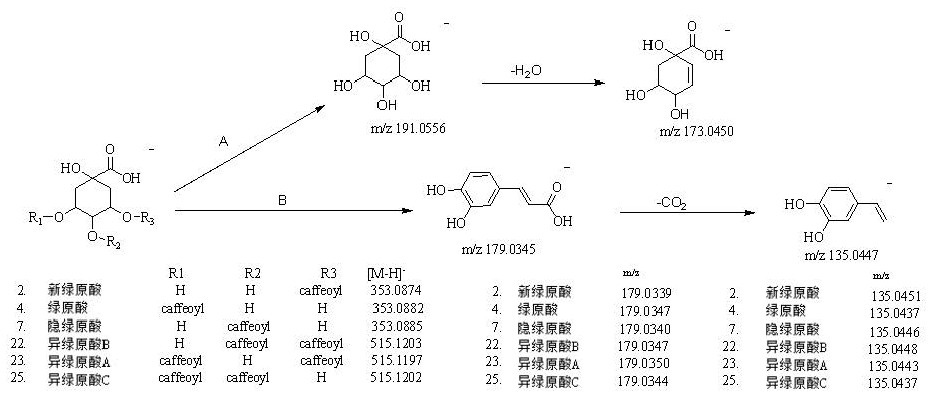
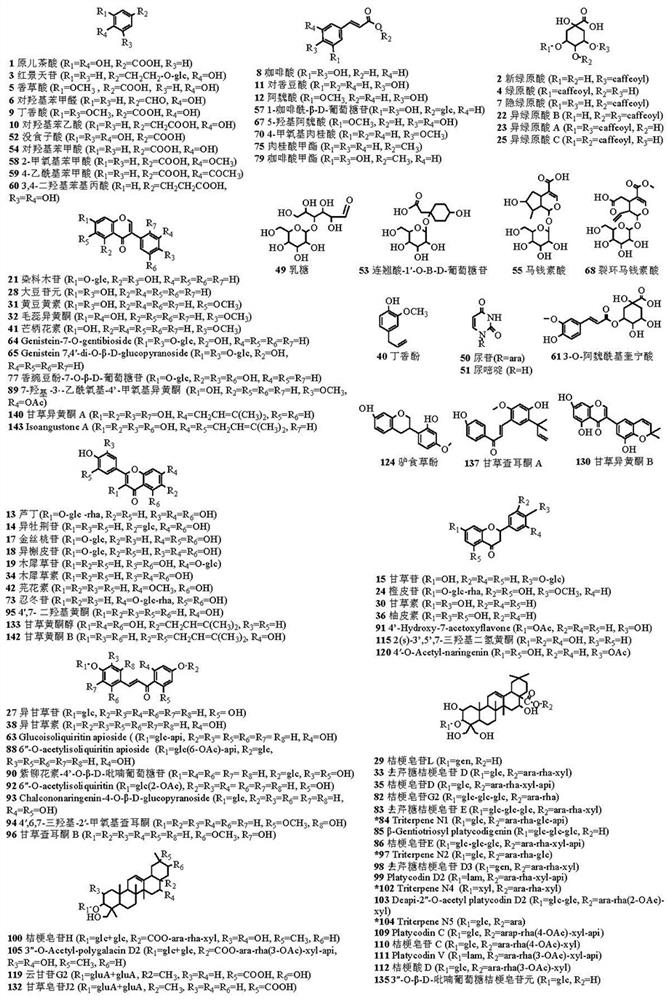
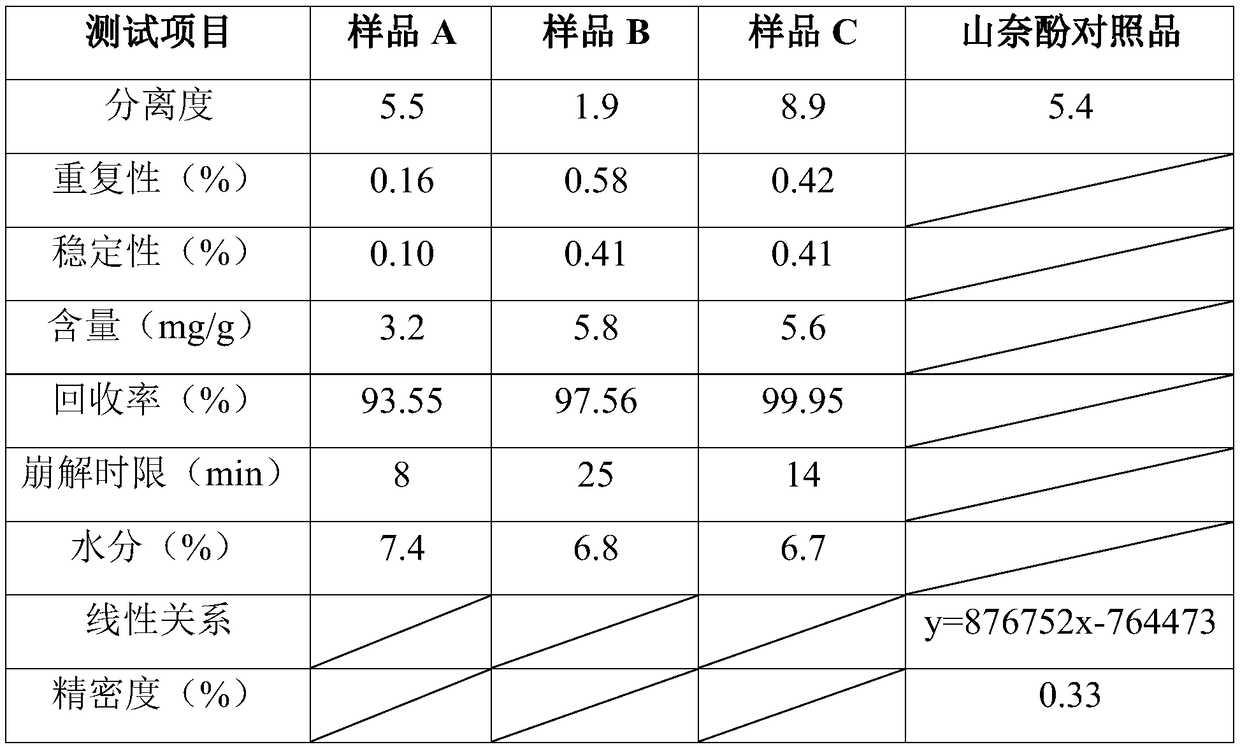
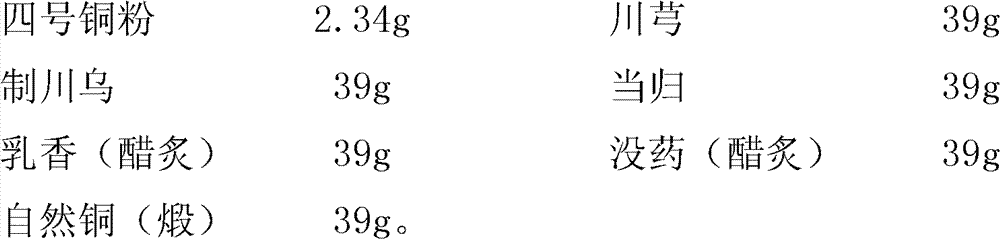Patents
Literature
42results about How to "Optimizing Chromatographic Conditions" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
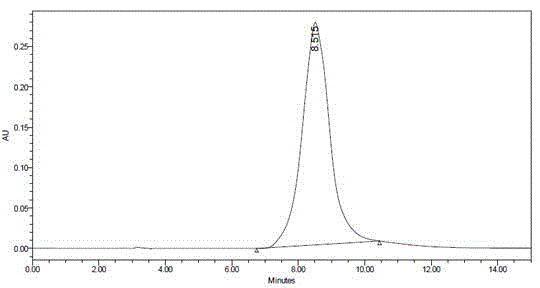
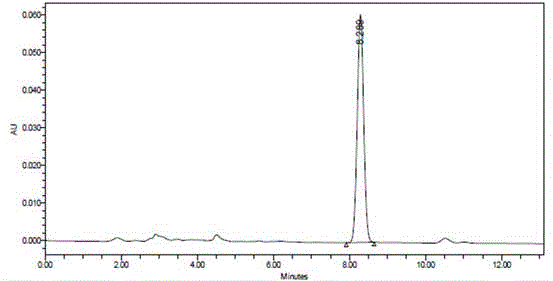
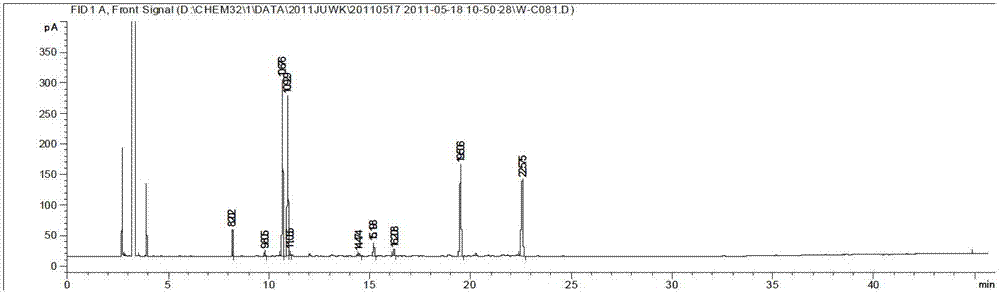
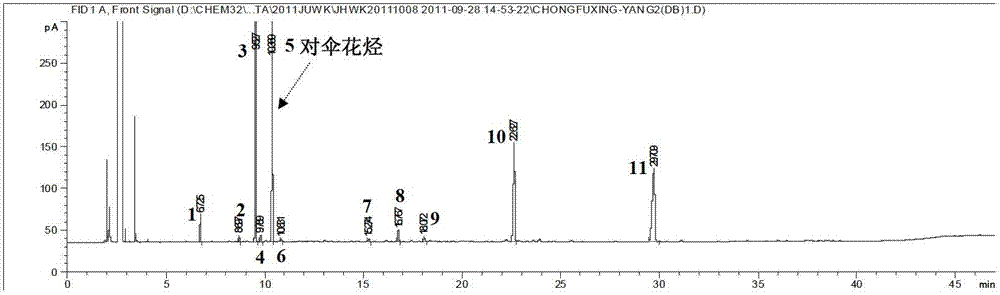
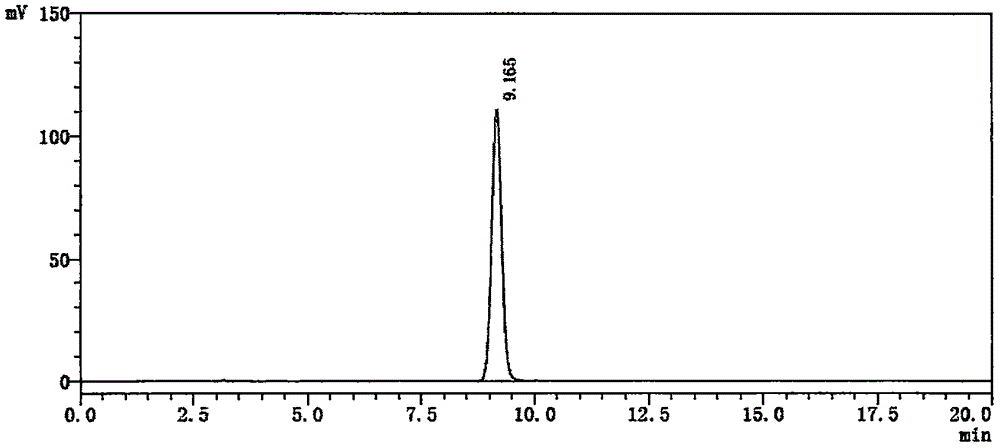
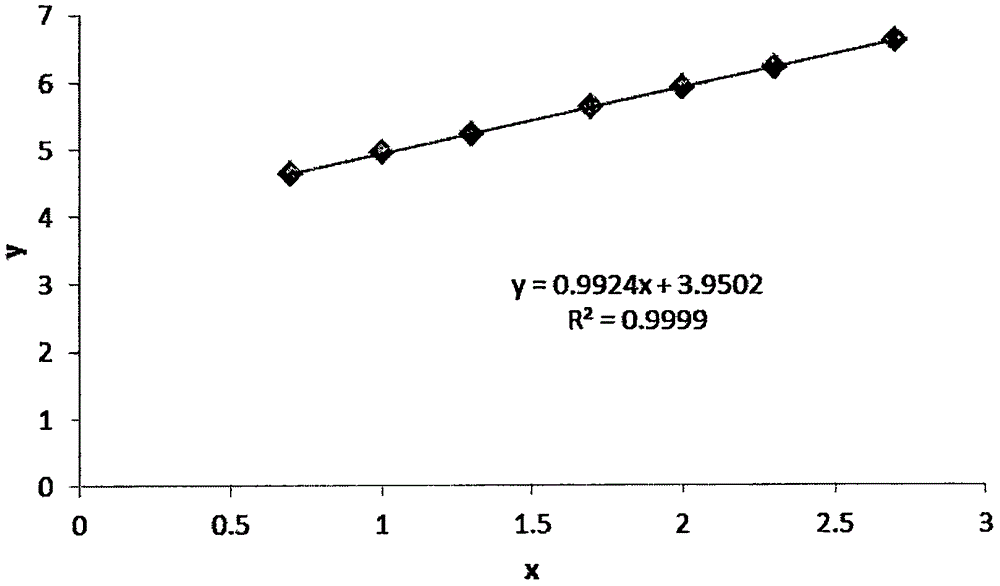
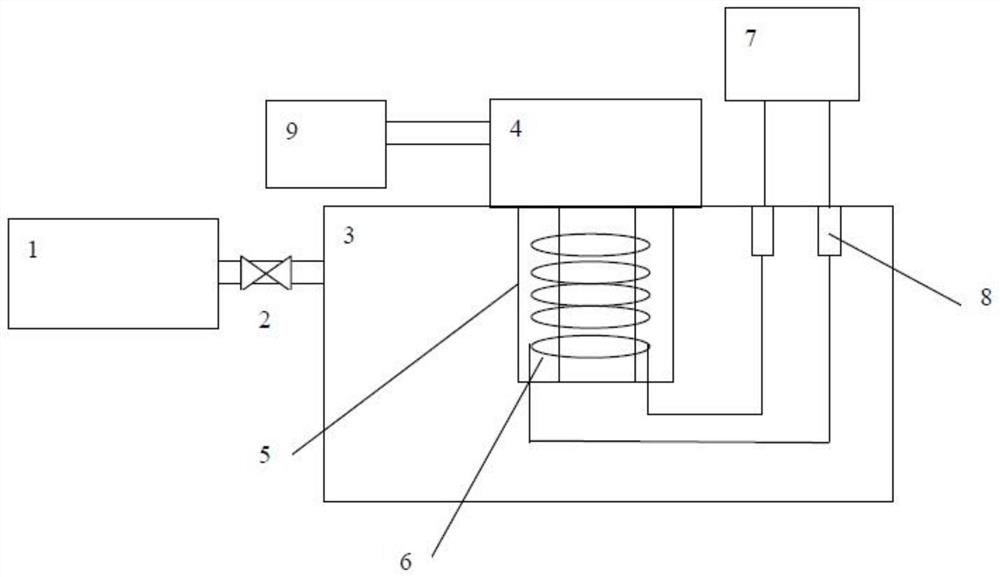
Method of simultaneously determining contents of 1-OHP ,3-OHB[a]P and 3-OHB[a]A in urine
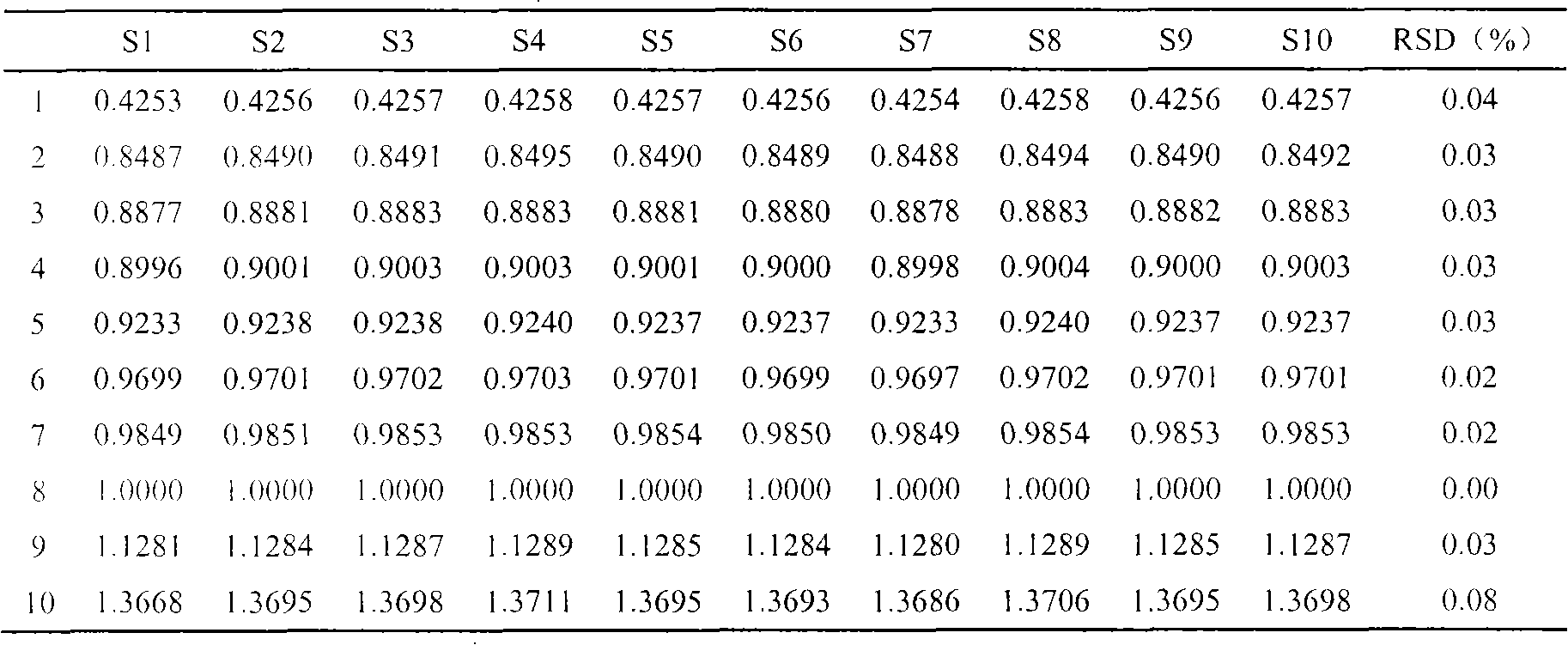
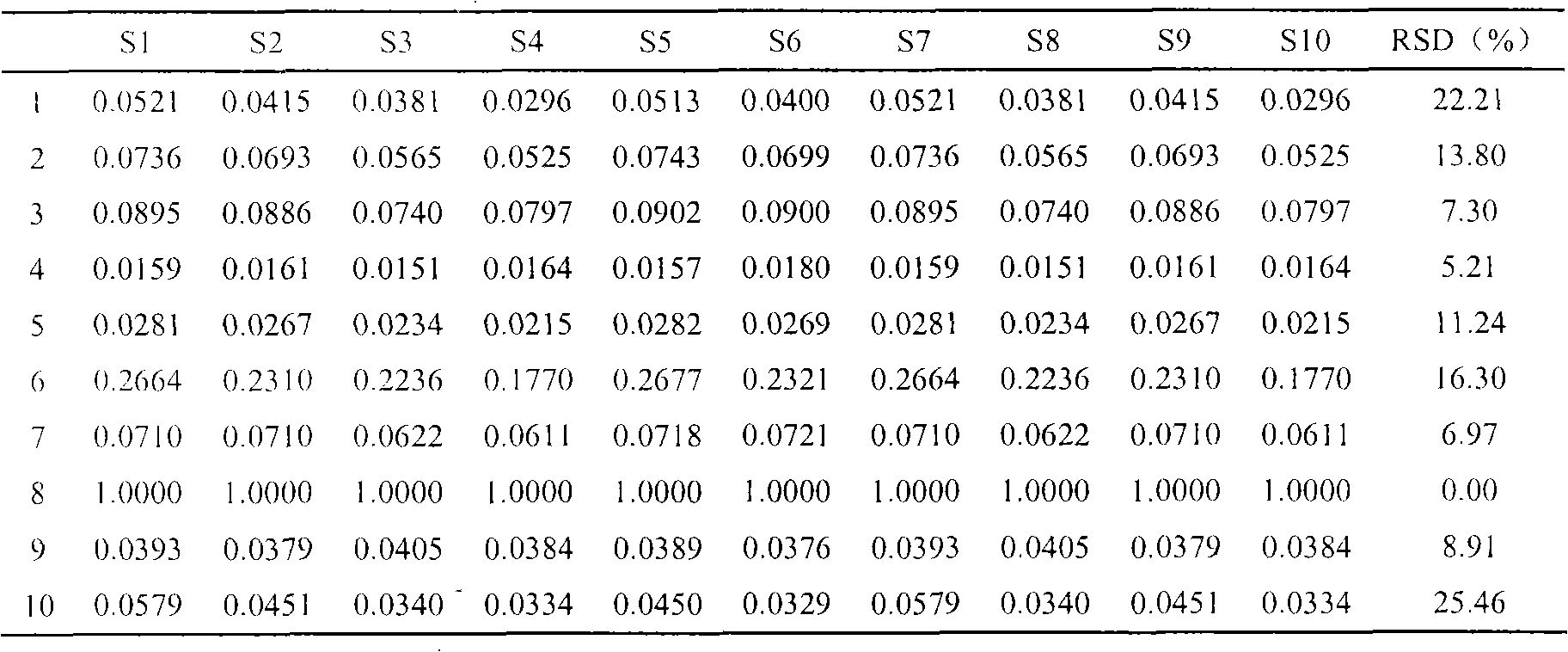
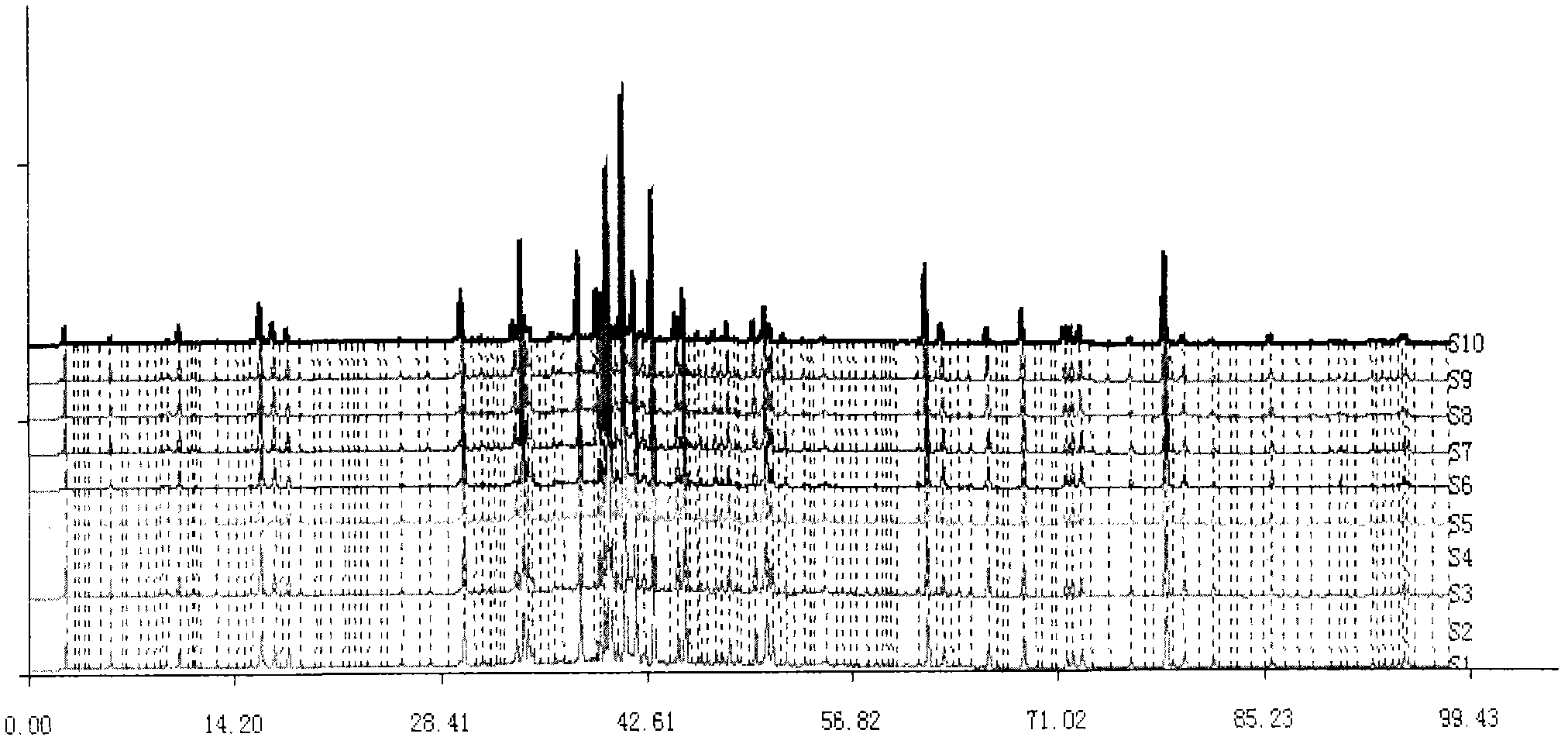
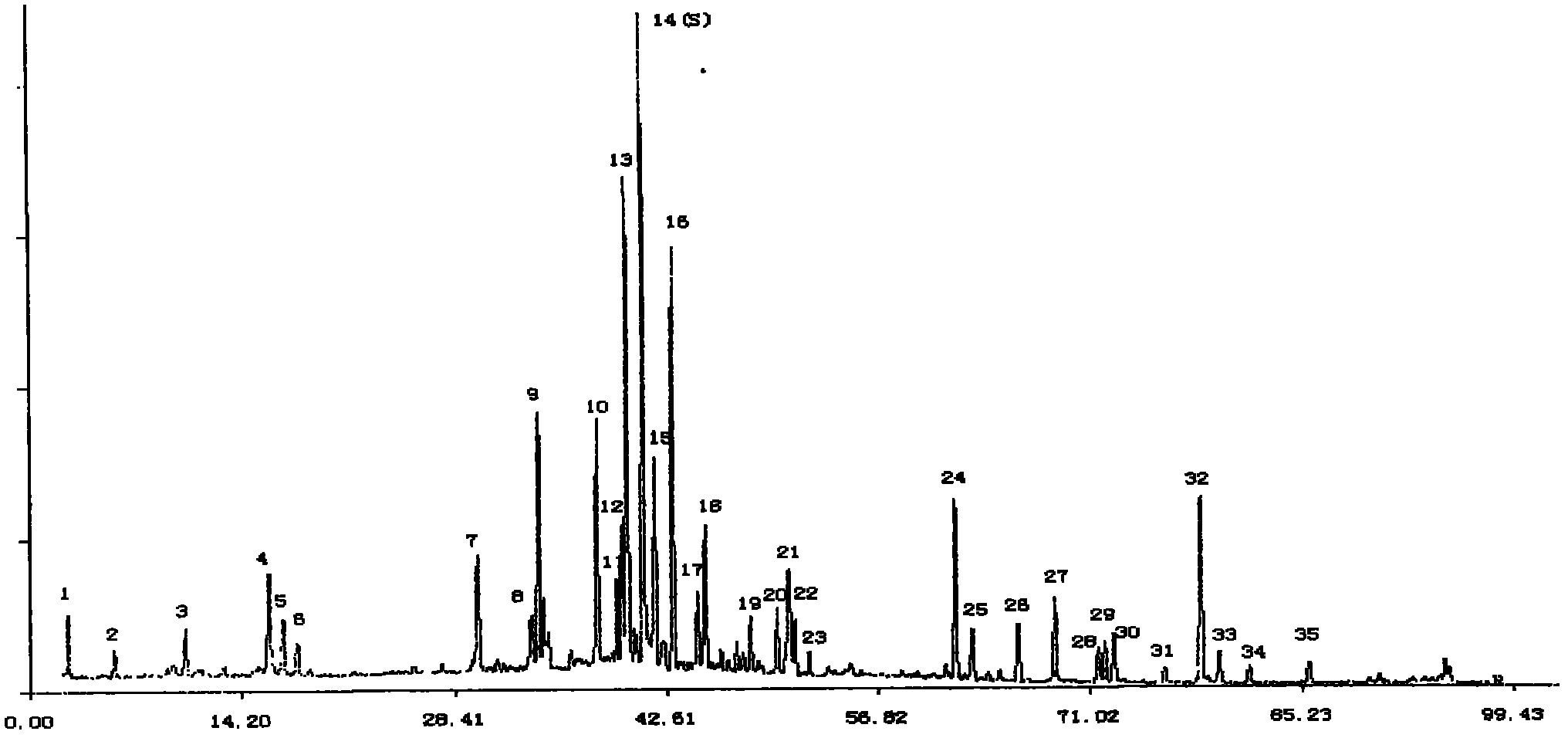
InactiveCN103063791AOptimizing enzymatic conditionsOptimizing Solid Phase Extraction ConditionsComponent separationChromatographic separationRotary evaporator
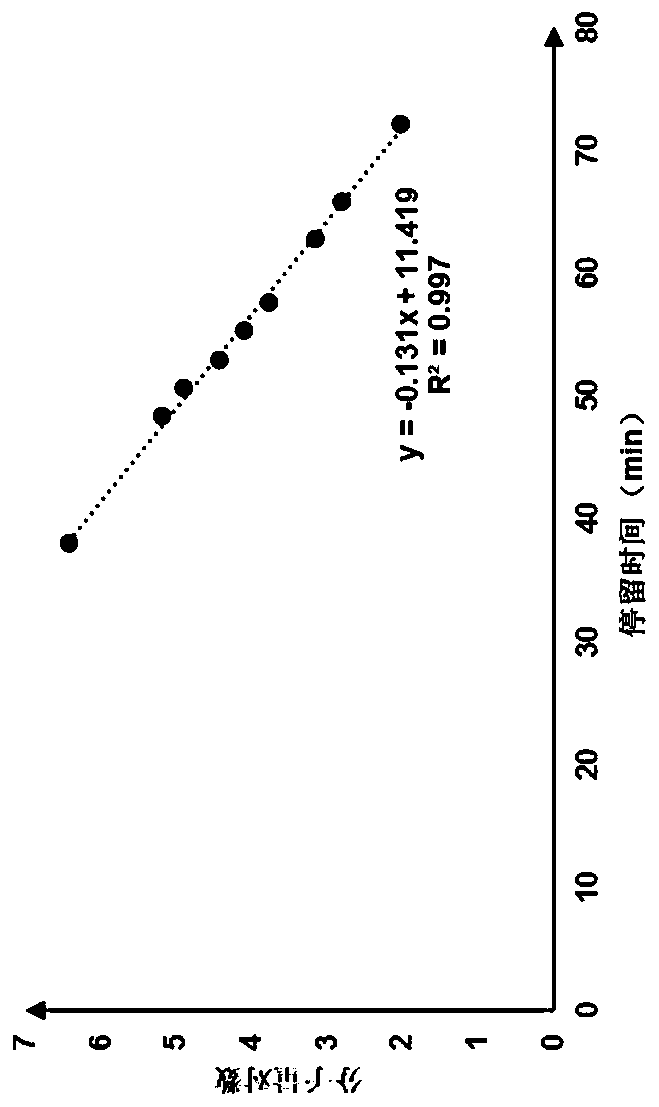
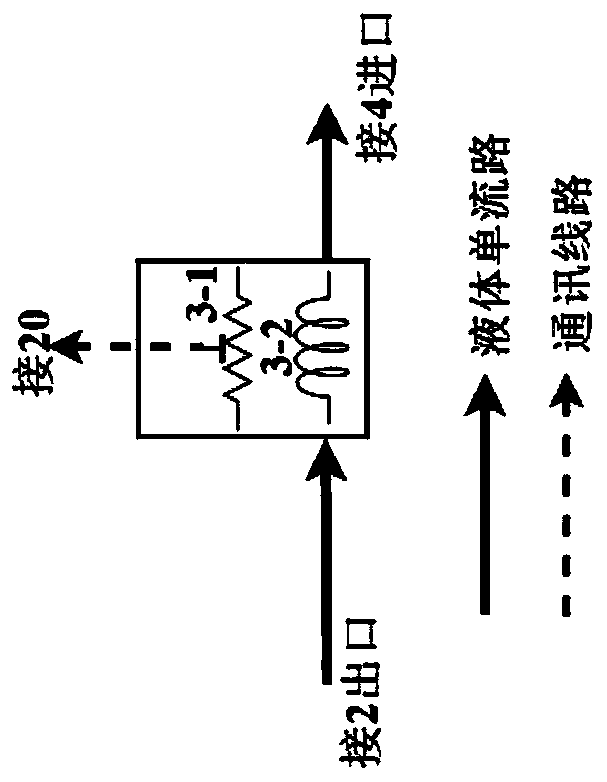
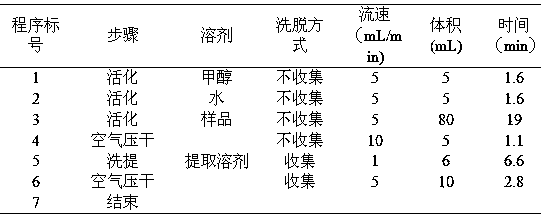
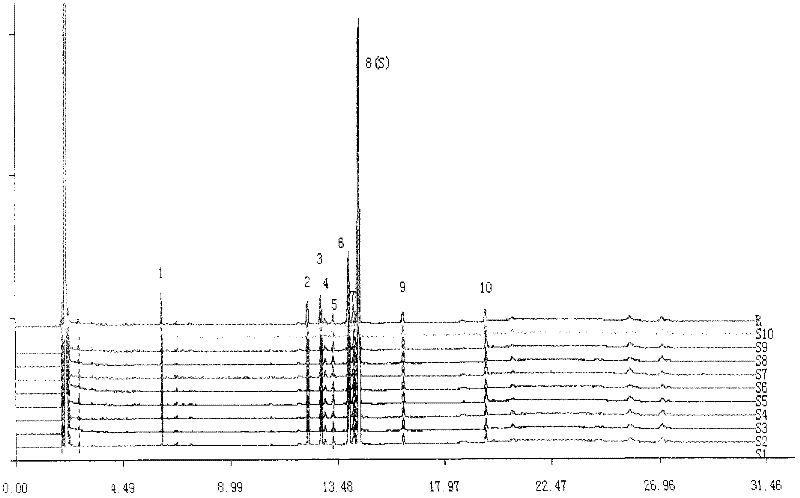
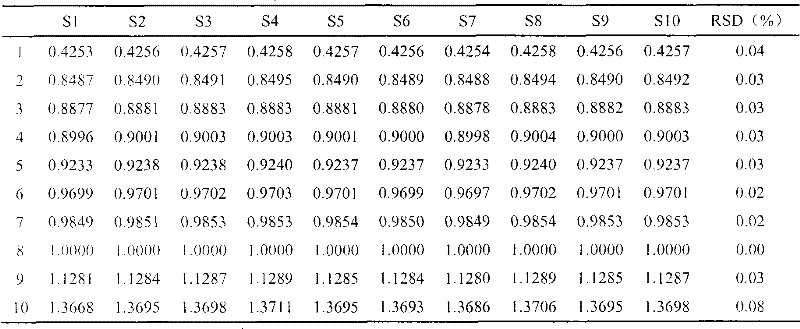
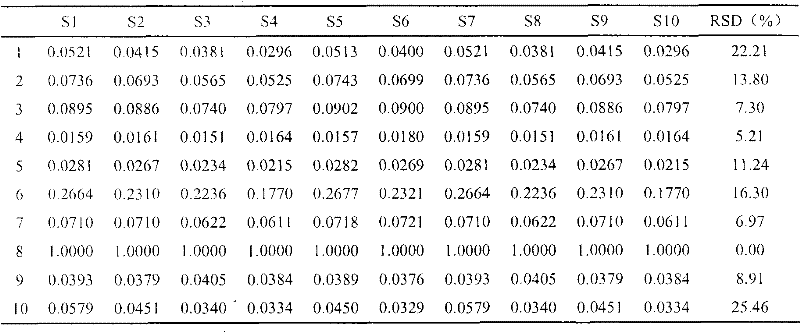
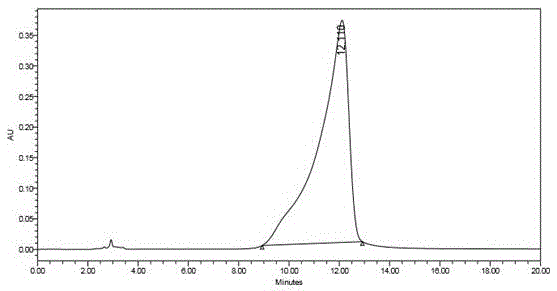
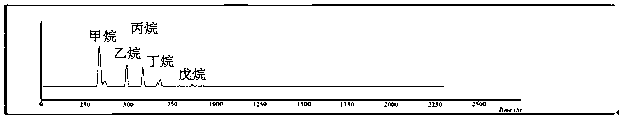
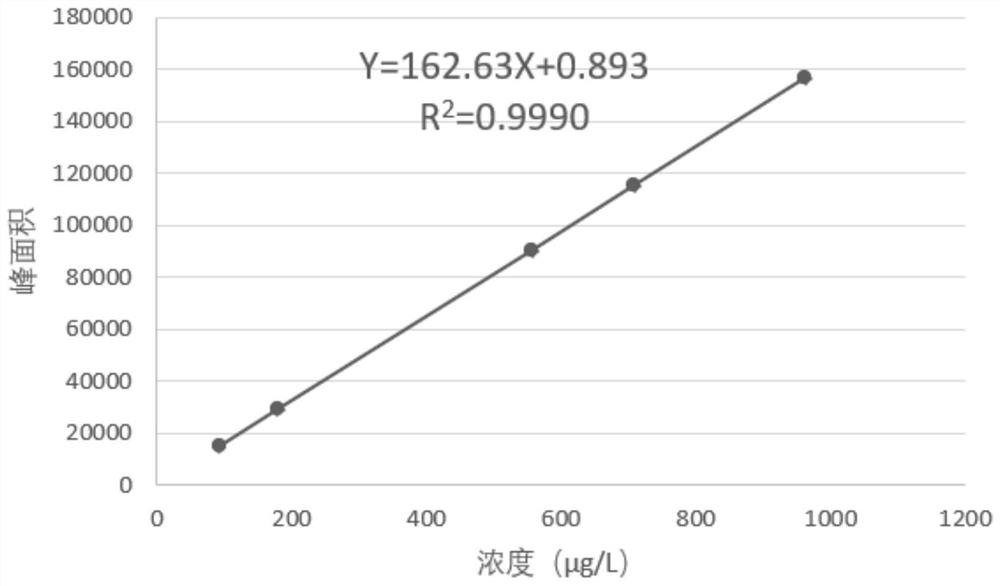
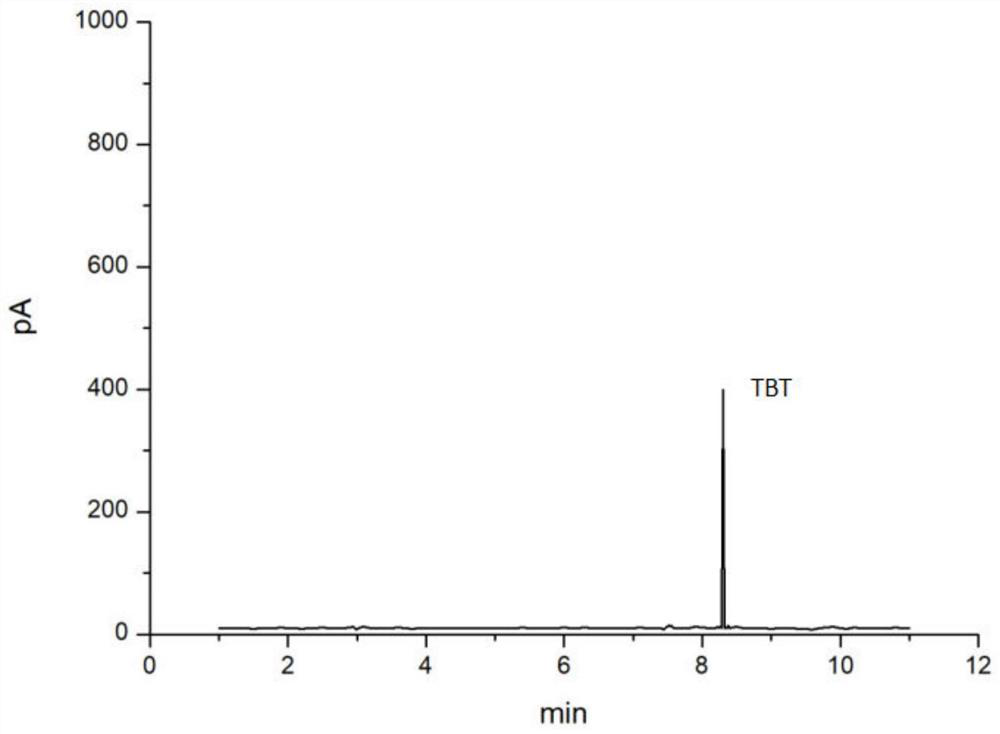
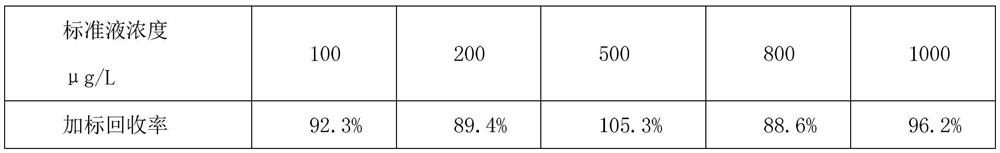
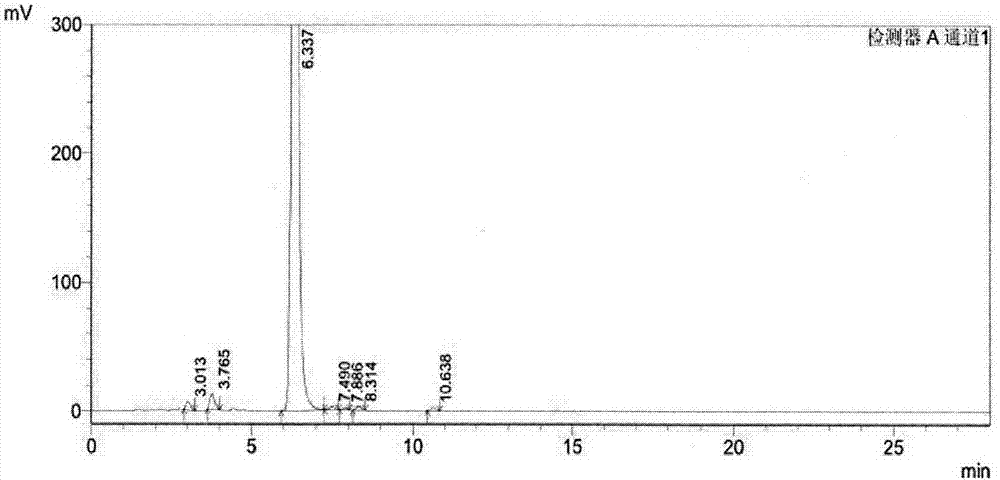
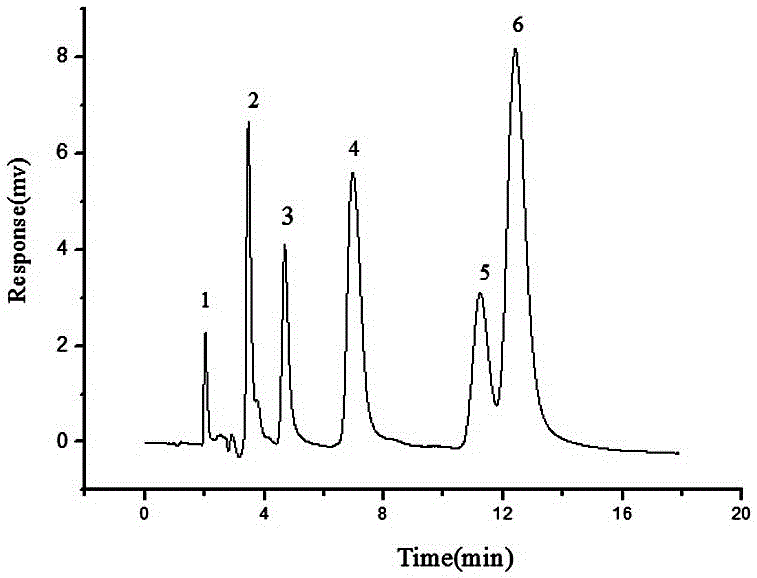
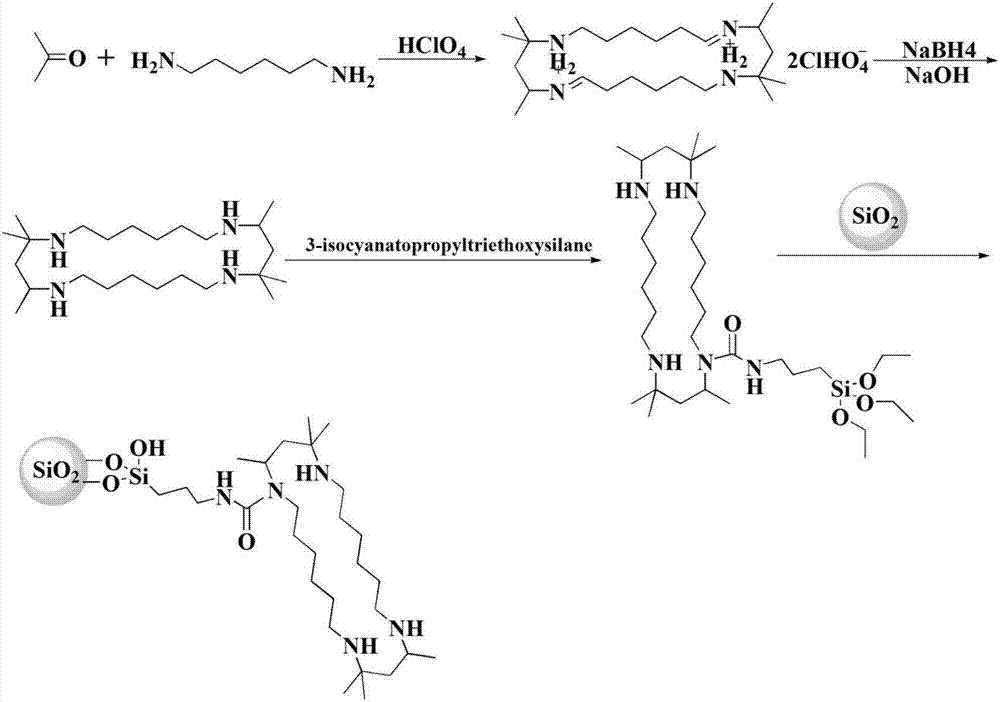
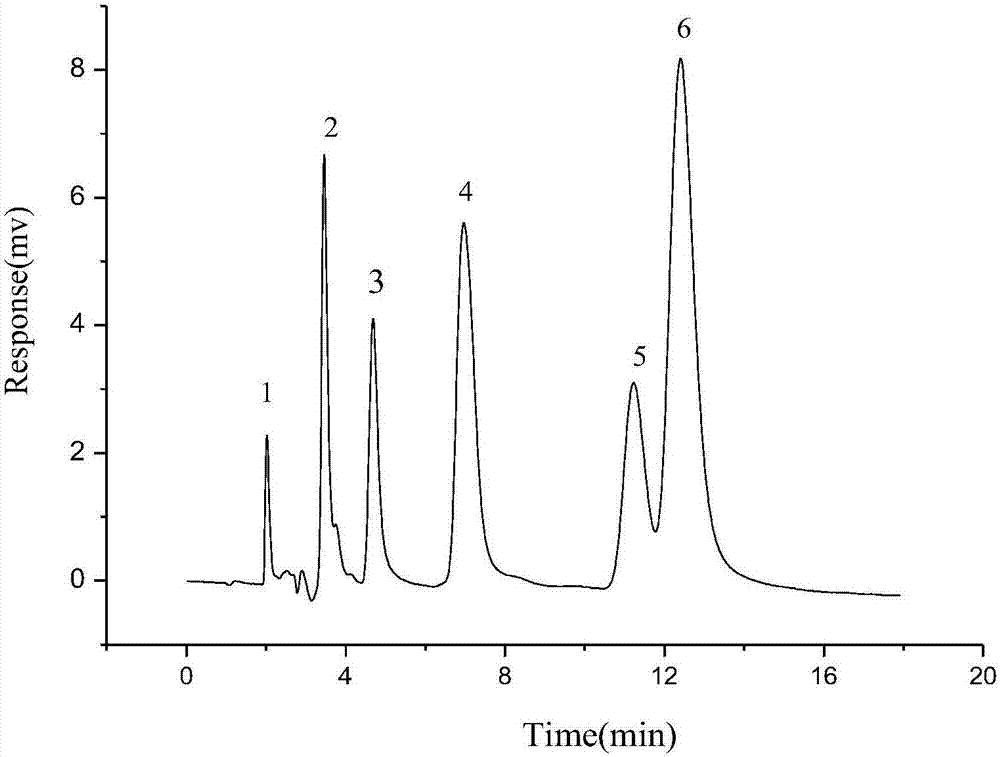
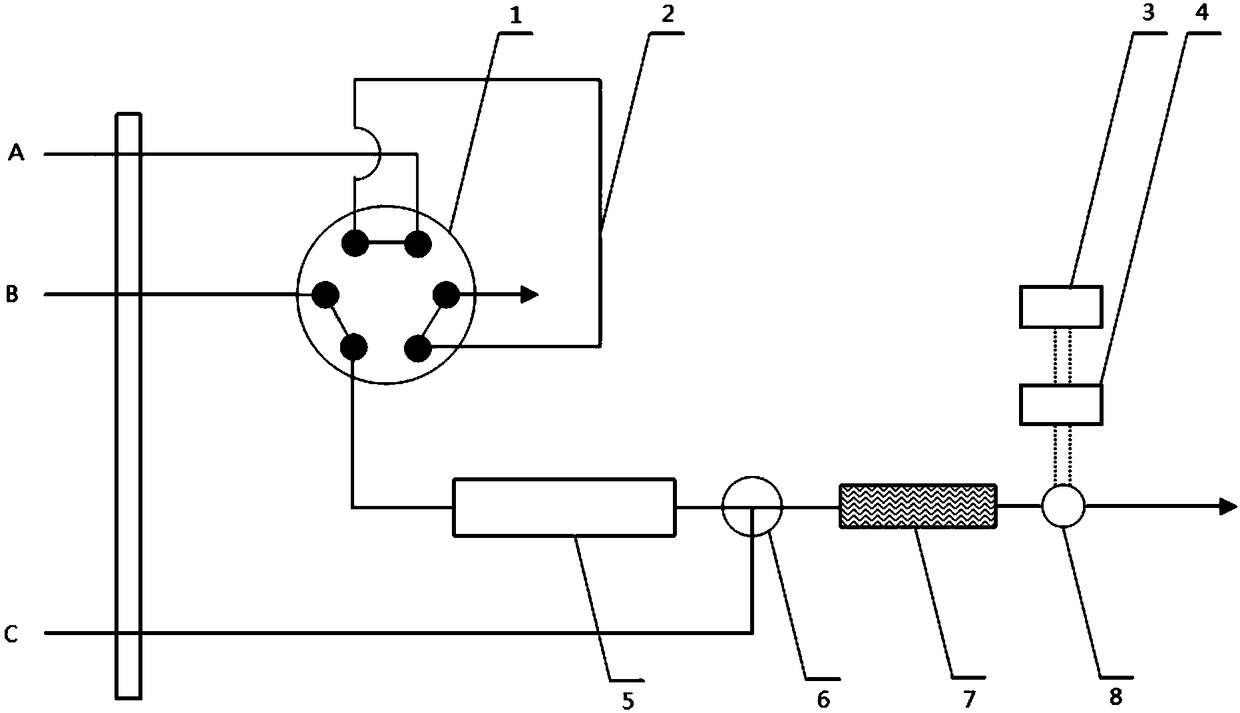
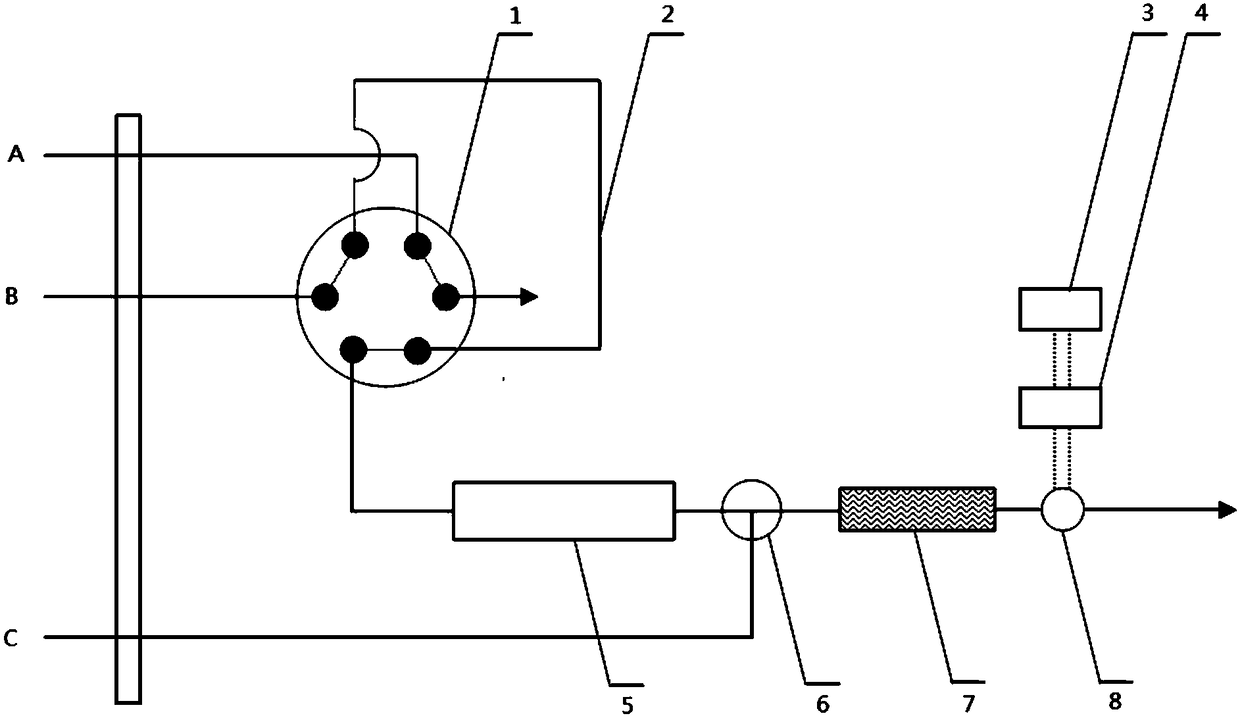
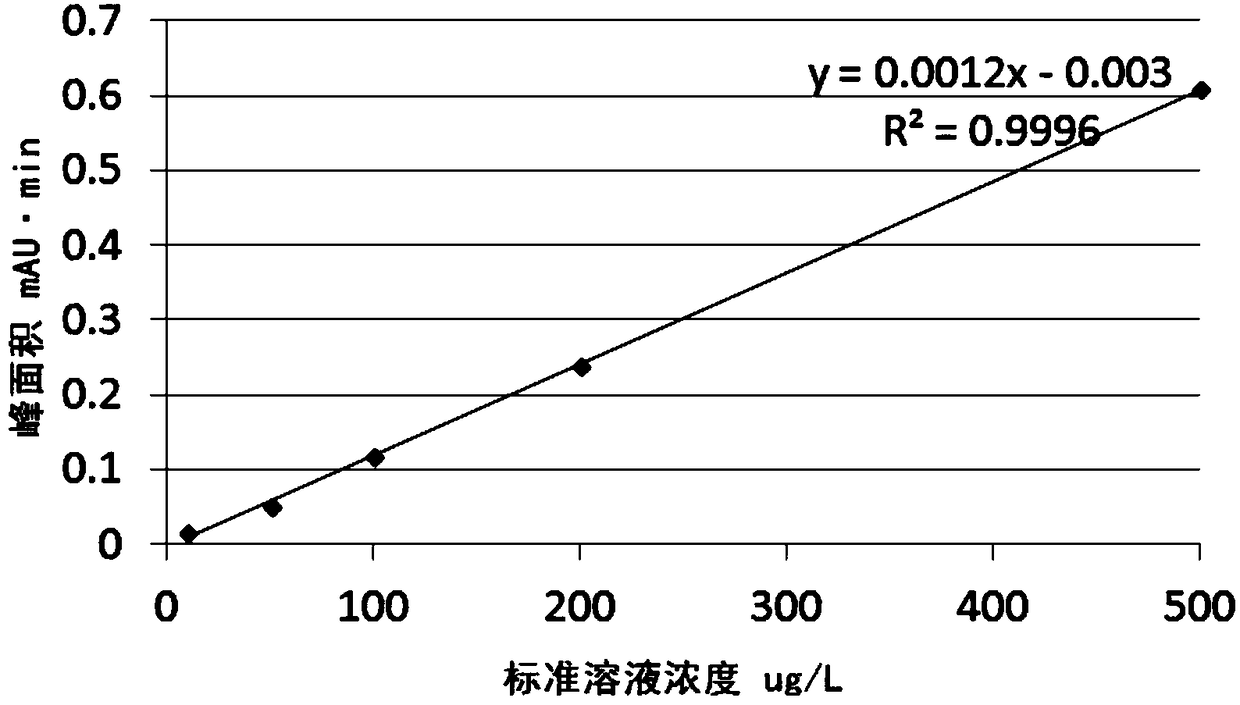
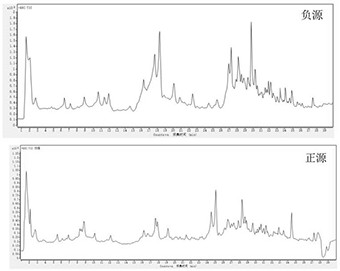
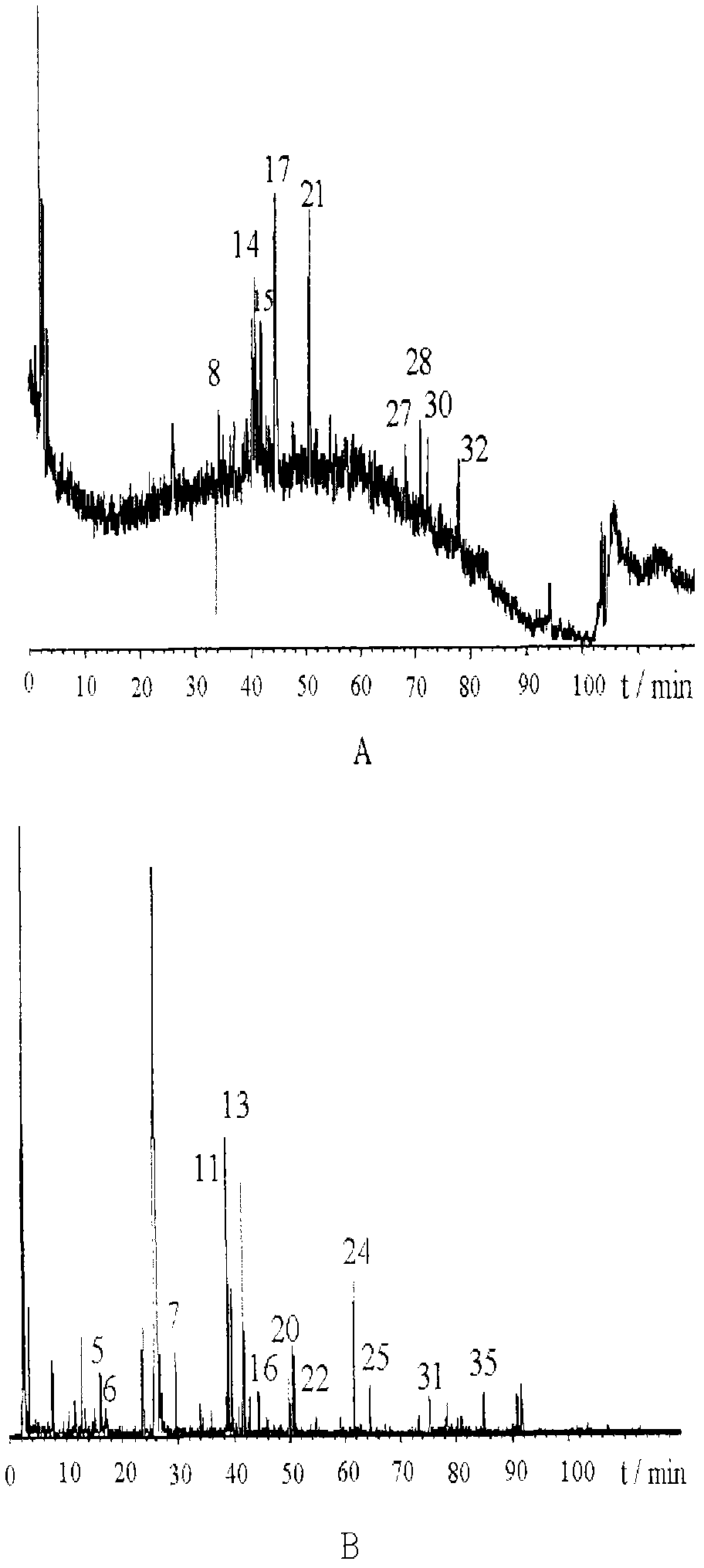
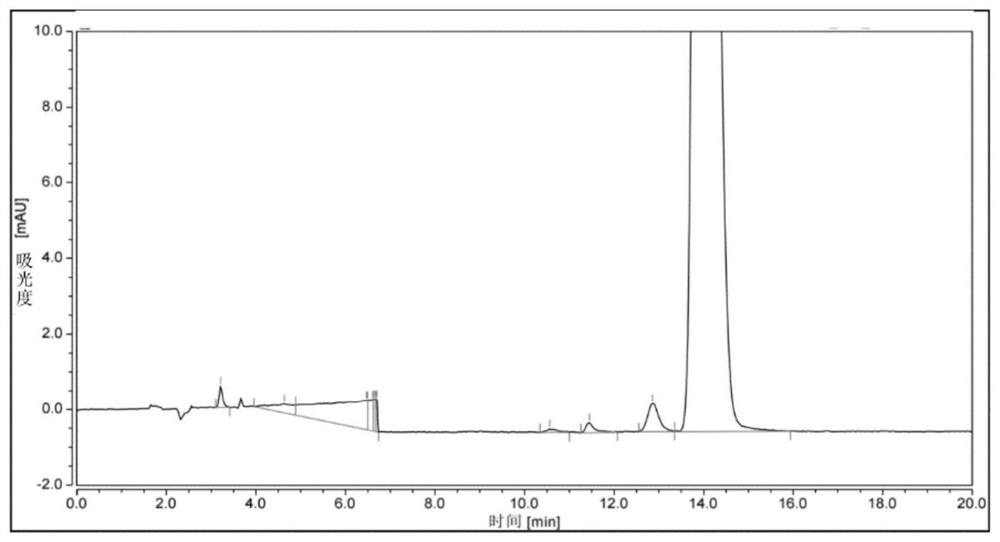
The invention discloses a method of simultaneously determining contents of 1-OHP ,3-OHB[a]P and 3-OHB[a]A in urine. The method comprises sequentially using beta-glucuronidase for enzymatic hydrolysis of samples, performing solid-phase extraction and purification, concentrating the samples with a vacuum rotary evaporator, and determining by using a liquid chromatography-tandem mass spectrometer, thereby rapidly, accurately and simultaneously detecting contents of 1-hydroxy pyrene (1-OHP), 3-hydroxy benzo [a] pyrene (3-OHB [a] P) and 3-hydroxy benzo [a] anthracene (3-OHB [a] A) in the urine. The method uses deuterated standards as quantitative analysis substances of internal standards and thus can reduce errors in a pretreatment process for the samples; uses a tandem mass spectrometer to relatively improve selectivity and accuracy of the method; and selects a method of preparing standards by matrices, wherein, compared with a method of preparing the standards by pure water, the method of preparing standards by matrices is relatively good in accuracy and can eliminate interference from matrix effects. Through selecting and optimizing chromatographic columns and gradient elution conditions, the method relatively improves a chromatogram separating process and shortens a chromatographic analysis time.
Owner:CHINA NAT TOBACCO QUALITY SUPERVISION & TEST CENT
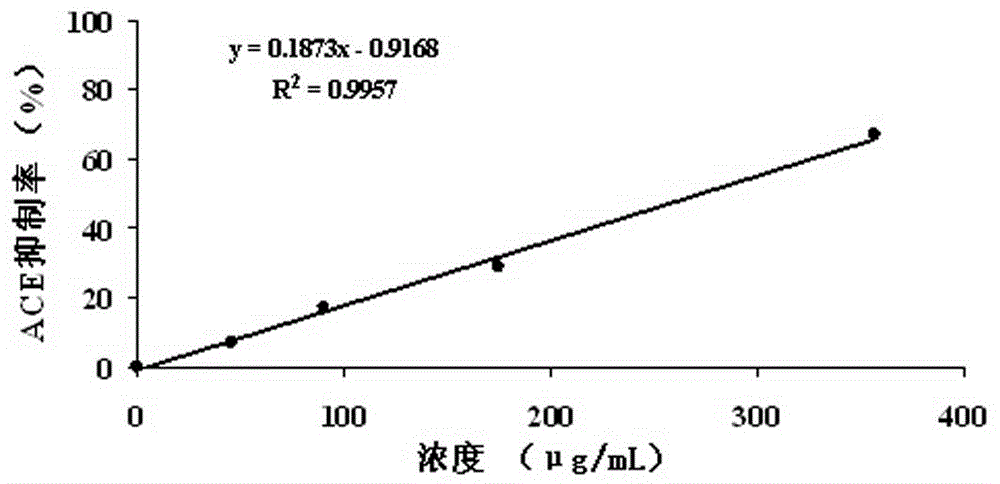
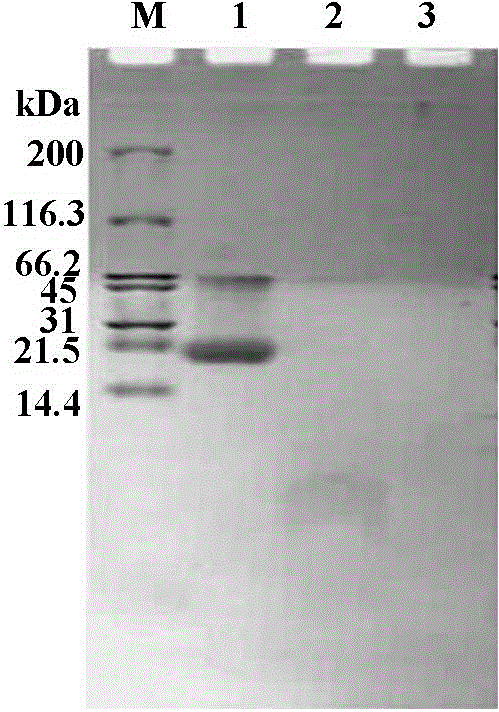
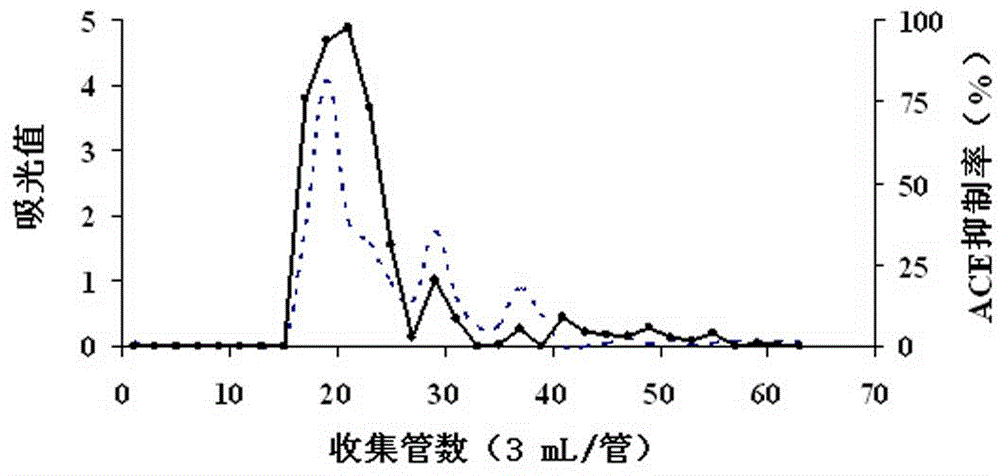
Preparation method for phycoerythrin ACE inhibitory peptide
ActiveCN104131055AOptimizing Chromatographic ConditionsEffective and feasible technical parametersPeptide preparation methodsAlgae/lichens peptidesMolecular biologyPepsin
Owner:JIMEI UNIV
Instrument for synchronously characterizing soluble organic matter structure/physicochemical/concentration characteristics of water sample
PendingCN110082447AAchieve structural/physicochemical/concentration propertiesAchieve comprehensive characterization of structure/physicochemical/concentration propertiesComponent separationFluorescence/phosphorescenceFluorescenceWater quality
The invention relates to an instrument for synchronously characterizing soluble organic matter structure / physicochemical / concentration characteristics of a water sample, and belongs to the technical field of environment detection. All instruments are in single-flow-path connection, and the purpose is that (1) the synchronism and the stability of the whole multi-detector detection system are ensured; and (2) the influence of nitrate ions in the water sample to be detected is removed on the organic nitrogen distribution of the water sample to realize the accurate detection of the organic nitrogen distribution with different molecular weights. According to the invention, ultraviolet absorption / fluorescence characteristics / organic carbon concentration / organic nitrogen concentration of organicmatters with different molecular weights in the water sample can be synchronously detected, and the comprehensive characterization of the structure / physicochemical / concentration characteristics of thewater sample to be detected is realized. Rich quantitative / qualitative information provided by the instrument and the method can be used for research and application of rapid water quality scanning,accurate identification of drinking water disinfection by-product precursors, membrane pollution mechanism and water treatment process optimization and the like in the field of water treatment.
Owner:TONGJI UNIV
Method for rapidly measuring content of bongkrekic acid in food by automatic solid phase extraction-ultra performance liquid chromatography
PendingCN108195954ASatisfy securityEnsure safetyComponent separationFood safetySolid phase extraction
The invention discloses a method for rapidly measuring a content of bongkrekic acid in food by automatic solid phase extraction-ultra performance liquid chromatography. The method comprises steps of pretreatment of food samples, purification of extracted solution and HPLC analysis to obtain the content of bongkrekic acid in the food samples; and results are proved. The method provided by the invention replaces a traditional manual column passing method with an automatic solid phase extraction instrument to realize automation, reduce labor costs and personal errors, realize controllability of solid phase extraction velocity, improve repeatability and accuracy of experiments and shorten a sample treatment cycle; the method optimizes extraction conditions, chromatographic conditions and the like to obtain an excellent separation degree and chromatographic peak, has advantages of high sensitivity, high recovery rate and relatively short pretreatment cycle, meets requirements on detection of the content of bongkrekic acid in food, provides a reference for a laboratory to carry out relevant detections and plays an active role in ensuring food safety.
Owner:ZHEJIANG INST FOR FOOD & DRUG CONTROL
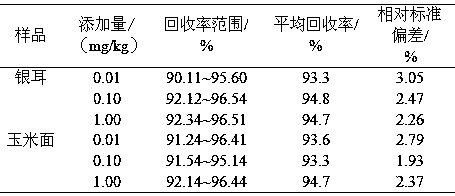
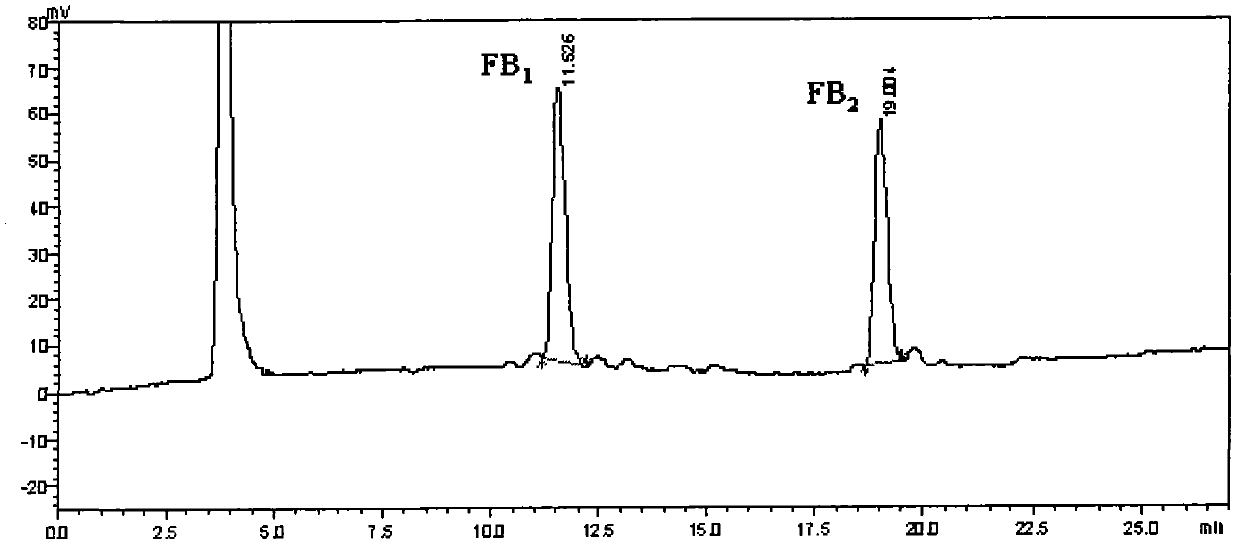
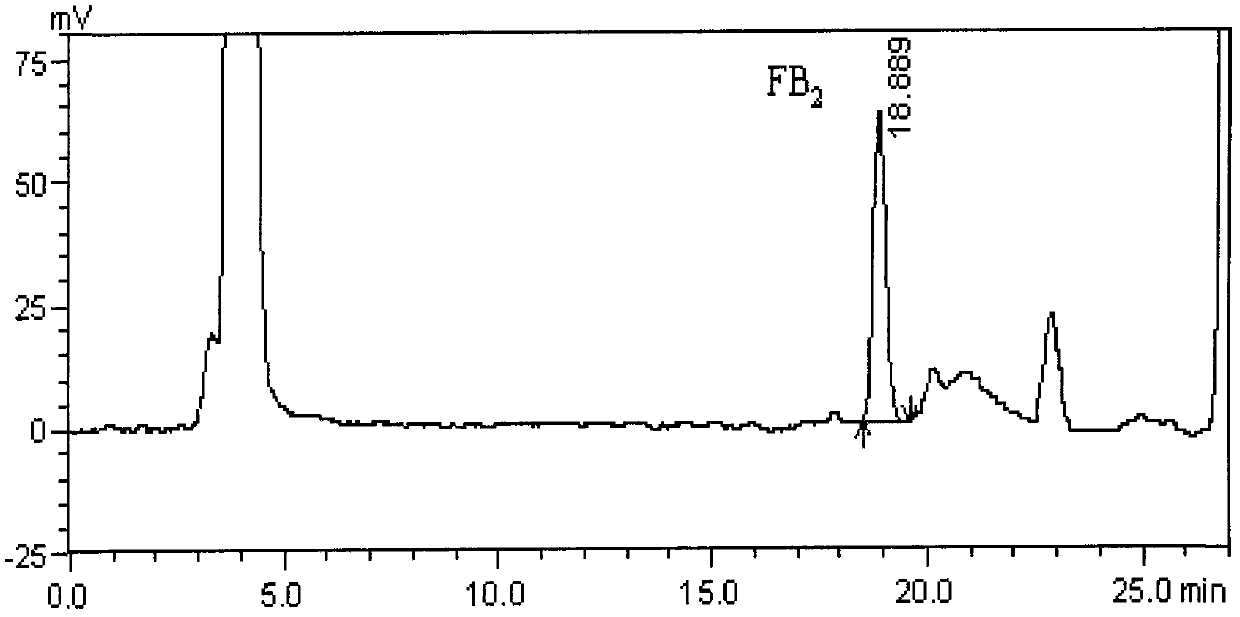
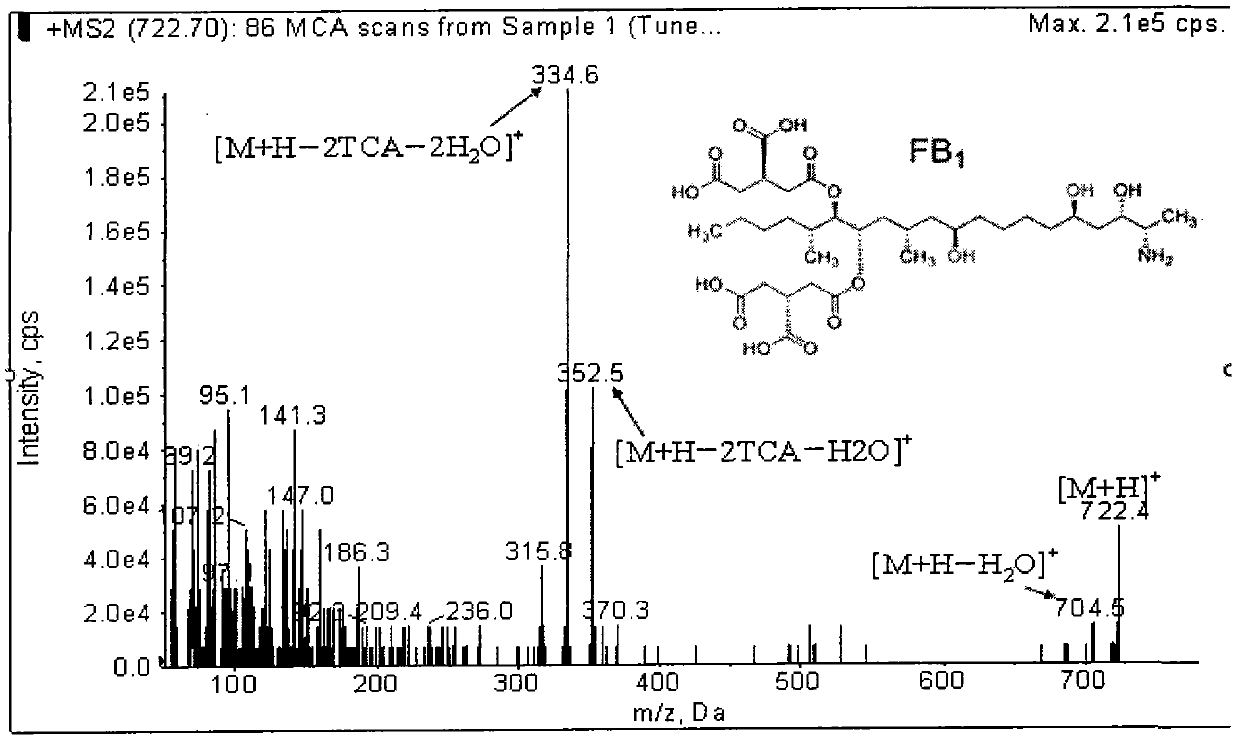
Method for simultaneously detecting fumonisins B1 and B2 in different matrix traditional Chinese medicines
InactiveCN102590364AEasy and fast handlingOptimizing Chromatographic ConditionsComponent separationFiltration membraneFluorescence
The invention relates to a method for simultaneously detecting fumonisins B1 and B2 in different matrix traditional Chinese medicines, namely an on-line derivation high-performance liquid phase fluorescent detection method. The method comprises the steps of extracting, purifying and detecting a sample and particularly comprises the following steps of: adding sodium chloride in the sample; performing oscillating extraction by using a horizontal table according to the volume ratio of methanol to water of 4:1; filtering; diluting a certain amount of filtrate by adding water; filtering by using a microfiber filtration membrane; purifying by using an immunoaffinity column; drying by using nitrogen; dissolving by using methanol; and sampling. A derivation agent is prepared by the following steps of: dissolving a proper amount of o-phthaldialdehyde by using methanol; adding into a proper amount of sodium tetraborate solution; adding 2-mercaptoethanol; mixing uniformly; and passing through a microfiltration membrane. The measurement method of the sample comprises the following steps of: separating by using C18 reversed phase chromatographic column; performing gradient elution by using methanol and sodium dihydrogen phosphate as mobile phase; reacting with the derivation agent; detecting by using a fluorescence detector; and confirming the positive sample by using high-performance liquid chromatography-mass spectrum.
Owner:INST OF MEDICINAL PLANT DEV CHINESE ACADEMY OF MEDICAL SCI
Gas chromatography fingerprint detection method for blood-nourishing brain-refreshing grain
ActiveCN102441058AOptimizing Chromatographic ConditionsThe measurement result is preciseNervous disorderComponent separationTest sampleGas phase
The invention provides a gas chromatography fingerprint detection method for blood-nourishing brain-refreshing grains, which comprises the following steps of: (1) preparing blood-nourishing brain-refreshing grain test sample soution: extracting the blood-nourishing brain-refreshing grain with a steam distillation method, and dissolving a volatile oil ingredient by ethyl acetate; and (2) injecting the volatile oil obtained in step (1) into a liquid chromatograph to measure, and obtaining the chromatogram map of the volatile oil. According to the self characteristics of the blood-nourishing brain-refreshing grain, the fingerprint of the blood-nourishing brain-refreshing grain is measured by the gas chromatography, an optimized chromatogram condition is found, a measured result is precise, and the receptivity and the stability are good.
Owner:TIANJIN TASLY PHARMA CO LTD
Method for measuring content of 5-fluorouracil in plasma and colorectal cancer cells based on high performance liquid chromatography
InactiveCN105588912AHigh recovery rateDetermination of precisionComponent separationTherapeutic effectDrug concentration
The invention relates to a method for measuring content of 5-fluorouracil in plasma and colorectal cancer cells based on high performance liquid chromatography and belongs to the technical field of liquid chromatography medical analysis. 5-fluorouracil (5-Fu) is a miazines antineoplastic drug widely applied clinically at present; the antineoplastic chemotherapy effect of 5-Fu is related to the concentration of 5-Fu in the cancer cells; other side effects are caused when the drug concentration in the body is ultrahigh. For the purpose of enhancing the drug therapeutic effect and reducing the side effects, the trend of the concentration of 5-Fu in the cancer cells and the drug concentration in human body shall be strictly monitored. The monitoring has significant clinical significance in promotion of drug therapeutic effect, reduction of toxicity and prevention of post-operation transfer. According to the method provided by the invention, 5-Fu in plasma and colorectal cancer cells is extracted in the manner of solid phase extraction, so that the extraction efficiency is increased; the chromatographic condition is optimized; the peak pattern is improved by adding a trifluoroacetic acid and methyl alcohol system into a detected flow phase; the number of theoretical plates is increased and the chromatographic condition is mild; under a selected optimal chromatographic condition, the measurement for 5-Fu is more precise and accurate than the measurement in literature reports.
Owner:JIANGSU INST OF NUCLEAR MEDICINE
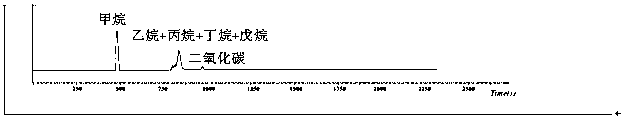
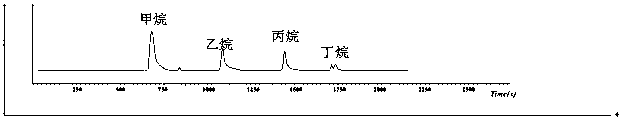
Method for analysis chromatographic separation of hydrocarbon compound carbon isotopes in natural gas
InactiveCN107831250AAccurate measurementFacilitates manual preparationComponent separationChromatographic separationGas phase
The invention discloses a method for analysis chromatographic separation of hydrocarbon compound carbon isotopes in natural gas, wherein the method comprises the following steps: (1) taking a naturalgas sample, injecting into a sample loop of a gas chromatography instrument, allowing to enter a filling column, carrying out chromatographic separation, and setting a chromatographic temperature-raising program: setting the initial temperature to 40 DEG C, making the temperature constant for 1 minute, then heating to 165 DEG C at a speed of 5 DEG C / min, making the temperature constant for 15 minutes, taking helium gas as a carrier gas, setting the flow rate at 13 mL / min, and taking the filling column as propack Q filling column; and collecting a separated hydrocarbon compound; and (2) carrying out carbon isotope analysis. The method combines the chromatographic separation method and the program switching valve; during chromatographic separation, suitable peak appearing intervals exist between chromatographic peaks; with cooperation of the program switching valve, subsequent manual preparation of hydrocarbon gas is facilitated. The problem that accurate and effective separation and enrichment of hydrocarbon gas in natural gas is difficult is solved, and then accurate determination of the hydrocarbon gas isotopes is realized.
Owner:CHINA PETROLEUM & CHEM CORP +1
Method for detecting organic tin in water body
PendingCN111948322AMeet analysis requirementsShort elution timeComponent separationPhosphateCitrate salt
The invention discloses a method for detecting organic tin in a water body. The method specifically comprises the following steps: carrying out liquid-liquid extraction reflux on a to-be-detected sample solution; adding a certain amount of extract liquor into a phosphate citrate buffer solution, normal hexane and a sodium tetraethylborate solution for derivatization; separating the extract liquorthrough a chromatographic column, and comparing with a standard solution by using a flame photometric method to obtain a standard curve, so as to calculate and detect the organic tin in the water body. According to the method, a liquid-liquid extraction gas chromatography flame photometry detector is adopted to determine the concentration of the organic tin compound in the water, derivatization and extraction are performed at the same time in the step S4; therefore, compared with solid-phase extraction in the background art, complex elution steps are not needed, the elution time is saved, theextraction and derivatization time is shortened, operation is easy and rapid. In addition, the actual sample analysis requirements can be met.
Owner:HOHAI UNIV
Separation preparation method of bazedoxifene acetate impurity A
InactiveCN104774170AEfficient separationOptimizing Chromatographic ConditionsOrganic chemistryMedicinal chemistryBazedoxifene
The invention relates to a method for preparing an impurity A having the purity of more than 97% from a bazedoxifene raw material, can be applied in research of reference substances and belongs to the field of medicine.
Owner:JIANGSU CAREFREE PHARM CO LTD
Tetranitrodocosyl heterocyclic ring chromatographic stationary phase and preparation method and application thereof
ActiveCN105664889AImprove hydrophobicityImprove hydrophilic abilityOther chemical processesHexamethylenediaminePesticide residue
The invention discloses a tetranitrodocosyl heterocyclic ring chromatographic stationary phase. A preparation method includes: using hydrochloric acid to acidize porous spherical silica gel for chromatography, and water washing to neutral to realizing activation of the surface of silica gel; utilizing closed ring reaction to enable docosyl heterocyclic ring ligand generated by reaction of acrylic aldehyde and hexamethyldiamine to react with gamma-isocyanatepropyltriethoxy silane, and modifying a synthetic silane heterocyclic ring on the surface of a silica gel carrier to realize high efficiency and high selectivity of the stationary phase. The tetranitrodocosyl heterocyclic ring chromatographic stationary phase prepared by the method has excellent physical structure of silica gel matrix and has special chromatographic performance of nitrogen containing micro-ring; a structure of multi-nitrogen multi-alkyl heterocyclic ring long chain is bonded on the surface of silica gel, so that the stationary phase has high hydrophobicity and has certain hydrophilicity, thereby having high separating performance on pesticide residue (polycyclic aromatic hydrocarbons, phenols and amine); chromatographic analysis conditions after optimization are simpler and more convenient and easy to operate, so that application prospect is quite good.
Owner:ZHENGZHOU UNIV
Tetradocosyl heterocycle bonded silica gel chromatographic support, and preparation method and application thereof
InactiveCN107486187AImprove physical structureImprove hydrophobicityComponent separationOther chemical processesHexamethylenediamineSilanes
The invention discloses a preparation method of a tetradocosyl heterocycle chromatographic support, and belongs to the field of preparation and application of high performance liquid chromatographic support. The method is operated according to the following steps: firstly performing acidizing on the surface of porous spherical silica gel for chromatography, washing the surface to neutral, realizing silica gel surface activation, then ensuring that acrolein and hexamethylenediamine are reacted through simple ring-closure reaction to produce docosyl heterocycle ligand to be reacted with gamma-isocyanatopropyltriethoxysilane, and ensuring that silane heterocycle formed through synthesis is modified to the surface of a silica gel carrier, so as to obtain a tetradocosyl heterocycle bonded silica gel chromatographic support (Sil-4N-22R) with excellent separation and selection properties. High efficiency and high selectivity of a stationary phase are realized.
Owner:GANSU AGRI UNIV
Determination method of formaldehyde-DNA adducts in saliva
InactiveCN105938124AOptimizing Chromatographic ConditionsOptimize detection conditionsComponent separationChromatographic separationHydrolysate
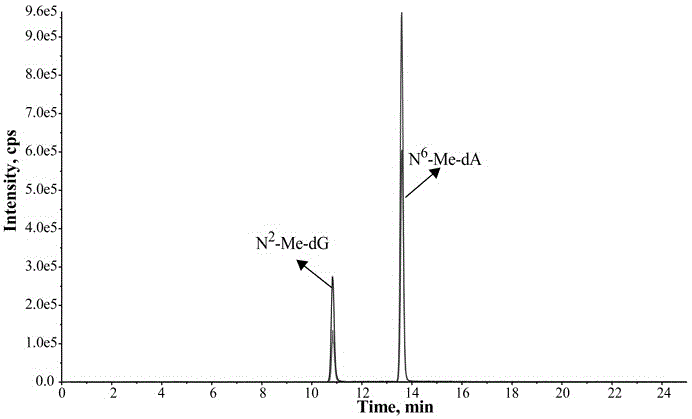
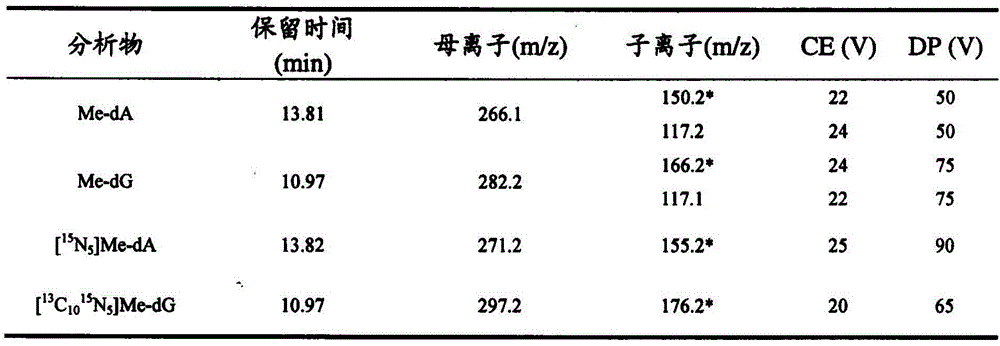
Provided is a determination method of formaldehyde-DNA adducts in saliva, namely the determination method of HOMe-dA and HOMe-dG in the saliva; the determination method includes the steps: collecting the saliva by using an OG-500 saliva collection tube, carrying out DNA extraction on the saliva, carrying out enzyme hydrolysis of the DNA solution, and successively adding a stable isotope internal standard, a reducing agent NaBH3CN and a DNA hydrolase; and carrying out solid-phase extraction of the hydrolysate with a Strata-X small column, collecting an eluted liquid, nitrogen-blowing to be dried at room temperature, redissolving in a methanol aqueous solution, introducing into an LC-MS / MS system, analyzing, and accurately detecting the content levels of Me-DA and Me-dG. The stable isotope is used as an internal standard quantitative analysis material, so the error caused by the sample pretreatment process can be reduced, and the selectivity, accuracy and sensitivity of the method can be improved by tandem mass spectrometry. Through selection and optimization of the chromatographic column and the elution conditions, the chromatographic separation process is relatively improved, the time of chromatographic analysis is shortened, and the consumption amount of an organic solvent is decreased.
Owner:CHINA NAT TOBACCO QUALITY SUPERVISION & TEST CENT
A kind of detection method of polyethylene glycol content
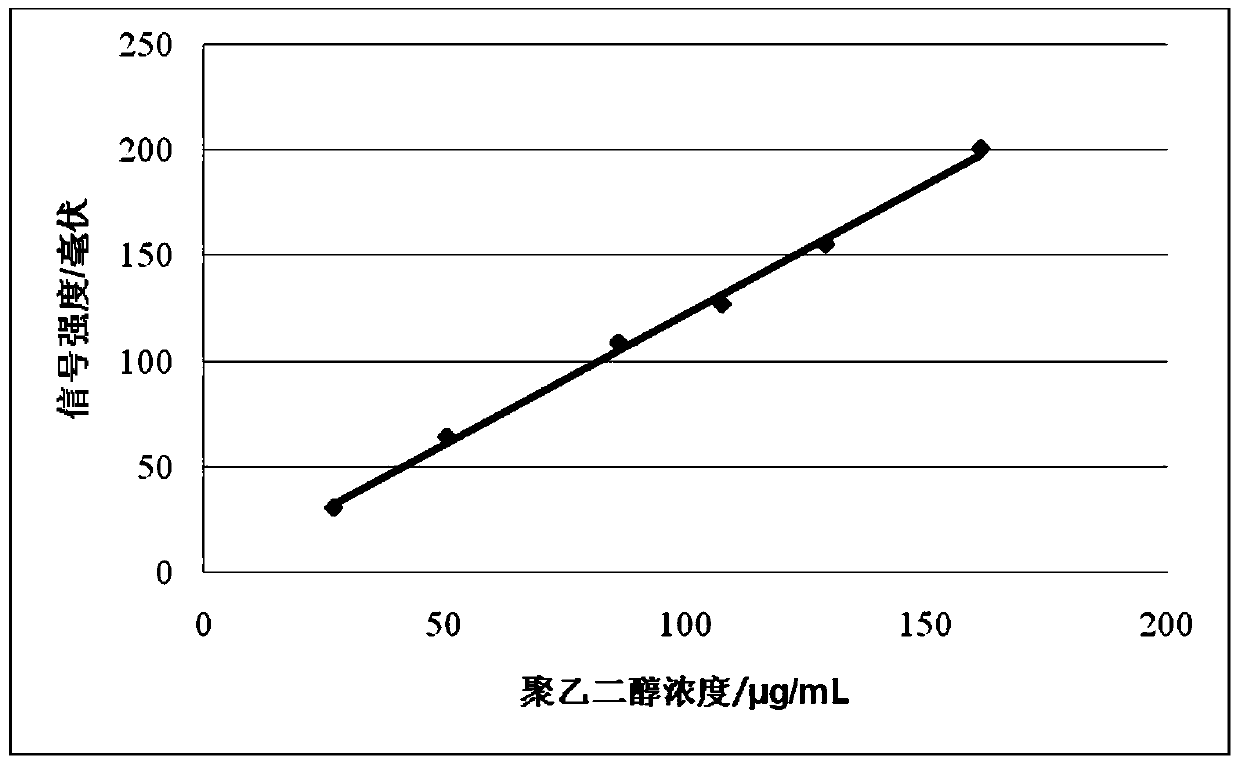
ActiveCN105548422BExtended elution timeEasy to separateComponent separationMedical equipmentPolythylene glycol
The invention discloses a detection method for polyethylene glycol content. The detection method utilizes the differences among the hydromechanical volumes of all components in a sample and, by optimizing chromatographic conditions, chooses a size exclusion chromatography column with appropriate grain size and aperture of chromatographic column packing to carry out high-quality separation, the chromatographic peak of polyethylene glycol is identified by a conventional common detector for laboratories, regression is carried out by an external standard method, and the absolute content of the polyethylene glycol in the sample is then quantified. The detection method disclosed by the invention solves the limitation in conventional methods, such as poor separation degree, low trace content test accuracy and test results only being capable of being expressed by percentage content, and the detection method is particularly suitable for detecting polyethylene glycol content or polyethylene glycol residue quantity in drugs or drug-carrying medical equipment, food, health-care products and other biological products.
Owner:LIFETECH SCI (SHENZHEN) CO LTD
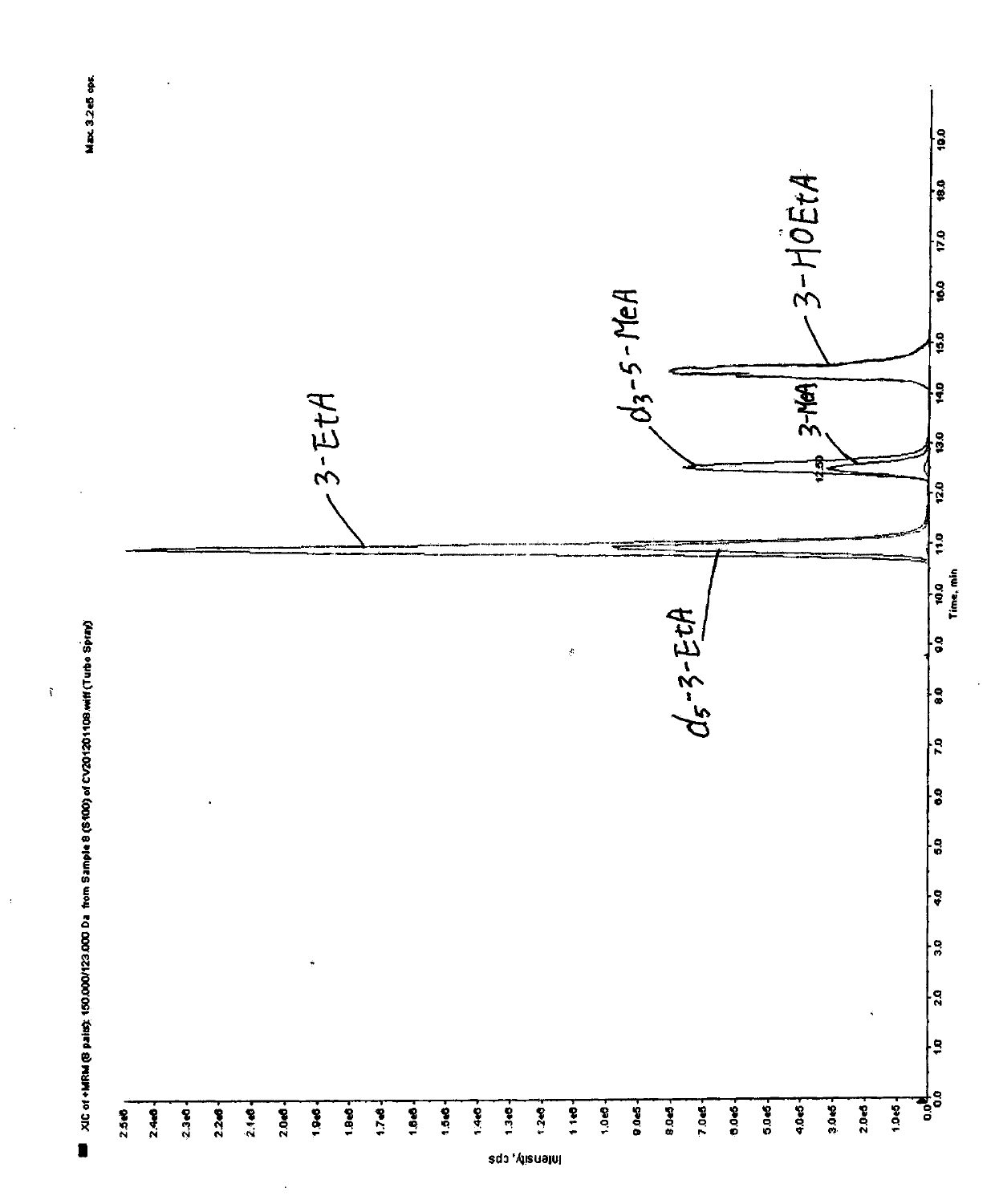
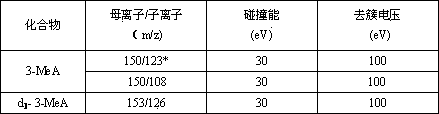
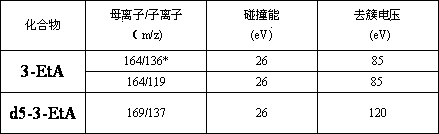
Determination method of 3-alkylated adenine DNA adducts in urine
ActiveCN103808836AOptimizing Chromatographic ConditionsOptimize detection conditionsComponent separationChromatographic separationAnalyte
The invention discloses a determination method of 3-alkylated adenine DNA adducts in urine, namely the determination method of 3-methyl adenine (3-MeA), 3-ethyl adenine (3-EtA) and 3-hydroxyethyl adenine (3-HOEtA). The determination method is characterized in that the test process comprises the following steps: unfreezing urine within 24 hours at room temperature, uniformly mixing and centrifugally filtering, purifying and enriching through an OasisMCX solid phase extraction small column, mixing to be uniform and then introducing into an LC-MS / MS system to analyze so as to accurately detect the content level of 3-MeA, 3-EtA and 3-HOEtA in the urine. The invention relates to the determination method of the 3-alkylated adenine DNA adducts in fresh urine, a deuterated standard product is used as an interior standard quantitive analyte so as to reduce the error caused in the pre-treatment process of the sample, and a tandem mass spectrometry well improves the selectivity, accuracy and sensitivity of the method. The chromatographic separation process is better improved through the selection and optimization of a chromatographic column and an elution condition, the chromatographic analysis time is shortened, and the consumption of an inorganic solvent is reduced.
Owner:CHINA NAT TOBACCO QUALITY SUPERVISION & TEST CENT +1
System and method for simultaneously determining content of trivalent chromium and hexavalent chromium through ion chromatography post-column derivation
InactiveCN109001366AMeet the test requirementsLow detection limitComponent separationIon chromatographyWater quality
The invention discloses a system and method for simultaneously determining content of trivalent chromium and hexavalent chromium through ion chromatography post-column derivation. The system comprisesa sample flow path, an eluent flow path, a post-column derivation liquid flow path, an analysis flow path, an injection valve, an injection ring, an ultraviolet-visible detector and a computer processing system, wherein the analysis flow path is formed by sequentially connecting a cation exchange chromatographic column, a three-way valve, a braided reaction tube and an optical flow cell in series. According to the method disclosed by the invention, the content of trivalent chromium and hexavalent chromium is simultaneously determined by adopting an IC-UV-Vis method, the method has existing application in water quality detection, trivalent chromium and hexavalent chromium in drinking water can be simultaneously determined by existing researchers by ion chromatography, the detection limit is low, and the method is excellent in reproducibility and sensitivity. Since the trivalent chromium and hexavalent chromium in toy materials need to be leached and tested, the leaching method in EN71-3 is used in the research, the chromatographic conditions are optimized after leaching liquor treatment, and the content of the trivalent chromium and hexavalent chromium in the leaching liquor is tested. The method is high in sensitivity and excellent in repeatability.
Owner:INSPECTION AND QUARANTINE TECHNOLOGY CENTER ZHONGSHAN ENTRY EXIT INSPECTION AND QUARANTINE
Comprehensive analysis method for chemical components of compound Xiling detoxification preparation
ActiveCN112485345AReduce sensitivityComprehensive analysisComponent separationColor/spectral properties measurementsChemical compoundChemical composition
The invention aims at taking phenolic acids, flavonoids and triterpenoids chemical components in a compound Xiling detoxification capsule or a compound Xiling detoxification tablet as research objectsand realizing comprehensive and rapid separation and identification of the chemical components of the compound Xiling detoxification capsule or the compound Xiling detoxification tablet on the basisof a UPLC-Q-TOF-MS analytical instrument and an analysis strategy of target precursor ions. The research comprises the following steps: firstly, carrying out information integration on phenolic acid,flavone and triterpenoids in the compound Xiling detoxification capsule or tablet according to literature, and summarizing structural characteristics and structural change rules of the compound Xilingdetoxification capsule or tablet; and performing mass spectrometry on each representative compound with different structural characteristics, and summarizing a mass spectrometry cracking rule and a neutral loss rule; then, taking a representative compound as a core, and constructing a target precursor ion list according to the structure change rule of each type of compound; and finally, analyzingthe cracking mode and the neutral loss rule of the target precursor ions by adopting secondary mass spectrometry, verifying the correctness of the target precursor ions, and comprehensively analyzingthe mass spectrometry, the retention time and other information to comprehensively and quickly analyze the chemical components in the compound Xiling detoxification capsule or tablet.
Owner:山东宏济堂制药集团股份有限公司
Quality analysis method of Thesium chinense tablets
InactiveCN108802208AEffective quality controlImprove standardizationComponent separationPhosphoric acidSilica gel
The invention belongs to the technical field of pharmacy and particularly relates to a quality analysis method of Thesium chinense tablets. The quality analysis method includes: providing the standardsolution of kaempferol and the test solution of the Thesium chinense tablets; performing liquid chromatography on the standard solution and the test solution, wherein chromatographic conditions include: octadecyl silane bonded silica gel is used as the filling agent, methanol-0.5% phosphoric acid solution with the volume ratio being 62:38 is used as the flowing phase, detection wavelength is 368nanometers, column temperature is 30 DEG C, flow rate is 1.0ml / minute, and theoretical plate number calculated according to kaempferol peak is not lower than 4000; performing quality analysis on the standard solution and the test solution. The quality analysis method has the advantages that the method is good in effect as compared with existing Thesium chinense particle standards, pharmacopeia standards and Thesium chinense tablet standards, the chromatographic conditions are improved, and the quality of the Thesium chinense tablets can be evidently controlled.
Owner:HONG KONG JOWA & HUAYUAN GRP CHUZHOU PHARMA CO LTD
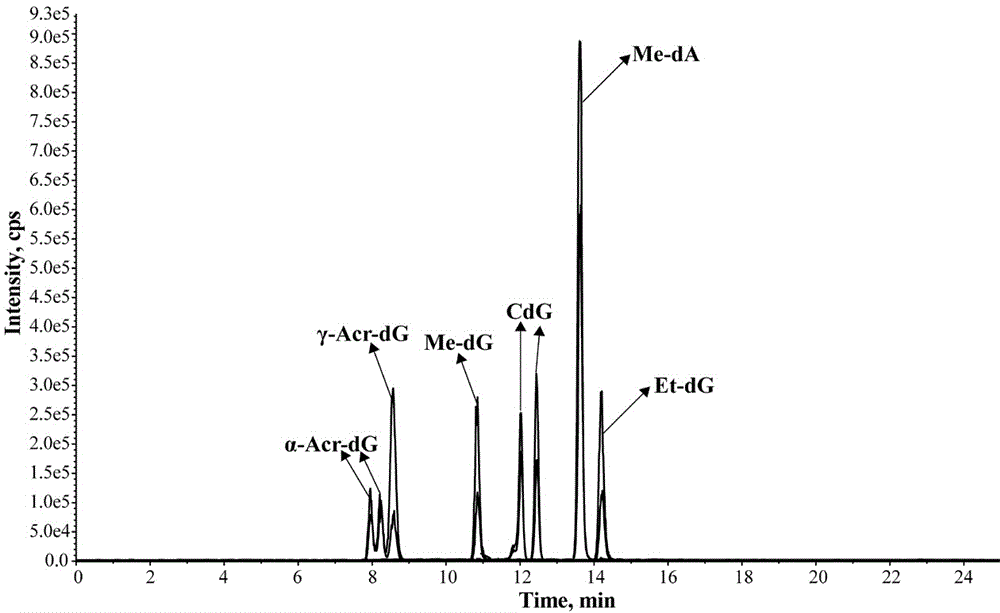
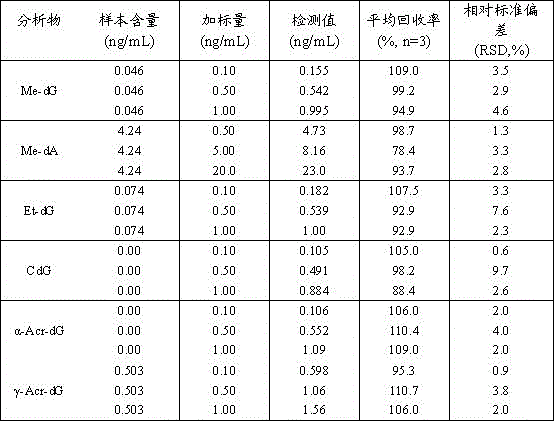
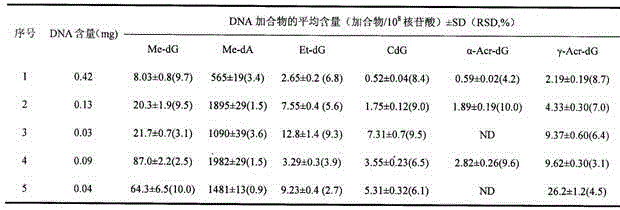
Method for measuring six aldehyde-DNA adducts in saliva
The invention discloses a method for measuring six aldehyde-DNA adducts in saliva, namely a method for measuring HOMe-dA, HOMe-dG, Ethylidene-dG, CdG, alpha-Acr-dG and gamma-Acr-dG in saliva. The method comprises the following steps: collecting saliva by an OG-500 saliva collecting tube, performing DNA extraction on the saliva, performing enzyme hydrolysis on a DNA solution, and sequentially adding stable isotope internal standards, a reducing agent, NaBH3CN and DNA hydrolase; and performing solid-phase extraction on hydrolysate via Strata-X columella, collecting an eluant, nitrogen-blowing at room temperature till the product is dried, re-dissolving into a water solution of methyl alcohol, and introducing LC-MS / MS system analysis, thereby accurately detecting the content levels of the six substances. With stable isotope as internal standard quantitative analyte, errors caused in pretreatment of samples can be reduced, and the selectivity, accuracy and sensitivity of the method are better improved by LC-MS / MS. By selecting and optimizing chromatographic columns and elution conditions, the chromatographic separation process is better improved, the time for analysis is shortened, and the consumption of a solvent is reduced.
Owner:CHINA NAT TOBACCO QUALITY SUPERVISION & TEST CENT
Method for detecting residual quantity of organic solvent in formoterol bulk drug
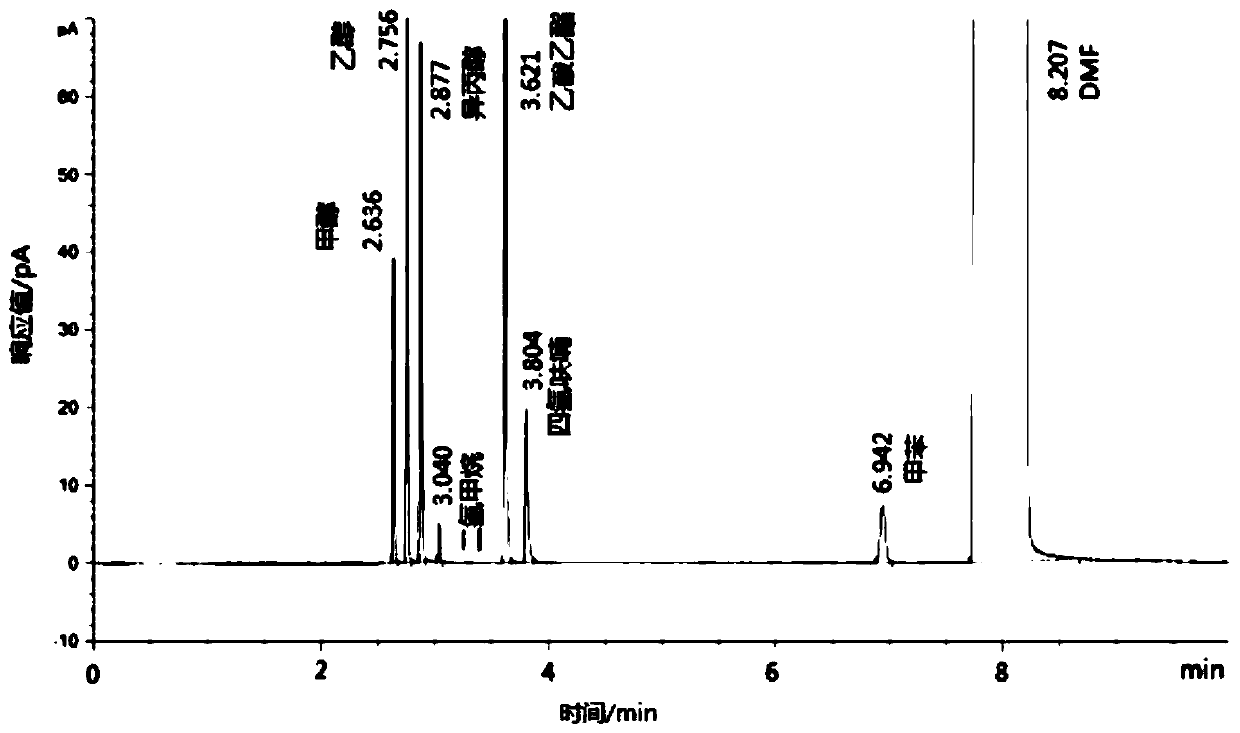
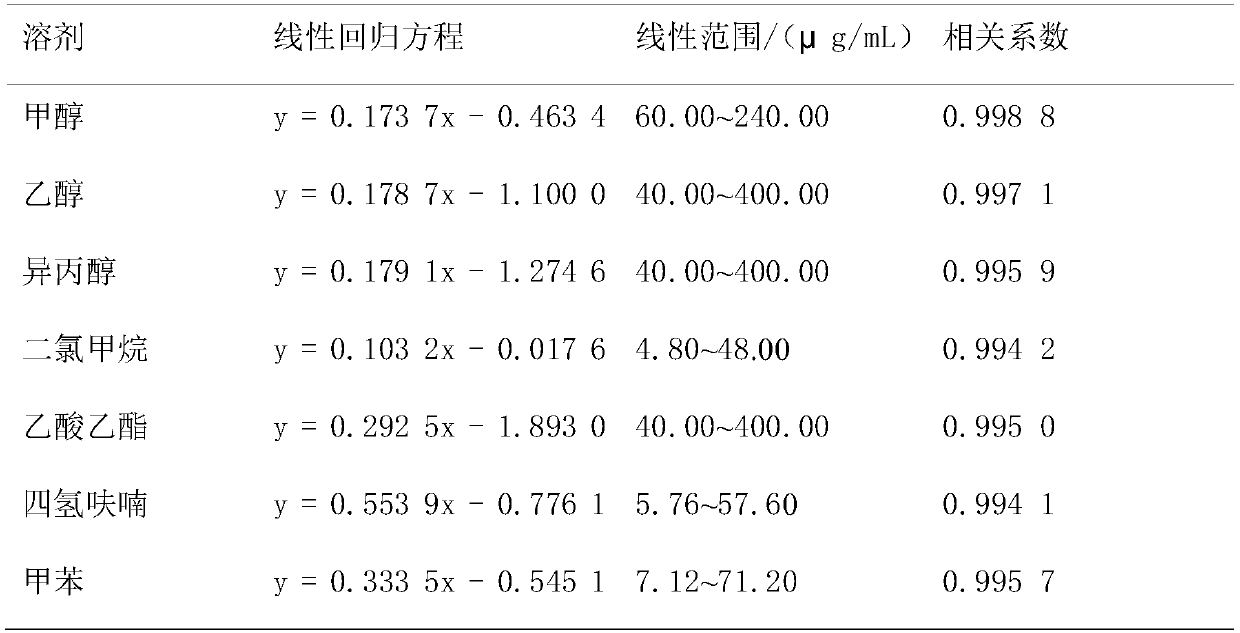
InactiveCN111380992AHigh sensitivityOptimizing Chromatographic ConditionsComponent separationGas liquid chromatographicNitrogen gas
The invention relates to a method for detecting the residual quantity of an organic solvent in a formoterol bulk drug, and belongs to the technical field of drug analysis. The detection method comprises the following steps: preparing a reference substance solution and a test sample solution, and detecting by using a gas chromatography external standard method under the chromatographic conditions:an HP-5 capillary column and a hydrogen flame ionization detector are adopted, nitrogen is used as carrier gas, and the flow rate is 0.8-1.2 mL / min; the temperature of a sample inlet is 195-205 DEG C,and the split ratio is 1: 1, the detector temperature is 245-255 DEG C, the hydrogen flow rate is 30 mL / min, the air flow rate is 300 mL / min, and the tail blowing rate is 25 mL / min; the heating temperature of the headspace bottle is 100 DEG C, the quantitative loop is 110 DEG C, the transmission line is 120 DEG C, the heating time of the headspace bottle is 30 minutes, and the pressurizing time is 0.2 minute. The method has the characteristics of simplicity, convenience, quickness and accuracy, and can be used for simultaneously detecting the residual quantity of seven organic solvents in formoterol tartrate.
Owner:广州卫生职业技术学院
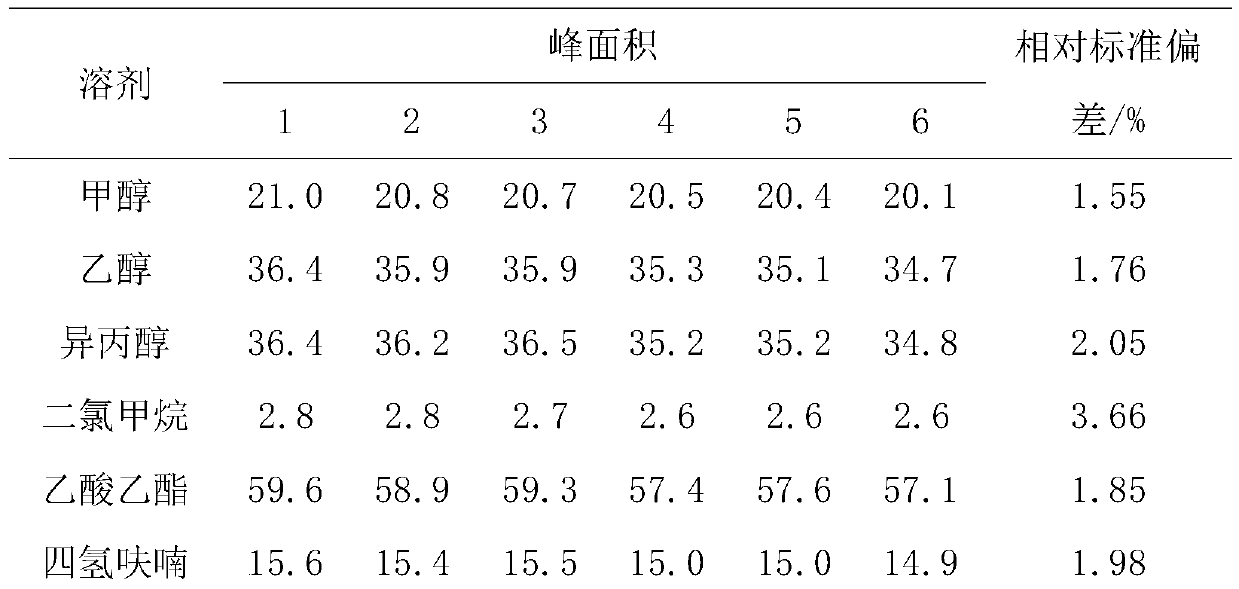
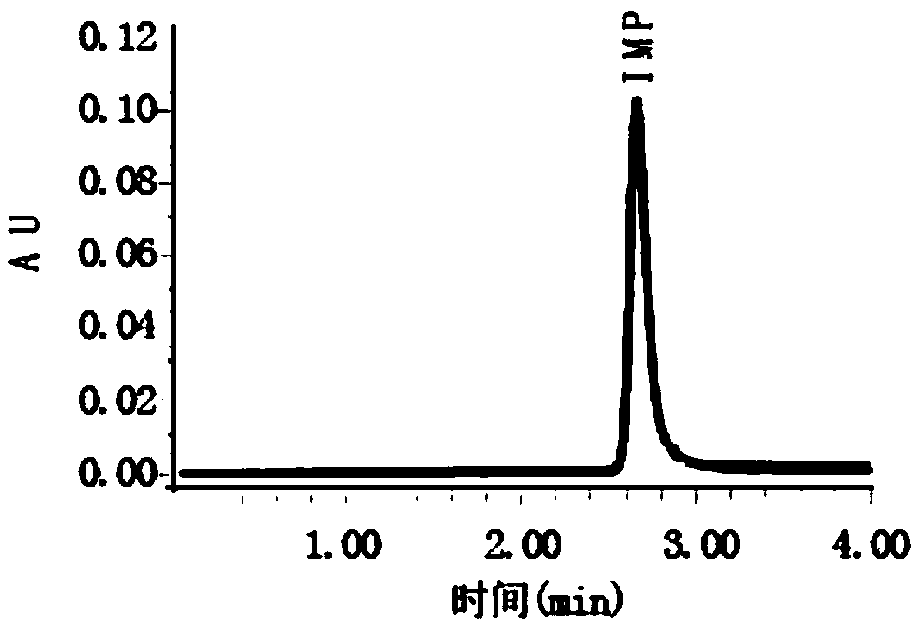
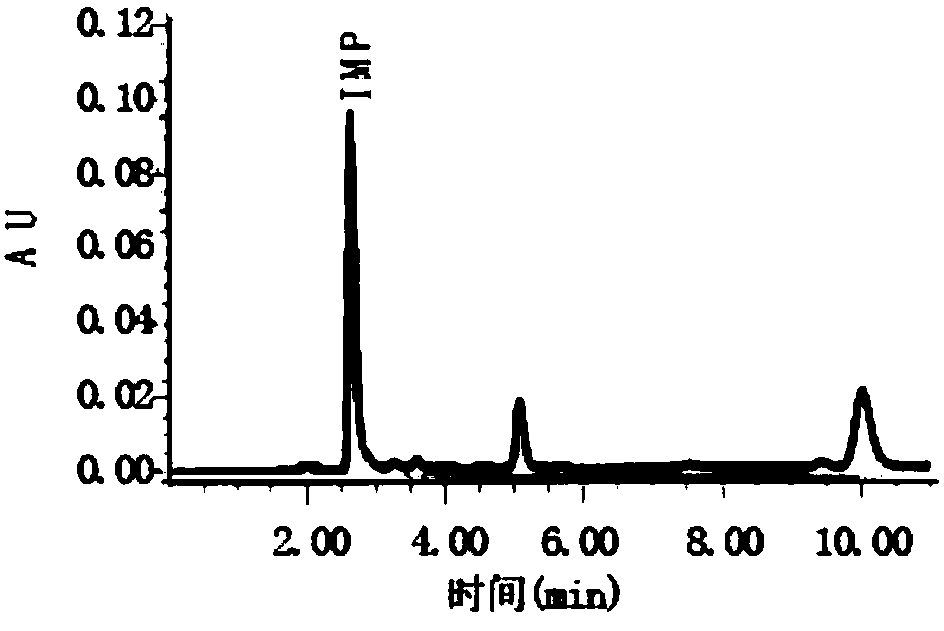
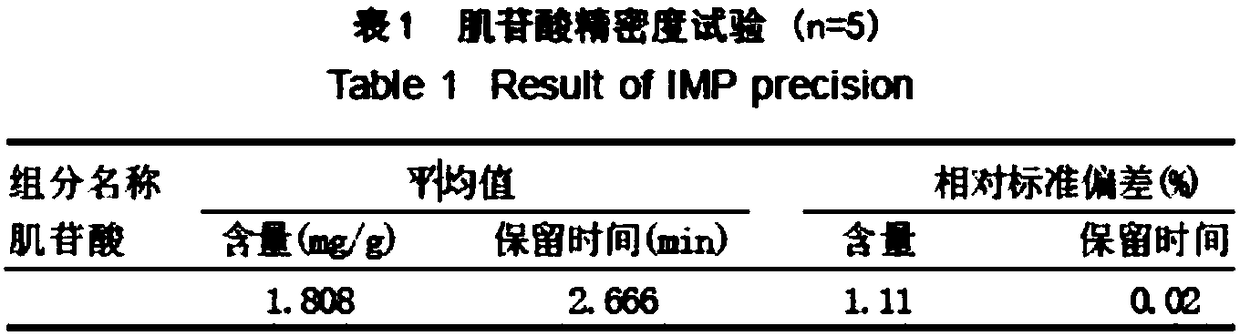
Liquid chromatography detection method for inosinic acid content in chicken
The invention discloses a liquid chromatography detection method for inosinic acid content in chicken. The method comprises the following steps: 1, selecting raw materials; 2, selecting reagents and main instruments; 3, preparing a main solution; 4, treating the raw materials; 5, setting chromatographic conditions; and 6, analyzing and calculating detection results. According to the method disclosed by the invention, ammonium formate adopted in a mobile phase is a water-soluble weak acid salt, and chemical characteristics of the ammonium formate are possibly favorable for prolonging the service life of a chromatographic column, so that the method is applicable to determination of lots of samples. The ammonium formate buffer solution serves as the mobile phase for performing reversed-phaseliquid chromatography, the chromatographic conditions are optimized, the determination precision and accuracy of inosinic acid content in muscle tissue samples are increased, and the aim of accuratelyand rapidly determining the inosinic acid content is achieved. The liquid chromatography detection method can be applied to detection and analysis of the inosinic acid content in fresh meat foods.
Owner:LANZHOU INST OF ANIMAL SCI & VETERINARY PHARMA OF CAAS
Novel chlorine-enhanced ionization reagent for liquid chromatography-mass spectrometry detection and application thereof
ActiveCN112255323AHigh detection sensitivityHigh selectivityComponent separationFluid phaseLiquid chromatography mass spectroscopy
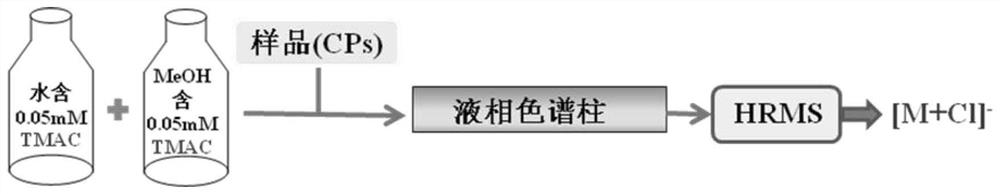
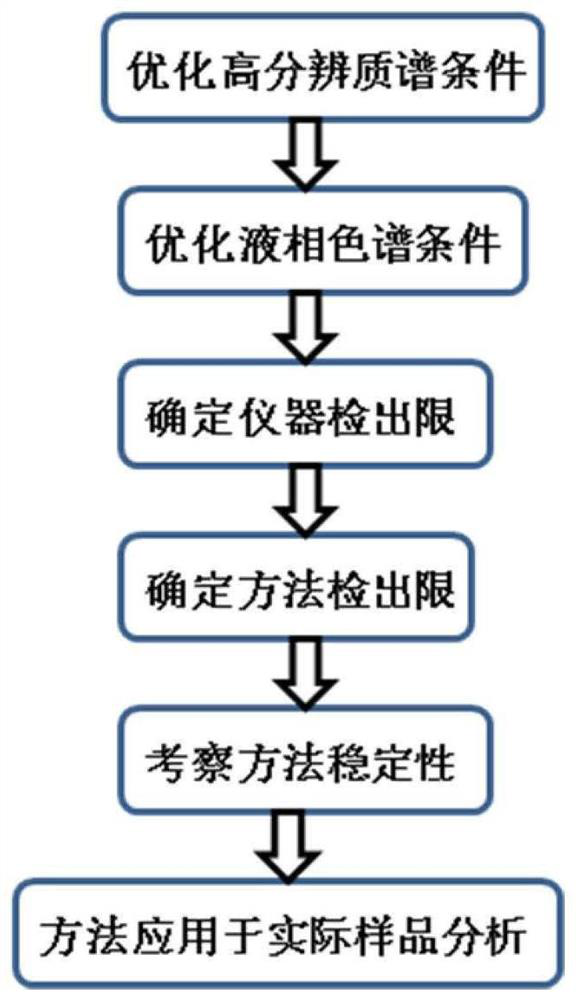
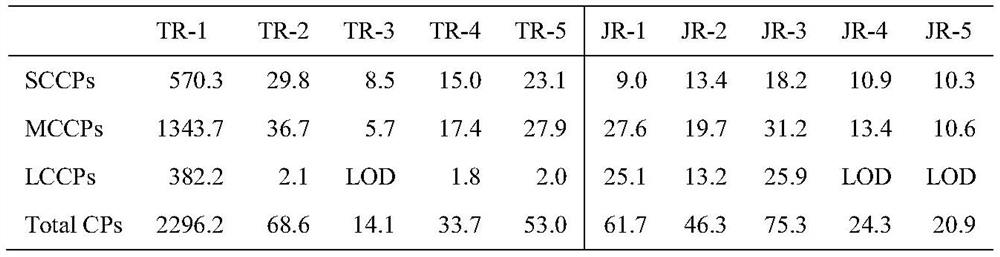
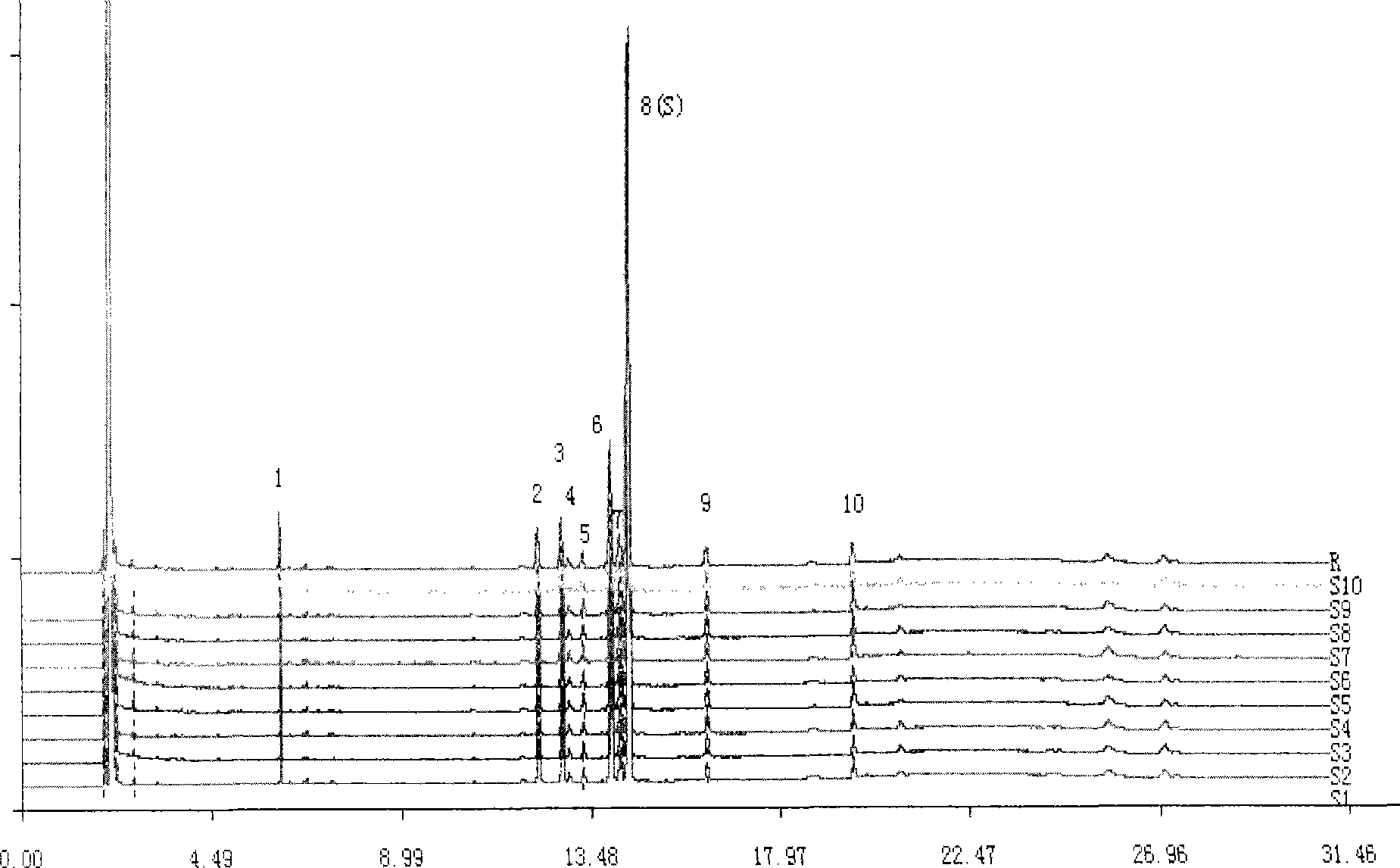
The invention discloses a novel chlorine-enhanced ionization reagent for liquid chromatography-mass spectrometry detection. The novel chlorine-enhanced ionization reagent is organic chlorine salt-tetramethylammonium chloride (TMAC) with volatility, and has no corrosion to a liquid chromatography system and a mass spectrometry system. The invention provides application of the chlorine-enhanced ionization reagent in liquid chromatography-high-resolution mass spectrometry detection of chlorinated paraffin, the result shows that TMAC is used as a mobile phase additive, the CPs detection sensitivity and selectivity can be remarkably improved under the condition of original configuration of an instrument, and the method is not influenced by the CPs carbon chain length and the chlorine content.
Owner:广东省农业科学院农业质量标准与监测技术研究所
Gas chromatography fingerprint detection method for blood-nourishing brain-refreshing grain
ActiveCN102441058BOptimizing Chromatographic ConditionsThe measurement result is preciseNervous disorderAntipyreticTest sampleGas phase
The invention provides a gas chromatography fingerprint detection method for blood-nourishing brain-refreshing grains, which comprises the following steps of: (1) preparing blood-nourishing brain-refreshing grain test sample soution: extracting the blood-nourishing brain-refreshing grain with a steam distillation method, and dissolving a volatile oil ingredient by ethyl acetate; and (2) injecting the volatile oil obtained in step (1) into a liquid chromatograph to measure, and obtaining the chromatogram map of the volatile oil. According to the self characteristics of the blood-nourishing brain-refreshing grain, the fingerprint of the blood-nourishing brain-refreshing grain is measured by the gas chromatography, an optimized chromatogram condition is found, a measured result is precise, and the receptivity and the stability are good.
Owner:TIANJIN TASLY PHARMA CO LTD
High performance liquid chromatography (HPLC) fingerprint detection method for blood-nourishing brain-refreshing grain
ActiveCN102441057BOptimizing Chromatographic ConditionsThe measurement result is preciseNervous disorderComponent separationHplc fingerprintAlcohol
The invention discloses a high performance liquid chromatography (HPLC) fingerprint detection method for blood-nourishing brain-refreshing grains, which is characterized by comprising the following steps of: 1) preparing blood-nourishing brain-refreshing grain test sample soution, precisely weighing the blood-nourishing brain-refreshing grain, and preparing into solution by 3 parts of methyl alcohol and 1 part of water to serve as preparation test sample solution; and 2) injecting the solution obtained in step 1) into a high performance liquid chromatograph for measuring to respectively obtain the chromatogram map of the solution. According to the self characteristics of the blood-nourishing brain-refreshing grain, the fingerprint of the blood-nourishing brain-refreshing grain is measured by the high performance liquid chromatography, an optimized chromatogram condition is found, a measured result is precise, and the receptivity and the stability are good.
Owner:TIANJIN TASLY MORDEN TCM RESOURCES
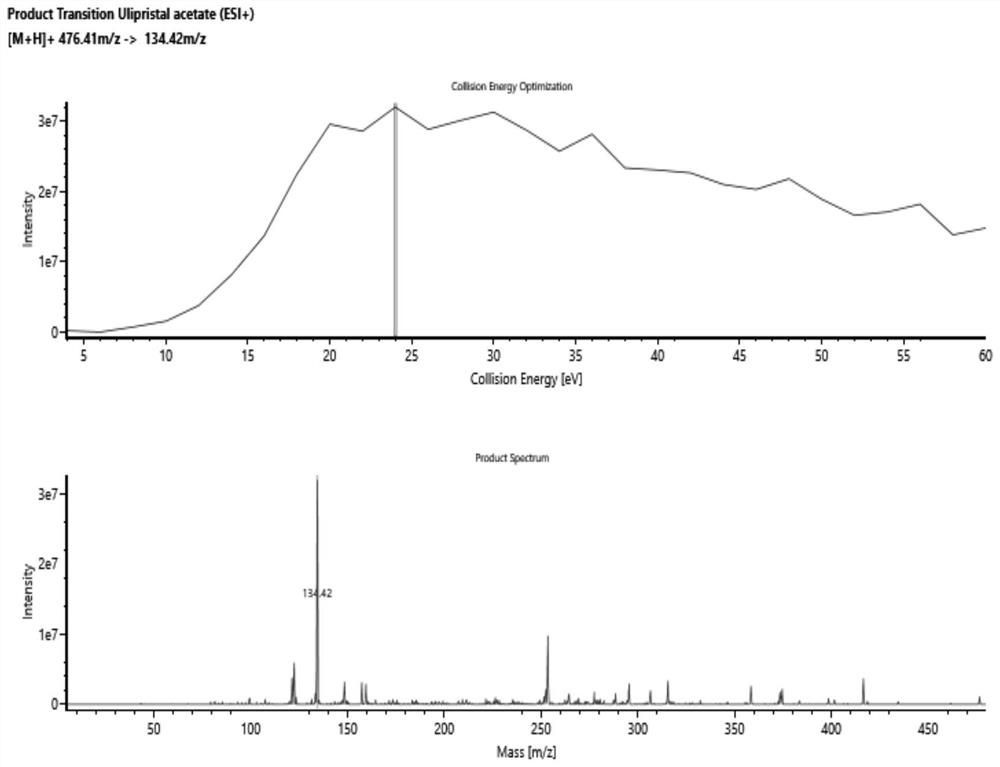
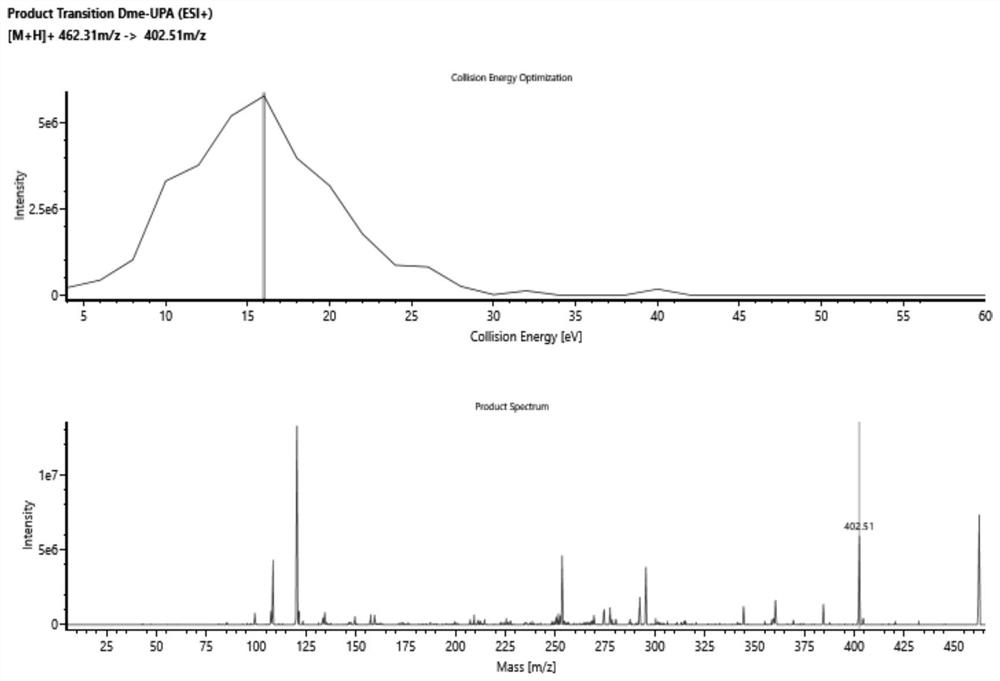
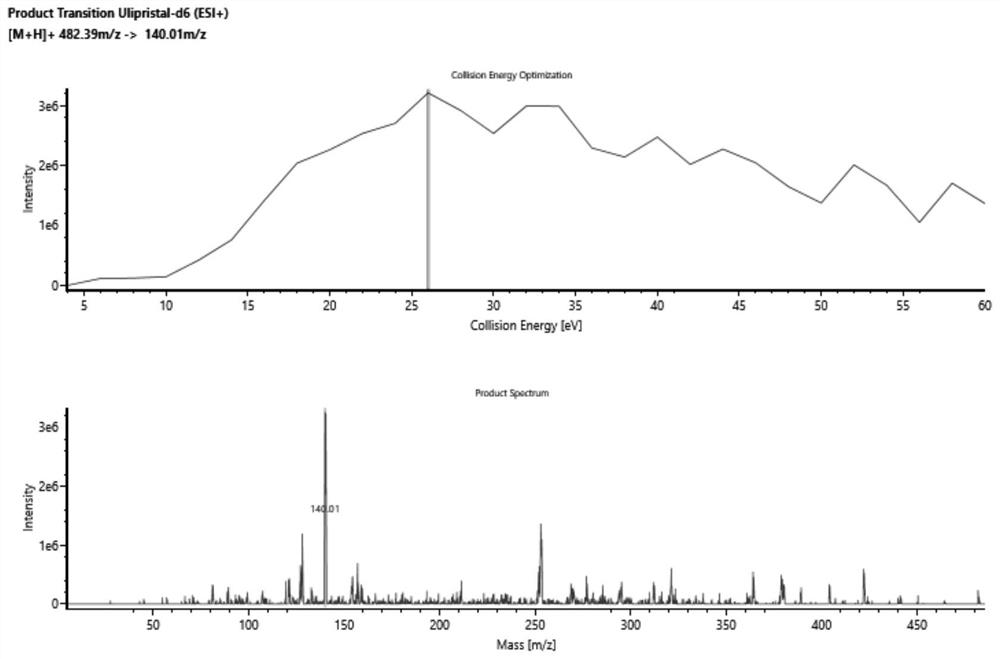
Method for detecting ulipristal acetate and metabolites thereof in blood plasma by LC-MS (liquid chromatography-mass spectrometry) method
ActiveCN114137115AReduce usageAvoid interferenceComponent separationMass Spectrometry-Mass SpectrometryBlood plasma
The invention discloses a method for detecting ulipristal acetate and an active metabolite mono-demethylation-ulipristal acetate in blood plasma by adopting an LC-MS (Liquid Chromatography-Mass Spectrometry) method. The method comprises the following steps: extracting a blood plasma sample by using dichloromethane / isopropanol, and performing LC-MS / MS detection. The method can be used for detecting ulipristal acetate, can also be used for detecting an active metabolite monodemethylation-ulipristal acetate, and has the characteristics of rapidness, high sensitivity and capability of simultaneously monitoring two components by using a small amount of plasma; the method can be applied to research on pharmacokinetics and bioequivalence of ulipristal acetate and active metabolite monodemethylation-ulipristal acetate.
Owner:四川尚锐分析检测有限公司
MMB4 DMS nano suspension and preparation method and application thereof
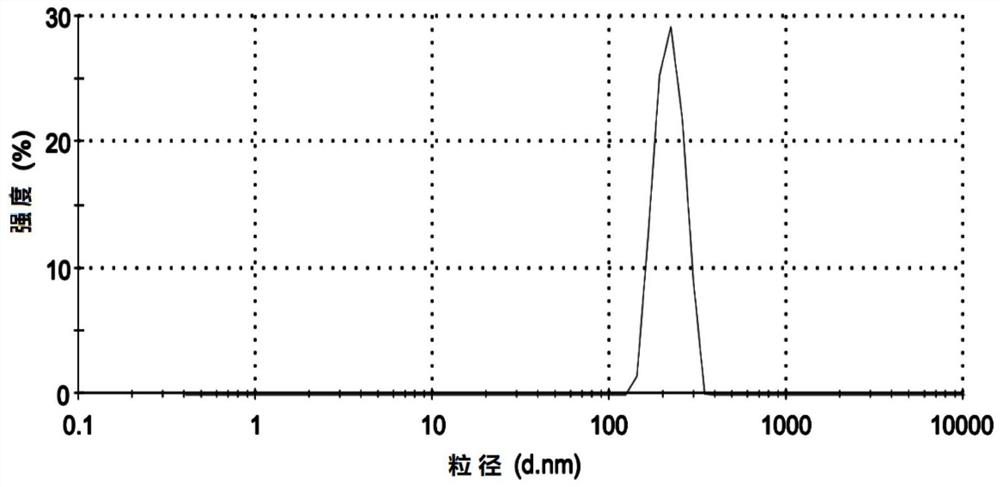
PendingCN112107540AUniform particle size distributionGood dispersionOrganic active ingredientsAntinoxious agentsChemistryParticle aggregation
The invention provides an MMB4 DMS nano suspension and a preparation method and application thereof. The nano suspension is composed of 1.0%-25.0% (w / w) of MMB4 DMS and a dispersion medium, wherein the viscosity of the nano suspension is 200-650 mPa.s, the polydispersity index (PDI) of nanocrystal particles of the nano suspension is 0.1-0.7, and the dispersion medium is oil. The preparation provided by the invention is proper in viscosity, not only cannot cause grinding difficulty due to an over-large viscosity, but also cannot cause sedimentation due to an over-small viscosity. The preparation process of the MMB4 DMS nano suspension is stable and suitable for the industrial production, and the nano suspension obtained by the process is free of particle aggregation and layering phenomena,and is good in stability.
Owner:ACADEMY OF MILITARY MEDICAL SCI
Bone-setting tablet, method thereof for identifying and measuring content
ActiveCN102359992BAdd identification methodThe identification method is simpleComponent separationMaterial analysis by observing effect on chemical indicatorMyrrhCurative effect
The invention relates to a method for preparing and detecting traditional Chinese medicine, in particular to a bone-setting tablet,a method thereof for identifying and measuring contents. The method comprises the following steps of: identifying olibanum, rhizoma ligustici wallichii, angelica and myrrh; and measuring the ferulic acid content. Through sifting of parameter tests, the bone-setting tablet has better chromatographic condition, favorable specificity and repeatability, quickness, economy and practicability; the added method for measuring the ferulic acid content ensures that the method has the advantages of strong specificity and high accuracy, easiness for control as a quality control method of rhizoma ligustici wallichii and angelica, therefore, the product quality of the bone-setting tablet is effectively improved, and the curative effect of the bone-setting tablet product is better ensured.
Owner:JILIN ZHENGHE PHARMA GRP
A method for analyzing fingerprints of Jinghuaweikang capsules
ActiveCN103940916BOptimizing Chromatographic ConditionsThe measurement result is preciseComponent separationGas phaseRepeatability
The invention provides a fingerprint analysis method of Jinghuaweikang capsules, which comprises preparation of a reference substance solution and a test solution, and then analyzing by gas chromatography to obtain a fingerprint. According to the characteristics of the Jinghua Weikang capsules, the analysis method of the present invention uses gas chromatography to measure the fingerprints of the Jinghua Weikang capsules, finds optimized chromatographic conditions, and makes the measurement results precise, repeatable and stable. The invention also provides a fingerprint of the Jinghuaweikang capsule.
Owner:TIANJIN TASLY PHARMA CO LTD
Hepatitis B virus core antigen content detection method
InactiveCN106248812ANo driftShort retention timeComponent separationHepatitis B virus core AntigenParallax
The present invention provides a hepatitis B virus core antigen content detection method. According to the technical scheme, hepatitis B virus core antigen is detected by using a HPLC method, a detector and chromatographic conditions are optimized, the detection precision is remarkably improved, and the trace HBcAg can be detected after the serum is directly taken out without the addition of a shell opening agent; the mobile phase and the solvent of an ELSD detector can evaporate completely during a detection process, such that the obtained chromatogram does not have the solvent peak, and the gradient elution does not have the reflected light parallax effect and cannot generate the baseline drift so as to significantly simplify the subsequent quantitative analysis; the retention time of the C8 column is short, and the separation effect is good, such that the detection time is shortened; and the detection conditions are mild, such that the denaturation deactivation of proteins, nucleic acids and other components cannot be caused so as to effectively ensure the accuracy of the detection. According to the present invention, the excellent technical effect is achieved by using the innovative technical improvement while the advantages of high sensitivity and easy operation are provided, and the accurate quantitation can be achieved by using the internal standard method after the detection.
Owner:林海燕
Low-temperature control device for analyzing hydrogen isotope by gas chromatography
ActiveCN112834668AOptimizing Chromatographic ConditionsComponent separationGas analysisTemperature control
The invention relates to a low-temperature control device for analyzing hydrogen isotope by gas chromatography. The low-temperature control device comprises a refrigeration mechanism, a cold head kit, a chromatographic column and a chromatographic instrument. The chromatographic instrument is connected with the chromatographic column; a cold head of the refrigeration mechanism is connected with the chromatographic column to provide a cold source; the chromatographic column is used for separating gas components; and the chromatographic column is arranged in the cold head kit. The low-temperature control device for analyzing the hydrogen isotope by the gas chromatography has the beneficial effects that the structure of the cold head, the chromatographic column and the connecting pipe of the pulse tube type refrigerator is adopted, a cold source is provided by the pulse tube type refrigerator, a mode of maintaining relatively low heat leakage under vacuum is adopted, and the temperature of the chromatographic column can be set to be in a range of 40K to room temperature through the controller; liquid nitrogen does not need to be supplemented, and possibility is provided for automatic control of the ash discharge gas analysis process. And meanwhile, the temperature is adjusted within the range of -40K to room temperature , so that the possibility is provided for optimizing the chromatographic condition of hydrogen isotope analysis.
Owner:CHINA INSTITUTE OF ATOMIC ENERGY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Method of simultaneously determining contents of 1-OHP ,3-OHB[a]P and 3-OHB[a]A in urine Method of simultaneously determining contents of 1-OHP ,3-OHB[a]P and 3-OHB[a]A in urine](https://images-eureka.patsnap.com/patent_img/1ddef151-5fe8-4064-a572-cbb073af4b27/HDA00002539623800011.PNG)
![Method of simultaneously determining contents of 1-OHP ,3-OHB[a]P and 3-OHB[a]A in urine Method of simultaneously determining contents of 1-OHP ,3-OHB[a]P and 3-OHB[a]A in urine](https://images-eureka.patsnap.com/patent_img/1ddef151-5fe8-4064-a572-cbb073af4b27/HDA00002539623800012.PNG)
![Method of simultaneously determining contents of 1-OHP ,3-OHB[a]P and 3-OHB[a]A in urine Method of simultaneously determining contents of 1-OHP ,3-OHB[a]P and 3-OHB[a]A in urine](https://images-eureka.patsnap.com/patent_img/1ddef151-5fe8-4064-a572-cbb073af4b27/HDA00002539623800021.PNG)