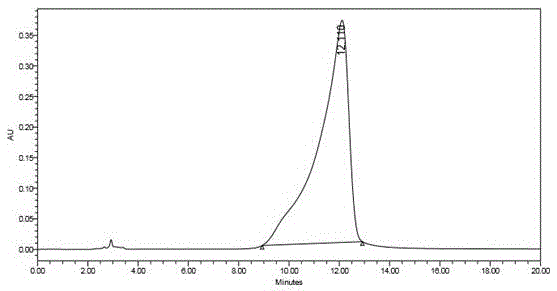
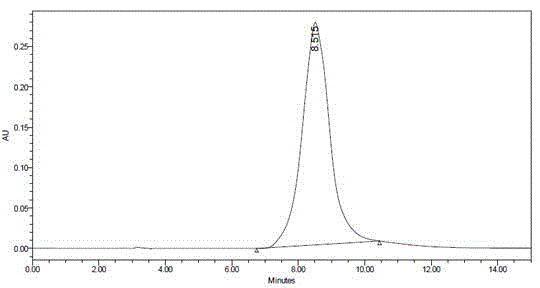
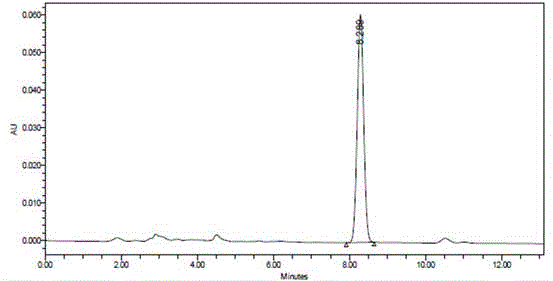
Method for measuring content of 5-fluorouracil in plasma and colorectal cancer cells based on high performance liquid chromatography
A high-performance liquid chromatography, cancer cell technology, applied in measurement devices, instruments, scientific instruments, etc., can solve the problems of affecting accuracy, large interference, low drug content, etc., to improve peak shape, optimize chromatographic conditions, and improve recovery. rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Example 1 Biological sample pretreatment
[0053] Plasma sample processing
[0054] Activate the solid-phase extraction column StyreScreen?HP with 1mL ethanol and 1mL deionized water (containing 0.1% trifluoroacetic acid in mass concentration) sequentially; absorb 0.5mL plasma sample, add it to the solid-phase extraction column at a speed of 1mL / min, wait until the sample is enriched After collection, rinse with 1mL deionized water and drain; elute with ethanol-deionized water containing 0.1% trifluoroacetic acid (V / V, 75︰25) solution, and drain; use a vacuum centrifugal evaporation concentrator to dry for 20min , remove the organic solvent; dry and concentrate in a freeze dryer, and dilute to an appropriate concentration with mobile phase during determination;
[0055] Colorectal cancer cell (SW480-B7 and SW480-NC) sample processing
[0056] The solid-phase extraction column StyreScreen?HP was activated with 1mL ethanol and 1mL deionized water (containing 0.1% triflu...
Embodiment 2
[0058] Embodiment 2 chromatographic condition optimization
[0059] 1. Selection of extraction column (comparison of different extraction methods)
[0060] ODS extraction column, StyreScreen? HP solid-phase extraction column and the common ethyl acetate extraction method and ether-isopropanol extraction method reported in the literature were used respectively. The column was first activated according to the above method, and the preparation containing 5-Fu1.0, 4.0, 12.0μg / mL low, medium and high concentrations of plasma samples and colorectal cancer cells samples were processed by different extraction methods, and the content of 5-Fu was determined by high performance liquid chromatography.
[0061] Extraction efficiency=measured value of C / standard value of C×100%. Using StyreScreen? HP extraction, the efficiency is greater than 85%, higher than ordinary organic solvent extraction.
[0062] The results of the extraction efficiency are shown in Table 1.
[0063] Table 1 Mea...
Embodiment 3
[0073] Embodiment 3 standard curve and detection limit:
[0074] plasma standard curve
[0075] Take an appropriate amount of 5-Fu standard substance dissolved in water, make 1mg / mL standard solution, dilute to 500, 100, 50, 10, 5, 1, 0.5μg / mL, take 100μL respectively, add healthy person’s unadministered Dilute the blank plasma to 2mL, mix well, configure the standard blood drug concentration of 25, 5, 2.5, 0.5, 0.25, 0.05, 0.025μg / mL, perform HPLC determination after solid phase extraction according to the above plasma sample processing method , record the peak area; take the 5-Fu concentration as the abscissa, and the corresponding peak area as the ordinate to perform linear regression, and the linearity is: Y=34783x+34562, R 2 =0.9991, the results showed that the mass concentration of 5-Fu showed a good linear relationship between 0.025~25μg / mL. According to the signal-to-noise ratio (S / N) of 3, the minimum detection concentration of 5-Fu is 0.02 μg / mL.
[0076] Colorect...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com