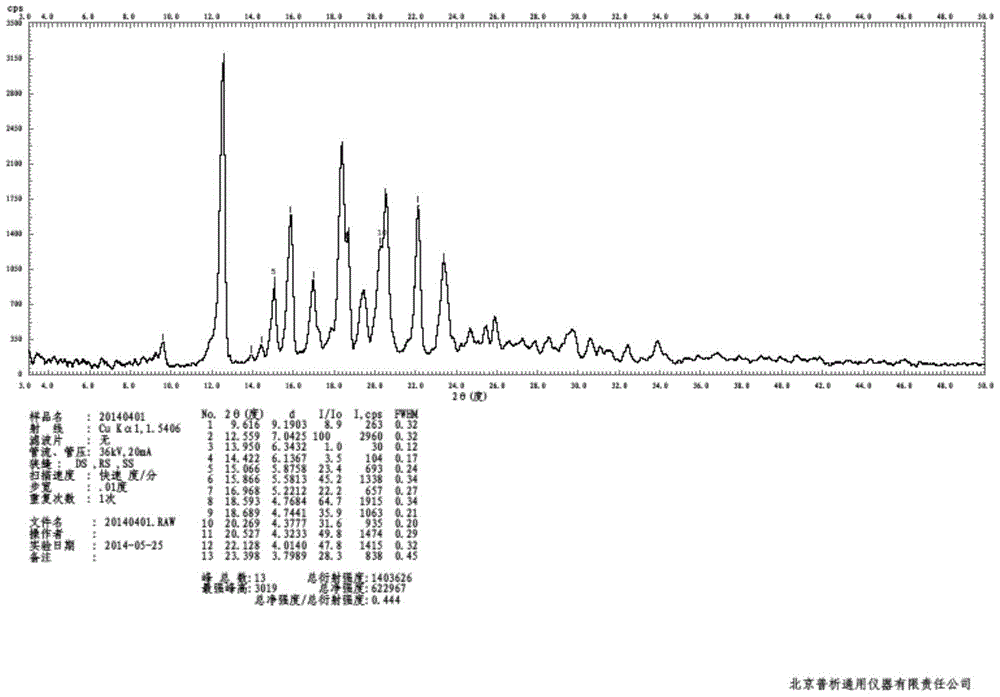
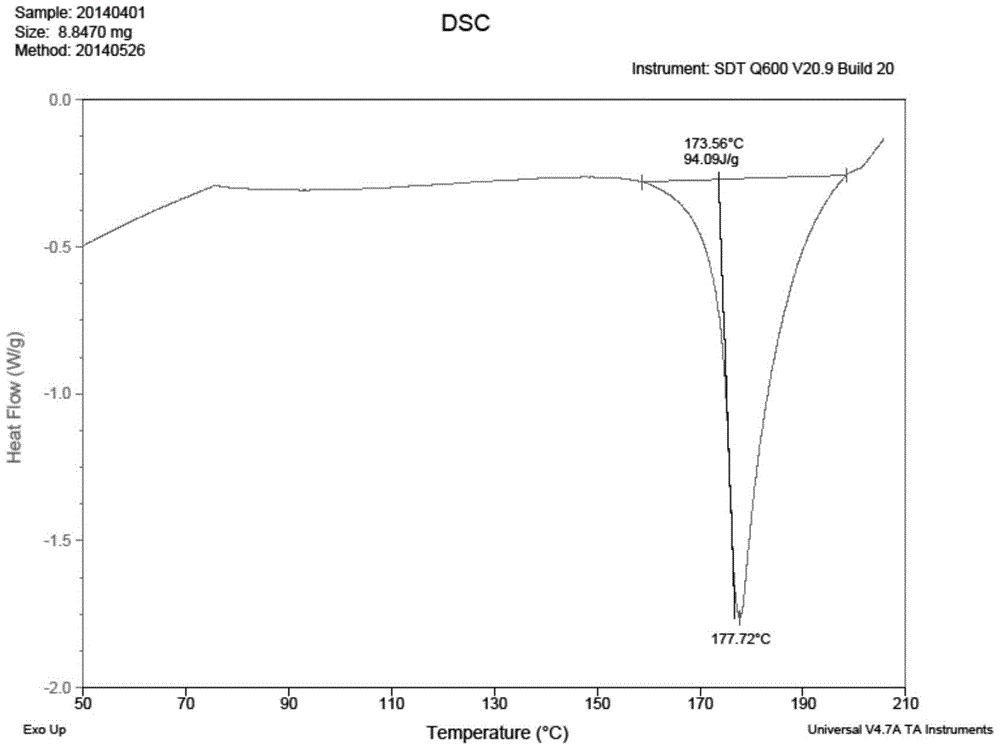
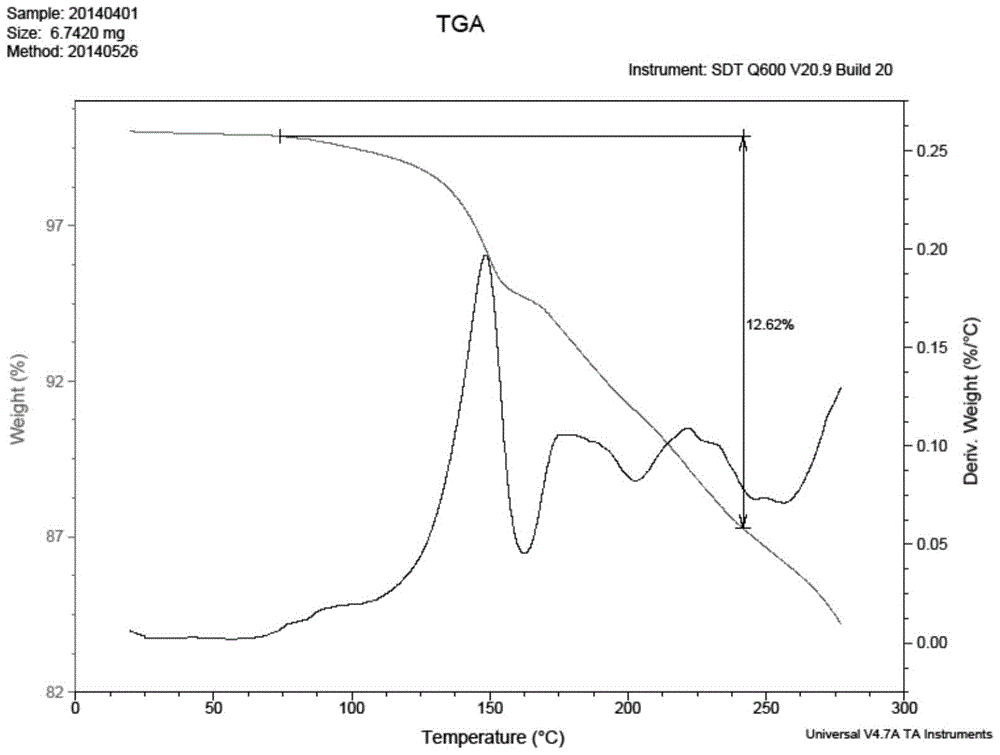
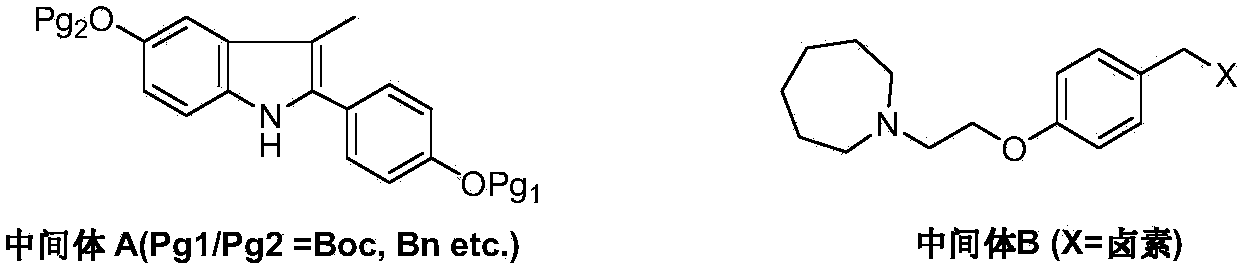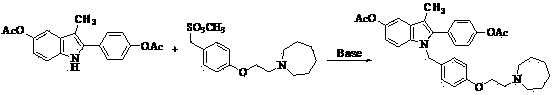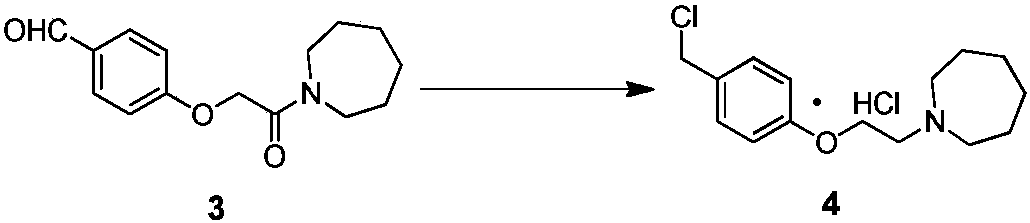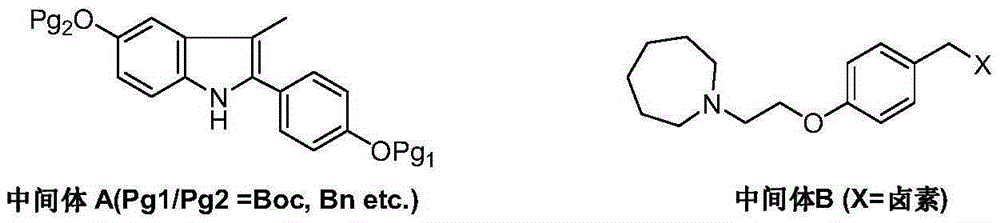Patents
Literature
54 results about "Bazedoxifene acetate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
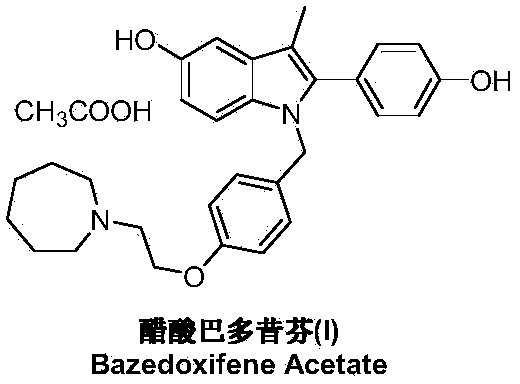
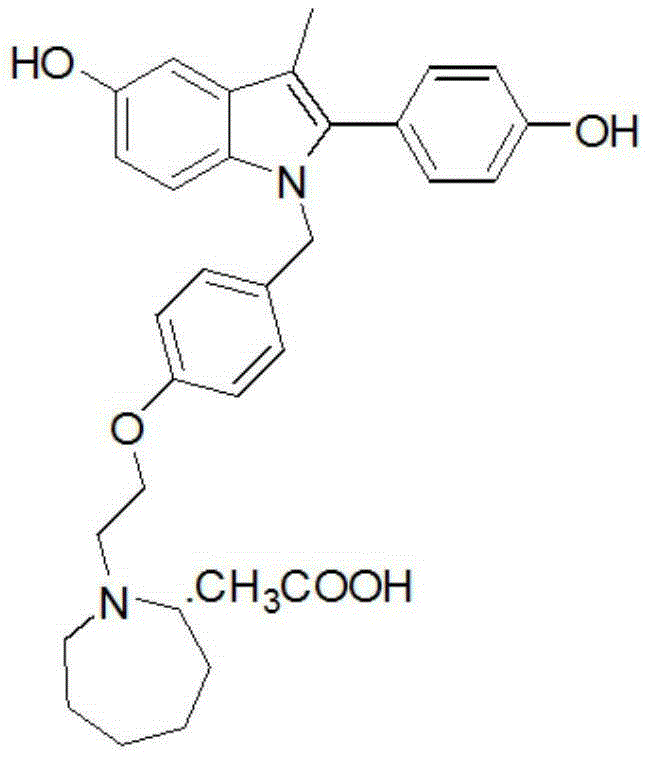
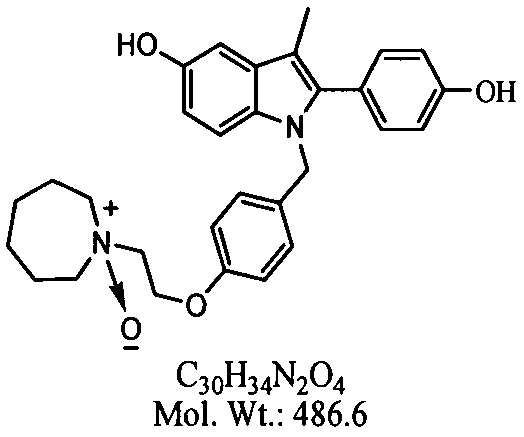
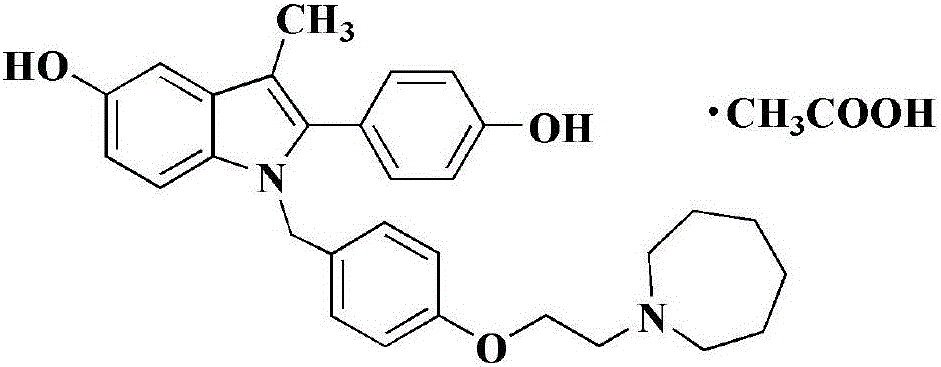
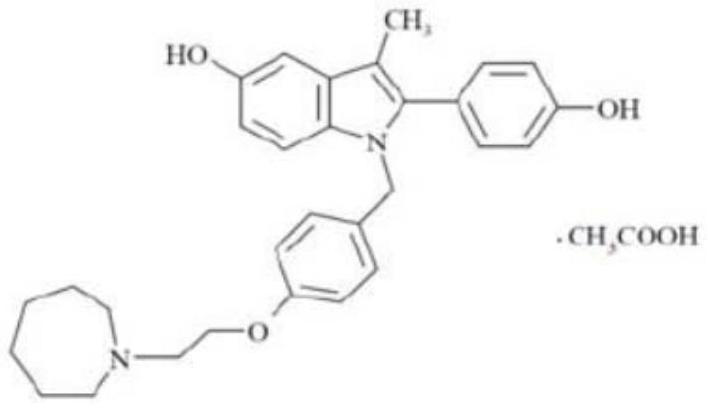
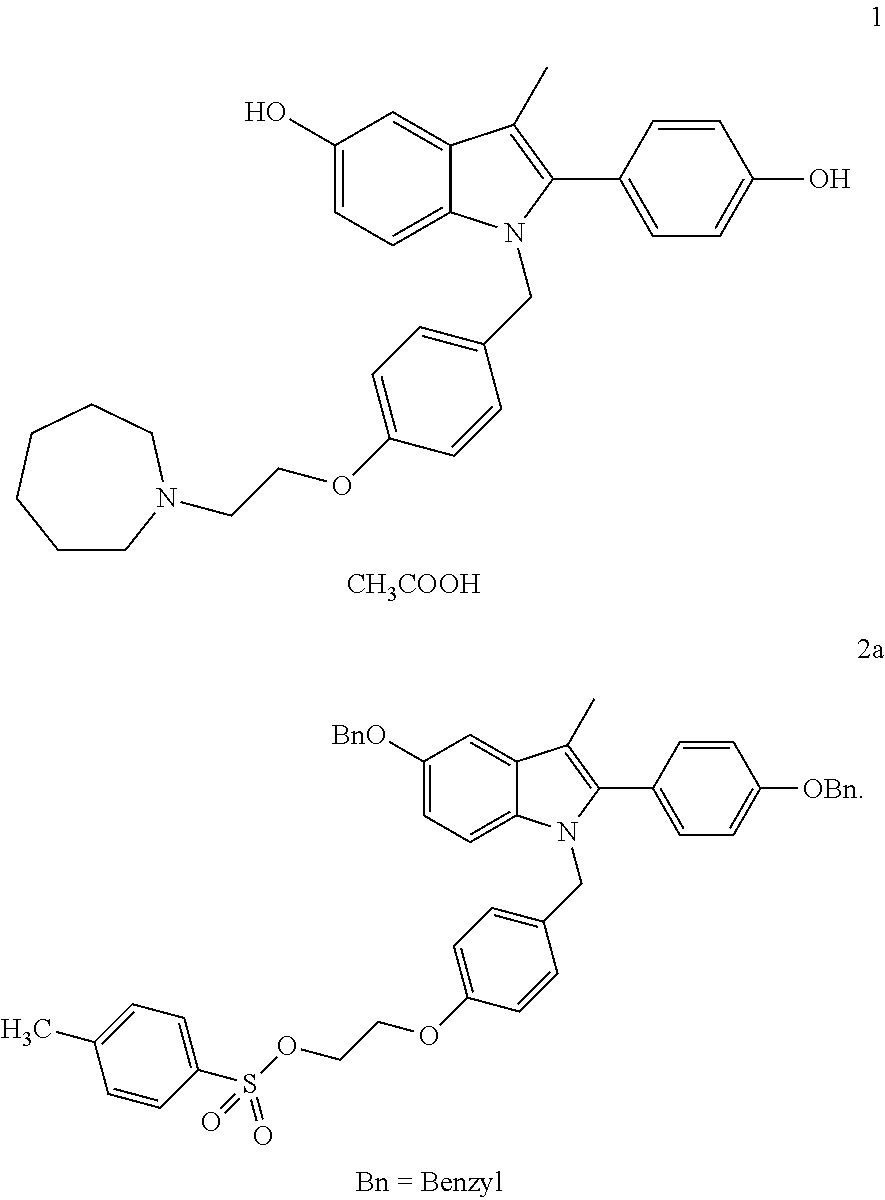
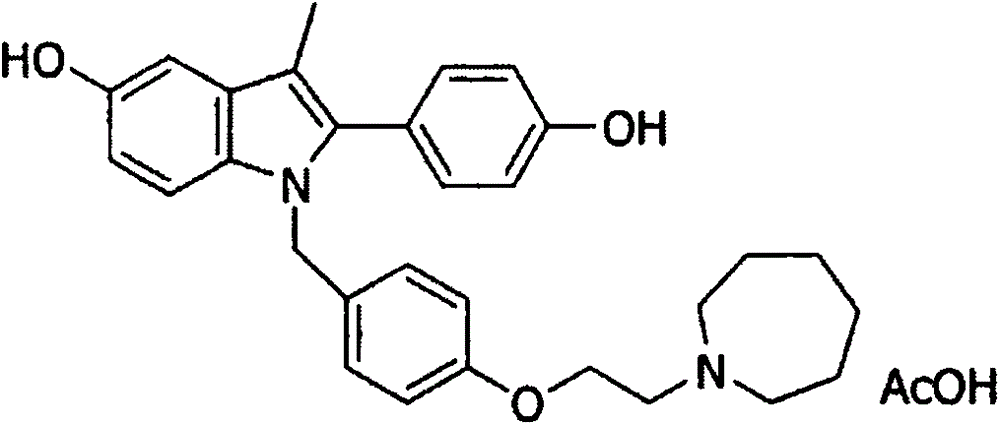
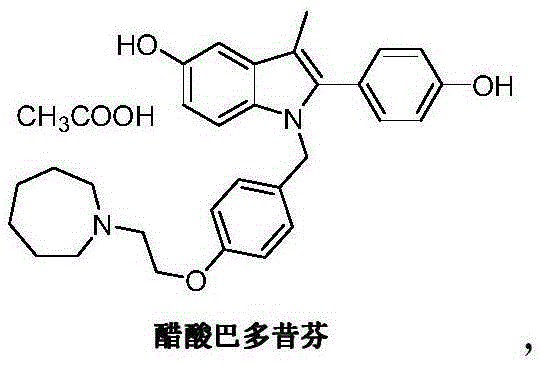
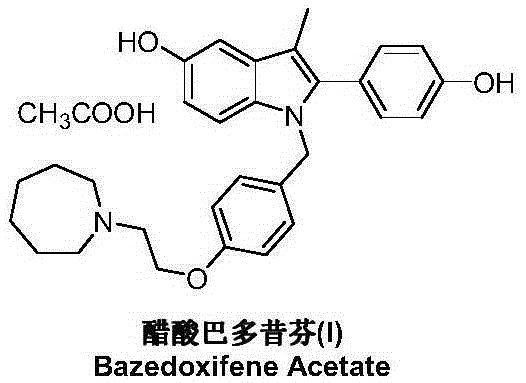
Bazedoxifene, or bazedoxifene acetate, is a medication for bone problems and possibly (pending more study) for cancer. It is a third-generation selective estrogen receptor modulator (SERM).
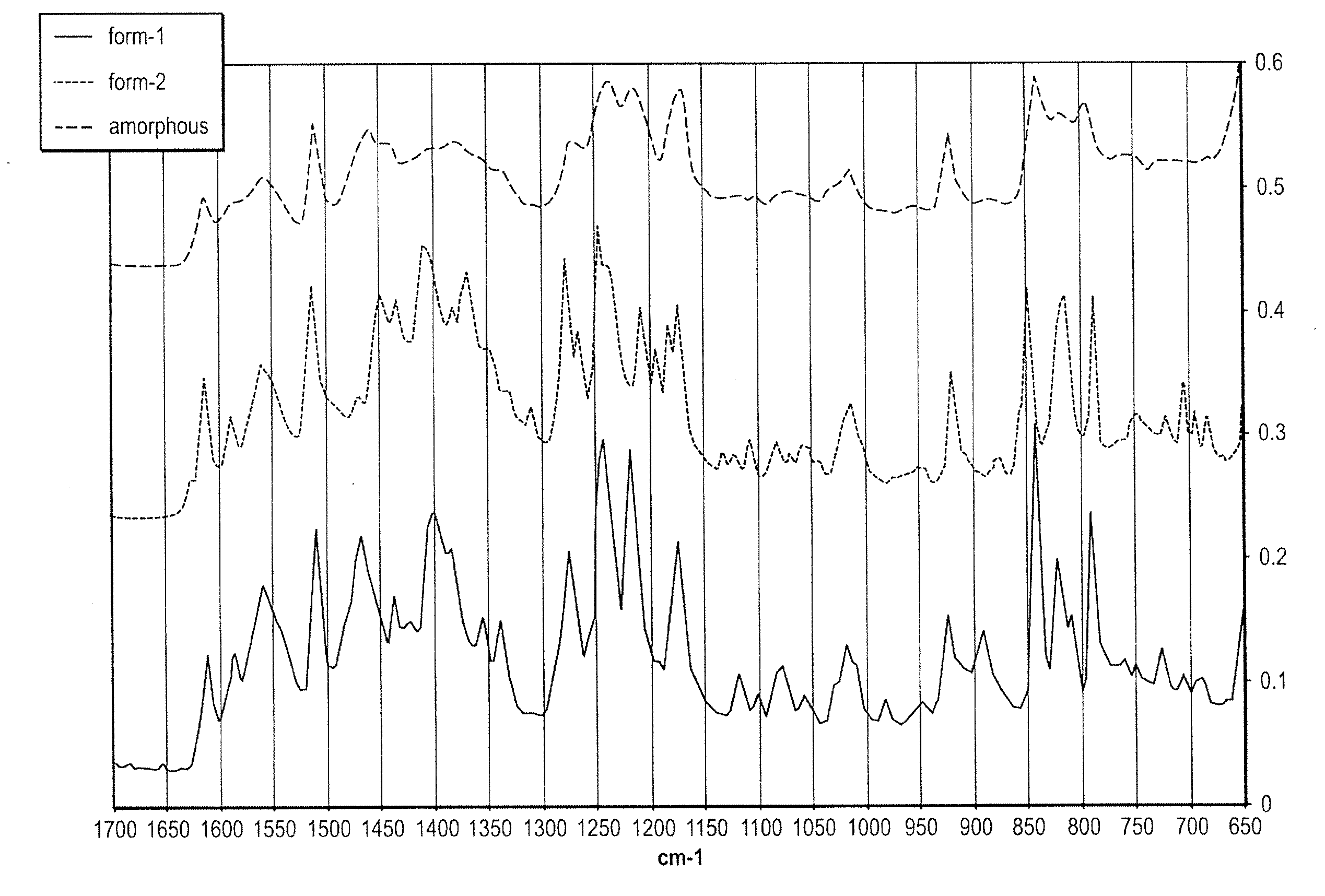
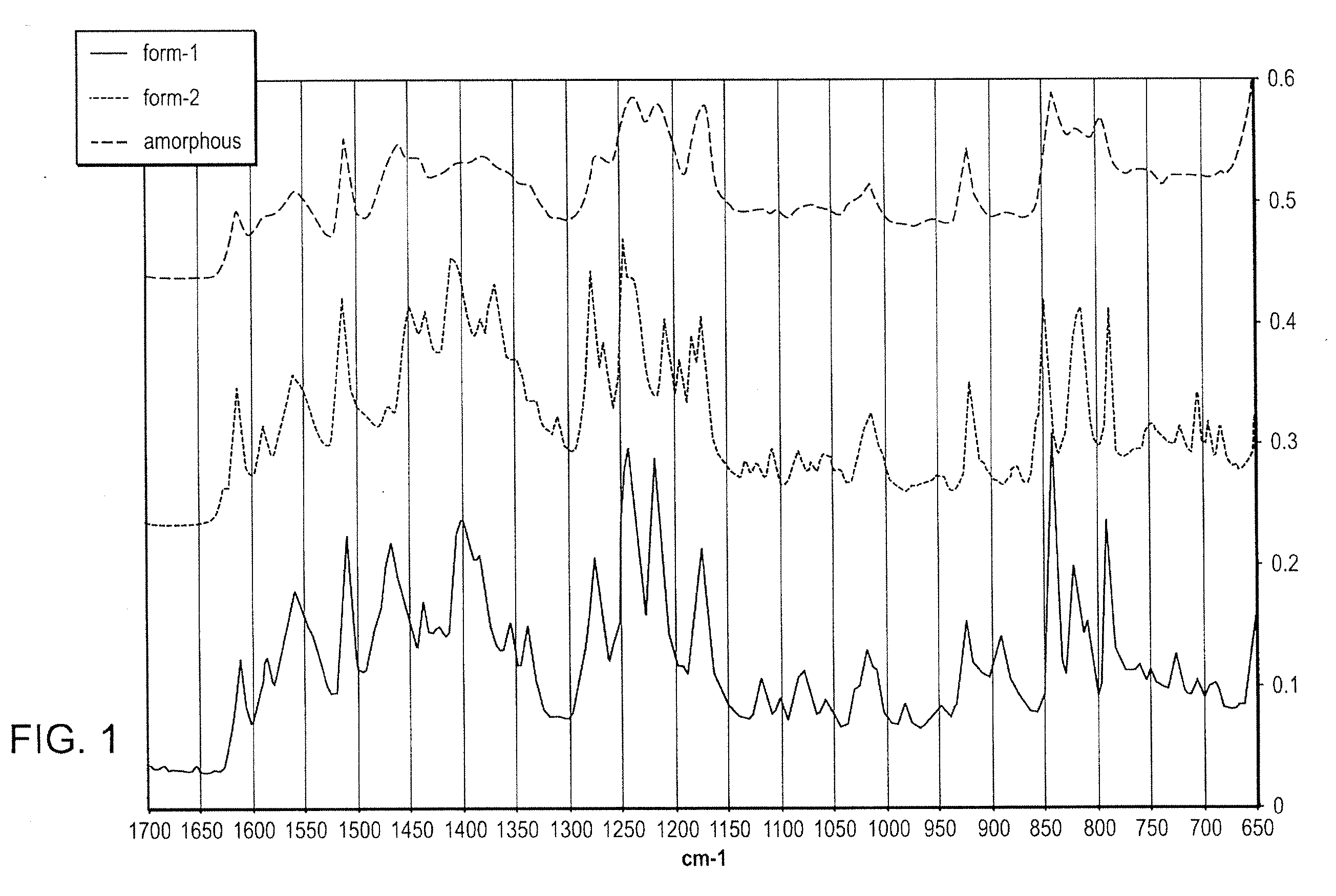
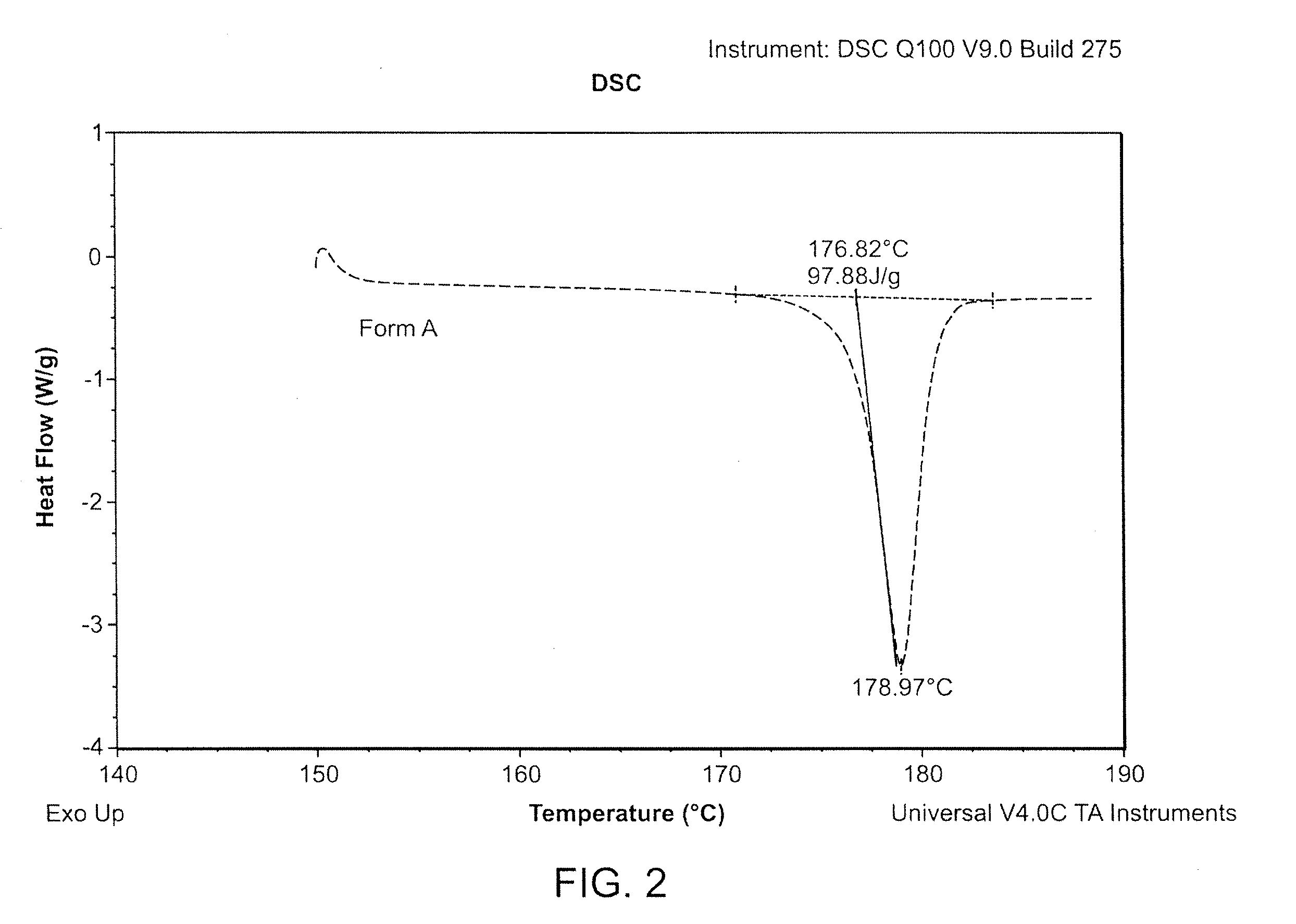
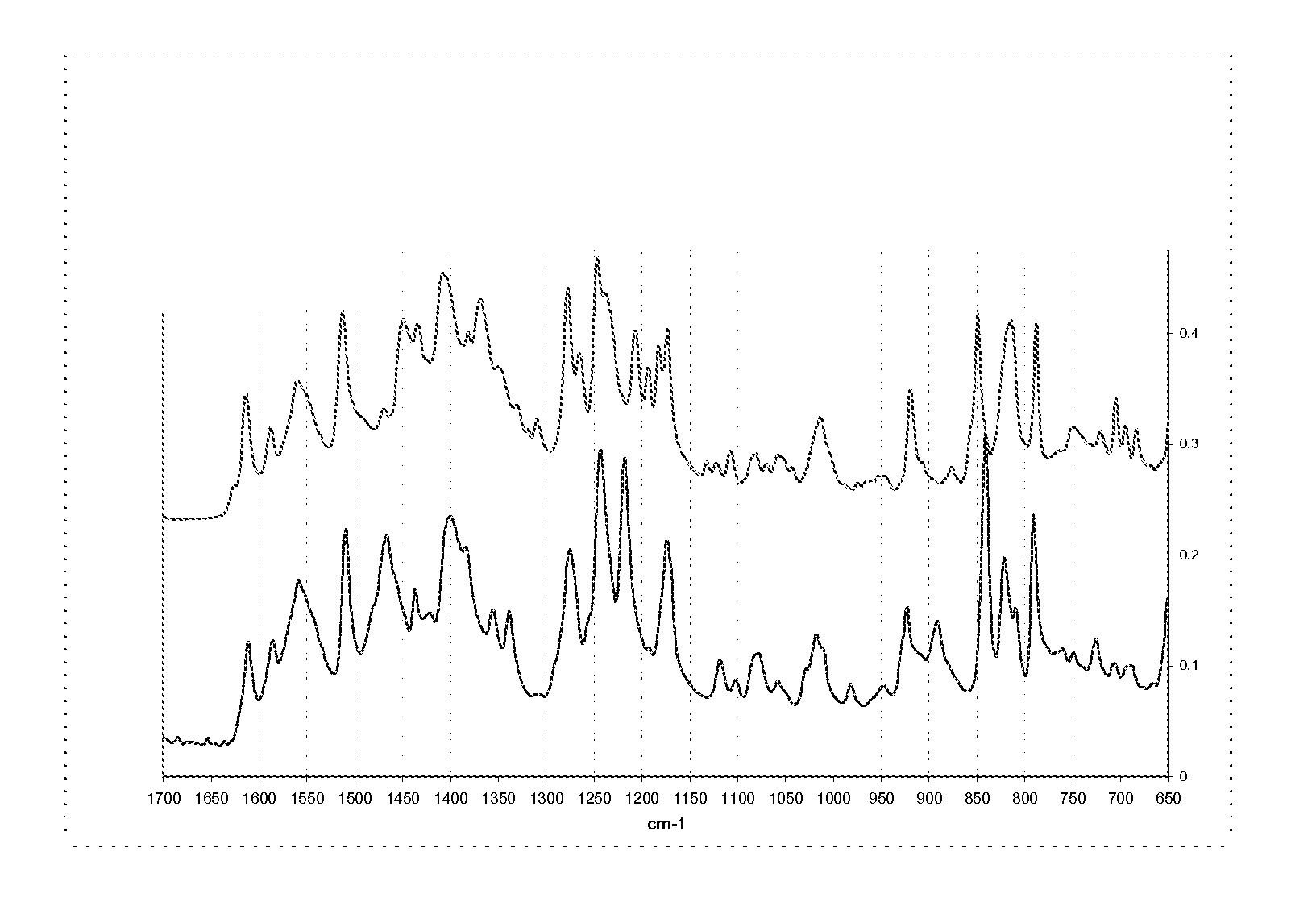
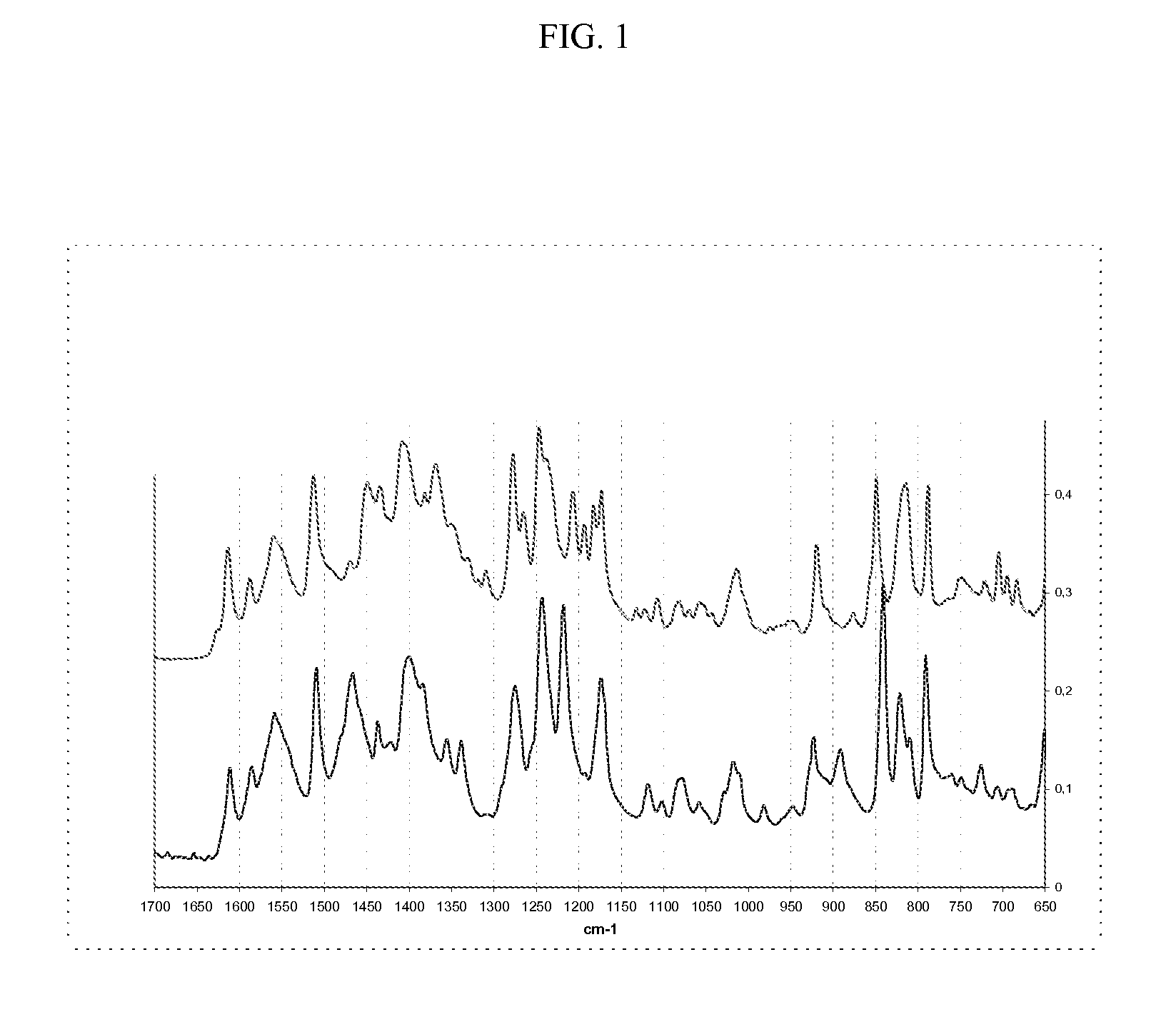
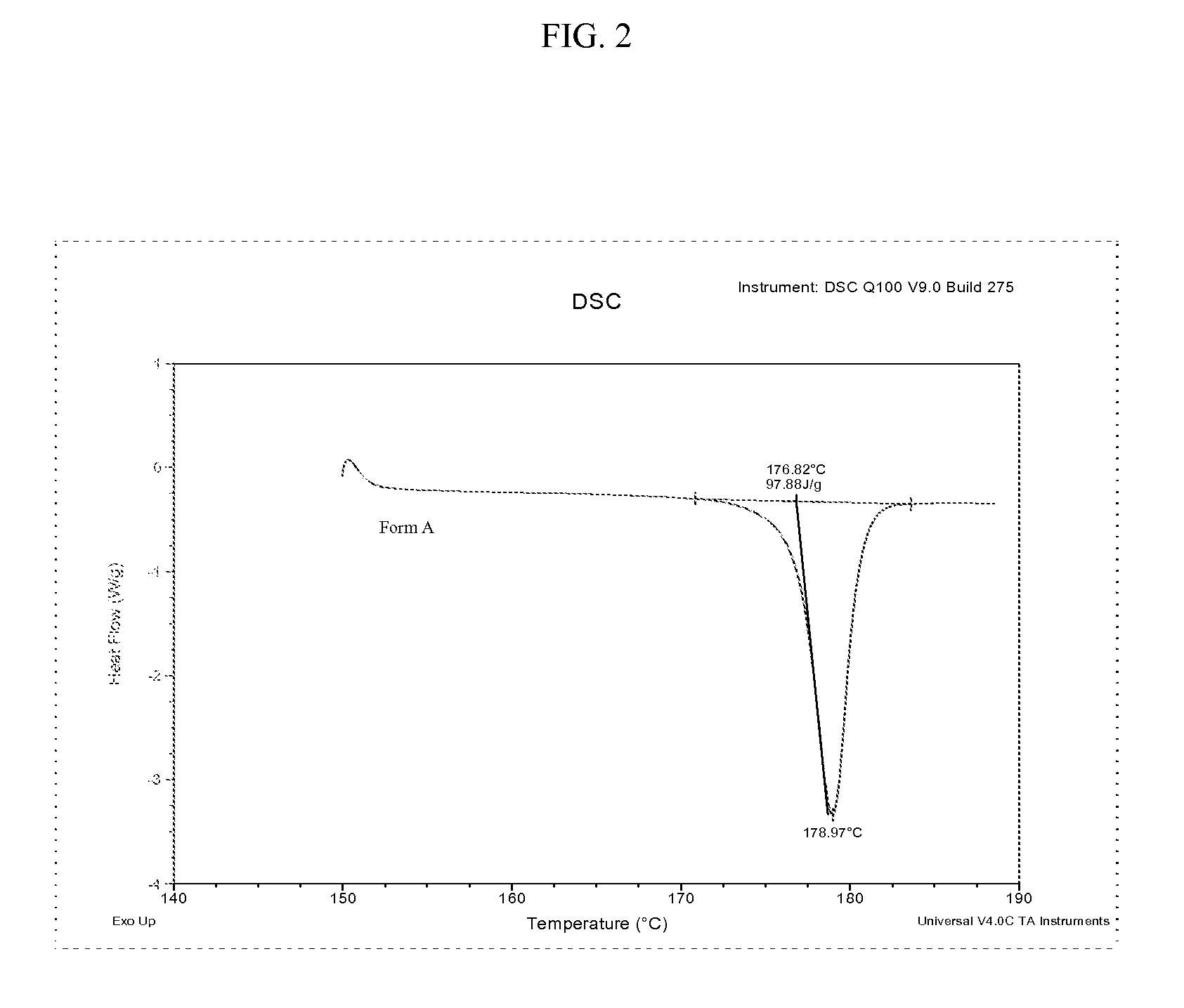
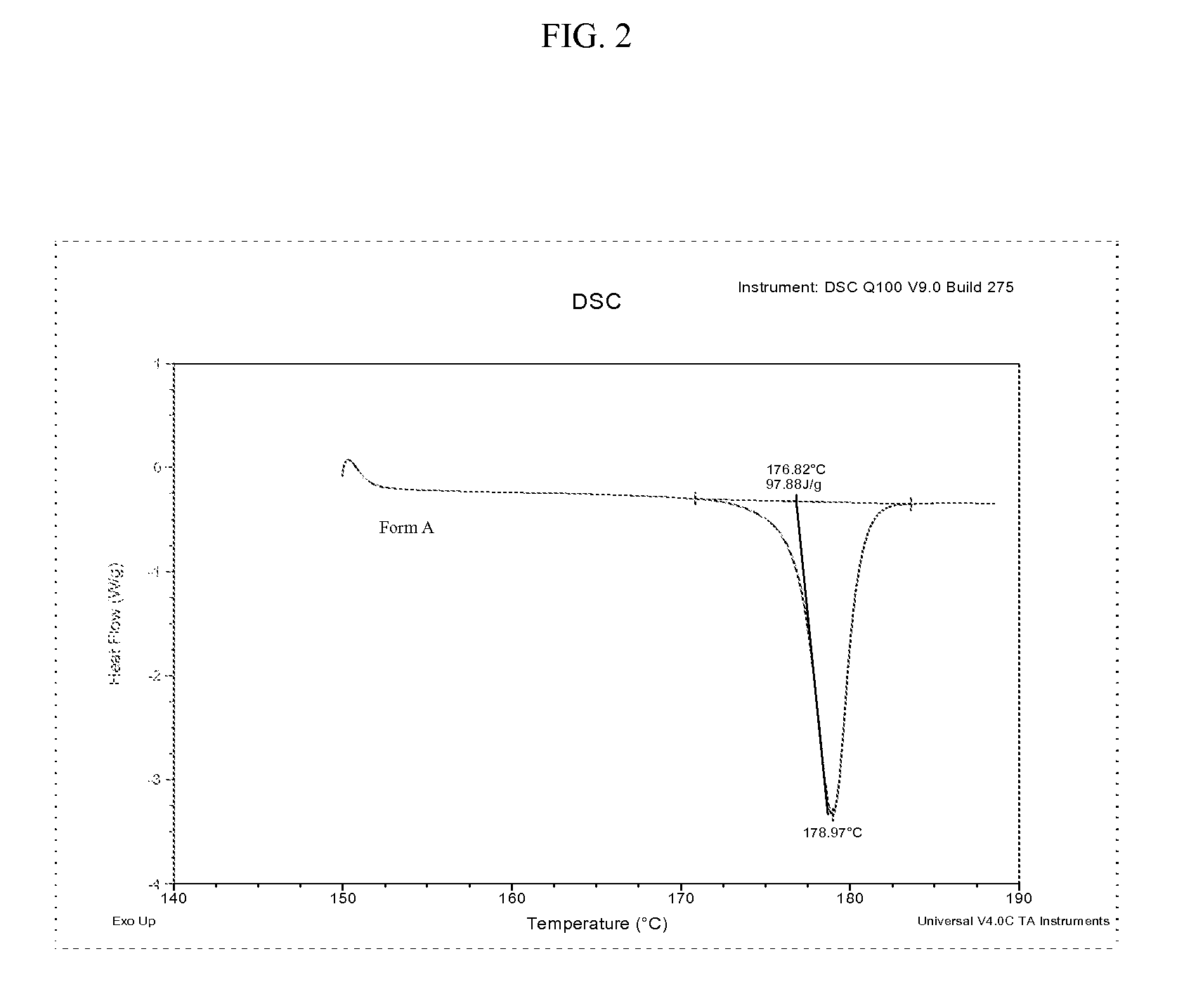
Methods of converting polymorphic form b of bazedoxifene acetate to polymorphic form a of bazedoxifene acetate
The present invention relates to methods of preparing polymorphic Form A of bazedoxifene acetate and polymorphic Form A prepared by such methods.
Owner:WYETH LLC
Methods of preparing polymorphic form a of bazedoxifene acetate
ActiveUS20100016581A1Stable formPreventing dry formOrganic chemistrySkeletal disorderBazedoxifene acetateUrology
The present invention relates to methods of preparing polymorphic Form A of bazedoxifene acetate and polymorphic Form A prepared by such methods.
Owner:WYETH LLC
Preparation method of bazedoxifene acetate
ActiveCN103864665AEase of industrial productionPromote the development of economy and technologyOrganic chemistryAcetic acidAlcohol
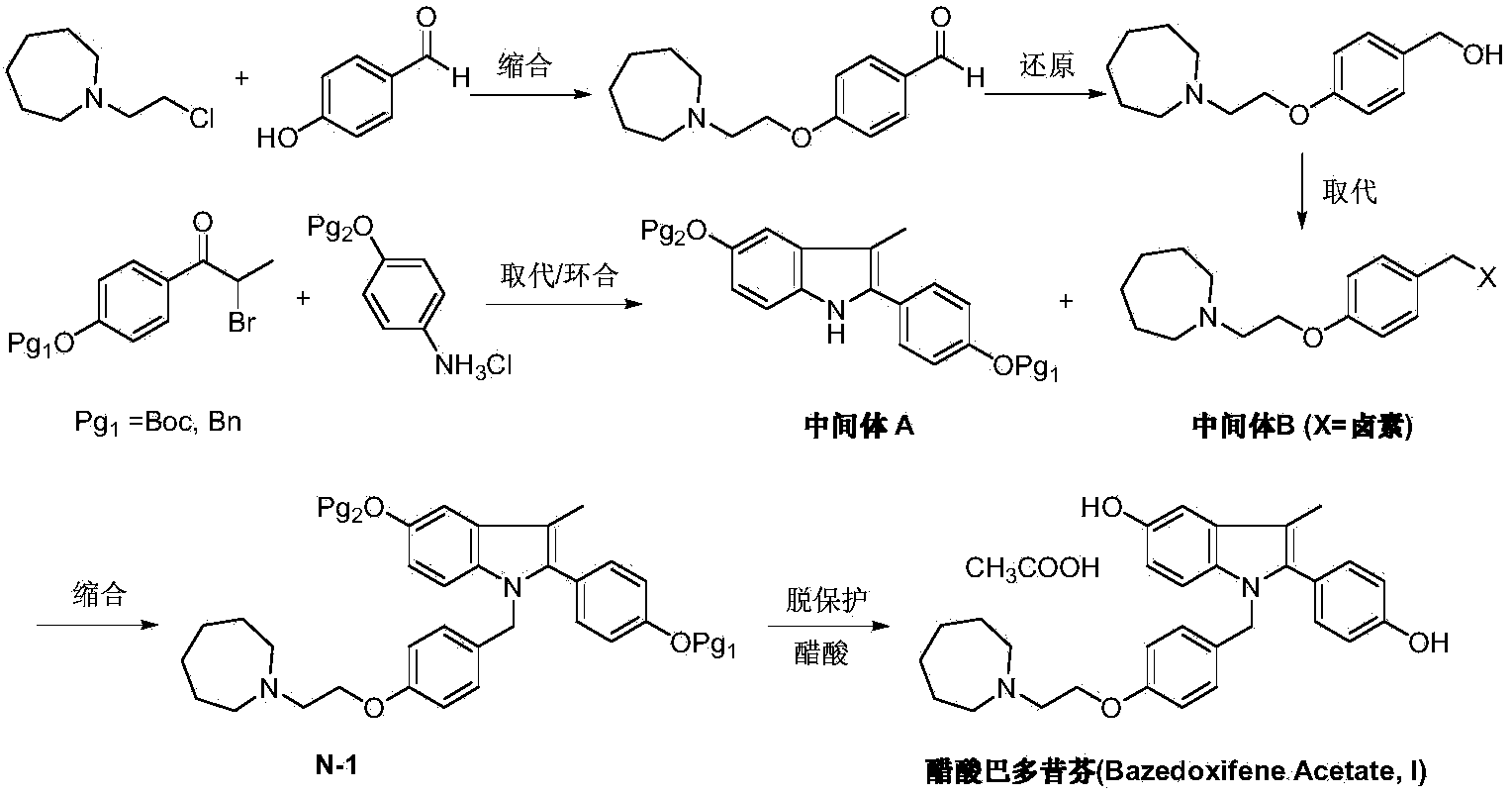
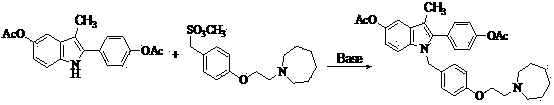
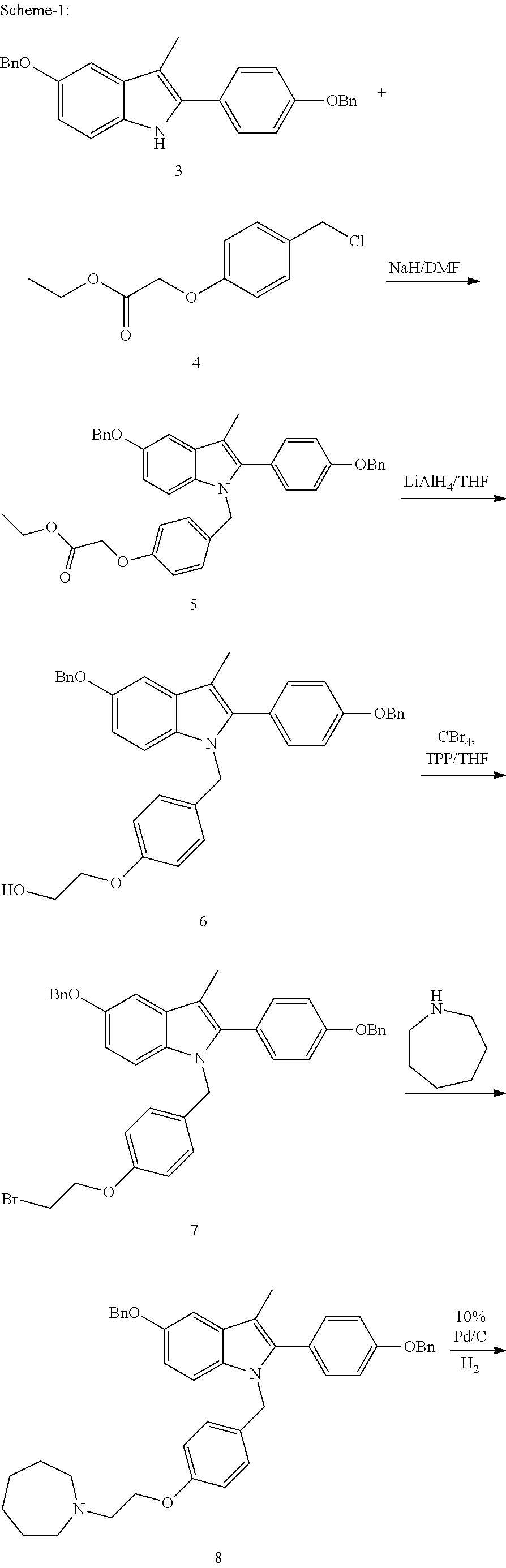
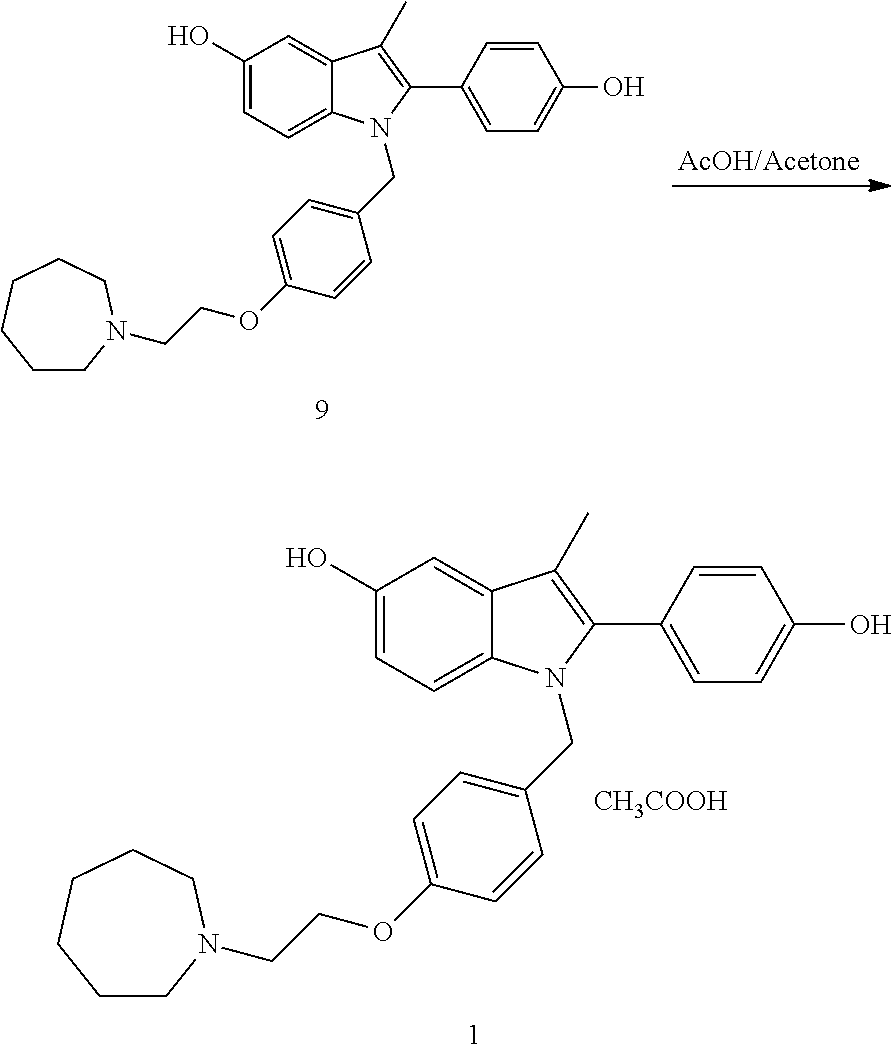
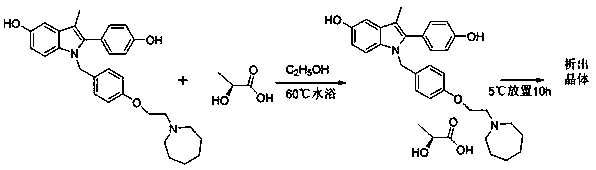
The invention discloses a preparation method of bazedoxifene acetate. The method comprises the following steps: carrying out a condensation cyclization reaction on 1-(4-Pg1 oxygroup phenyl) propyl alcohol (II) and N-{4-(2-azacycloheptane-1-yl-ethyoxyl-benzyl)}N-{4-(Pg2 oxygroup phenyl)} hydrazine (III) so as to obtain 1-{4-(2-azacycloheptane-1-yl-ethyoxyl-benzyl)}-2-(4-Pg1 oxygroup-phenyl)-3-methyl-5-(Pg2 oxygroup)-1H-benzpyrole (IV); and carrying out deprotection on the intermediate (IV) and salifying the intermediate (IV) with acetic acid so as to obtain the bazedoxifene acetate (I). The preparation method is concise in process, high in yield, economical and environment-friendly, thereby providing a novel preparation way for the industrial production of the bazedoxifene acetate.
Owner:苏州特瑞药业股份有限公司
Novel process for the preparation of bazedoxifene acetate and intermediates thereof
ActiveUS20120330008A1Conveniently preparedHigh purityOrganic compound preparationSulfonic acid esters preparationSulfonateBenzene
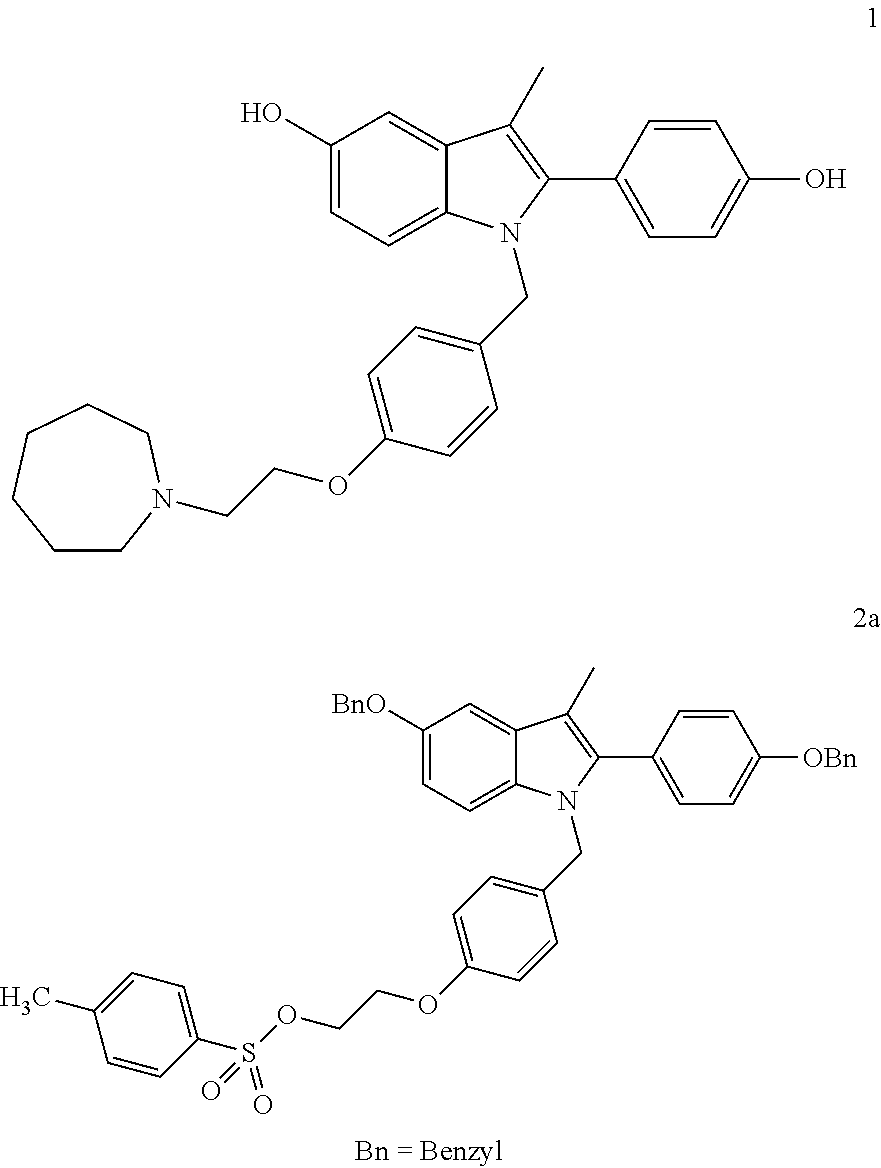
A novel process is described for the preparation of pharmaceutically useful compounds such as 1-{4-[2-(azepan-1-yl)ethoxy]benzyl}-2-(4-hydroxyphenyl)-3-methyl-1H-indol-5-ol acetic acid commonly known as bazedoxifene acetate of the formula-1 using 2-(4-{[5-(benzyloxy)-2-[4-(benzyloxy)phenyl]-3-methyl-1H-indol-1-yl]methyl}phenoxy)ethyl-4-methylbenzenzene-1-sulfonate (formula 2a)
Owner:DIVI S LAB LTD
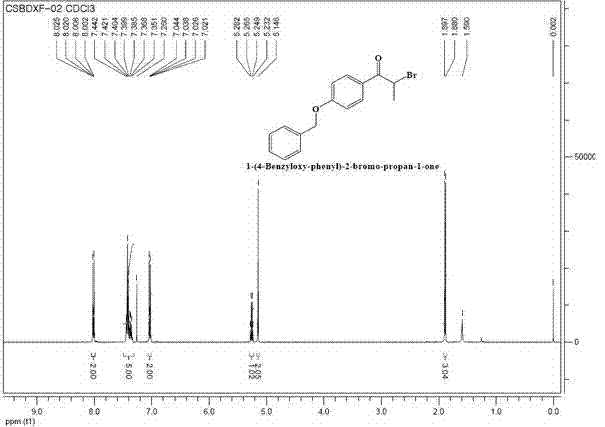
Preparation method for intermediate (namely 1-(4-benzyloxy-phenyl)-2-bromo-propan-1-one) for bazedoxifene acetate
InactiveCN103483173AHigh selectivityImprove efficiencyOrganic compound preparationCarbonyl compound preparationOrganosolvEthylic acid
The invention discloses a preparation method for an intermediate (namely 1-(4-benzyloxy-phenyl)-2-bromo-propan-1-one) for bazedoxifene acetate. The preparation method comprises the following steps: adding 1-(4-benzyloxy-phenyl)-2-propan-1-one and a metal bromine salt into an organic solvent, heating for reflux, and after completion of reaction, cooling to obtain a reaction solution; treating the reaction solution to obtain a liquid crude product, adding the liquid crude product into an organic solvent, heating for reflux for 0.5-2 hours, and after completion of reflux, treating to obtain a product. According to the preparation method, the easily obtained metal bromine salt substitutes for elementary bromine for bromination reaction, so that the selectivity of reaction is improved; the reaction condition is gentle, the selectivity is high, the intermediate (1-(4-benzyloxy-phenyl)-2-bromo-propan-1-one) for the bazedoxifene acetate is synthesized efficiently, and the yield and purity of the obtained product are high, so that the method is suitable for industrial production.
Owner:河南海汇药物研究有限公司
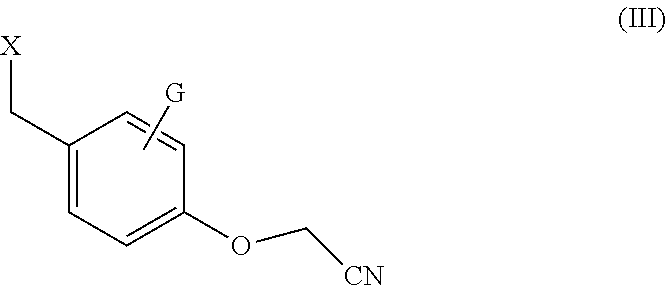
Processes for the synthesis of bazedoxifene acetate and intermediates thereof
Efficient processes for the synthesis of pharmaceutically useful compounds such as (1-[4-(2-azepan-1-yl-ethoxy)-benzyl]-2-(4-hydroxy-phenyl)-3-methyl-1H-indol-5-ol acetic acid commonly known as bazedoxifene acetate (Formula IX) using cyanomethoxybenzyl halides of Formula III, where X=Halogens e.g., Cl, F, Br, I; G=Any electron donating or electron withdrawing substituent.
Owner:SANDOZ AG
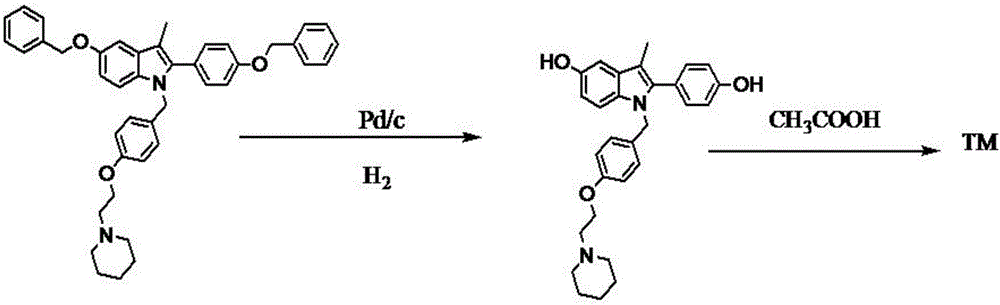
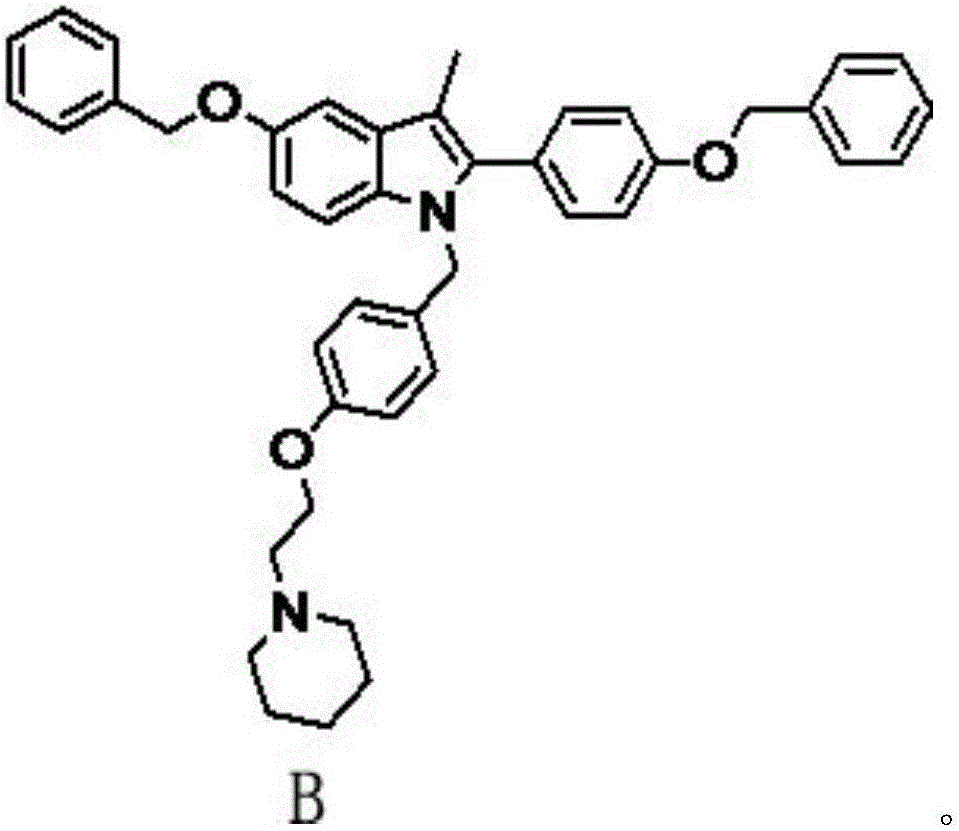
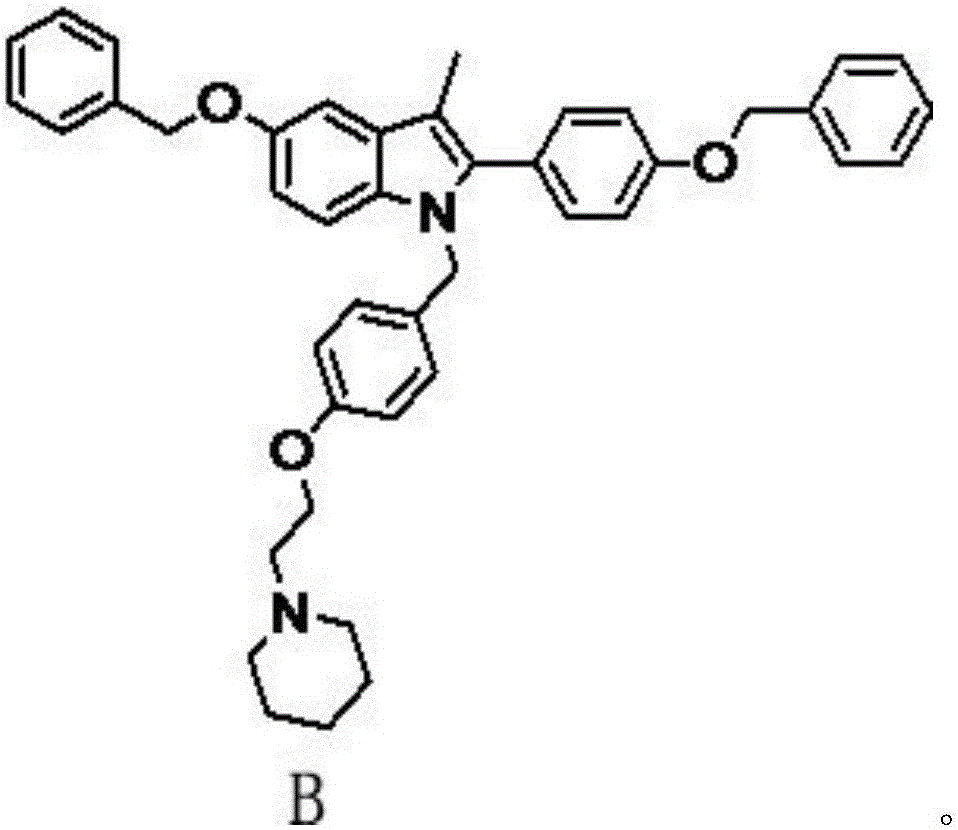
Preparation method of bazedoxifene acetate and key intermediate thereof
The invention belongs to the field of medicinal chemistry, and discloses a preparation method of bazedoxifene acetate. The method comprises the following steps: carrying out condensation reaction on a compound I and a compound II in an organic solvent under an alkaline condition, so as to obtain an intermediate III; hydrolyzing and salifying the intermediate III, so as to obtain the target compound, namely bazedoxifene acetate (TM). By adopting the method, the productivity and the quality of bazedoxifene acetate can be obviously improved.
Owner:万全万特制药江苏有限公司
Preparation method of bazedoxifene acetate crystal form A
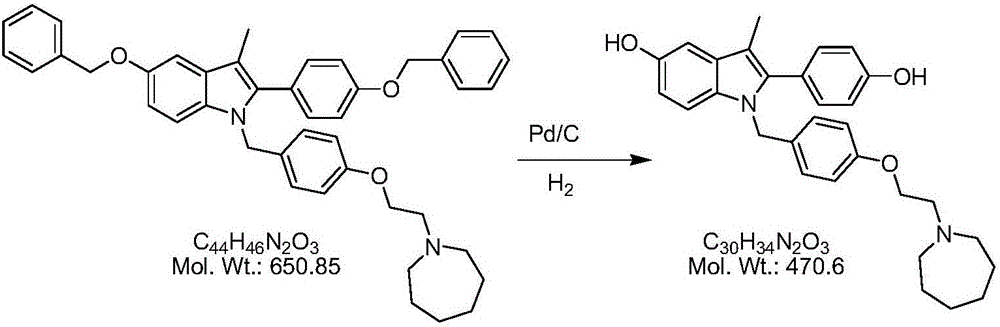
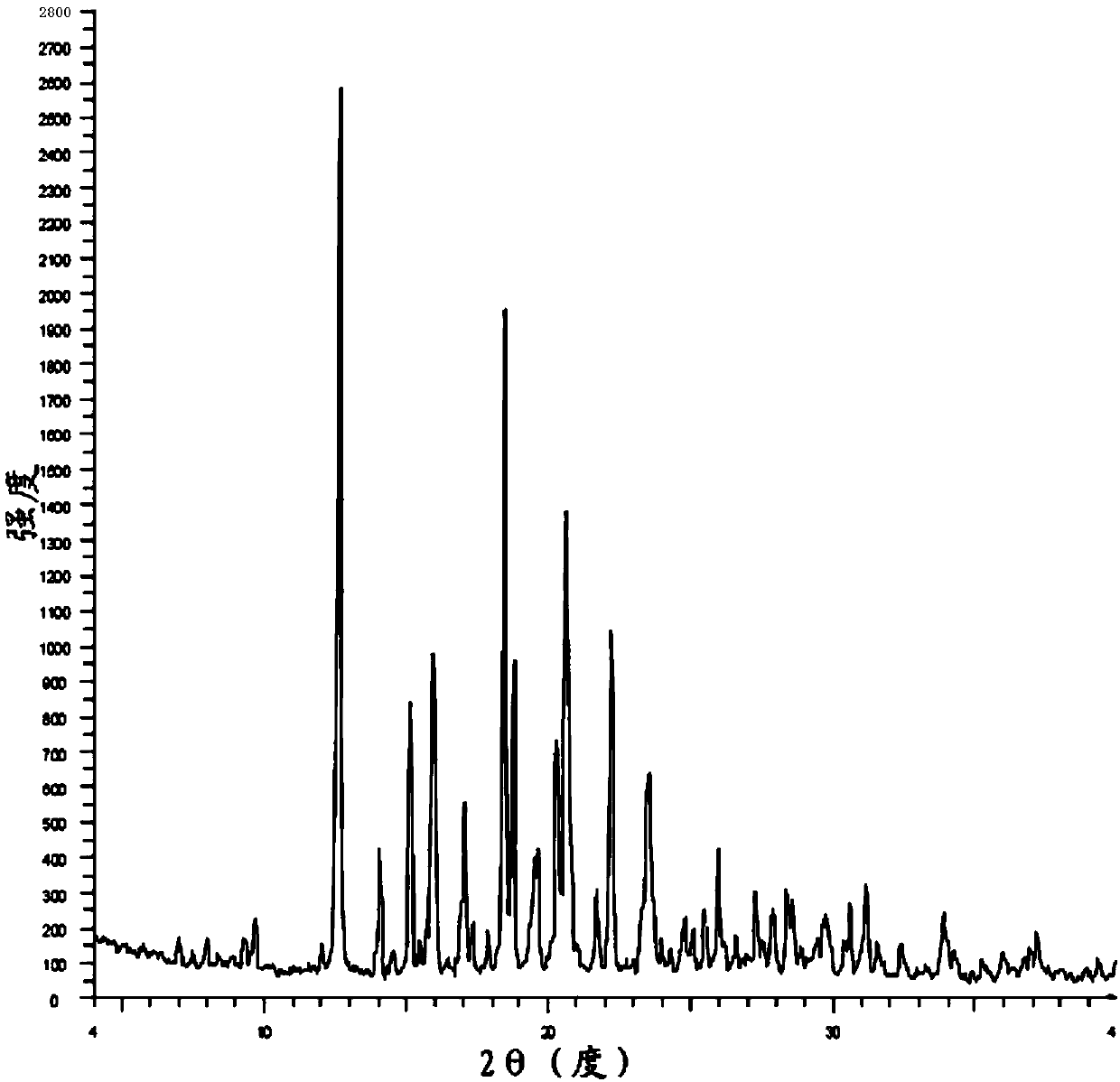
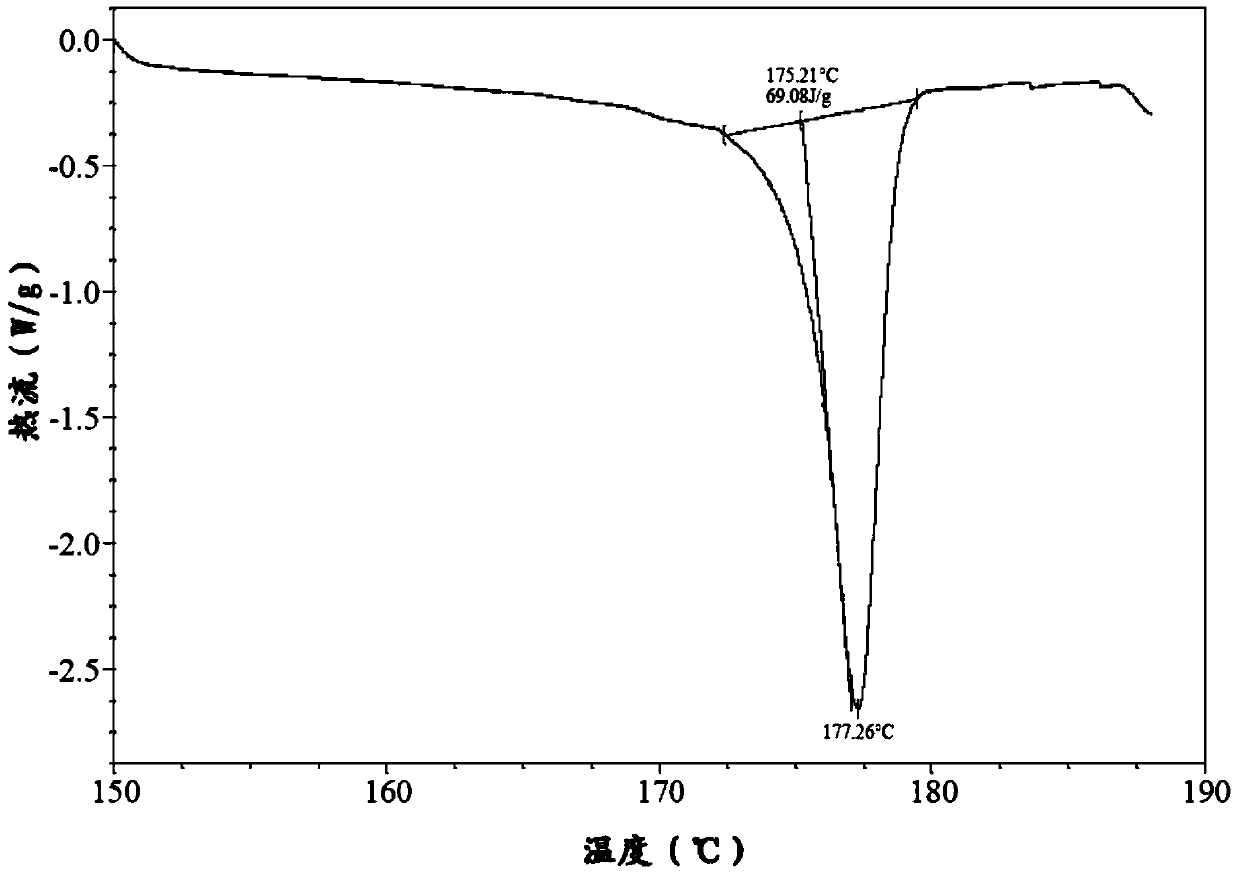
The invention belongs to the technical field of chemical pharmacy and relates to a preparation method of a bazedoxifene acetate crystal form A. The preparation method of the bazedoxifene acetate crystal form A comprises the following steps: step 1, in the presence of palladium / carbon serving as a catalyst, dissolving hexamethyleneimine benzyloxy benzpyrole and ammonium formate in a benign organic solvent to have reaction; step 2, after the reaction is finished completely, filtering the palladium on activated carbon, cooling the filtrate, adding acetic acid and a toxic inorganic solvent, stirring, crystallizing, filtering and drying to obtain a crude crystal form A product; step 3, under the protection and presence of inert gas, dissolving the crude crystal form A product in the benign organic solvent, heating and dissolving; step 4, thermally filtering the solution, cooling the filtrate, dropwise adding the toxic inorganic solvent and crystallizing; step 5, after the crystallization is ended, filtering the solution and drying the solid to obtain the bazedoxifene acetate crystal form A.
Owner:CHINA RESOURCES SAIKE PHARMA
Preparation method of bazedoxifene acetate and crystal form A
ActiveCN105669518AReduce usageReduce construction costsOrganic chemistry methodsPalladium on carbonAcetic acid
A preparation method of bazedoxifene acetate is characterized by including the following steps: (1) performing a reaction by mixing and suspending a compound represented in the formula (A), ammonium formate or cyclohexadiene, and a palladium-carbon catalyst in an organic solvent, reaction terminal being detected with TLC or HPLC; (2), filtering and washing a reaction product, adding acetic acid with stirring, and filtering and drying the mixture to prepare the bazedoxifene acetate. The invention also provides a preparation method of a bazedoxifene acetate crystal form A. The preparation method is free of hydrogen and avoids usage of special devices, such as a hydrogenation kettle, while a safe hydrogen donor is employed, so that the method can be carried out even with a common reaction kettle and a reaction workshop, thereby reducing dangerousness of the synthesis and building and operation cost of a special device workshop. The preparation method of the crystal form A, compared with the prior art, is simple in operation, is free of a crystal seed for inducing crystallization, is high in crystal form purity and is simple in solvent system which can be recycled and reused.
Owner:上海医药集团(本溪)北方药业有限公司
Bazedoxifene acetate dispersing tablet and preparation method thereof
The invention discloses a bazedoxifene acetate dispersing tablet, and a preparation method and application thereof. The bazedoxifene acetate dispersing tablet comprises the following components in percentage by weight: 5-40 percent of bazedoxifene acetate, 10-50 percent of filler, 10-50 percent of disintegrating agent, 10-50 percent of acidifying agent, 0.1-15 percent of adhesive and 0.1-20 percent of lubricant and glidant. Compared with a common tablet, the bazedoxifene acetate dispersing tablet does not contain surfactant, has excellent solubility, dispersibility and disintegration, and can be completely disintegrated in 1 minute. The bazedoxifene acetate dispersing tablet prepared by adopting the method has the advantages of high solubility, good bioavailability, quick in-vivo distribution, stable quality and good taste; the preparation method is simple and practical, and is suitable for industrial production.
Owner:王志刚
Separation preparation method of bazedoxifene acetate impurity A
InactiveCN104774170AEfficient separationOptimizing Chromatographic ConditionsOrganic chemistryMedicinal chemistryBazedoxifene
The invention relates to a method for preparing an impurity A having the purity of more than 97% from a bazedoxifene raw material, can be applied in research of reference substances and belongs to the field of medicine.
Owner:JIANGSU CAREFREE PHARM CO LTD
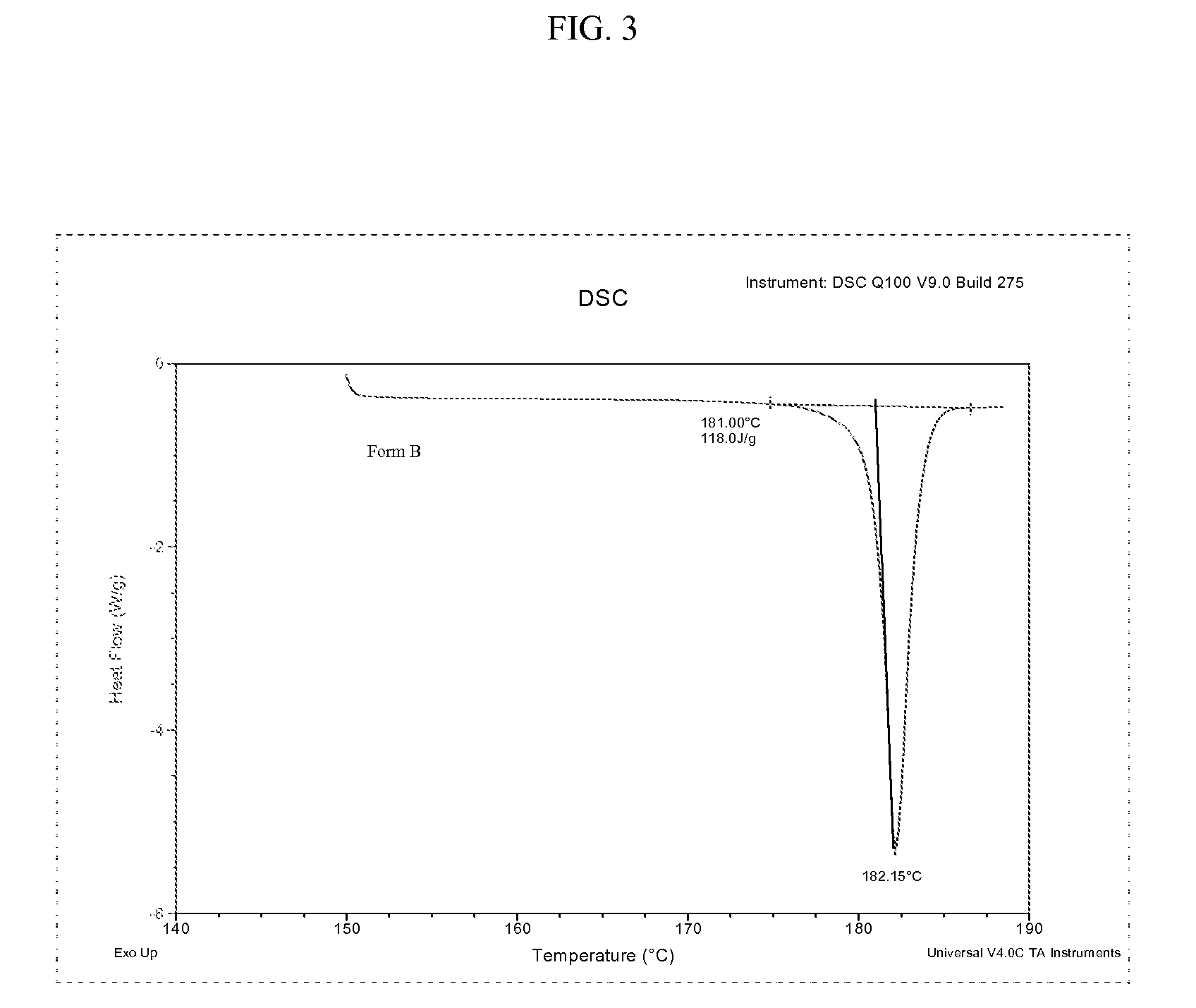
Preparation method of bazedoxifene acetate polycrystalline type B
ActiveCN104370796AEasy to prepareSuitable for industrial productionOrganic chemistry methodsAcetic acidAntioxidant
The invention discloses a preparation method of bazedoxifene acetate polycrystalline type B. Bazedoxifene free alkali and glacial acetic acid are taken as raw materials and salified to obtain the bazedoxifene acetate polycrystalline type B. The operation conditions of the preparation process are simple and do not need to be carried out under the protection of inert gas; no antioxidant is used in the preparation process; the yield and purity of the prepared product are high, and therefore the product is suitable for industrial production.
Owner:YANGTZE RIVER PHARM GRP CO LTD
Methods of preparing polymorphic form A of bazedoxifene acetate
The present invention relates to methods of preparing polymorphic Form A of bazedoxifene acetate and polymorphic Form A prepared by such methods.
Owner:WYETH LLC
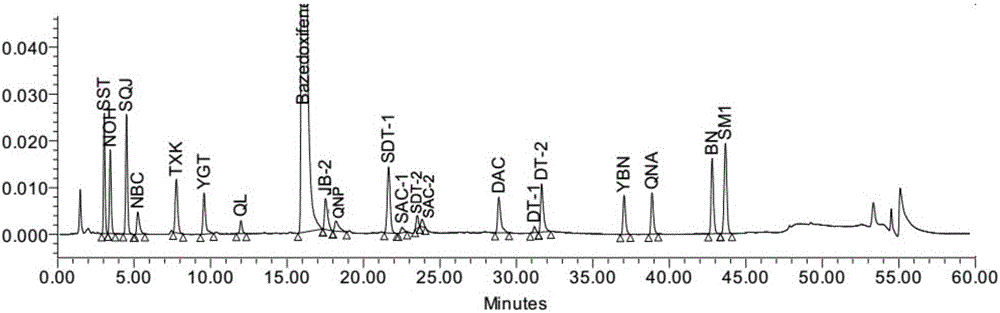
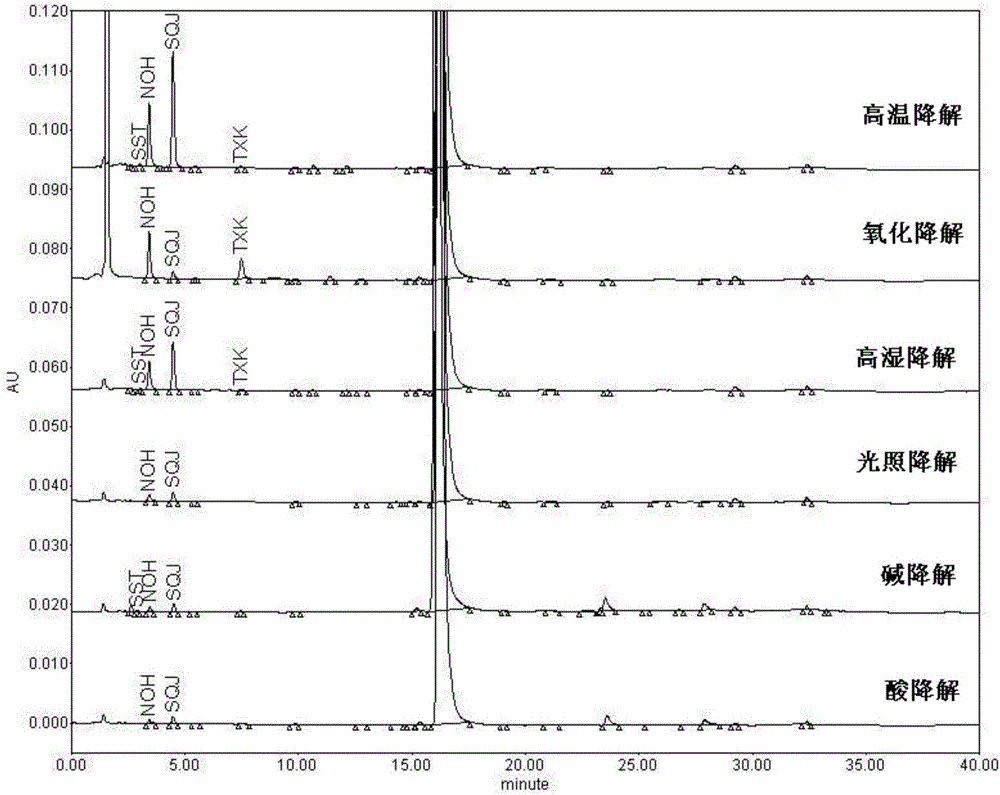
Pharmaceutical analysis method for efficiently measuring bazedoxifene acetate and impurities of bazedoxifene acetate
ActiveCN106198827AEfficient separationStrong specificityComponent separationFiller ExcipientPhosphoric acid
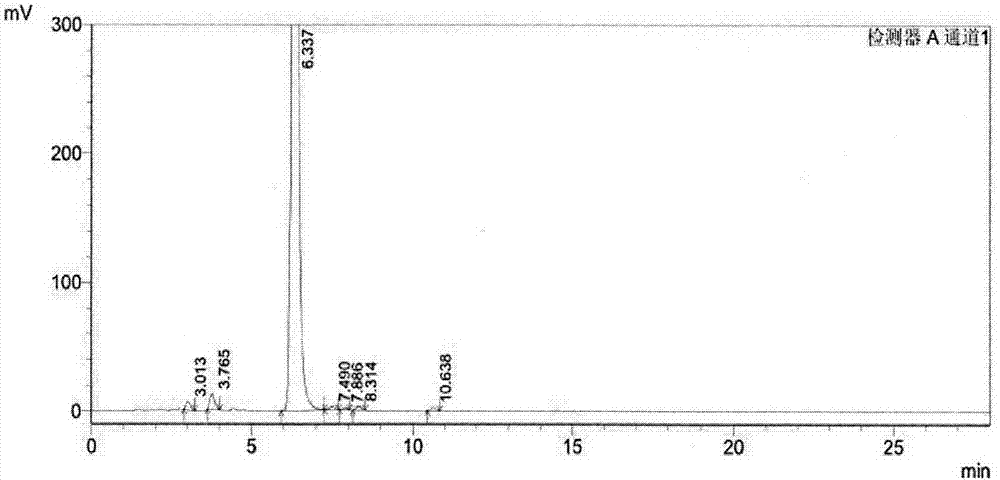
The invention provides a pharmaceutical analysis method for efficiently measuring bazedoxifene acetate and impurities of the bazedoxifene acetate and belongs to the technical field of pharmaceutical analysis. A chromatographic column with octadecylsilane chemically bonded silica as filling agent serves as a solid phase, a mixed solution of phosphate buffer solution and acetonitrile serves as a mobile phase A, a mixed solution of phosphoric acid and acetonitrile serves as a mobile phase B, effective separation can be achieved in a gradient elution mode, and the content of bazedoxifene acetate and the impurities of the bazedoxifene acetate can be effectively measured.
Owner:QILU PHARMA CO LTD
Industrial production method for bazedoxifene acetate
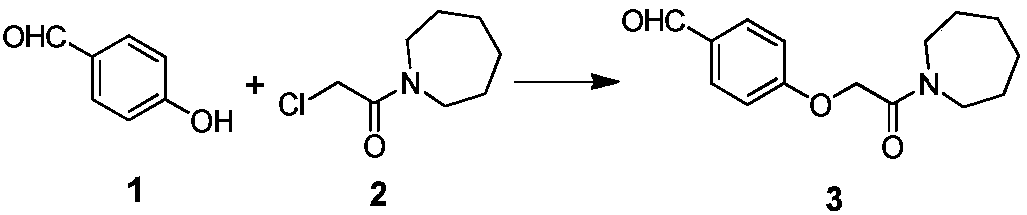
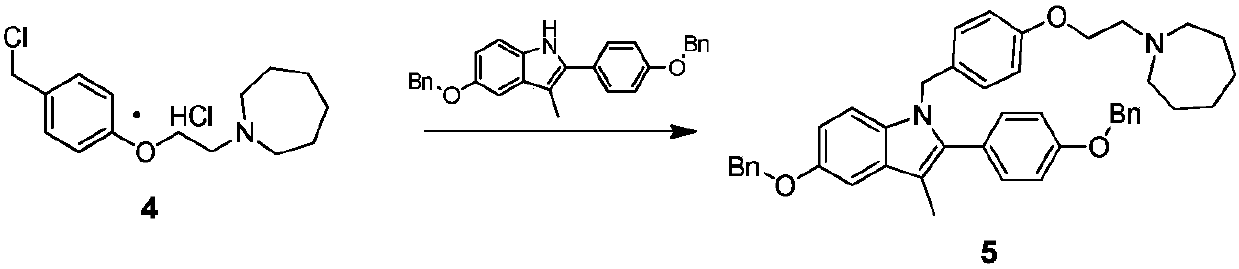
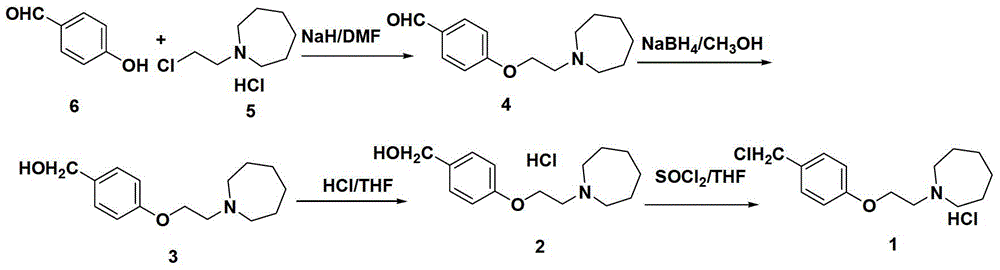
ActiveCN107793344AQuality improvementHigh yieldCarboxylic acid salt preparationAcetic acidBenzaldehyde
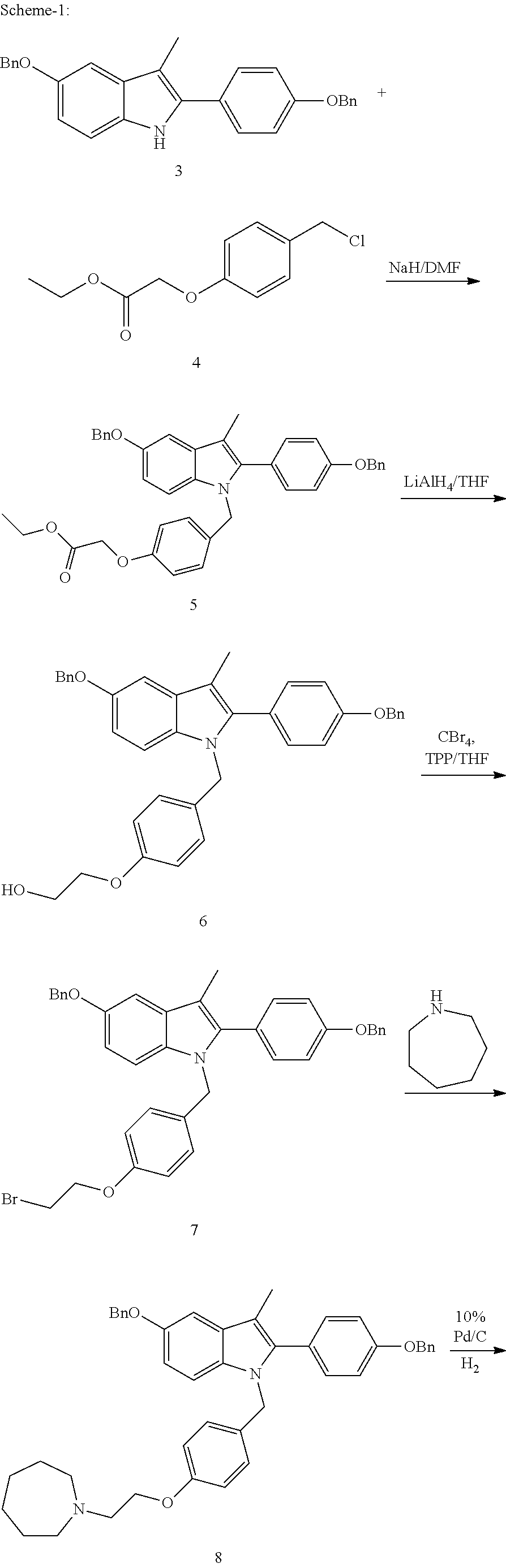
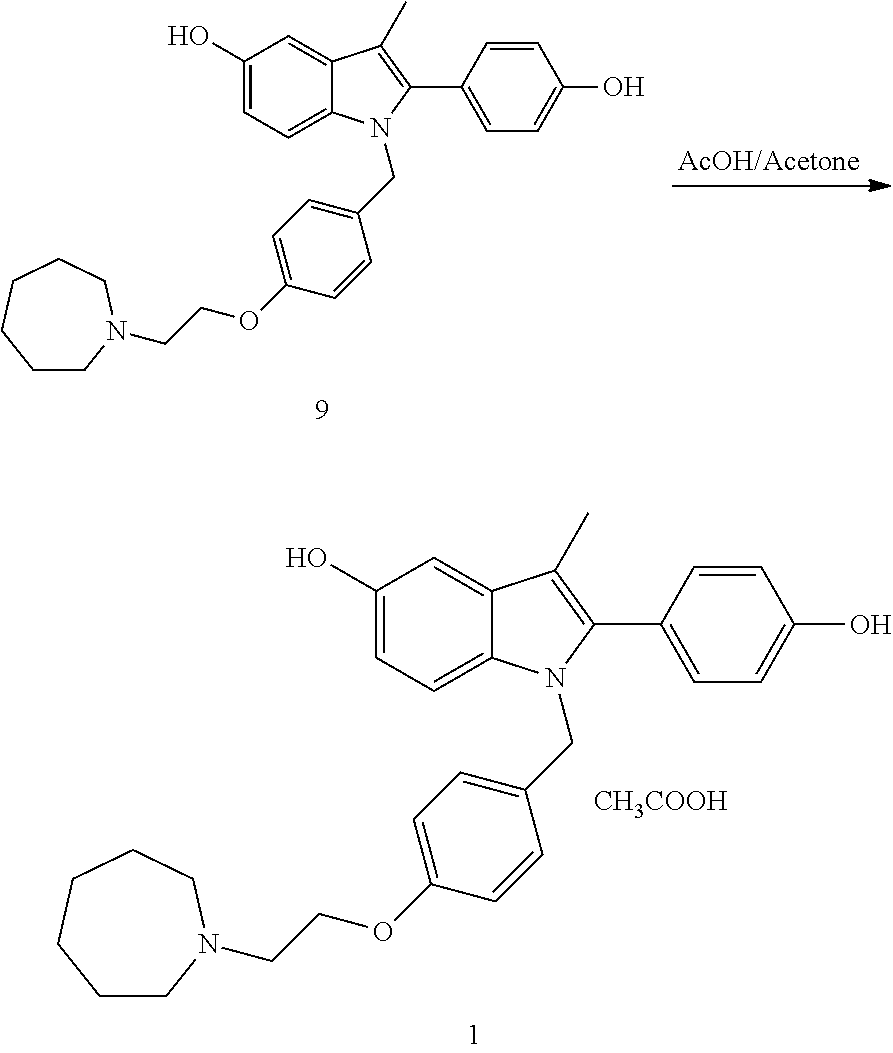
The invention discloses an industrial production method for bazedoxifene acetate. The production method comprises the following steps: taking p-hydroxy benzaldehyde as a starting material, substituting with chloracetyl-hexamethyleneimine, reducing with borohydride and chlorinating with a chlorinating agent to obtain a compound 4; reacting the compound 4 with 5-(benzyloxy)-2-(4-(benzyloxy)phenyl)-3-methyl-1H-indole to obtain a compound 5; and carrying out debenzylation to obtain a compound 6, and salifying with acetic acid to obtain a target compound which is the bazedoxifene acetate. The defective workmanship of preparation in the prior art is solved, the used reagent is low in cost and easy to obtain, environmental pollution is small, safety is high, an operation process is simple, and thus, the bazedoxifene acetate is suitable for being produced industrially.
Owner:YANGTZE RIVER PHARM GRP CO LTD
Bazedoxifene acetate composition with excellent property
ActiveCN103845336AGood chemical stabilityFast drug releaseSkeletal disorderPharmaceutical non-active ingredientsSolid solutionTocopherol polyethylene glycol succinate
The invention discloses a bazedoxifene acetate composition with excellent properties. The composition comprises bazedoxifene acetate, polyethylene glycol (PEG) 6000-8000 and vitamin E tocopherol polyethylene glycol succinate (TPGS), wherein a solid solution and / or solid sol is formed by polyethylene glycol (PEG) 6000-8000 and vitamin E TPGS, and bazedoxifene acetate is dispersed into the solid solution and / or solid sol. The composition is high in chemical stability and medicine release speed.
Owner:JIANGSU SEMPOLL PHARMA
The preparation method of bazedoxifene acetate intermediate
ActiveCN104151265BFew reaction stepsSimple post-processingOrganic chemistryRaloxifeneAfter treatment
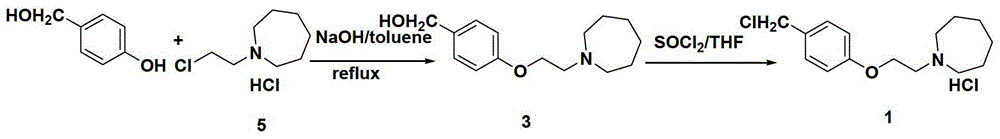
The invention discloses a preparation method of bazedoxifene acetate intermediate. The present invention provides a preparation method of bazedoxifene acetate intermediate compound 3, and the preparation method comprises the following steps: step 1, in a polar aprotic solvent in the presence of an alkali, intermediate compound 4 is obtained by condensation reaction of p-hydroxy benzaldehyde 6 and compound 5; and step 2, the reaction solution obtained in step 1, without after treatment, is directly mixed with a protic solvent, then is reduced by a reductant to obtain the bazedoxifene acetate intermediate compound 3. The preparation method has the advantages of short course, mild reaction conditions, safe operation, simple post treatment process, high conversion rate, high product yield, good purity, environmental friendliness and suitability for industrialized production.
Owner:SHANGHAI INST OF PHARMA IND CO LTD +2
Bazedoxifene acetate emplastrum
InactiveCN103860524ASimple preparation processGood treatment effectSkeletal disorderPharmaceutical non-active ingredientsTransdermal patchAntioxidant
The invention discloses a bazedoxifene acetate emplastrum preparation and a preparation method thereof. According to the preparation method, transdermal emplastrum is prepared from bazedoxifene acetate micro-emulsion, and is divided into four layers, namely an anti-adhesive layer, an adhesive layer, a lining layer and a drug-storage layer, wherein the effective component is bazedoxifene acetate micro-emulsion, and is prepared from bazedoxifene acetate serving as an active component, polysorbate 80 serving as a surfactant, propylene glycol serving as a cosurfactant, purified water or distilled water and the like. Except the effective component, the emplastrum also comprises a non-polar polymer, a plasticizer, a tackifier, a transdermal enhancer and an antioxidant. Compared with the existing preparations, such as tablets, the bazedoxifene acetate emplastrum has the prominent advantages of durable drug effect, safety and low toxicity and accurate administration dosage, is convenient to use, and is simple and sanitary.
Owner:王志刚
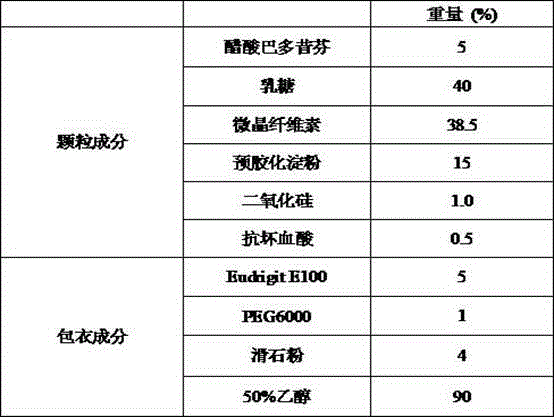
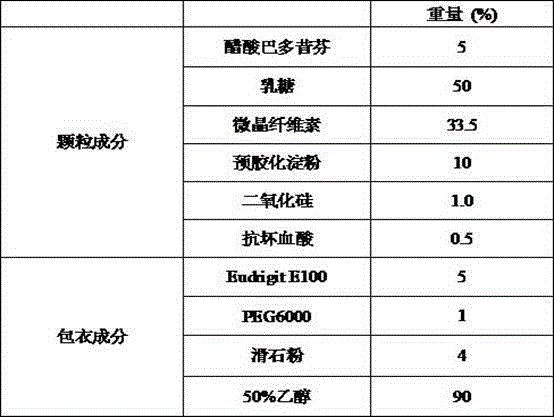
Bazedoxifene acetate composition and bazedoxifene acetate film-coated tablet preparation method
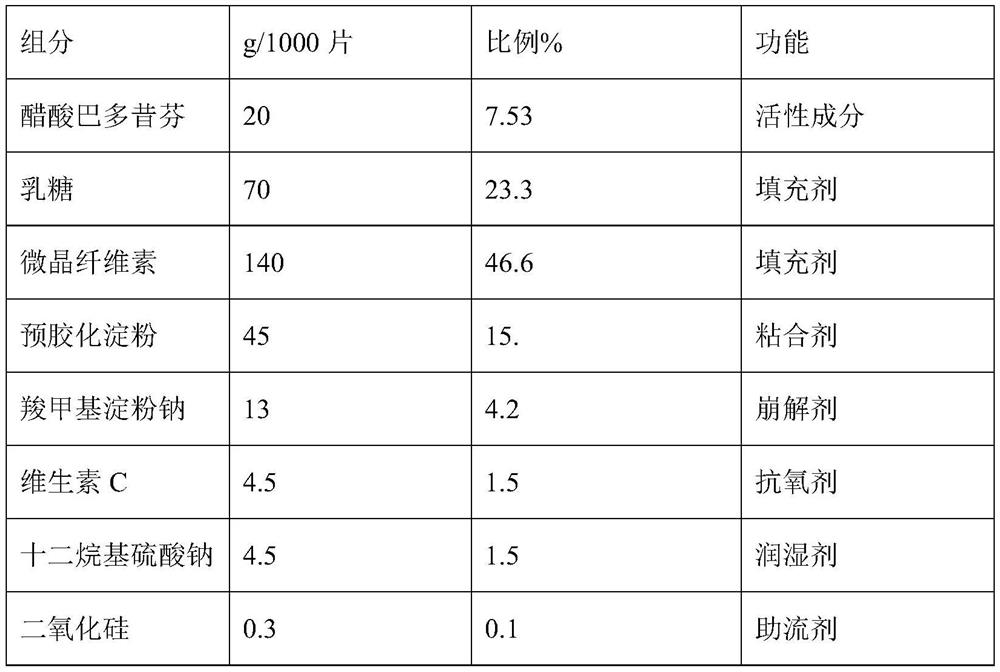
PendingCN112754999ASimple processImprove stabilitySkeletal disorderPharmaceutical non-active ingredientsCoated tabletsVitamin C
The invention discloses a bazedoxifene (Viviant) acetate composition and a bazedoxifene acetate film-coated tablet preparation method, and relates to the technical field of medicines. The bazedoxifene acetate composition comprises the following components by weight of 20-26 parts of bazedoxifene acetate, 250-350 parts of a filling agent, 30-50 parts of an adhesive, 8-15 parts of a disintegrating agent, 3-6 parts of an antioxidant, 2-6 parts of a flow aid, 0.1-0.5 part of a lubricant, 1-3 parts of a wetting agent, and a proper amount of a purified water solvent, wherein bazedoxifene acetate is a BCS II type medicine, and the particle size D90 is 30 microns; the antioxidant is vitamin C; and the filling agent is composed of lactose and microcrystalline cellulose, and the ratio of the lactose to the microcrystalline cellulose is 1: 2-2: 1 according to the weight fraction. The fluidized bed granulation technology adopting the process for preparation is simple in process, lower in cost and easy for industrial production.
Owner:ZHENGZHOU TAIFENG PHARMA CO LTD
Bazedoxifene acetate capsule and preparation method thereof
InactiveCN104546794AImprove stabilitySkeletal disorderPharmaceutical non-active ingredientsPharmaceutical SubstancesOsteoporosis
The invention discloses a bazedoxifene acetate capsule which is used for preventing the osteoporosis of postmenopausal women. The capsule is convenient to carry and take, and compared with a tablet and a pill, the bazedoxifene acetate capsule is dispersed in stomachs and dissolved out more quickly, and takes the effect more quickly. Meanwhile, the invention further discloses a preparation method of the bazedoxifene acetate capsule. Compared with a common capsule granulating method, capsule grains prepared by the preparation method can improve the stability of drugs and preparations of the drugs effectively.
Owner:AVENTIS PHARMA HAINAN
Preparation method of pure acetic acid bazedoxifene crystal form A
InactiveCN106748959AIncrease productivitySimple processOrganic chemistry methodsCarboxylic acid salt preparationAcetic acidHydrogen
The invention discloses a preparation method of a pure acetic acid bazedoxifene crystal form A. The preparation method comprises the following process steps of firstly, under the condition of using palladium carbon as a catalyst, dissolving raw materials into a good organic solvent, and introducing hydrogen to react to a non-raw material point; after reaction is completed, filtering to remove the palladium carbon, cooling filtrate, adding acetic acid and a poor organic solvent, stirring, crystallizing, filtering, and drying, so as to obtain a crude product of the crystal form A. The preparation method has the advantages that the technology is simple, the production efficiency is high, and the preparation cost of the pure acetic acid bazedoxifene crystal form A is effectively reduced.
Owner:成都归合科技有限公司
Process for the preparation of bazedoxifene acetate and intermediates thereof
ActiveUS8569483B2High purityHigh yieldOrganic compound preparationSulfonic acid esters preparationSulfonate3-methyl-1H-indol-5-ol
A novel process is described for the preparation of pharmaceutically useful compounds such as 1-{4-[2-(azepan-1-yl)ethoxy]benzyl}-2-(4-hydroxyphenyl)-3-methyl-1H-indol-5-ol acetic acid commonly known as bazedoxifene acetate of the formula-1 using 2-(4-{[5-(benzyloxy)-2-[4-(benzyloxy)phenyl]-3-methyl-1H-indol-1-yl]methyl}phenoxy)ethyl-4-methylbenzenzene-1-sulfonate (formula 2a)
Owner:DIVI S LAB LTD
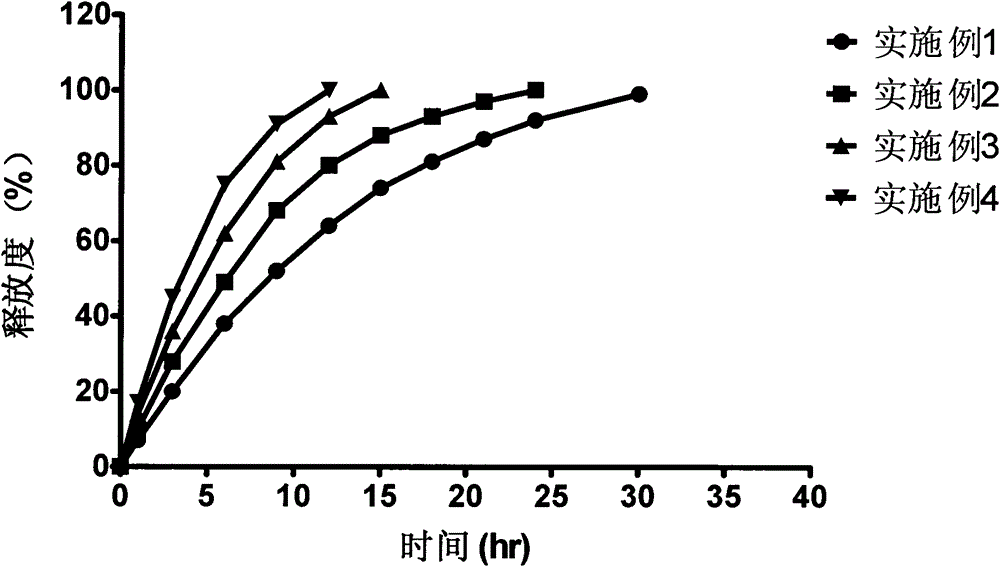
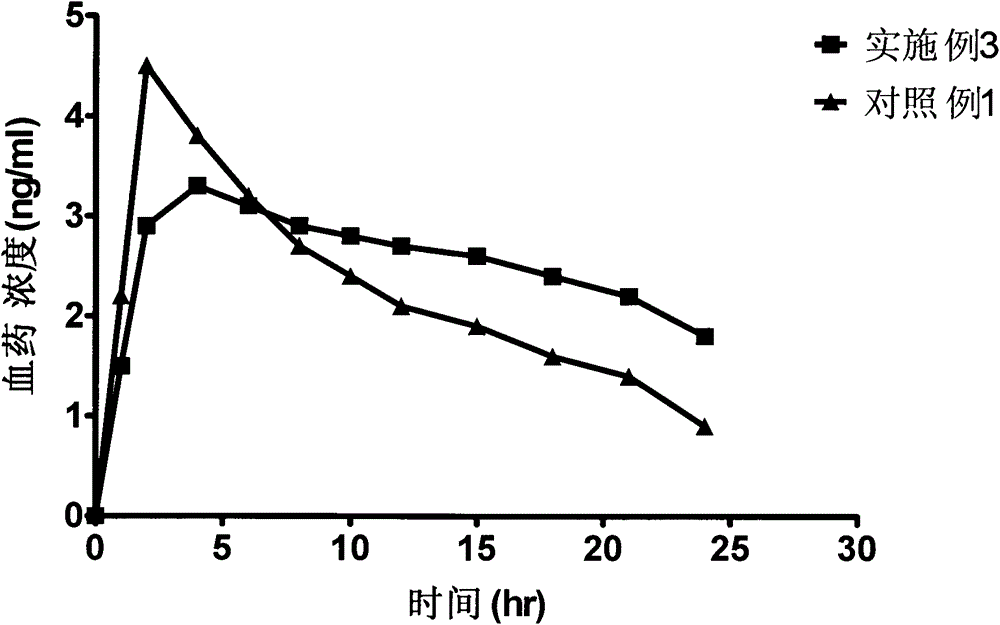
Bazedoxifene acetate sustained release preparation with excellent performance
ActiveCN103877581ABlood concentration is stableAvoid or reduce side effectsPharmaceutical non-active ingredientsCapsule deliveryMedicineHydrophilic matrix
The invention discloses a bazedoxifene acetate sustained release preparation with excellent performance and a preparation process thereof. The preparation is a sustained release dropping pill, which comprises the following components in percentage by weight: 1-25 percent of bazedoxifene acetate, 40-70 percent of hydrophobic dropping pill matrix, and 5-40 percent of hydrophilic matrix. The sustained release dropping pill has a higher chemical stability, physical stability (low-crystal form conversion rate) and in-vivo drug-release behavior (higher bioavailability and smooth and durable plasma concentration).
Owner:JIANGSU SEMPOLL PHARMA
Preparation method of bazedoxifene acetate
The invention discloses a preparation method of bazedoxifene acetate. The method is characterized in that raney nickel is used as a catalyst, and a compound I and hydrogen react in a microreactor to obtain bazedoxifene under the conditions that the temperature is 20 to 40DEG C and the hydrogen pressure is controlled to be 0.1 to 0.5Mpa. According to the method, the raney nickel with lower price is used to replace expensive palladium carbon, so that the production cost is effectively reduced, the microreactor is used and the production is in streamline operation; an obtained product is high in yield and good in quality; the preparation method has the advantages of high reaction efficiency, mild reaction conditions, safe and controllable operation, short reaction time and low cost; the method is easier for industrial production (The formula is shown in the description).
Owner:山东安信制药有限公司
A bazedoxifene acetate sustained-release preparation with excellent performance
ActiveCN103877581BBlood concentration is stableAvoid or reduce side effectsPharmaceutical non-active ingredientsCapsule deliveryMedicineHydrophilic matrix
The invention discloses a bazedoxifene acetate sustained release preparation with excellent performance and a preparation process thereof. The preparation is a sustained release dropping pill, which comprises the following components in percentage by weight: 1-25 percent of bazedoxifene acetate, 40-70 percent of hydrophobic dropping pill matrix, and 5-40 percent of hydrophilic matrix. The sustained release dropping pill has a higher chemical stability, physical stability (low-crystal form conversion rate) and in-vivo drug-release behavior (higher bioavailability and smooth and durable plasma concentration).
Owner:JIANGSU SEMPOLL PHARMA
Pharmaceutical composition of bazedoxifene acetate tablets and preparation method thereof
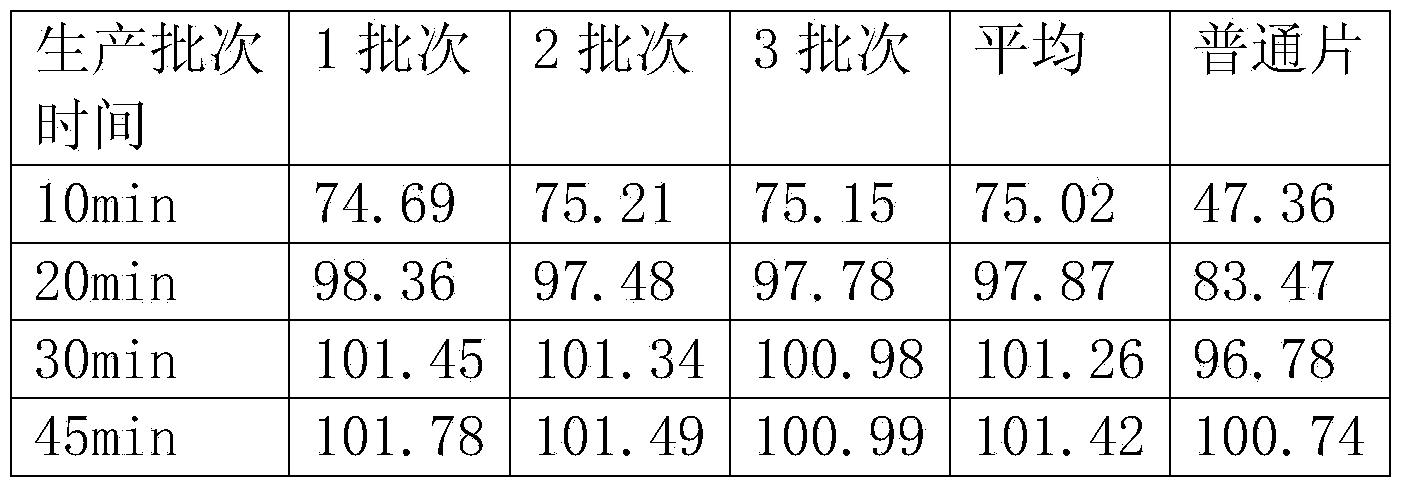
ActiveCN112076163AConsistent qualityGuaranteed content uniformitySkeletal disorderPill deliveryVitamin CPharmacology
The invention relates to a pharmaceutical composition containing bazedoxifene acetate as an effective component and a preparation method thereof. The composition comprises bazedoxifene acetate; lactose; microcrystalline cellulose; pregelatinized starch; low-substituted hydroxypropyl cellulose; colloidal silica; sodium dodecyl sulfate; vitamin C; and glyceryl behenate. The invention also relates toa preparation method of the composition. The pharmaceutical composition of the present invention has the effects of improving the inter-batch difference on drugs, and ensuring the content uniformityof drugs and consistent dissolution of different batches of drugs, and has medicinal safety and effectiveness.
Owner:SICHUAN KELUN PHARMA RES INST CO LTD
The preparation method of bazedoxifene acetate
ActiveCN103864665BEase of industrial productionPromote the development of economy and technologyOrganic chemistryAcetic acidAlcohol
The invention discloses a preparation method of bazedoxifene acetate. The method comprises the following steps: carrying out a condensation cyclization reaction on 1-(4-Pg1 oxygroup phenyl) propyl alcohol (II) and N-{4-(2-azacycloheptane-1-yl-ethyoxyl-benzyl)}N-{4-(Pg2 oxygroup phenyl)} hydrazine (III) so as to obtain 1-{4-(2-azacycloheptane-1-yl-ethyoxyl-benzyl)}-2-(4-Pg1 oxygroup-phenyl)-3-methyl-5-(Pg2 oxygroup)-1H-benzpyrole (IV); and carrying out deprotection on the intermediate (IV) and salifying the intermediate (IV) with acetic acid so as to obtain the bazedoxifene acetate (I). The preparation method is concise in process, high in yield, economical and environment-friendly, thereby providing a novel preparation way for the industrial production of the bazedoxifene acetate.
Owner:苏州特瑞药业股份有限公司
Preparation method of bazedoxifene acetate and a crystal form thereof
ActiveCN105669518BReduce usageReduce construction costsOrganic chemistry methodsPalladium on carbonAcetic acid
A preparation method of bazedoxifene acetate is characterized by including the following steps: (1) performing a reaction by mixing and suspending a compound represented in the formula (A), ammonium formate or cyclohexadiene, and a palladium-carbon catalyst in an organic solvent, reaction terminal being detected with TLC or HPLC; (2), filtering and washing a reaction product, adding acetic acid with stirring, and filtering and drying the mixture to prepare the bazedoxifene acetate. The invention also provides a preparation method of a bazedoxifene acetate crystal form A. The preparation method is free of hydrogen and avoids usage of special devices, such as a hydrogenation kettle, while a safe hydrogen donor is employed, so that the method can be carried out even with a common reaction kettle and a reaction workshop, thereby reducing dangerousness of the synthesis and building and operation cost of a special device workshop. The preparation method of the crystal form A, compared with the prior art, is simple in operation, is free of a crystal seed for inducing crystallization, is high in crystal form purity and is simple in solvent system which can be recycled and reused.
Owner:上海医药集团(本溪)北方药业有限公司
A kind of preparation method of bazedoxifene acetate crystal form a
The invention belongs to the technical field of chemical pharmacy and relates to a preparation method of a bazedoxifene acetate crystal form A. The preparation method of the bazedoxifene acetate crystal form A comprises the following steps: step 1, in the presence of palladium / carbon serving as a catalyst, dissolving hexamethyleneimine benzyloxy benzpyrole and ammonium formate in a benign organic solvent to have reaction; step 2, after the reaction is finished completely, filtering the palladium on activated carbon, cooling the filtrate, adding acetic acid and a toxic inorganic solvent, stirring, crystallizing, filtering and drying to obtain a crude crystal form A product; step 3, under the protection and presence of inert gas, dissolving the crude crystal form A product in the benign organic solvent, heating and dissolving; step 4, thermally filtering the solution, cooling the filtrate, dropwise adding the toxic inorganic solvent and crystallizing; step 5, after the crystallization is ended, filtering the solution and drying the solid to obtain the bazedoxifene acetate crystal form A.
Owner:CHINA RESOURCES SAIKE PHARMA
Crystal form of bazedoxifene L-lactate
InactiveCN108218760AGood water solubilityGood crystal stabilityOrganic chemistry methodsSolubilityActive ingredient
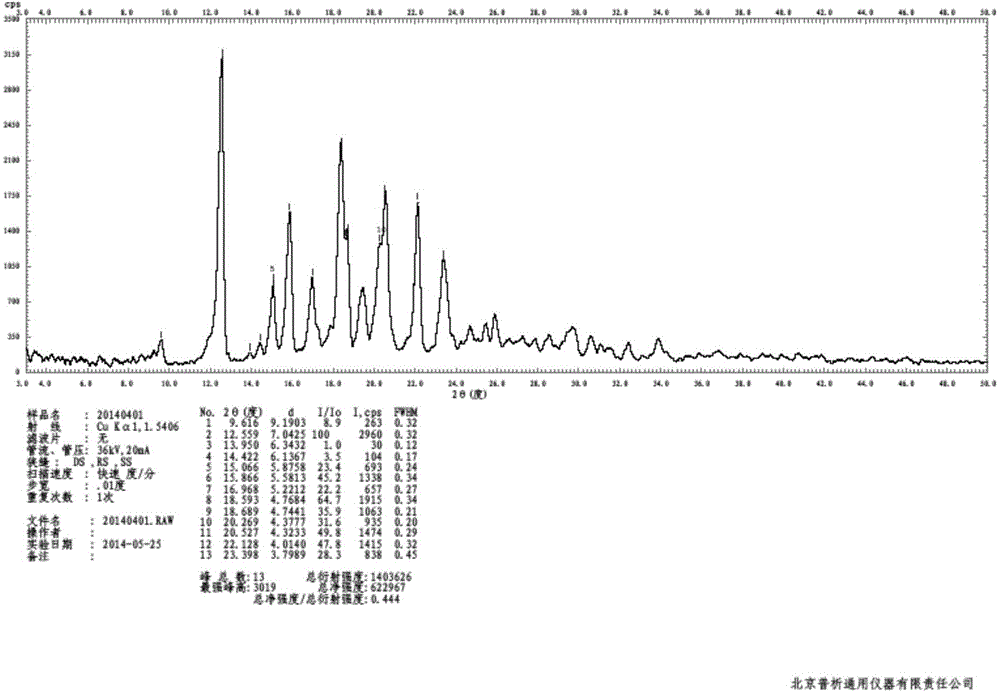
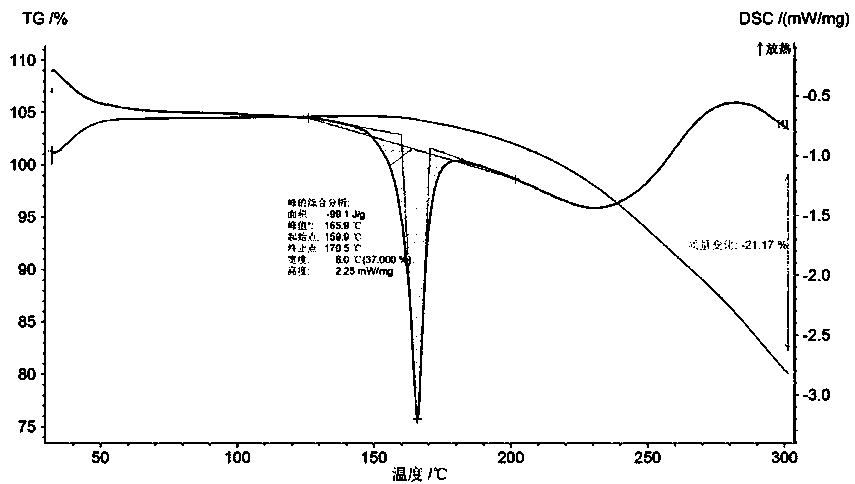
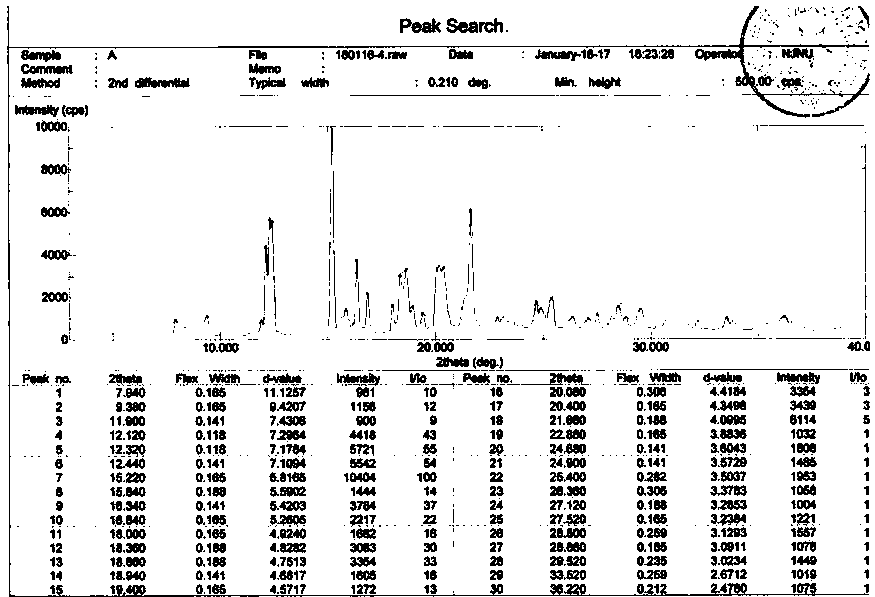
The invention discloses a crystal form of bazedoxifene L-lactate. An X-ray powder diffraction pattern displayed by the crystal form has peaks of the following spacing values: 21.66 angstroms, 19.40 angstroms, 18.94 angstroms, 18.66 angstroms, 18.36 angstroms, 18.00 angstroms, 16.84 angstroms, 16.34 angstroms, 15.22 angstroms, 12.44 angstroms, 12.32 angstroms, 12.12 angstroms, and 9.38 angstroms. According to the crystal form of the bazedoxifene L-lactate, disclosed by the invention, the water solubility is much higher than that of various crystal forms (A, B, C and D) of bazedoxifene acetate;crude drugs for preparing tablets do not need to be micronized and the crystal form has good stability; the risk of crystal form transformation does not occur in a medicine preparation process.
Owner:FUJIAN INST OF MICROBIOLOGY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com