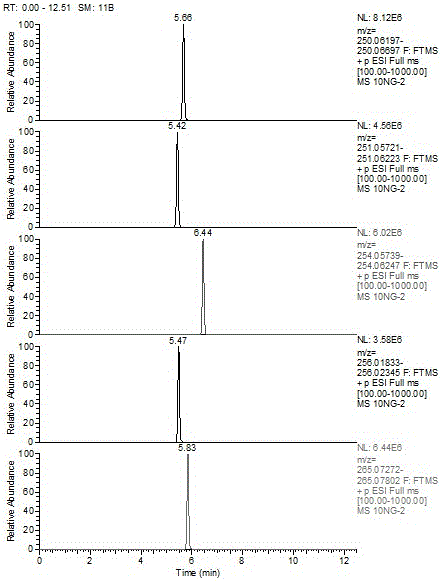
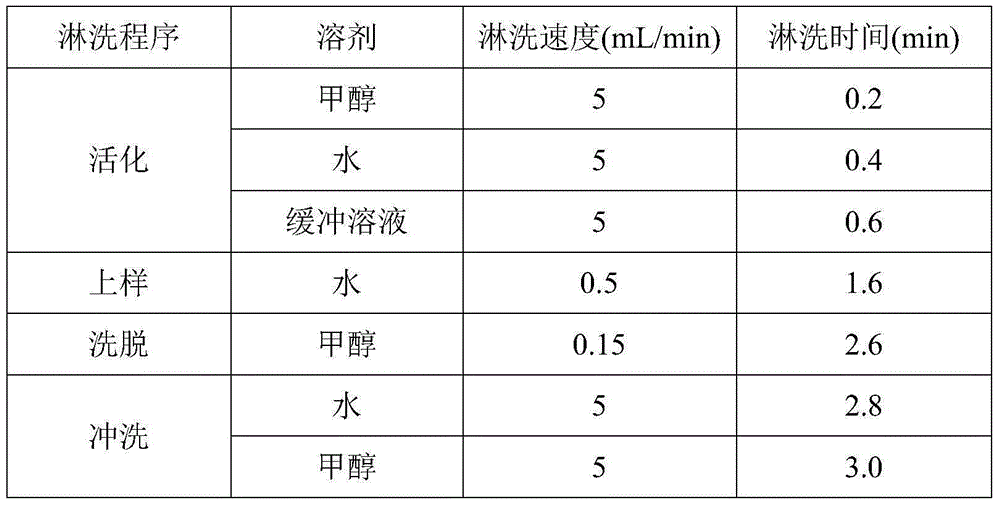
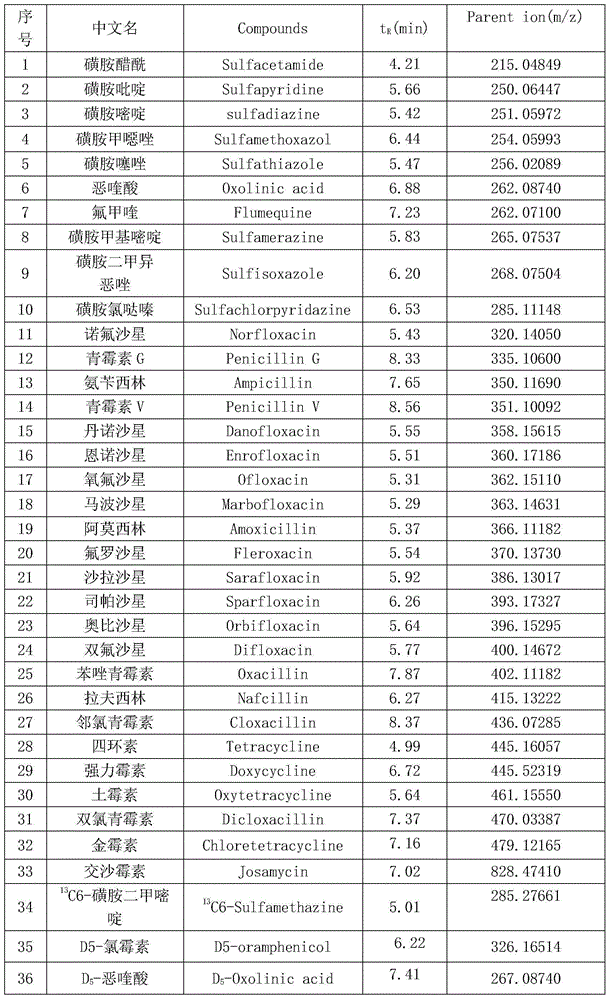
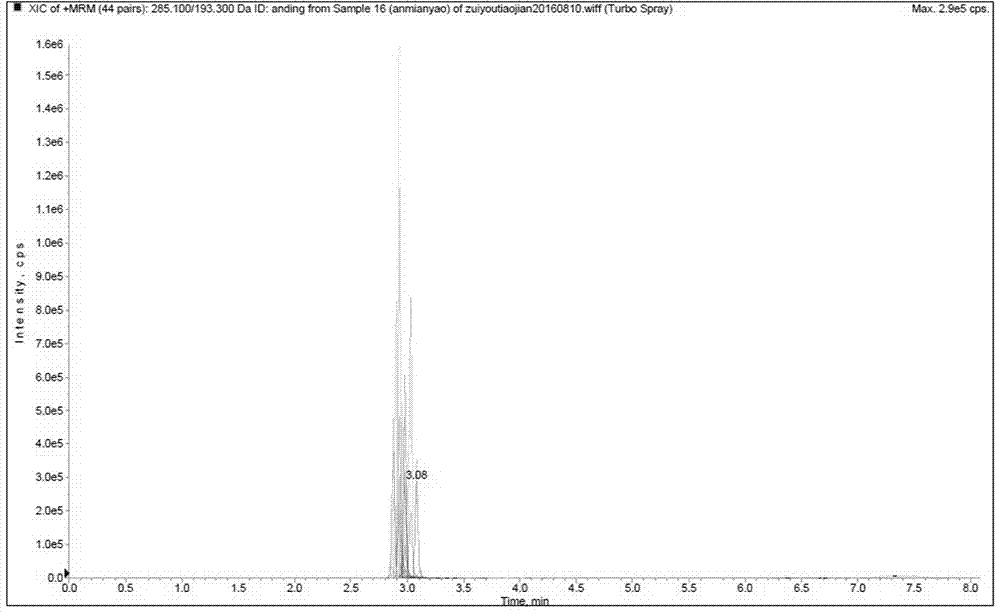
Patents
Literature
596 results about "Liquid chromatography mass spectroscopy" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Liquid chromatography–mass spectrometry (LC-MS) is an analytical chemistry technique that combines the physical separation capabilities of liquid chromatography (or HPLC) with the mass analysis capabilities of mass spectrometry (MS).
Method for detecting residual quantity of multiple alkaline drugs in animal derived food
The invention relates to the fields of analytical chemistry and food safety, in particular to a method for detecting the residual quantity of multiple alkaline drugs in animal derived food. Based on the vortex mixed extracting of acetonitrile, isopropanol and citric acid buffer solutions, the purification of a hydrophilic polystyrene-divinylbenzene solid phase extraction column and a cation exchange solid phase extraction column and the liquid phase chromatography-mass spectra determination, the method can detect the residual quantity of multiple alkaline drugs in pork, pork liver, eggs, shrimps and milk, such as beta-receptor agonists,sulfonamides, benzodiazepines, nitroimidazoles, benzimidazoles and triphenylmethanes. The method has the advantages of simple operation, fast and accurate detection and high efficiency.
Owner:SHANGHAI ANPEL SCI INSTR +1
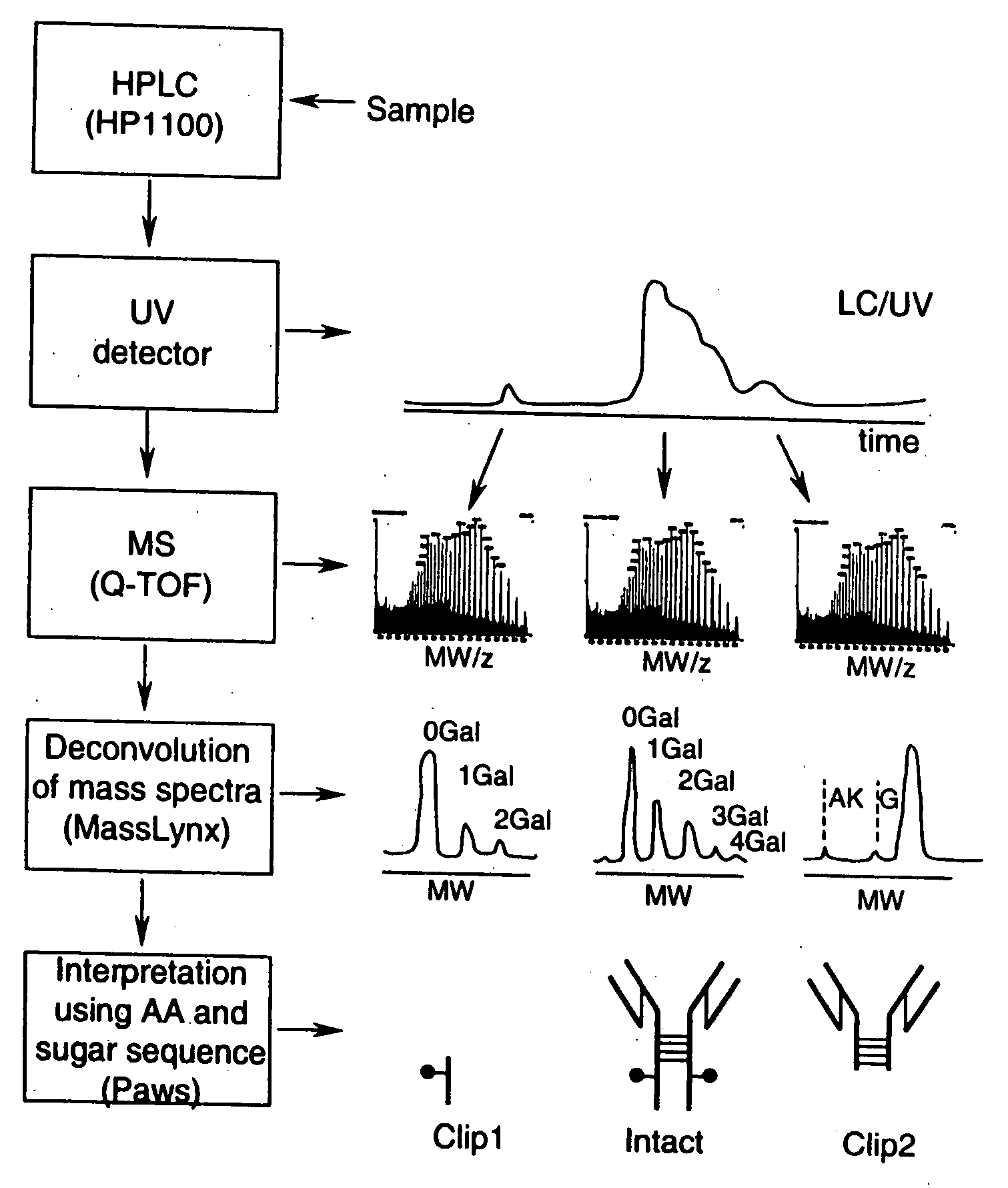
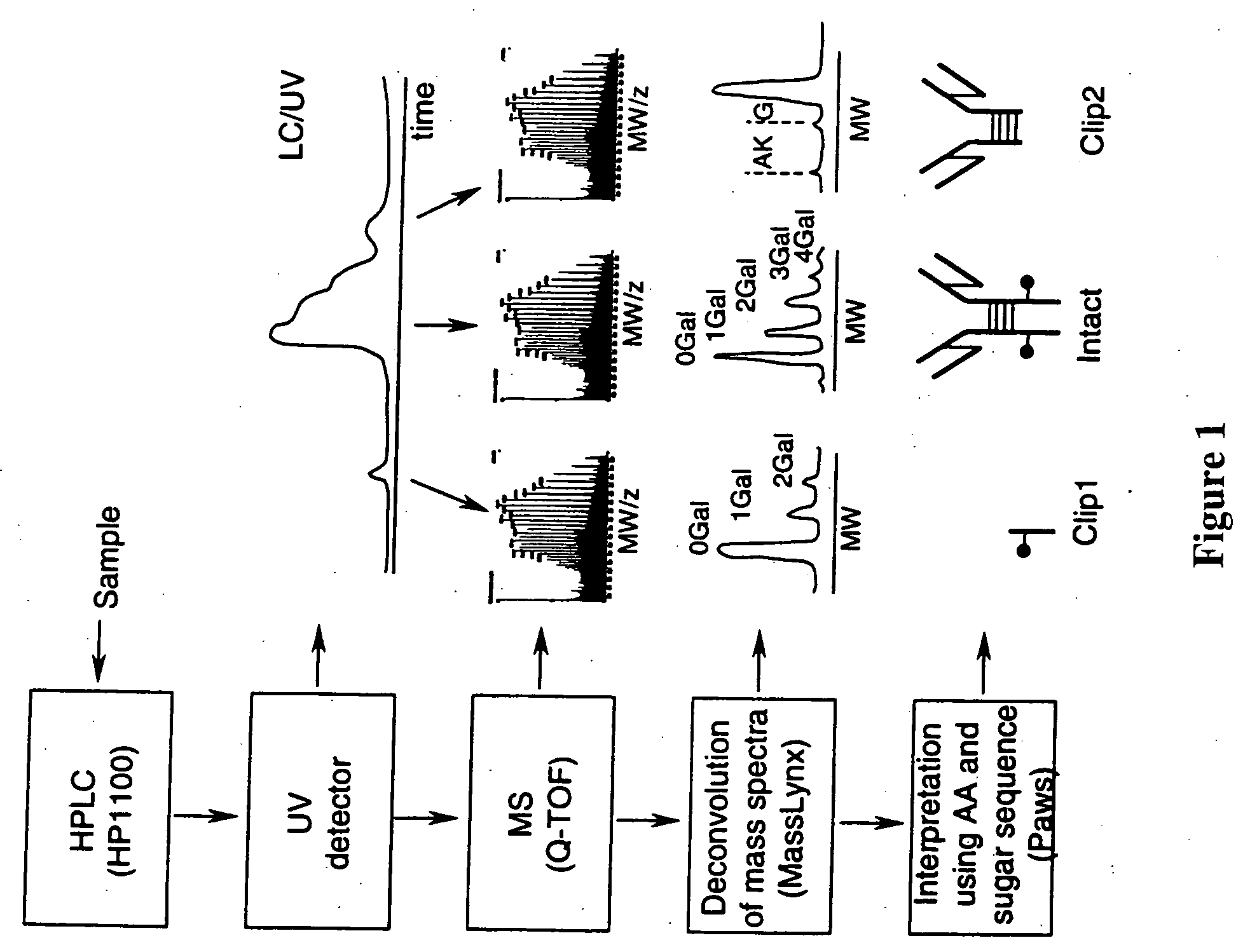
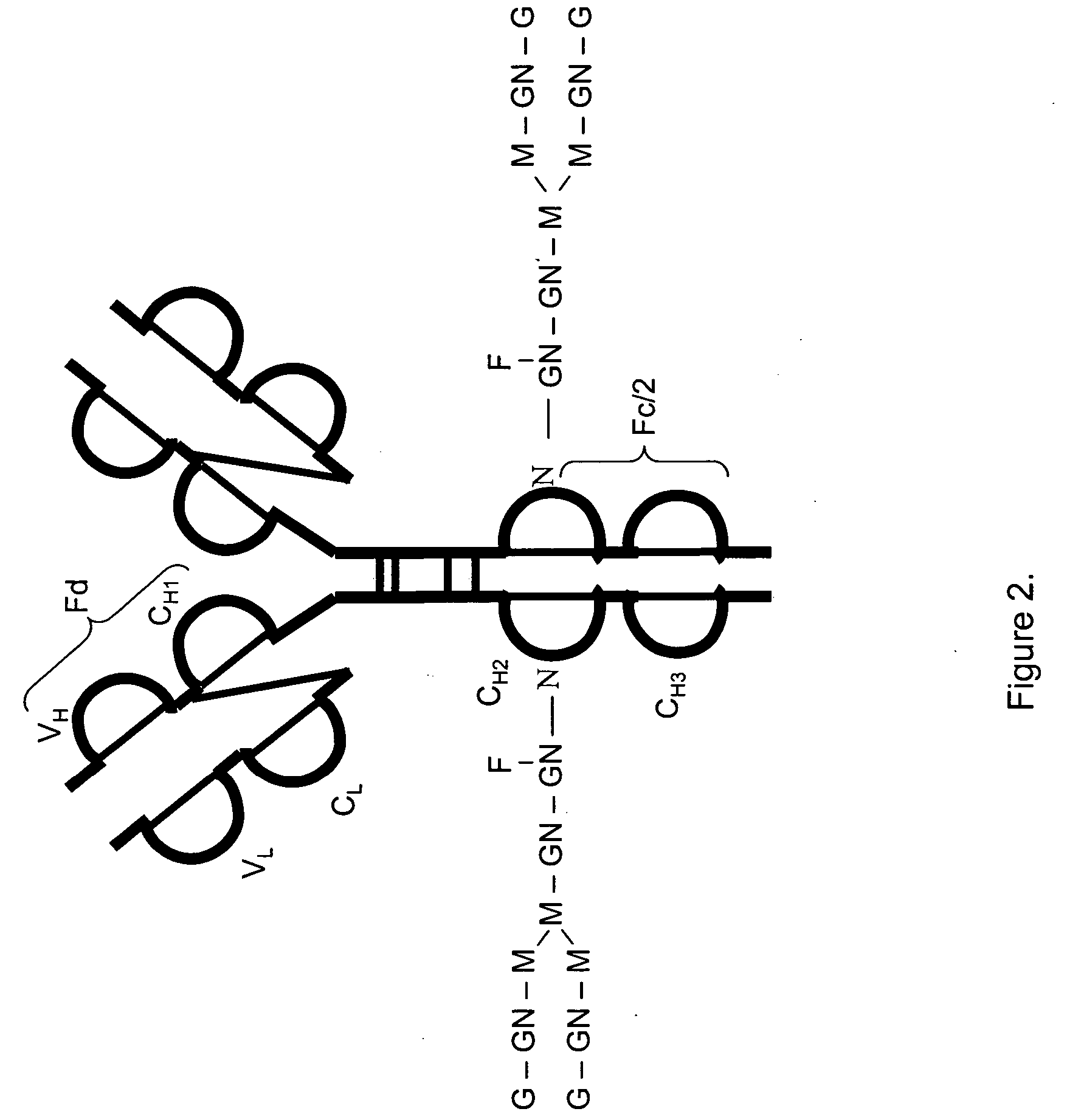
LC/MS method of analyzing high molecular weight proteins
InactiveUS20050161399A1Serum immunoglobulinsComponent separationLiquid chromatography mass spectroscopyBiochemistry
Owner:AMGEN INC
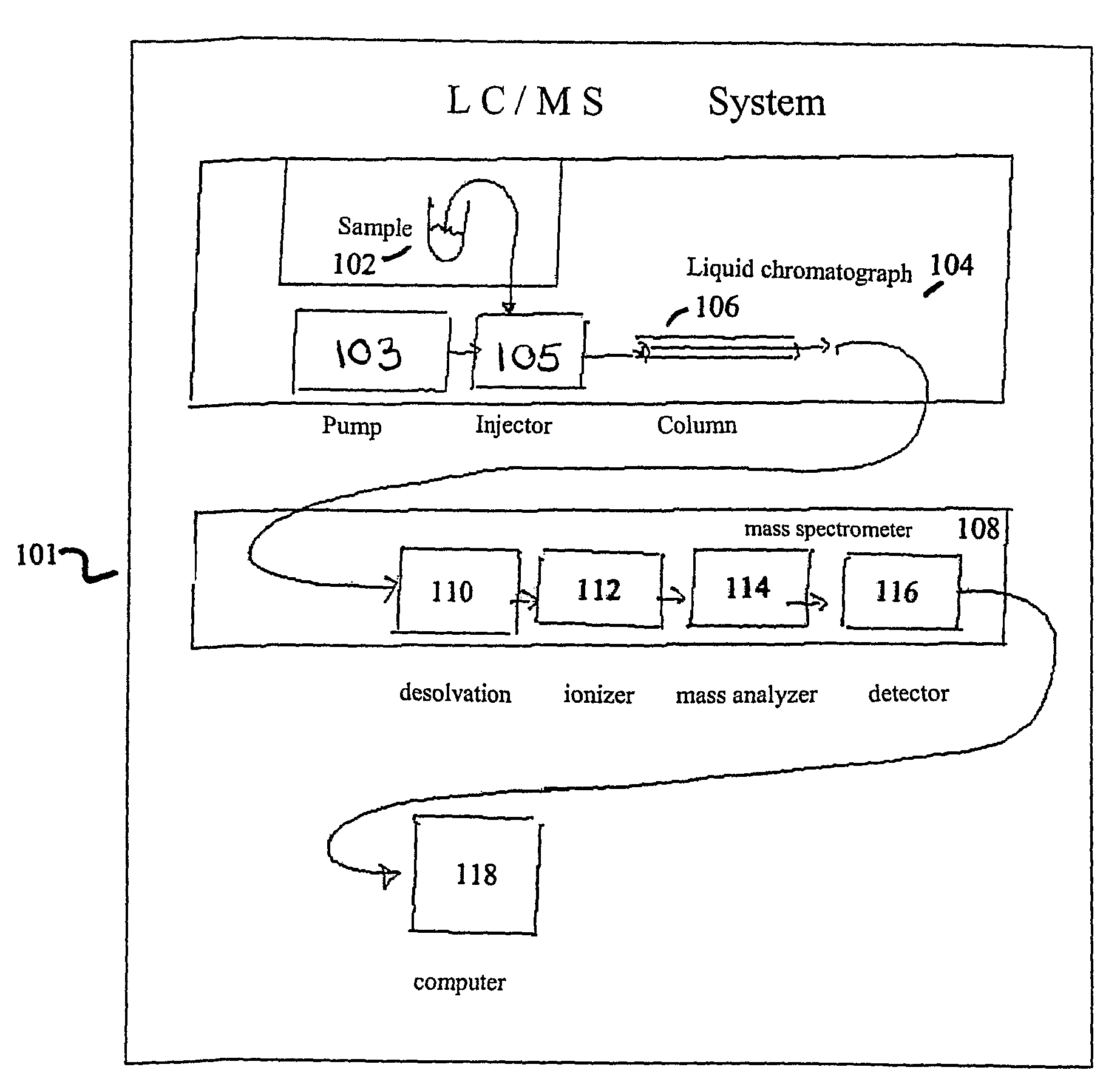
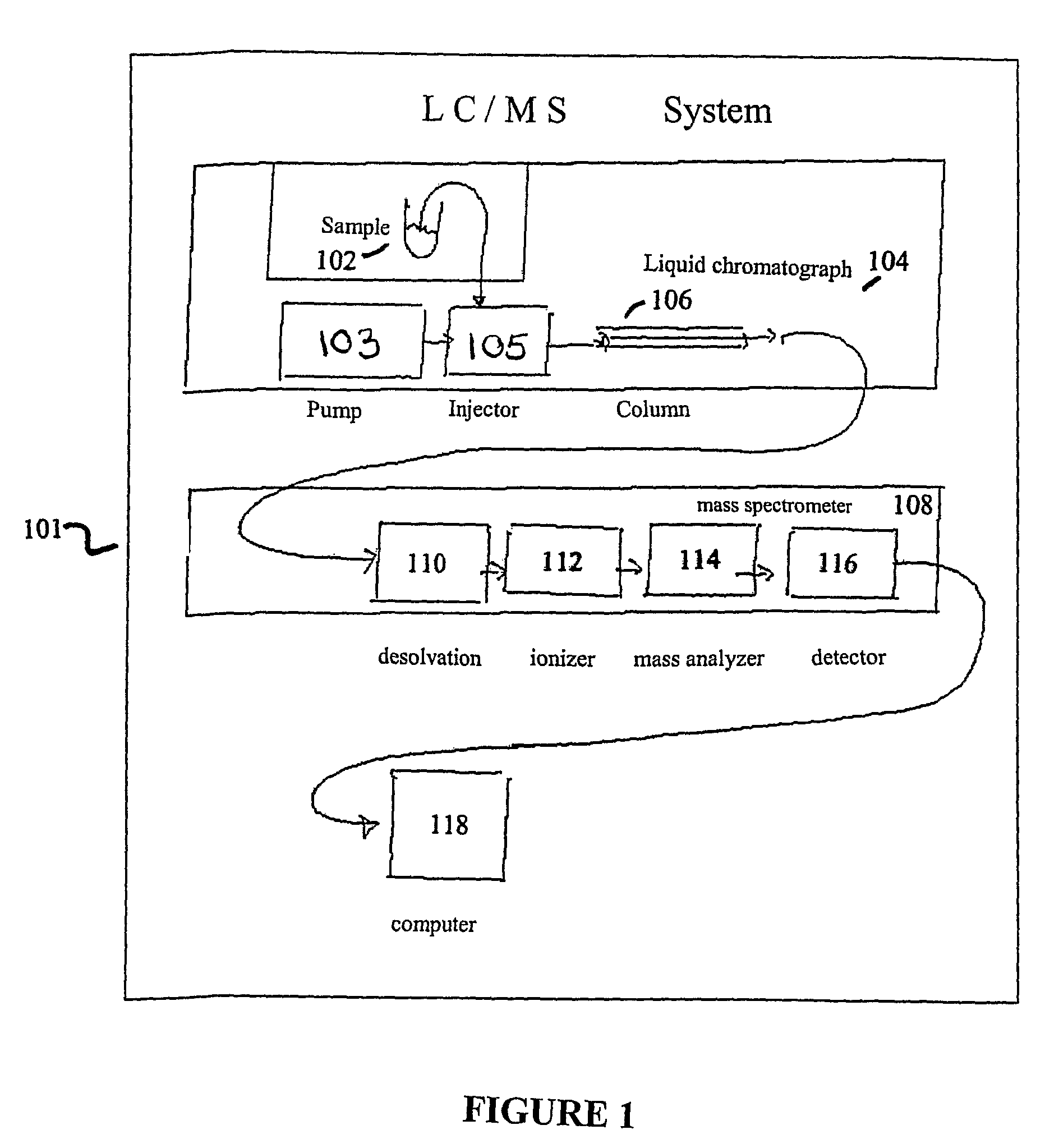
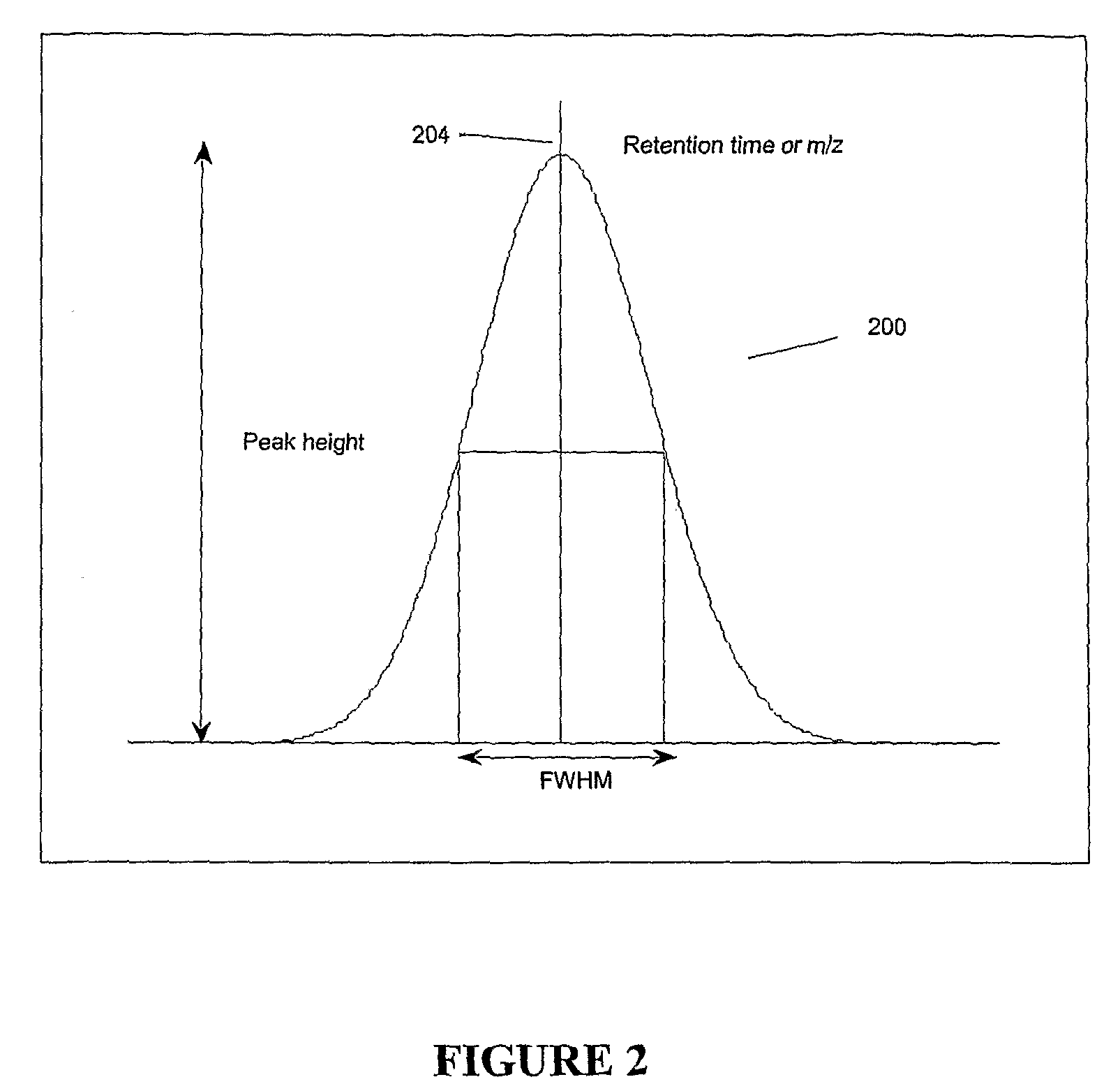
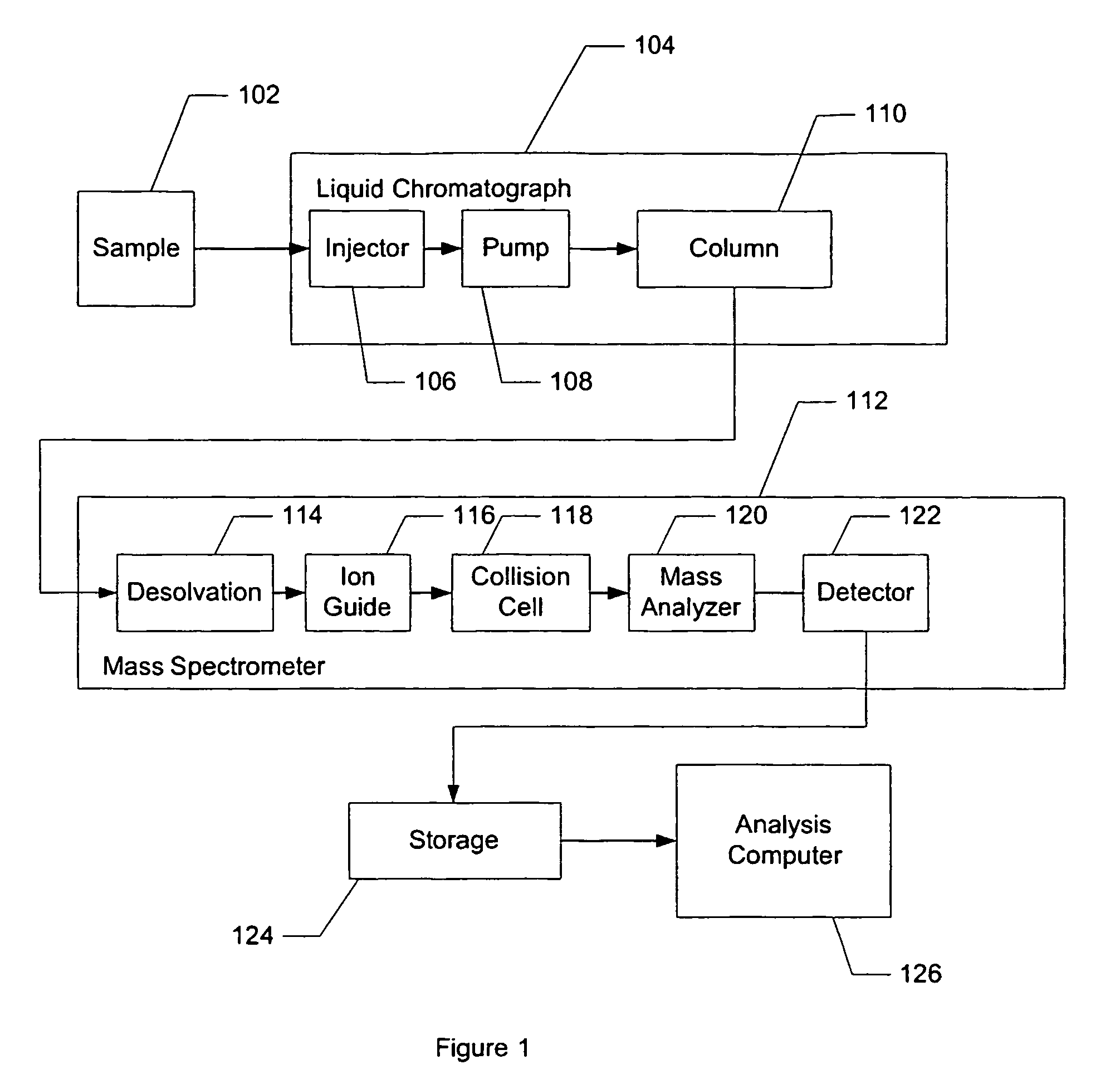
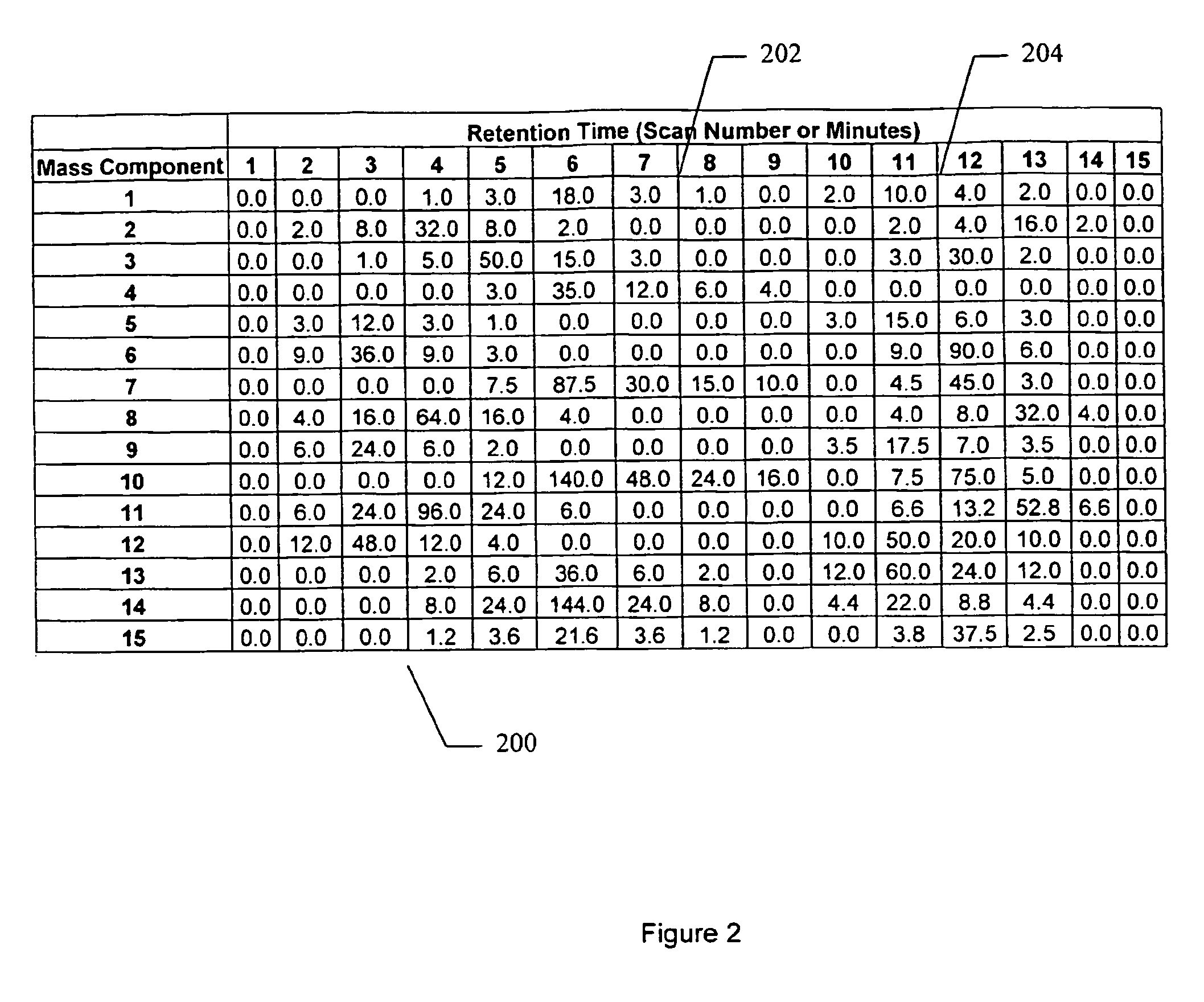
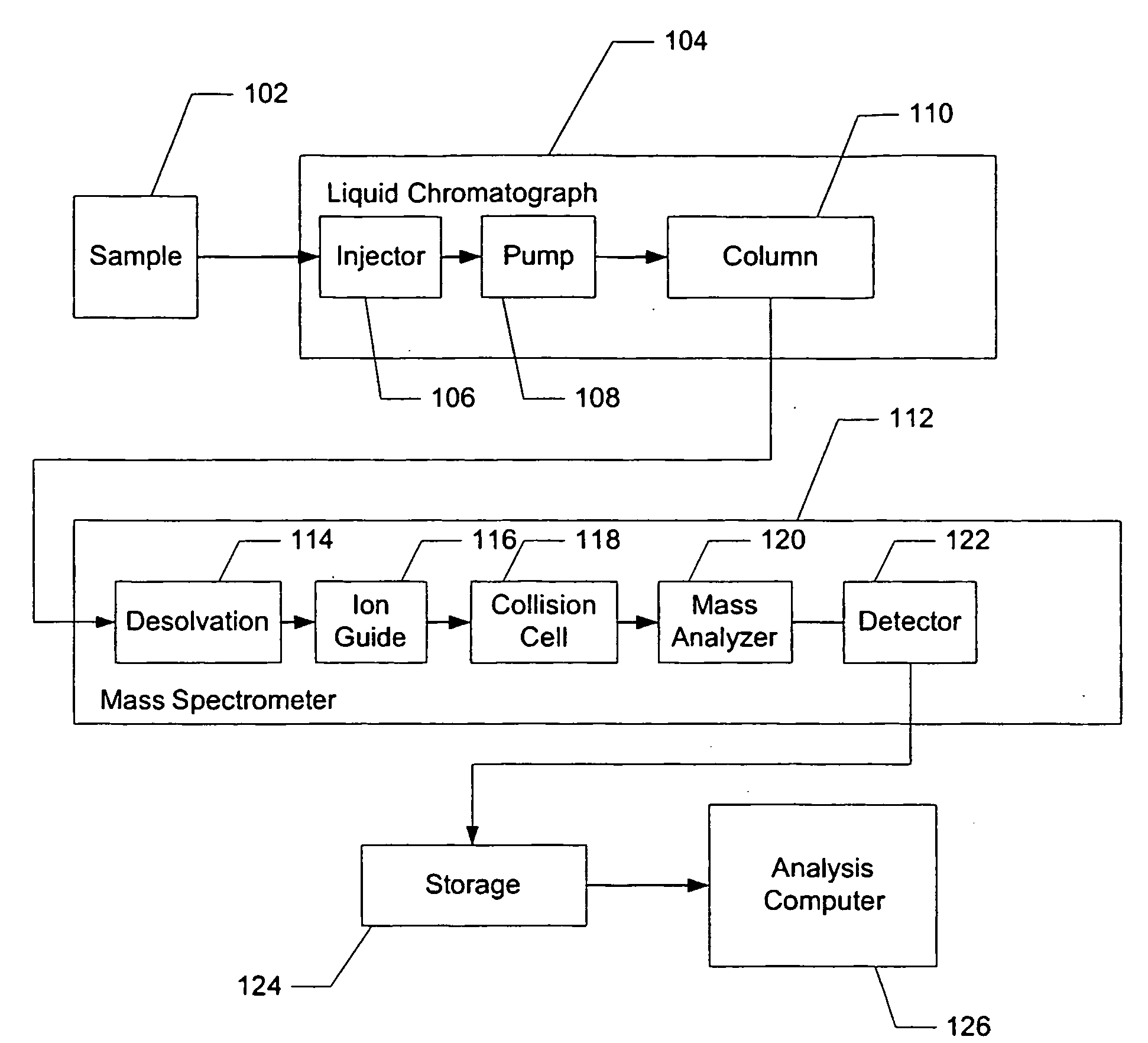
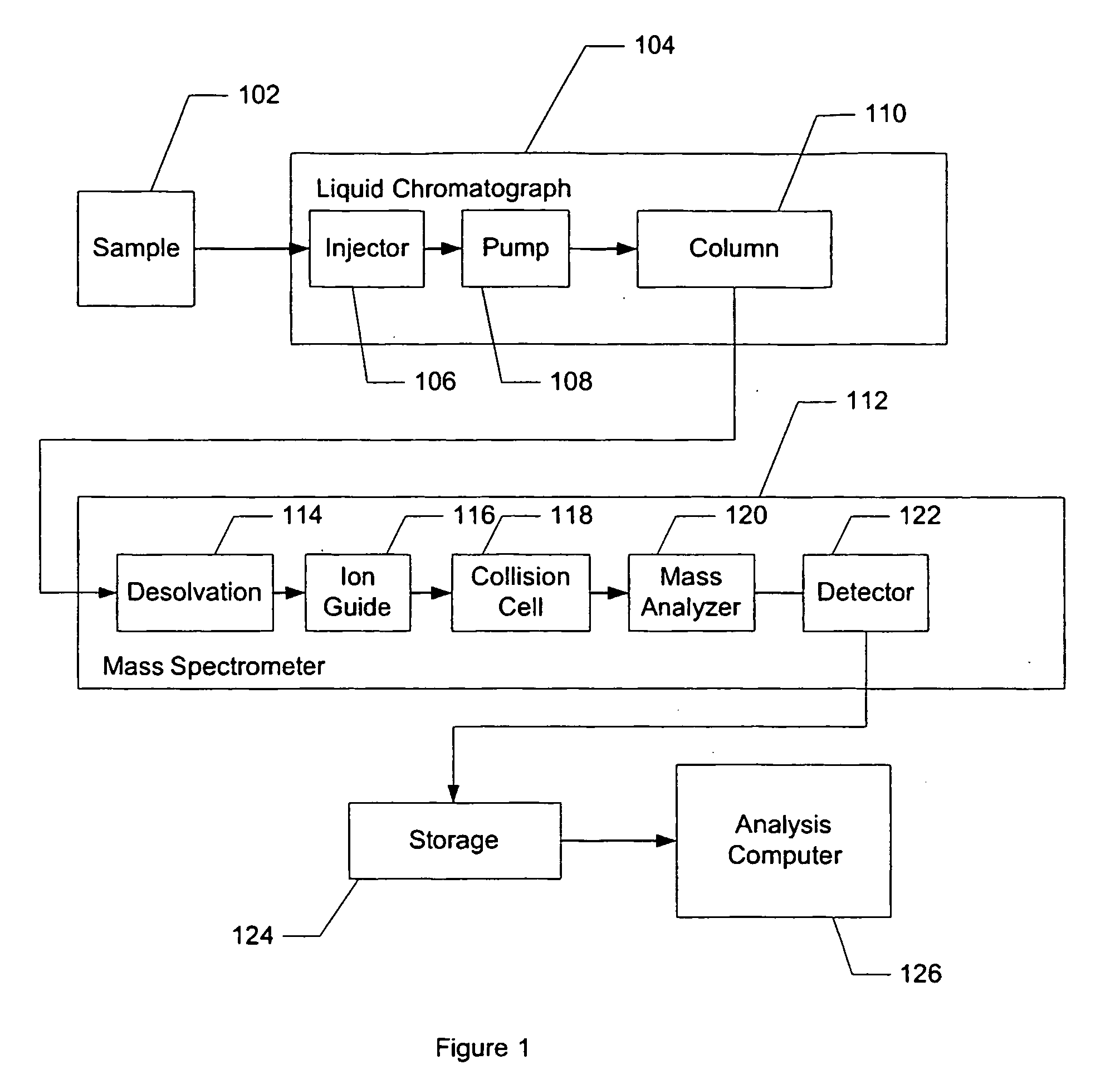
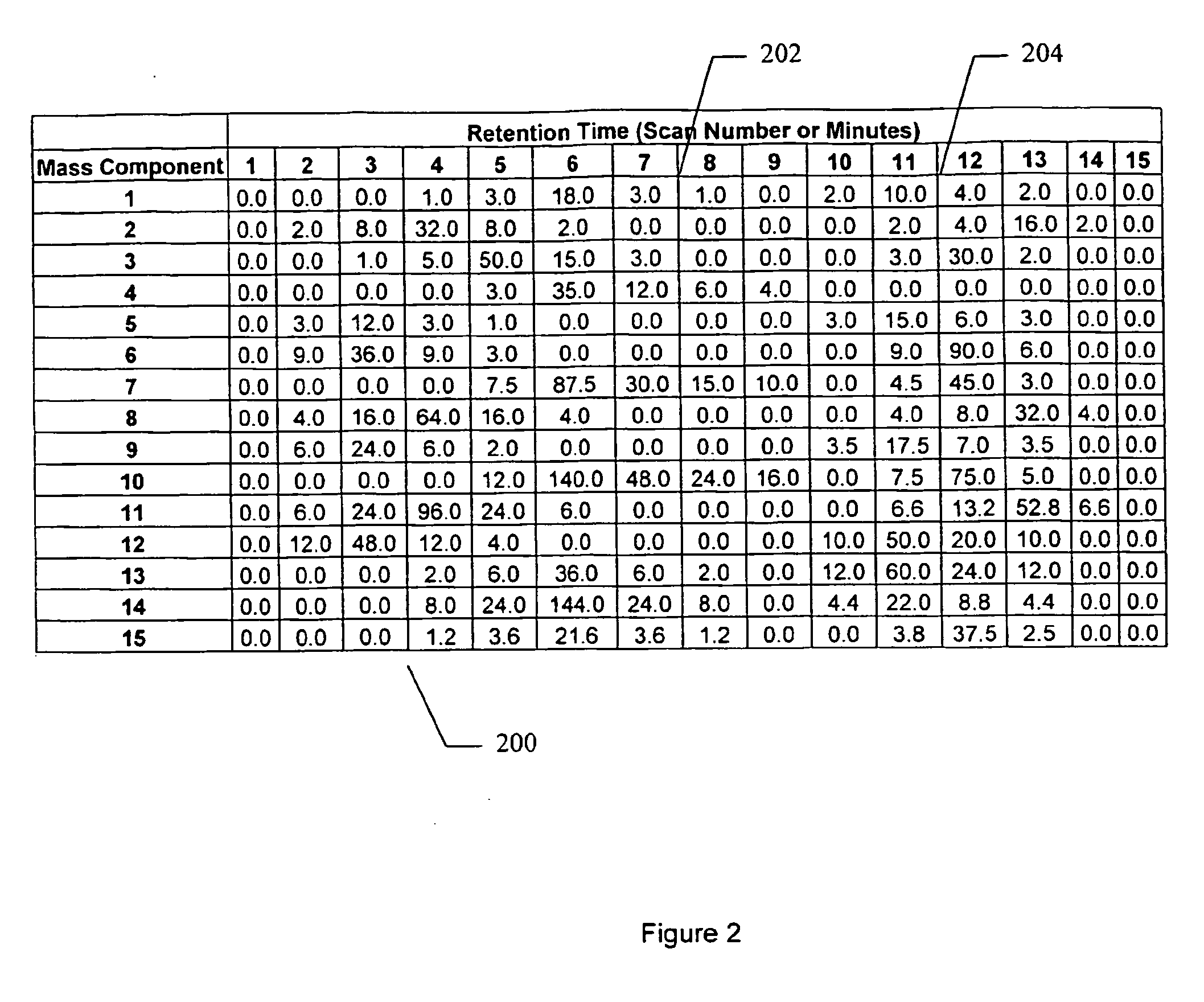
Apparatus and method for identifying peaks in liquid chromatography/mass spectrometry data and for forming spectra and chromatograms
ActiveUS7645984B2Accurately and optimally estimatedReduce impactThermometer detailsSpectral/fourier analysisRetention timeLiquid chromatography mass spectroscopy
Chromatograms and mass spectra produced by an LC / MS system are analyzed by creating a two-dimensional data matrix of the spectral and chromatographic data. The two-dimensional matrix can be created by placing the spectra generated by the mass spectrometer portion of the LC / MS system in successive columns of the data matrix. In this way, the rows of the data matrix correspond to chromatographic data and the columns of the data matrix correspond to the spectra. A two-dimensional filter is specified and applied to the data matrix to enhance the ability of the system to detect peaks associated with ions. The two-dimensional filter is specified according to desired criteria. Rank-1 and rank-2 filters can be specified to improve computational efficiency. One method of applying the two-dimensional filter is through convolution of the data matrix with the two-dimensional filter to produce an output data matrix. Peaks corresponding to detected ions are identified in the output data matrix. Parameters of the peaks are determined and stored for later processing including quantitation, or simplification of chromatograms or spectra by, for example, identifying peaks associating with ions having retention times falling within a specified retention time window or having mass-to charge ratios falling within a specified mass-to-charge ratio window.
Owner:WATERS TECH CORP
Micro-devices and analytical procedures for investigation of biological systems
InactiveUS20050118599A1Bioreactor/fermenter combinationsBiochemistry cleaning apparatusFiberLiving systems
Fibres with an extraction phase coated thereon in combination with a positioning device are described to perform adsorption of components of interest from an animal or animal tissue for the investigation of living systems. A number of interfaces to analytical instrumentation are disclosed including mass spectrometry, LC / MS, MALDI and CE as well as direct spectroscopic on-fibre measurement.
Owner:PAWLISZYN JANUSZ B
Micro-devices and analytical procedures for investigation of biological systems
InactiveUS7384794B2Bioreactor/fermenter combinationsBiochemistry cleaning apparatusFiberLiving systems
Fibres with an extraction phase coated thereon in combination with a positioning device are described to perform adsorption of components of interest from an animal or animal tissue for the investigation of living systems. A number of interfaces to analytical instrumentation are disclosed including mass spectrometry, LC / MS, MALDI and CE as well as direct spectroscopic on-fibre measurement.
Owner:PAWLISZYN JANUSZ B
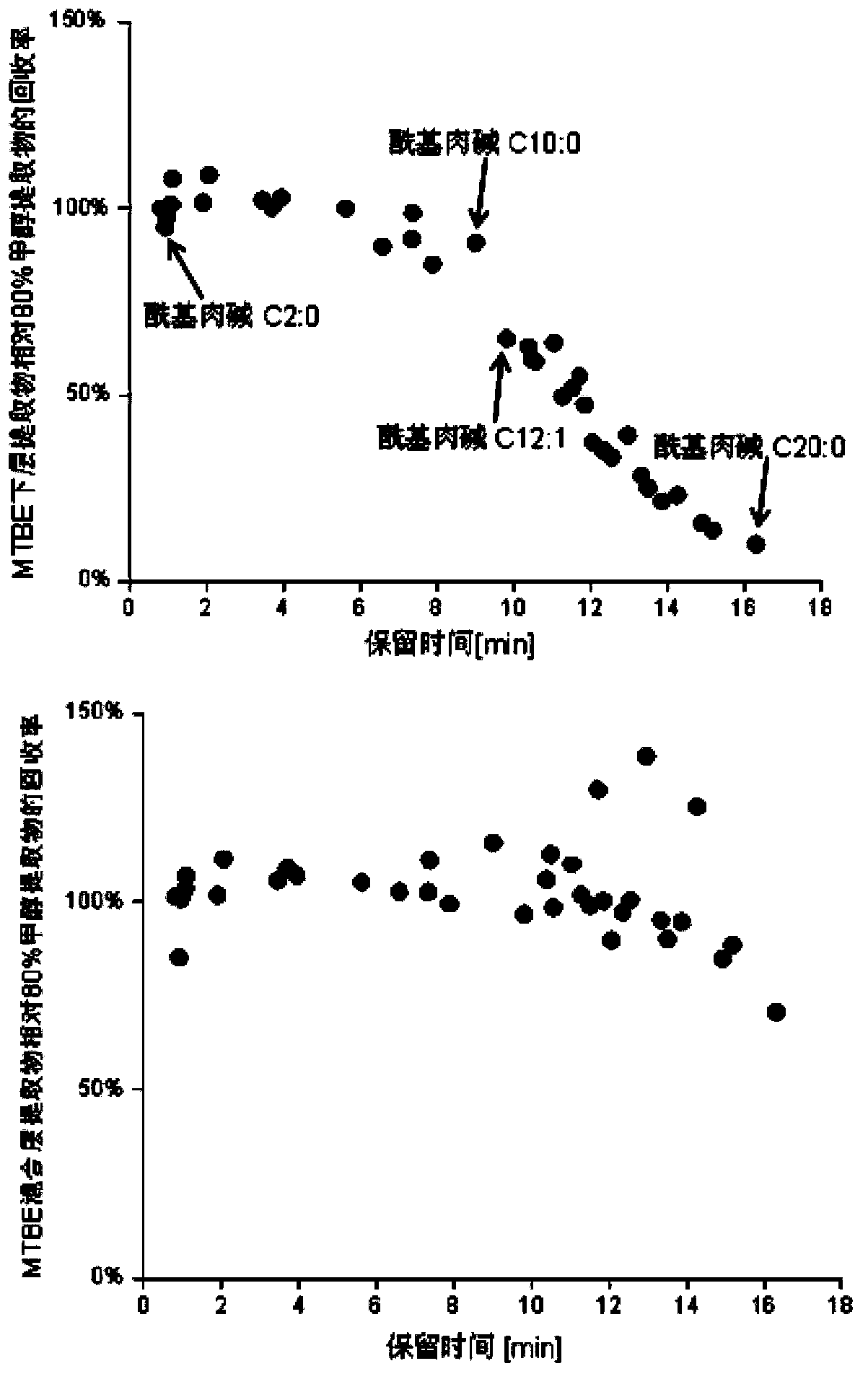
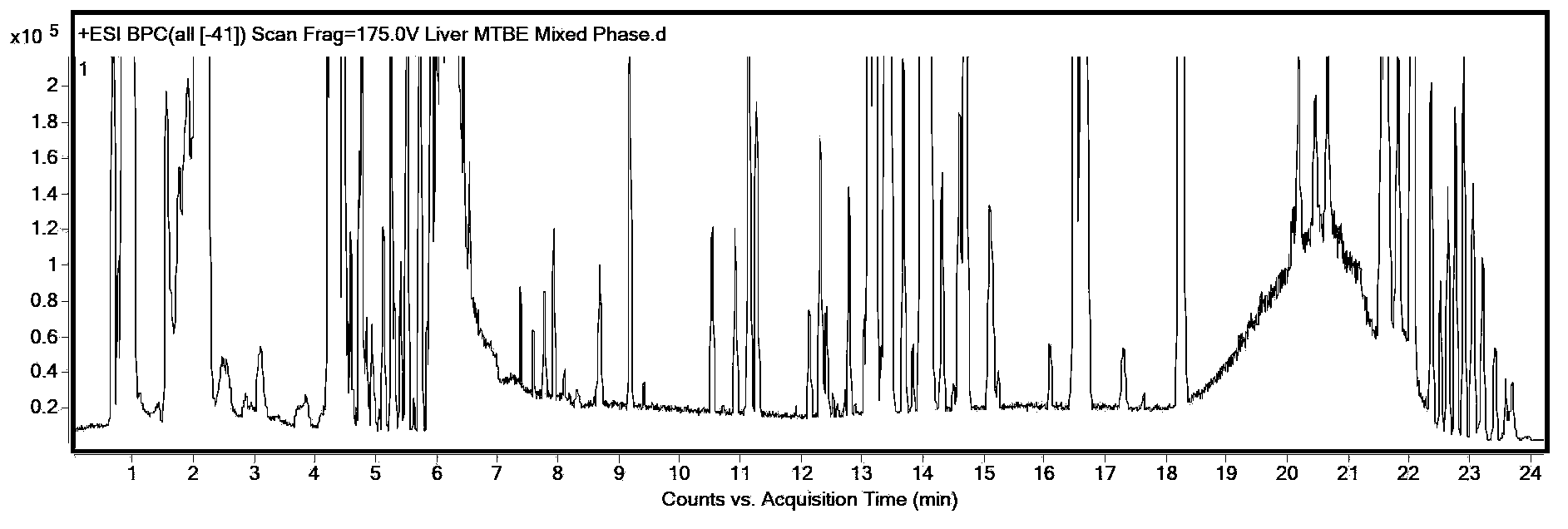
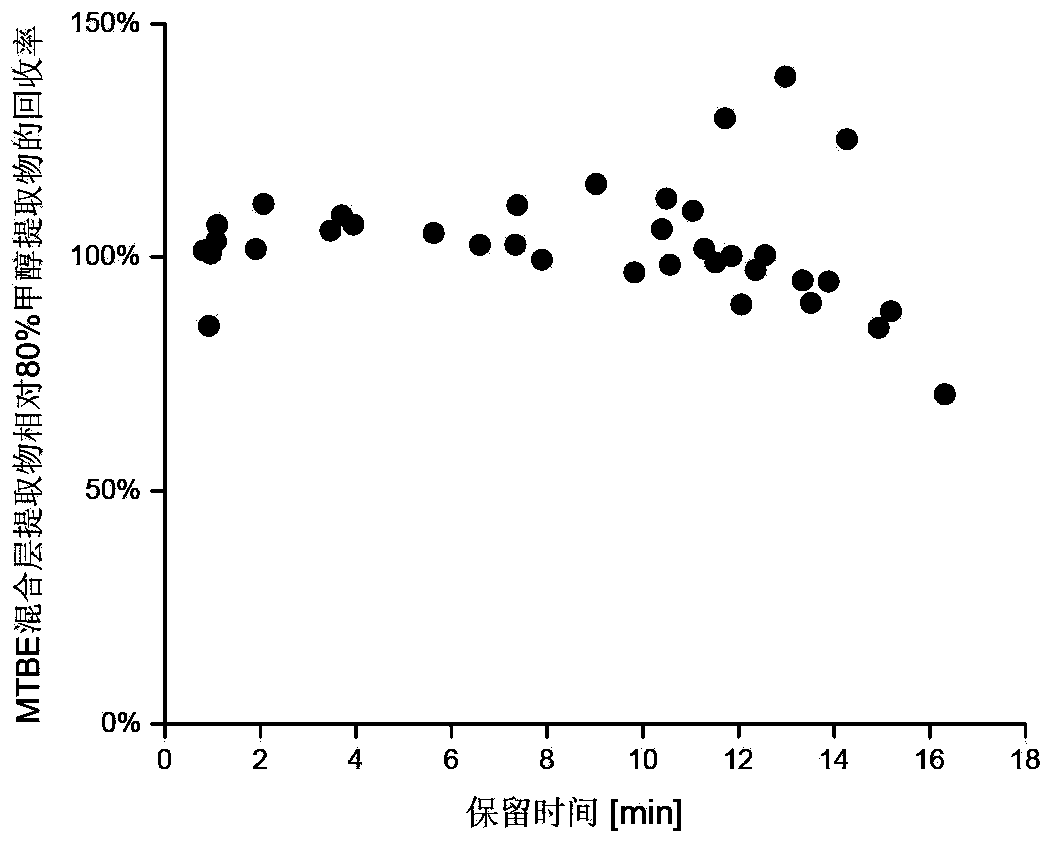
Method for simultaneous extraction and analysis of metabolite group and lipid group in microtissue
The invention discloses a method for simultaneous extraction and analysis of a metabolite group and a lipid group in microtissue. The method comprises the following steps: freeze drying to-be-analyzed microtissue, accurately weighing 1 to 25 mg of the freeze-dried microtissue and adding solvents like methanol (MeOH), methyl tert butyl ether (MTBE) and water in certain proportion for extraction; allowing a solution obtained after completion of extraction to be divided into two layers, wherein an upper layer mainly contains nonpolar metabolites and lipids, and the lower layer mainly comprises polar and medium-polar metabolites; and subjecting the upper-layer solution and the lower-layer solution to mixing in proportion and freeze-drying, then carrying out redissolving and then metabonomical analysis based on liquid chromatography-mass spectrometry, subjecting the upper-layer solution to freeze-drying and then to redissolving and carrying out lipidomical analysis based on liquid chromatography-mass spectrometry. The method has the following advantages: metabolites and lipids are extracted as many as possible through one extraction of a small amount of tissue, and through metabonomical and lipidomical analysis, the amount of a tissue sample is saved, which benefits other biochemical analysis of the tissue sample.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
LC/MS method of analyzing high molecular weight proteins
InactiveUS7329353B2Ion-exchange process apparatusComponent separationLiquid chromatography mass spectroscopyBiochemistry
Owner:AMGEN INC
System and method for grouping precursor and fragment ions using selected ion chromatograms
ActiveUS7800055B2Ion-exchange process apparatusParticle separator tubesLiquid chromatography mass spectroscopyRetention time
LC / MS data generated by an LC / MS system is analyzed to determine groupings of ions associated with originating molecules. Ions are grouped initially according to retention time, for example, using retention time or chromatographic peaks in mass chromatograms. After initial groupings are determined based on retention time, ion peak shapes are compared to determine whether ions should be excluded. Ions having peak shapes not matching other ions, or alternatively a reference peak shape, are excluded from the group.
Owner:WATERS TECH CORP
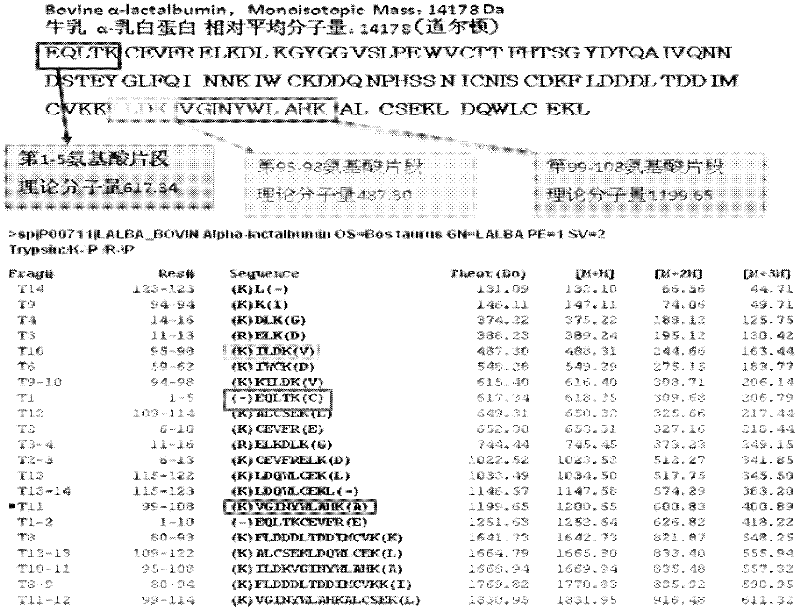
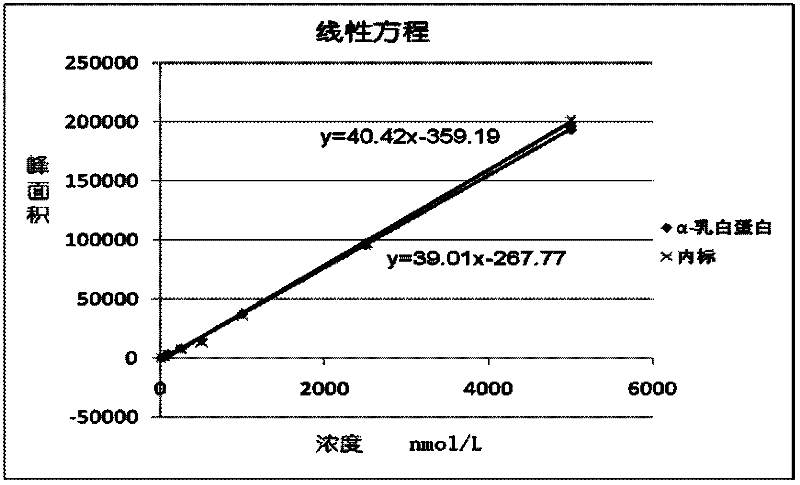
Quantitative detection method for bovine alpha-lactalbumin
InactiveCN102590413AEasy to operateGuaranteed reproducibilityComponent separationRelative standard deviationReversed-Phase Liquid Chromatography
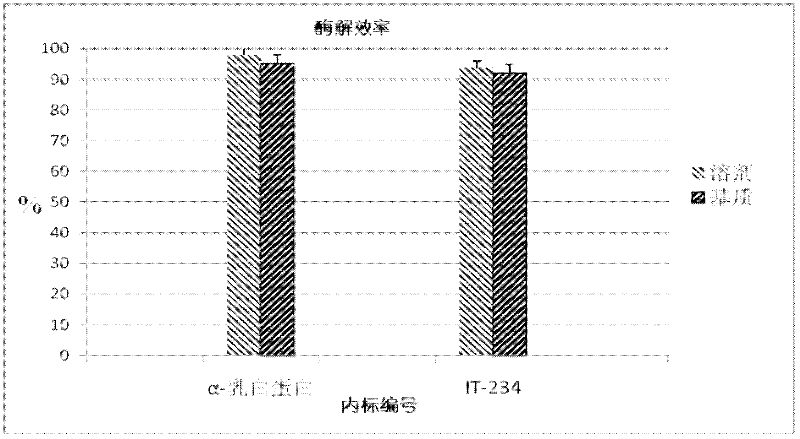
The invention relates to a quantitative detection method for thermal-denaturation and non-denaturation bovine alpha-lactalbumin in milk and milk products by applying an enzymolysis-liquid chromatography and mass spectrometry combination technology. The quantitative detection method comprises the steps as follows: taking a certain amount of milk or milk samples, dissolving and diluting the milk or milk samples in water to obtain solution with total protein content being about 1mg / mL; after volume metering, correctly sucking 500 mu L, adding an internal standard substance, reacting disulfide bond with dithiothreitol (DTT), alkylating to protect sulfydryl produced in reaction by iodoacetamide (IAA), and then conducting constant-temperature and constant-time enzymolysis with trypsin; and separating enzymolysis products by reversed phase liquid chromatography, conducting detection with a mass spectrum multiple reaction monitoring (MRM) manner, and calculating the result by an internal standard method. The quantitation limit of the method is 0.001g / 100g; when adding amount is 0.2, 1.7 and 5.0g / 100g, the recovery rate is 98.9-110.8% (n is equal to 6) and repeatability: RSD (Relative Standard Deviation) is smaller than 7.6%; and the quantitative detection method can be applicable to the quantitative detection of samples with different contents of bovine alpha-lactalbumin.
Owner:ZHEJIANG CENT FOR DISEASE CONTROL & PREVENTION
Bladder cancer patient urine specific metabolite spectrum, establishing method and application
The invention provides a bladder cancer patient urine specific metabolite spectrum, an establishing method and an application, and especially provides a method for establishing a spectrum of specific metabolites in urine of bladder cancer patients, and a method for screening a related specific biomarker. Through metabonomics, especially metabonomics based on liquid chromatography-mass spectrometry combined technology, the invention establishes a bladder cancer patient urine specific metabolite spectrum. The invention provides a basis for early diagnosis of bladder cancer and bladder cancer diseases with different pathological stages, and also provides a spectrum of specific metabolites in urine of bladder cancer patients and a related specific biomarker. The method provided by the invention has the characteristics of noninvasiveness, convenience, and rapidness, can accurately reflect the difference of metabolite spectra of bladder cancer patients and normal people, and has high specificity.
Owner:BGI GENOMICS CO LTD
System and Method for Grouping Precursor and Fragment Ions Using Selected Ion Chromatograms
ActiveUS20080272292A1Ion-exchange process apparatusParticle separator tubesLiquid chromatography mass spectroscopyRetention time
LC / MS data generated by an LC / MS system is analyzed to determine groupings of ions associated with originating molecules. Ions arc grouped initially according to retention time, for example, using retention time or chromatographic peaks in mass chromatograms. After initial groupings are determined based on retention time, ion peak shapes are compared to determine whether ions should be excluded. Ions having peak shapes not matching other ions, or alternatively a reference peak shape, are excluded from the group.
Owner:WATERS TECH CORP
LC/MS (liquid chromatography-mass spectrometer) metabonomics analysis method based on serum of GDM (gestational diabetes mellitus) patient
InactiveCN103592389AComprehensively reflect the variation statusNo noise disturbanceComponent separationMetaboliteOriginal data
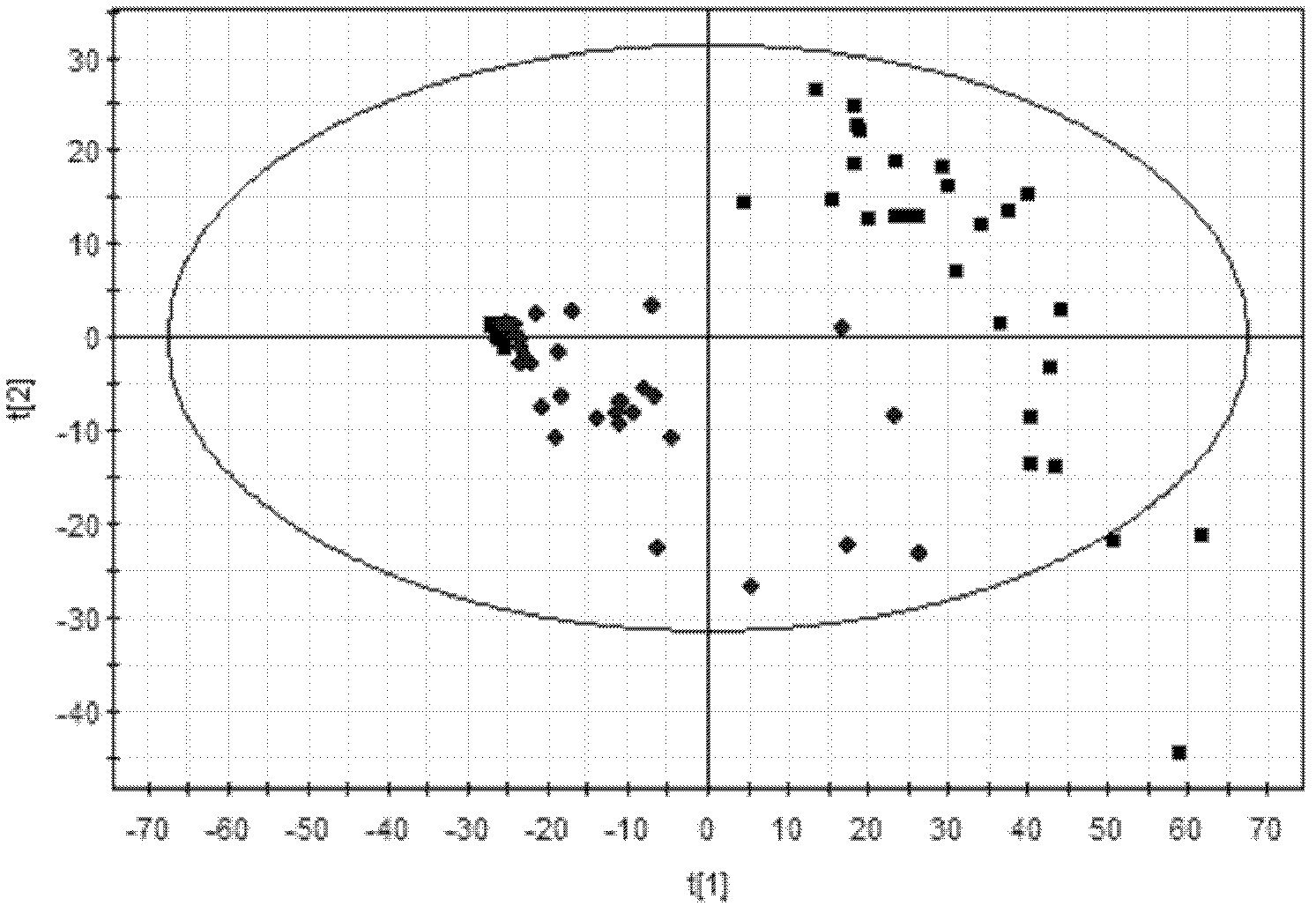
The invention discloses an LC / MS (liquid chromatography-mass spectrometer) metabonomics analysis method based on serum of a GDM (gestational diabetes mellitus) patient. The method comprises the steps as follows: serum samples of a normal pregnant woman and a pregnant woman with GDM are collected, processed and separated by a chromatographic column, and mass spectrometric detection analysis is adopted; original data of an instrument is extracted and aligned after LC / MS detection analysis, and data free of noise disturbance and capable of being used for statistical analysis is obtained; and a multi-dimensional statistical model is established and visually displays metabolic profiling difference between the two groups, so that differential metabolites of the two groups are obtained, and the substances are subjected to preliminary evaluation. With the adoption of the method, the variation condition of the metabolites of the GDM patient can be presented comprehensively and synthetically, and the method can be used for providing powerful technical support for early diagnosis and prognosis of GDM.
Owner:HUZHOU CENT HOSPITAL
Ionizable isotopic labeling reagents for relative quantification by mass spectrometry
ActiveUS20080050833A1Improve ETD analysisConvenient charging statusOrganic compound preparationComponent separationMetaboliteIsotopic labeling
Relative quantification of metabolites by Electrospray Ionization Mass Spectrometry (ESI-MS) requiring a mechanism for simultaneous analysis of multiple analytes in two or more samples. Labeling reagents that are reactive to particular compound classes and differ only in their isotopic kit facilitating relative quantification and providing tangible evidence for the existence of specific functional groups. Heavy and light isotopic forms of methylacetimidate were synthesized and used as labeling reagents for quantification of amine-containing molecules, such as biological samples. Heavy and light isotopic forms of formaldehyde and cholamine were also synthesized and used independently as labeling reagents for quantification of amine-containing and carboxylic acid-containing molecules, such as found in biological samples. Advantageously, the labeled end-products are positively charged under normal acidic conditions involving conventional Liquid Chromatography Mass Spectrometry (LC / MS) applications. Labeled primary and secondary amine and carboxylic acid end-products also generated higher signals concerning mass-spectra than pre-cursor molecules and improved sensitivity. Improved accuracy concerning relative quantification was achieved by mixing heavy and light labeled Arabidopsis extracts in different ratios. Labeling strategy was further employed to ascertain differences in the amounts of amine-containing metabolites for two strains of Arabidopsis seeds.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ILLINOIS +1
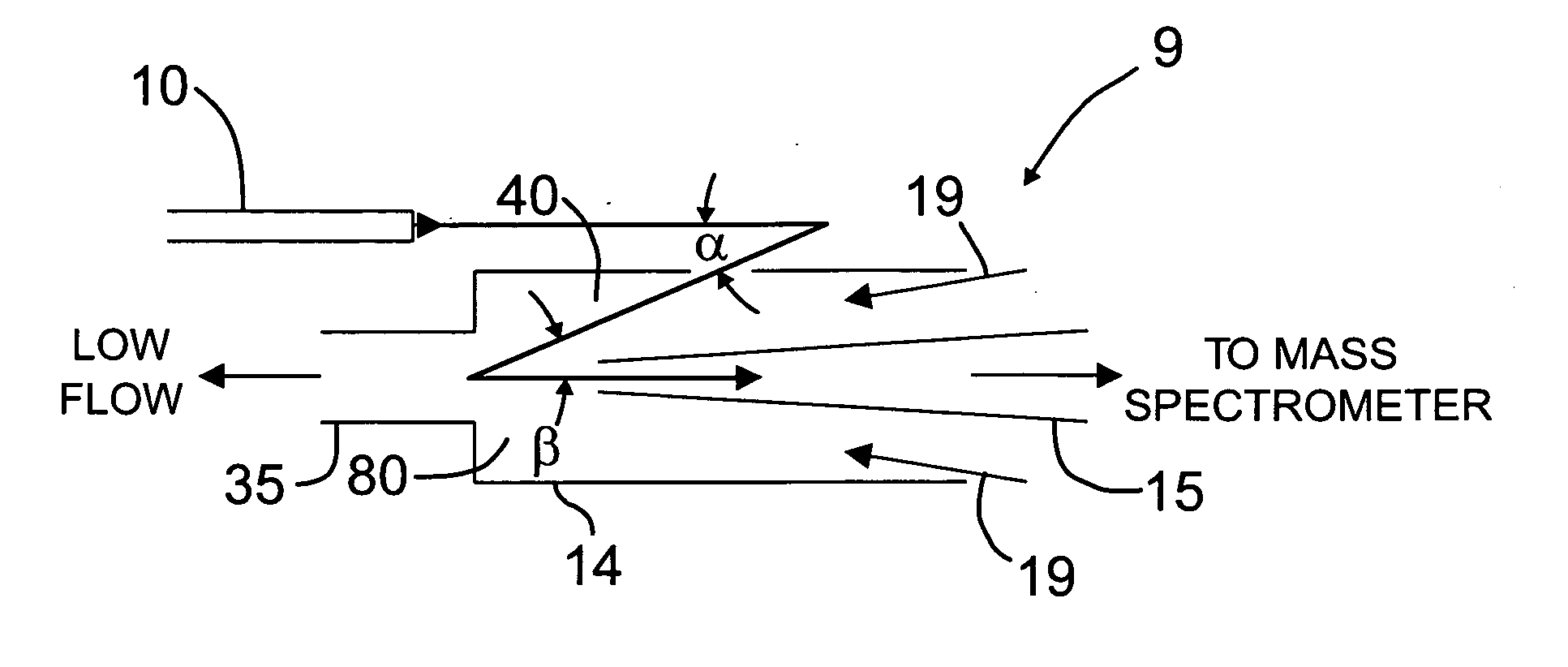
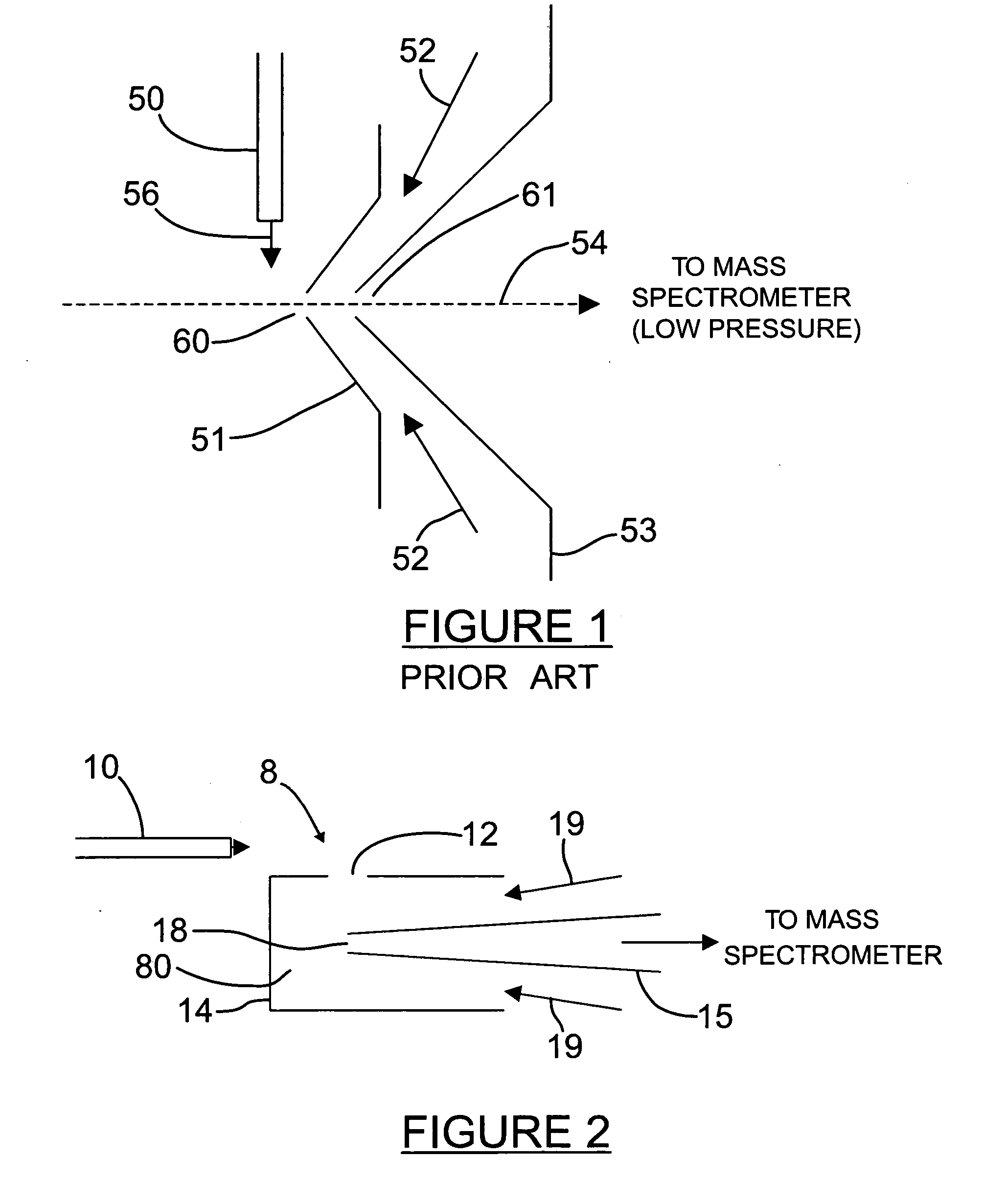
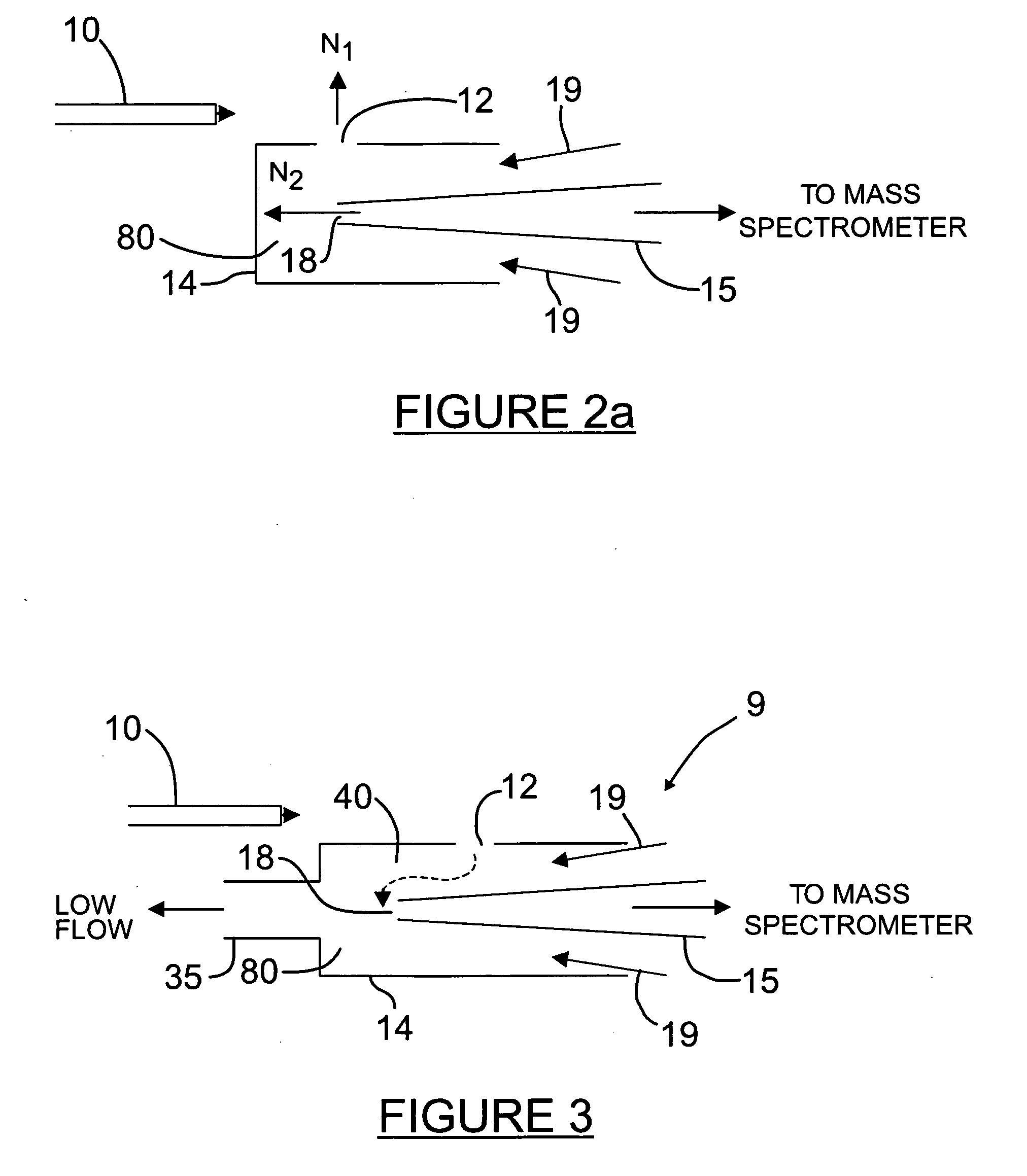
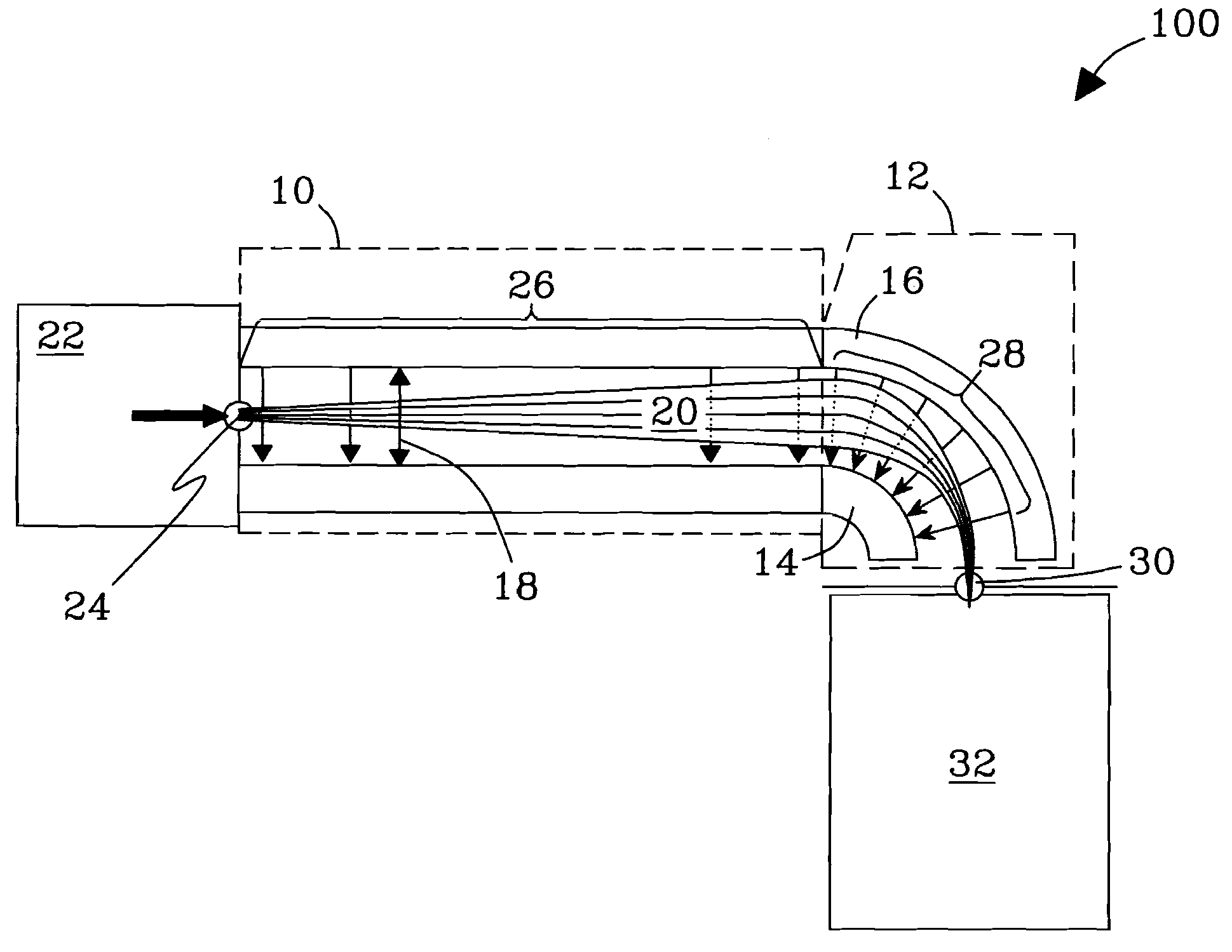
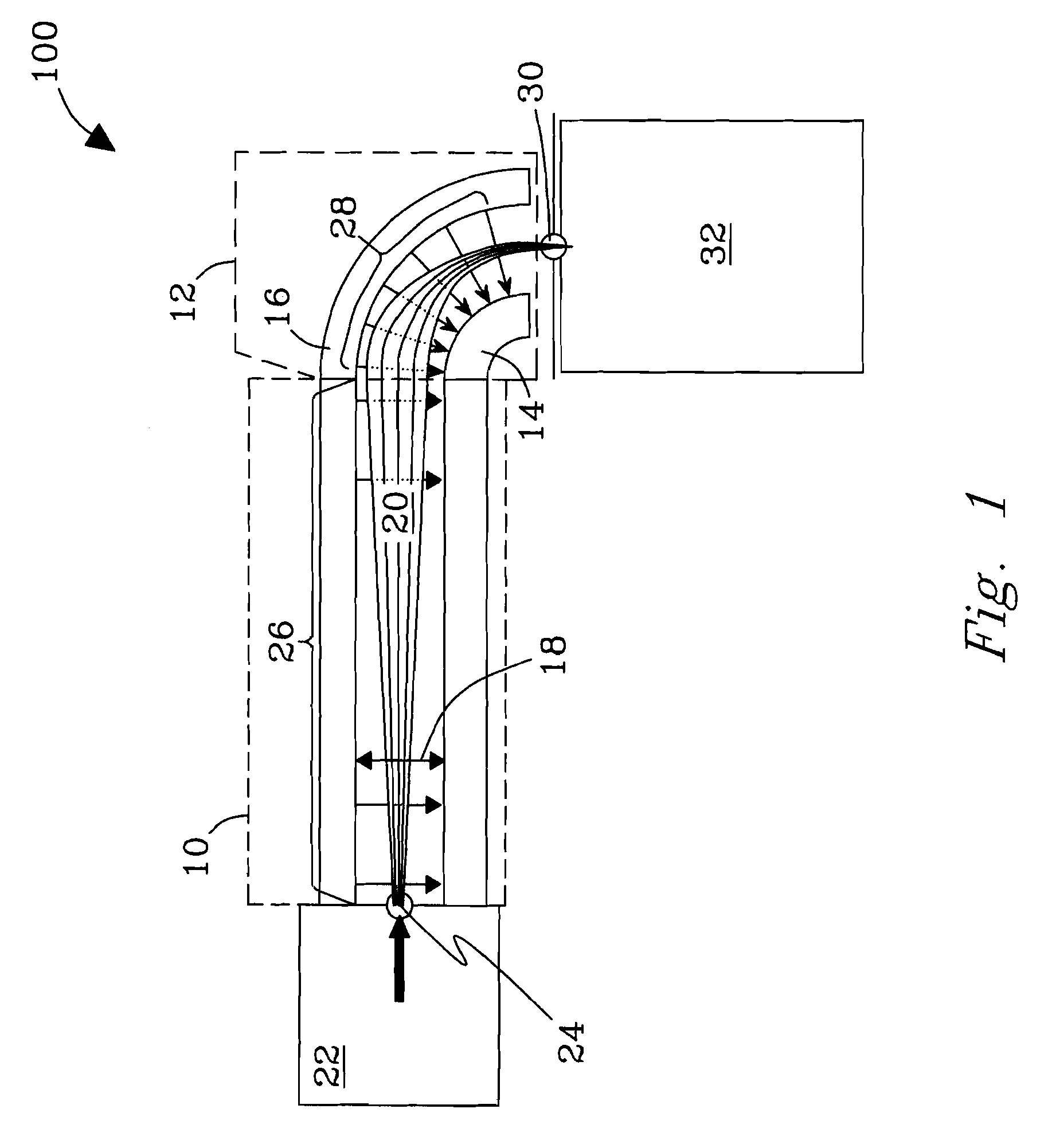
Orthogonal ion sampling for electrospray [LC/MS] mass spectrometry
InactiveUSRE36892E1Increased signal noiseLess sensitivityComponent separationSamples introduction/extractionAnalyteLiquid chromatography mass spectroscopy
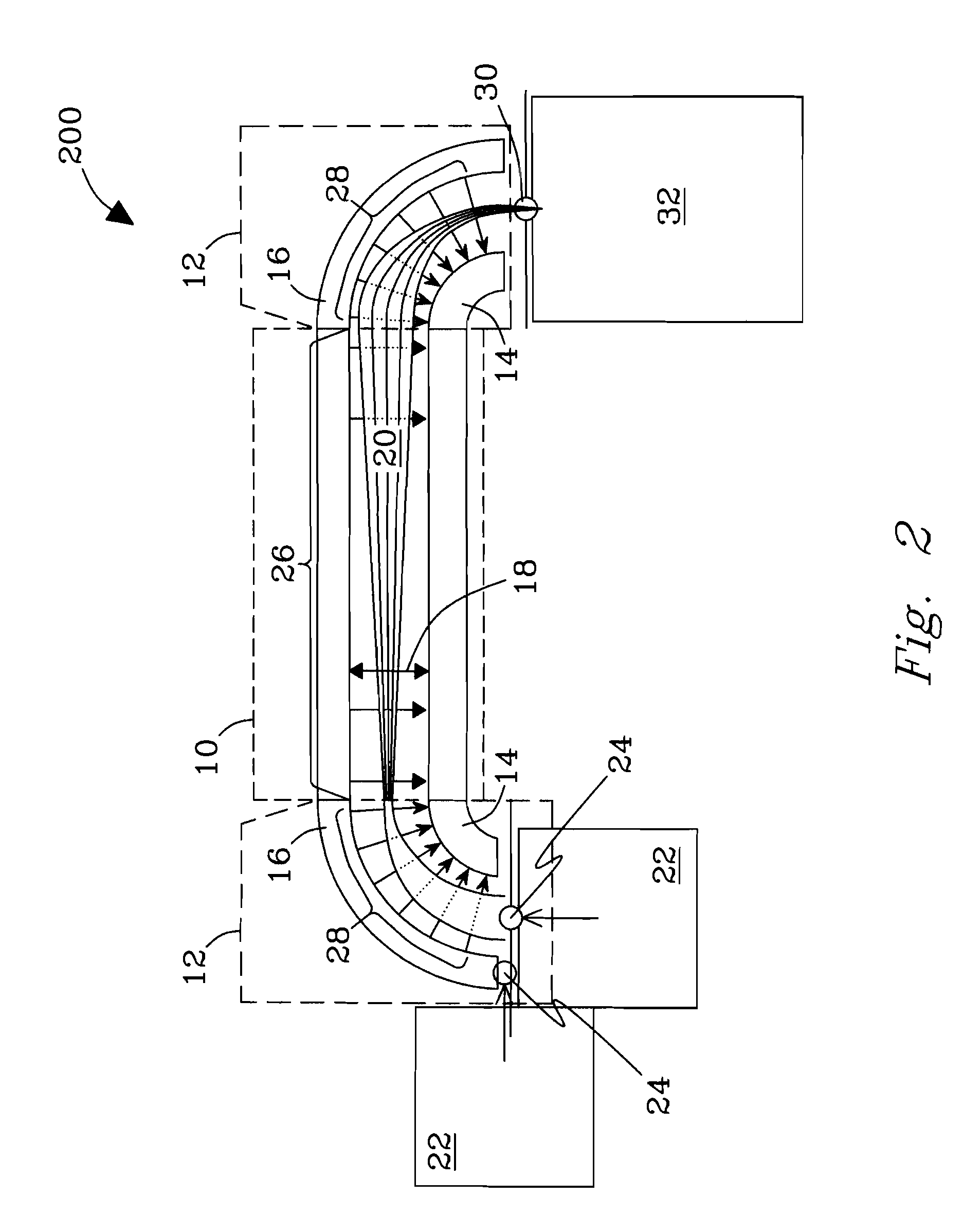
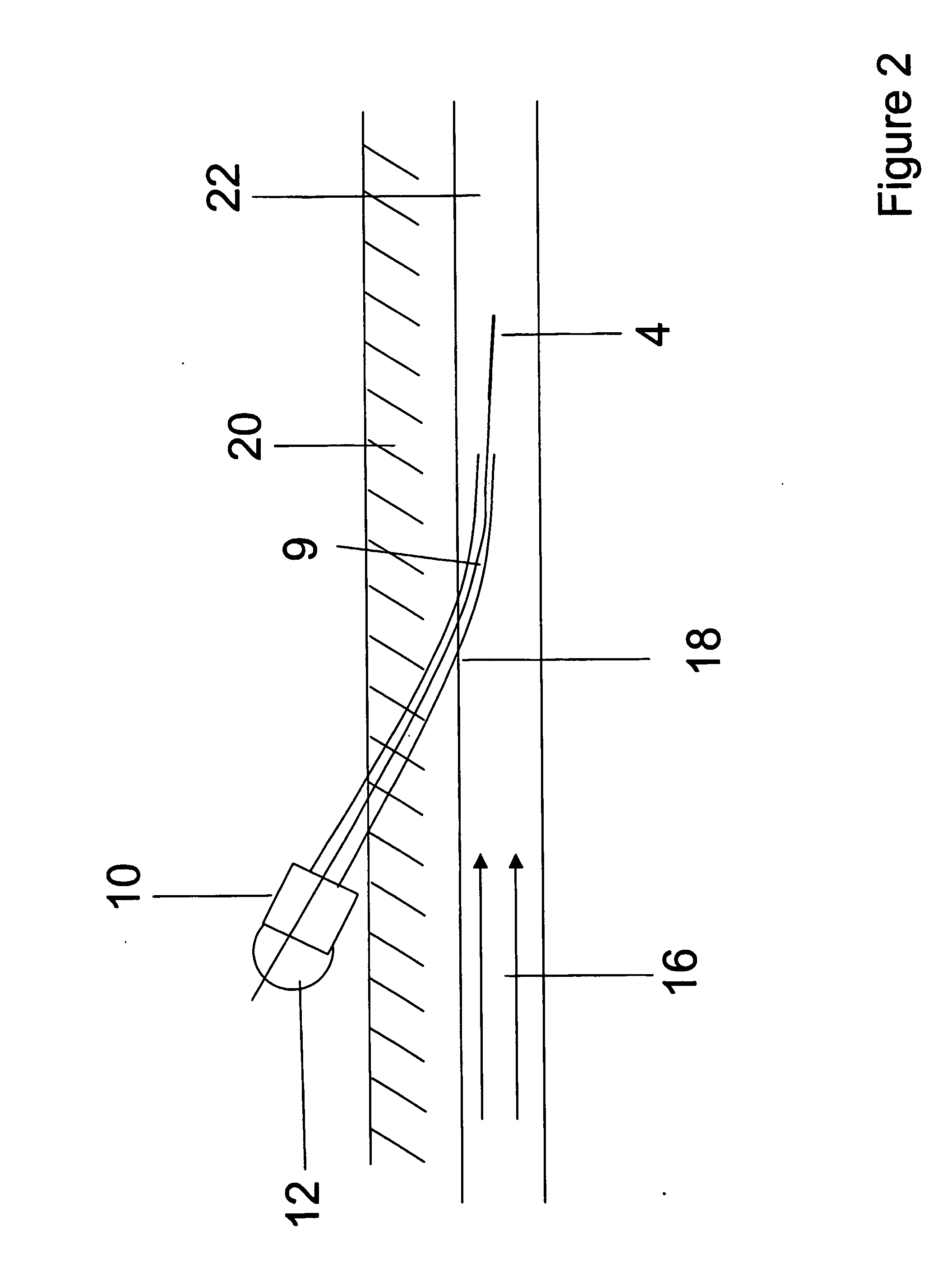
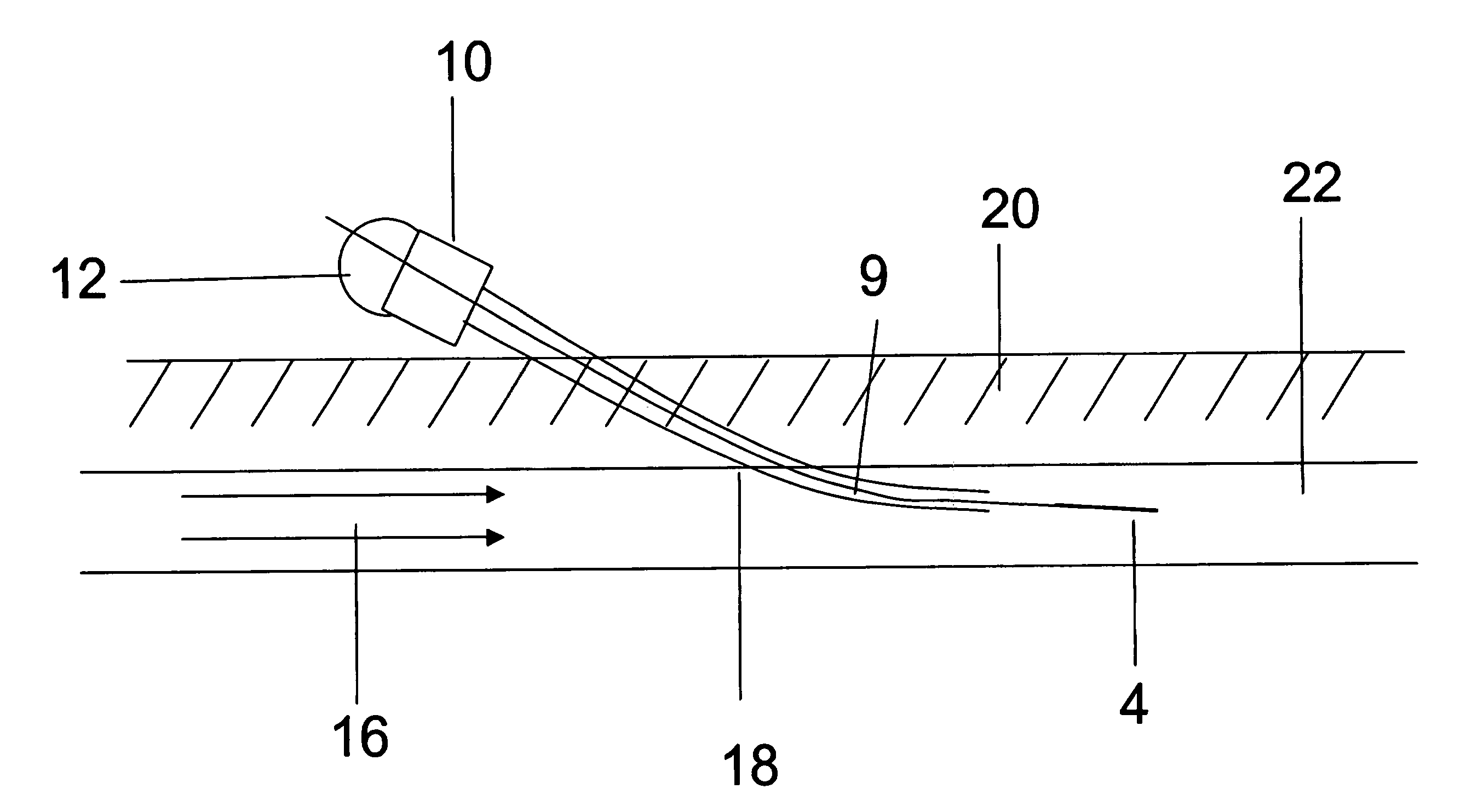
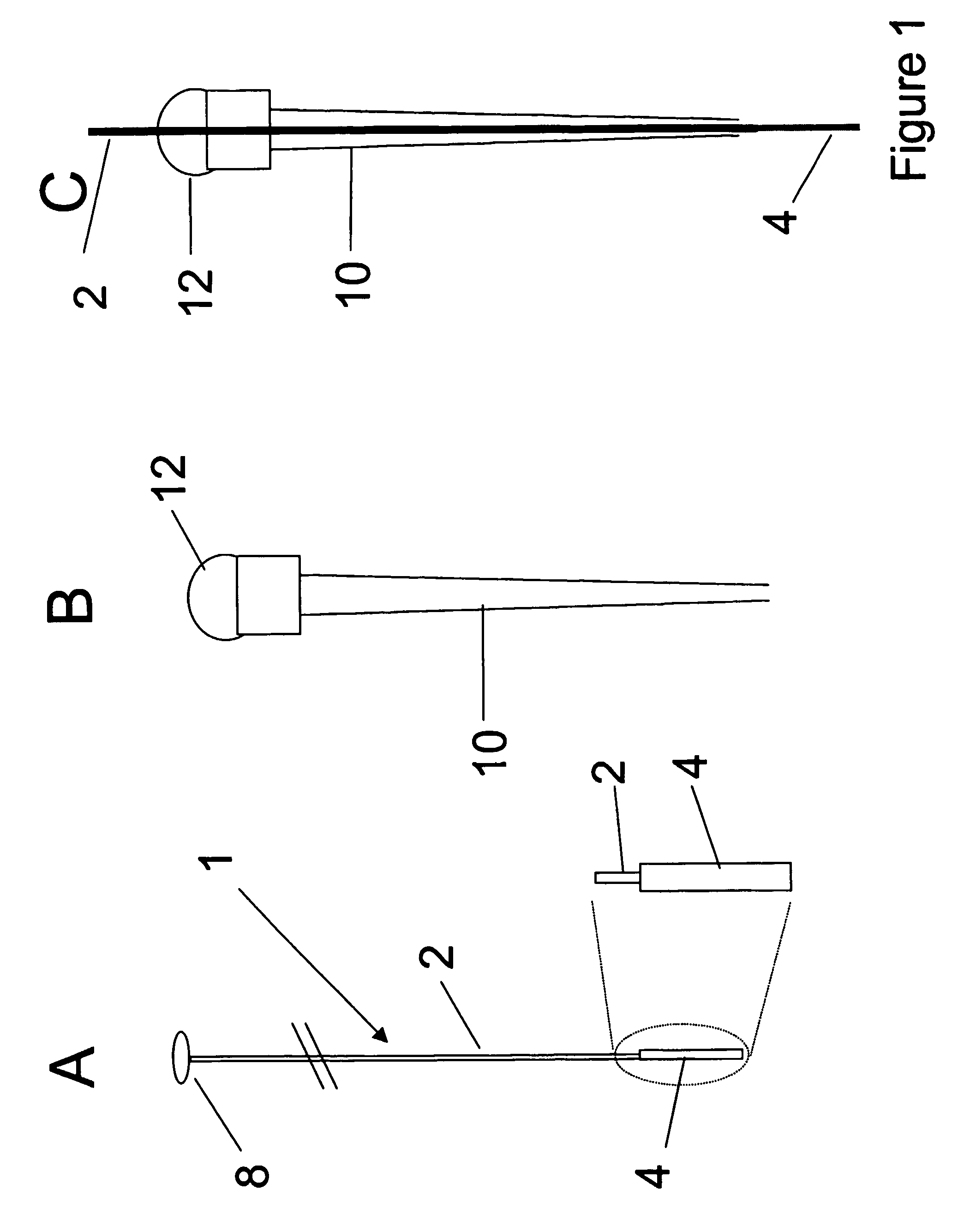
[The invention teaches the uses of a plurality of electric fields and of orthogonal spray configurations of vaporized analyte which combine so as to operate to enhance the efficiency of analyte detection and mass analysis with a mass spectrometer by reducing vapor in the vacuum system and concomitant noise. Several embodiments of the invention are described for purposes of illustration.] The invention relates to a method and apparatus for improving signal relative to noise without loss of ion collection efficiency in electrospray mass spectrometry, including liquid chromatography / mass spectrometry.
Owner:AGILENT TECH INC
Liquid chromatography for synchronously detecting 15 anabolic hormone residues in food
ActiveCN101551362ALow toxicityLow costComponent separationPreparing sample for investigationWater bathsHplc method
The invention relates to a liquid chromatography for detecting anabolic hormone drug residue in animal-derived food, which is characterized by first sampling: animal musculature is taken off the fat and connective tissue, then minced and evenly ground, and the sample is weighed; extracting: anabolic hormone extract is extracted from the weighed sample by methanol ultrasonic extraction, the extract is evaporated to dryness in water bath through a rotary evaporator, and the residue is dissolved by methanol aqueous solution; purifying: C18 Solidoid extraction column on the extract is carried out solid phase extraction; and testing by devices: the filtrate is tested by opposite phase high efficiency liquid chromatography, quantified by external reference method-peak area, and then carried out with binary gradient elution. In the invention, the detected sample is complex biological sample; the established method can complete one detection in about one hour and is simple and fast, with reliable sensitivity and low cost; the method can analyze more veterinary hormone drug species than the synchronous usage of the existing GC or HPLC methods with easier operation, and has lower cost than the existing GC / MS and LC / MS or LC / MS / MS methods with low solvent toxicity.
Owner:上海国矗生物科技有限公司
System and method for metabonomics directed processing of LC-MS or LC-MS/MS data
InactiveUS20060151688A1Reduce settingsParticle separator tubesComponent separationRetention timeLiquid chromatography mass spectroscopy
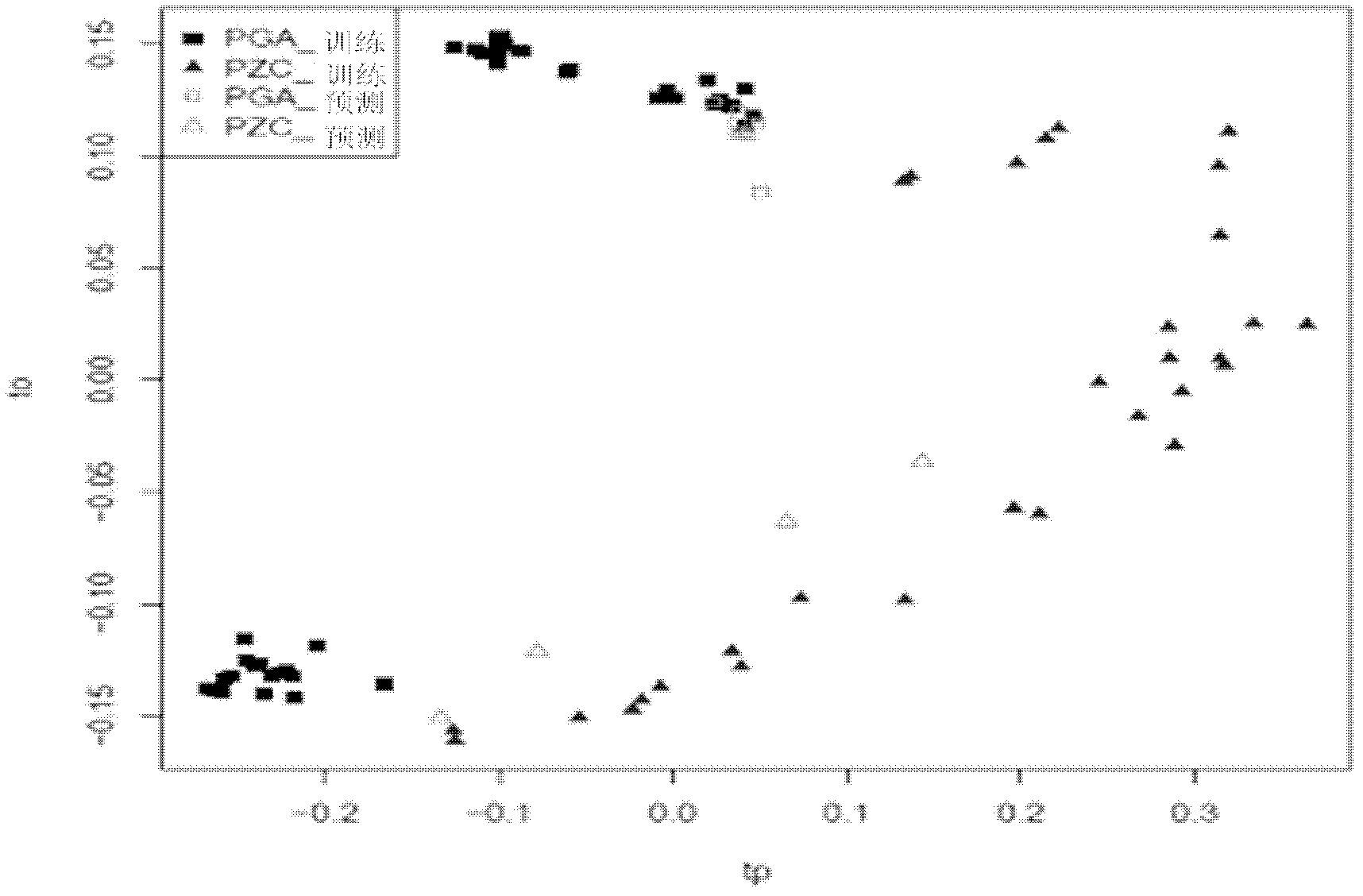
A method of programmatically reducing a set of collected LC-MS or LC-MS / MS data such that true chromatographic and MS peaks are identified for use in Metabonomics is disclosed. The identified peaks are used to create a list of LC / MS, GC / MS, DIOS-MS or MALDI-MS signals and responses for a batch of samples which appear in a Master Entity List. The samples in the Master Entity List are then subjected to isotope de-clustering and adduct removal prior to chemometrics being applied to automatically identify biomarkers. An LC-MS / MS or LC / MS, GC / MS, DIOS-MS or MALDI-MS acquisition list is generated for the signals identified as responsible for the PLS-DA or PCA separation. The LC or GC retention time, exact mass and MS / MS spectrum may be compared to databases of known compounds and identified compounds associated with biological parameters may be stored in a new compound database.
Owner:MICROMASSS UK LTD
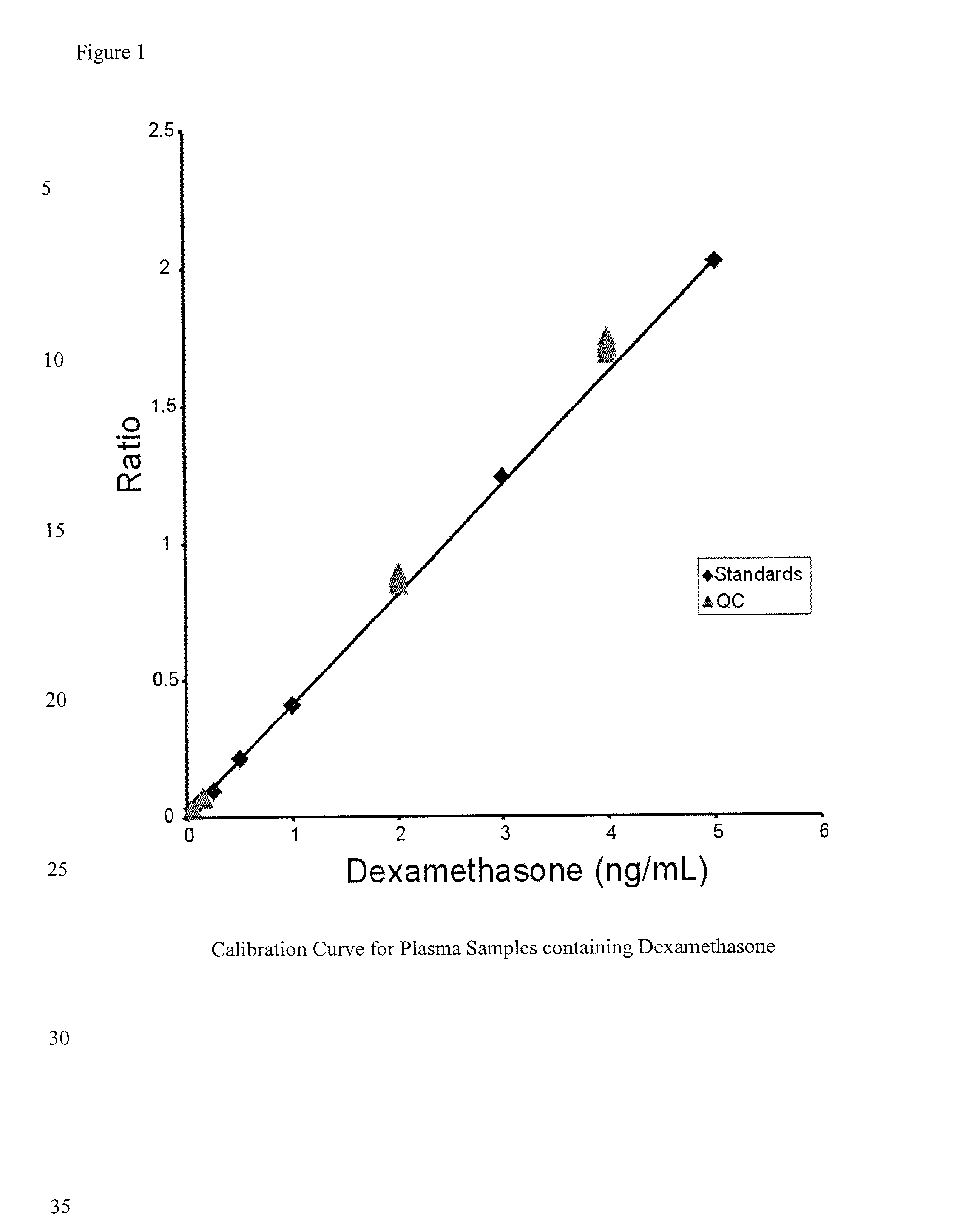
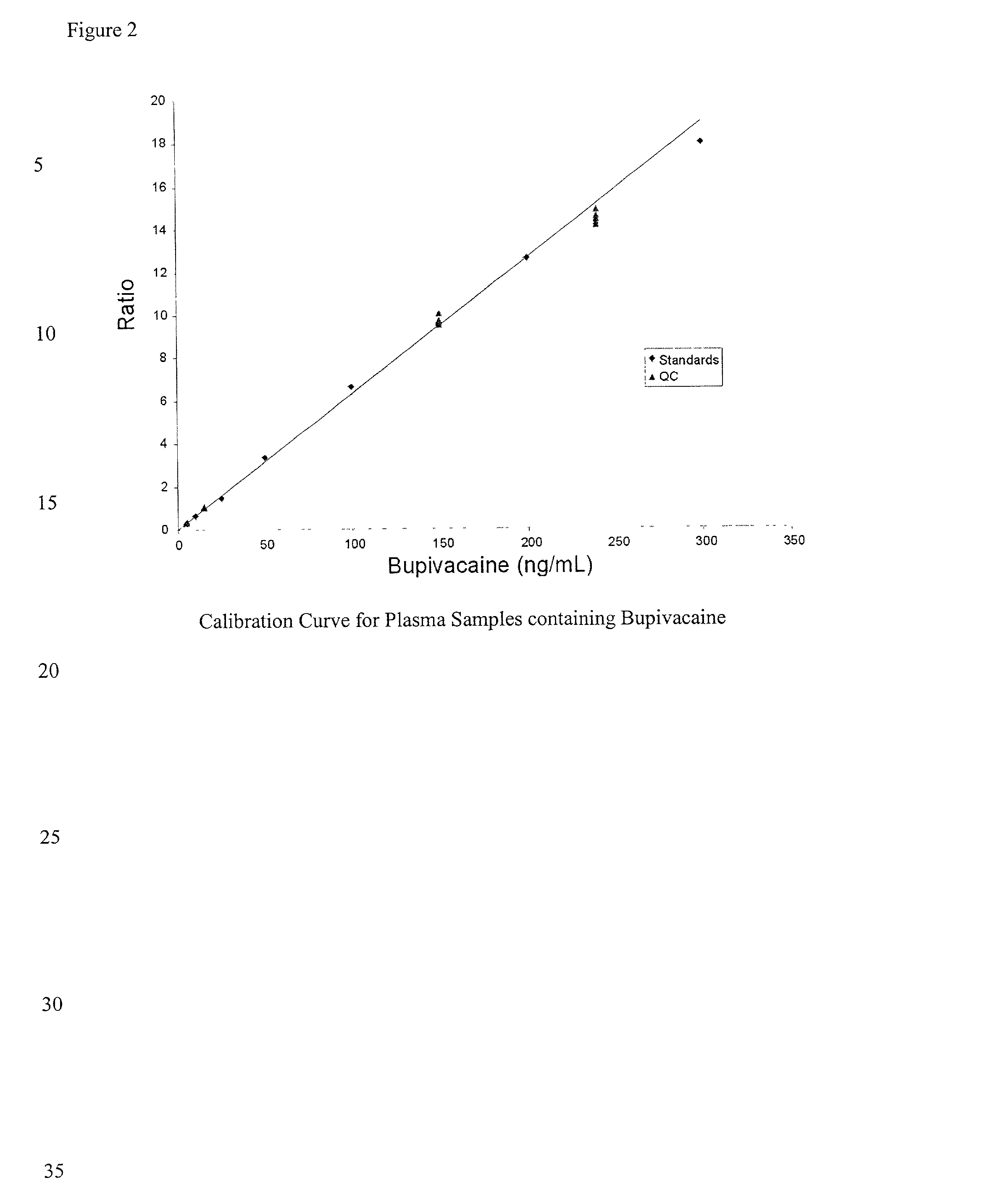
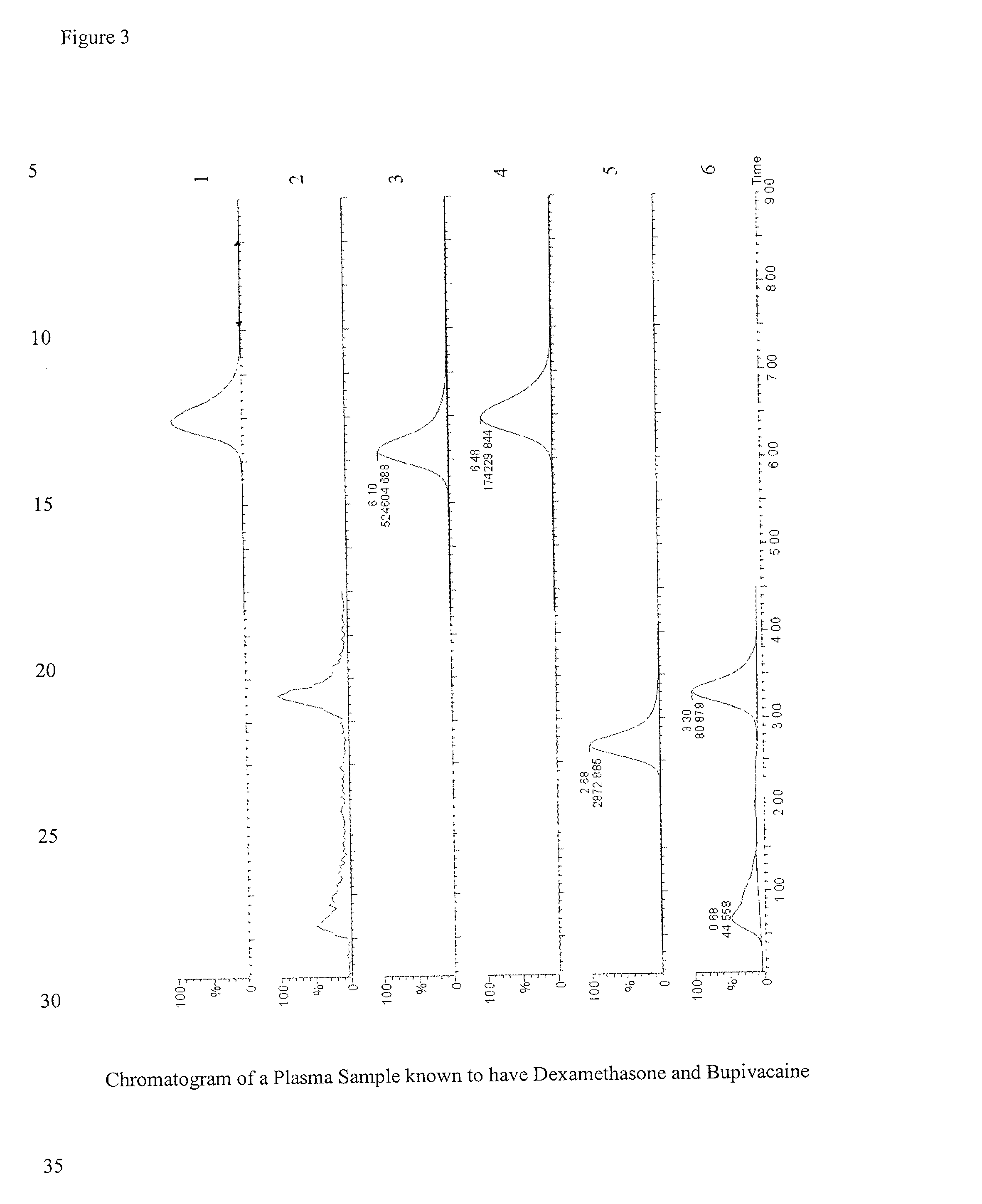
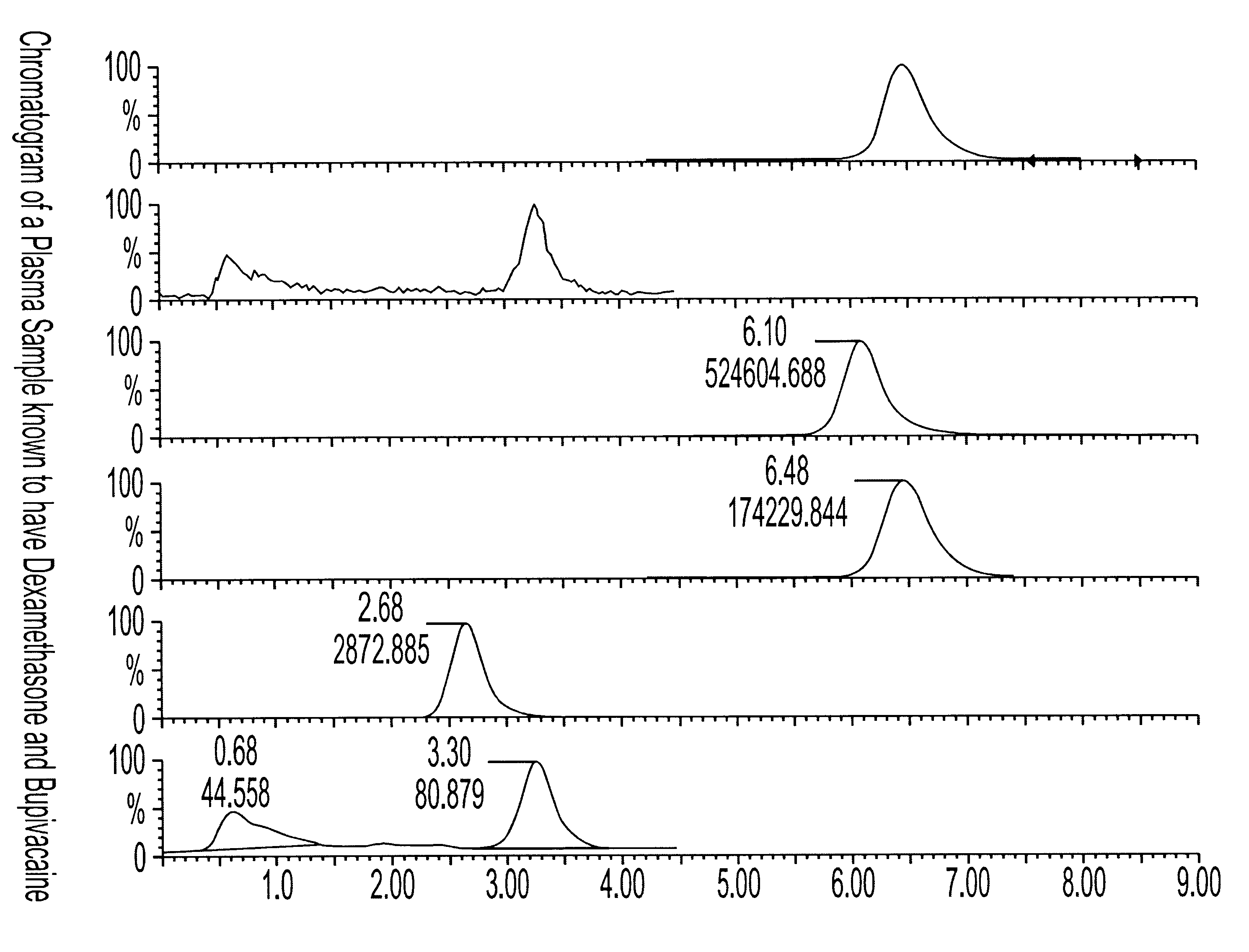
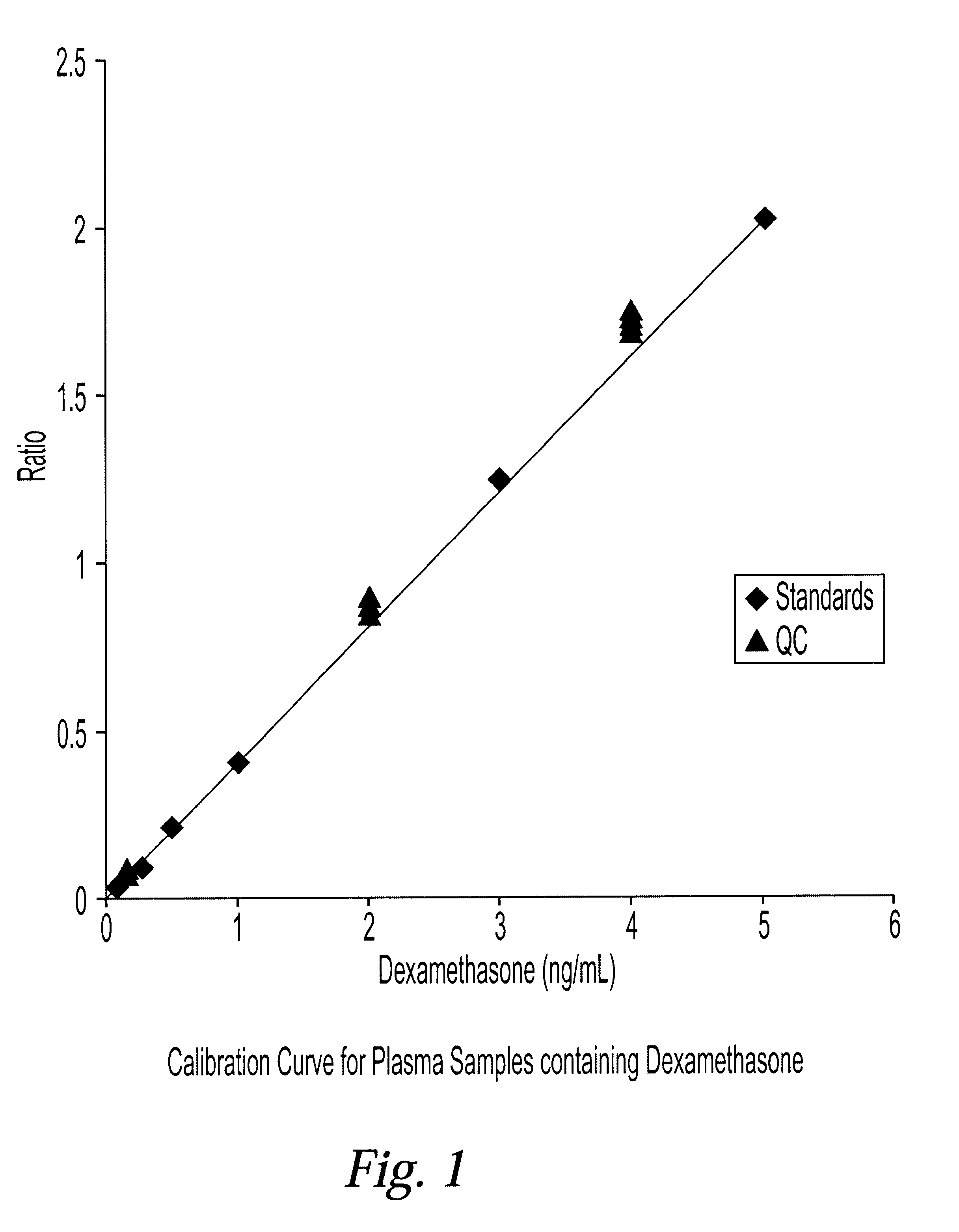
Determination of corticosteroids in human plasma using micromass LC/MS/MS/
InactiveUS20020168775A1Component separationBiological testingLiquid chromatography mass spectroscopyInternal standard
The present invention is directed to a method of detecting a corticosteroid in a sample by adding an internal standard to a sample suspected of containing a corticosteroid; removing interfering compounds from the sample; placing the sample on an HPLC column equilibrated with a NH4OAc:MeOH solution and collecting an eluent; and analyzing the eluent of the HPLC column with a MS, wherein if contained in the sample, the corticosteroid forms an adduct that is detected by the MS.
Owner:PURDUE PHARMA LP
High sensitivity mass spectrometer interface for multiple ion sources
InactiveUS20070181800A1High sensitivityImprove throughputComponent separationSamples introduction/extractionLiquid chromatography mass spectroscopyMass spectrometric
An interface for mass spectrometers. The interface uses non coaxial sampling pathways of the analyte ion beam prior to entering the entrance of a mass spectrometer for decreasing chemical background, and can be done in such a way as to permit multiple sprayers, increasing sample throughput and sensitivity for LC / MS (liquid chromatography / MS). The interface includes an ion source having an exit from which a beam of analyte ions are emitted, a curtain plate and an aperture in the curtain plate member, an orifice plate having an orifice therein. The orifice plate is being spaced from the curtain plate member defining a flow passageway therebetween, and the aperture in the orifice plate is aligned with a sample entrance to a first vacuum stage of a mass spectrometer maintained substantially lower than atmospheric pressure. The aperture in the curtain plate member is non coaxially aligned with the orifice in the orifice plate and the interface includes a gas flow mechanism for directing a counter flow gas into the flow passageway.
Owner:PERKINELMER HEALTH SCI CANADA INC
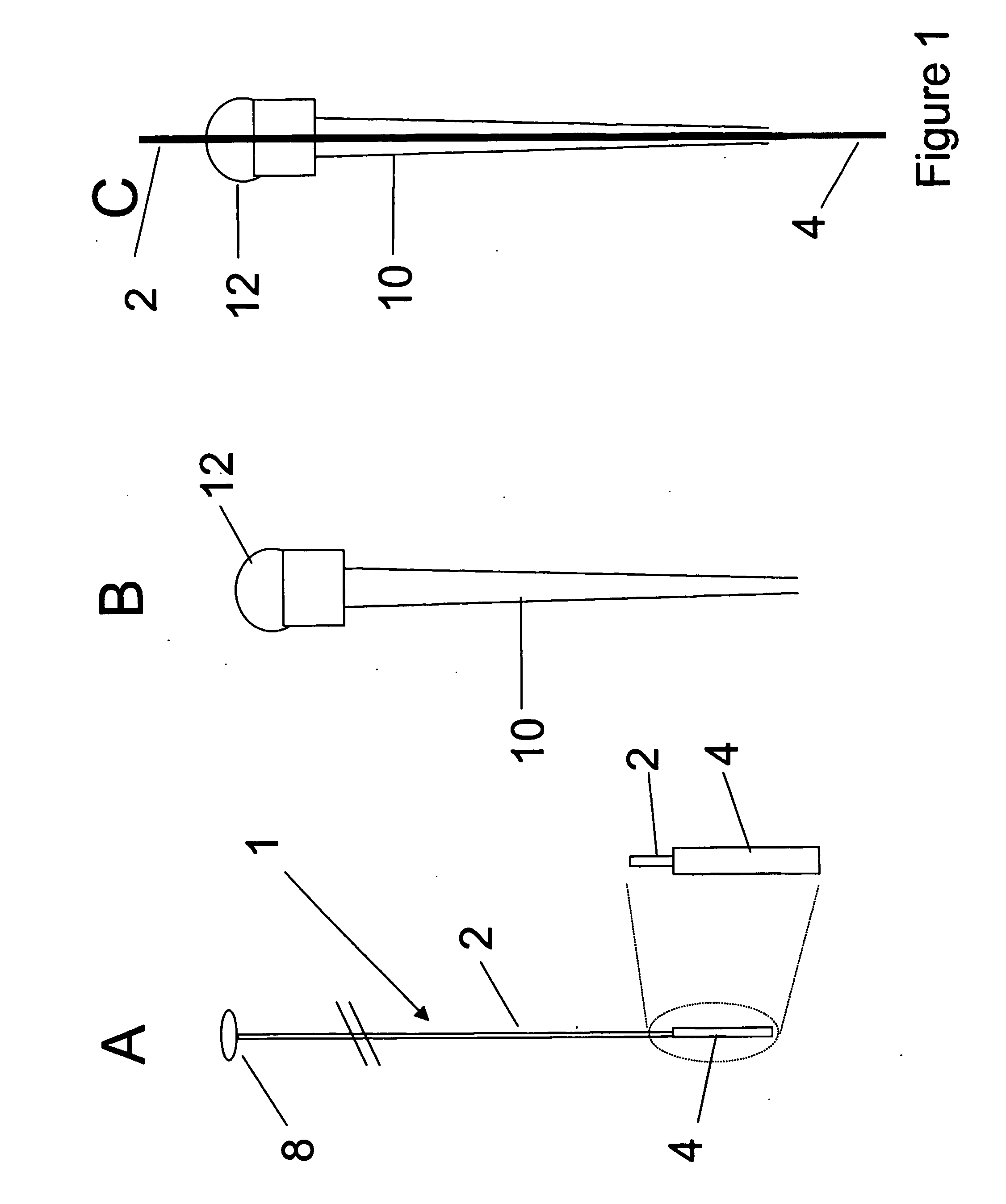
Hooked differential mobility spectrometry apparatus and method therefore
InactiveUS20080156978A1Improve ionization efficiencyHigh analytical sensitivityTime-of-flight spectrometersElectron/ion optical arrangementsLiquid chromatography mass spectroscopyIon beam
Disclosed are a device and method for improved interfacing of differential mobility spectrometry (DMS) or field asymmetric waveform ion mobility spectrometry (FAIMS) analyzers of substantially planar geometry to subsequent or preceding instrument stages. Interfacing is achieved using curved DMS elements, where a thick ion beam emitted by planar DMS analyzers or injected into them for ion filtering is compressed to the gap median by DMS ion focusing effect in a spatially inhomogeneous electric field. Resulting thinner beams are more effectively transmitted through necessarily constrained conductance limit apertures to subsequent instrument stages operated at a pressure lower than DMS, and / or more effectively injected into planar DMS analyzers. The technology is synergetic with slit apertures, slit aperture / ion funnels, and high-pressure ion funnel interfaces known in the art which allow for increasing cross-sectional area of MS inlets. The invention may be used in integrated analytical platforms, including, e.g., DMS / MS, LC / DMS / MS, and DMS / IMS / MS that could replace and / or enhance current LC / MS methods, e.g., for proteomics research.
Owner:BATTELLE MEMORIAL INST
Oregano and mint Anti-inflammatory compositions and methods
InactiveUS20120135094A1Facilitates frameworkReduce painFood ingredient as antioxidantBiocideAdditive ingredientLiquid chromatography mass spectroscopy
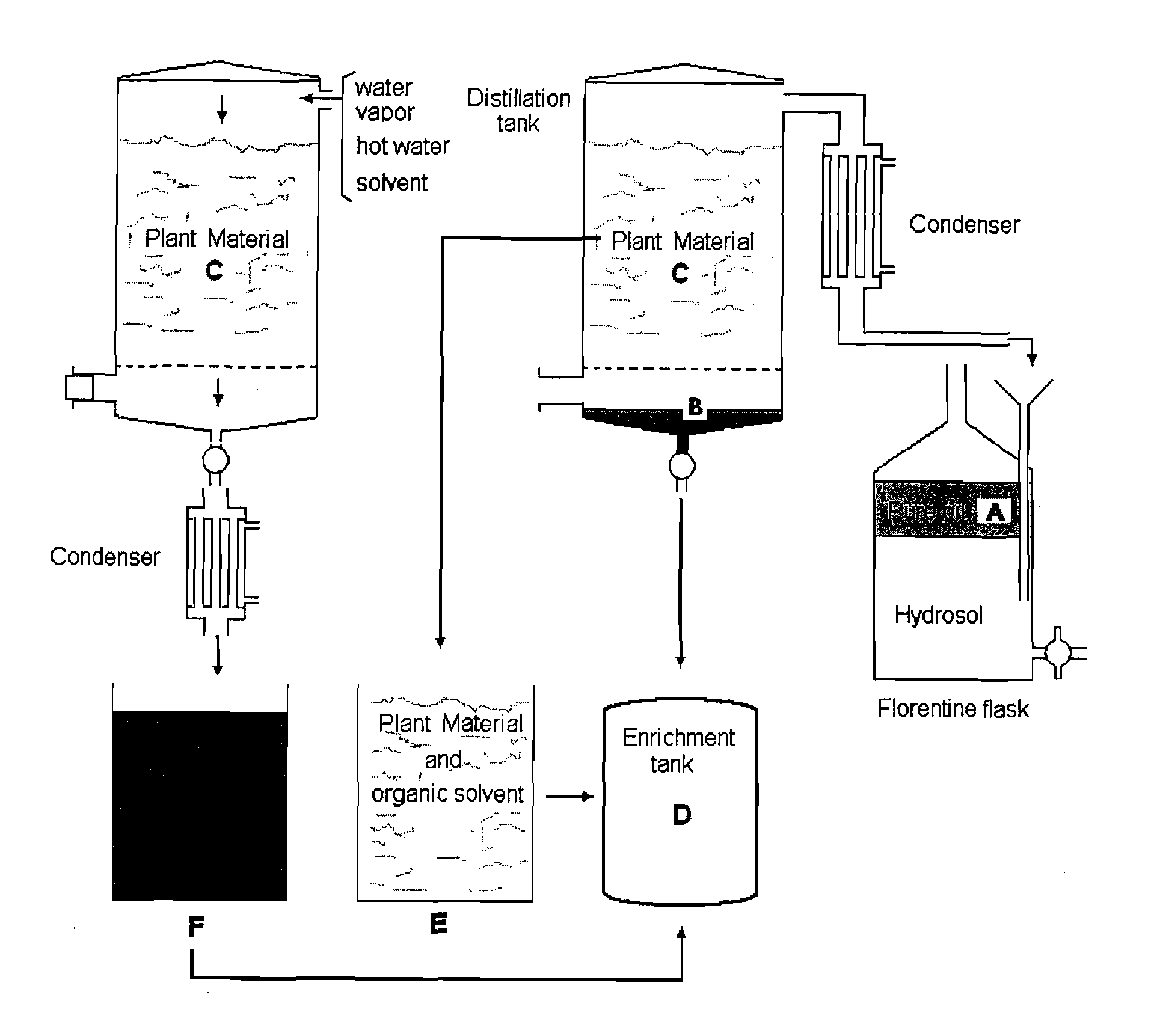
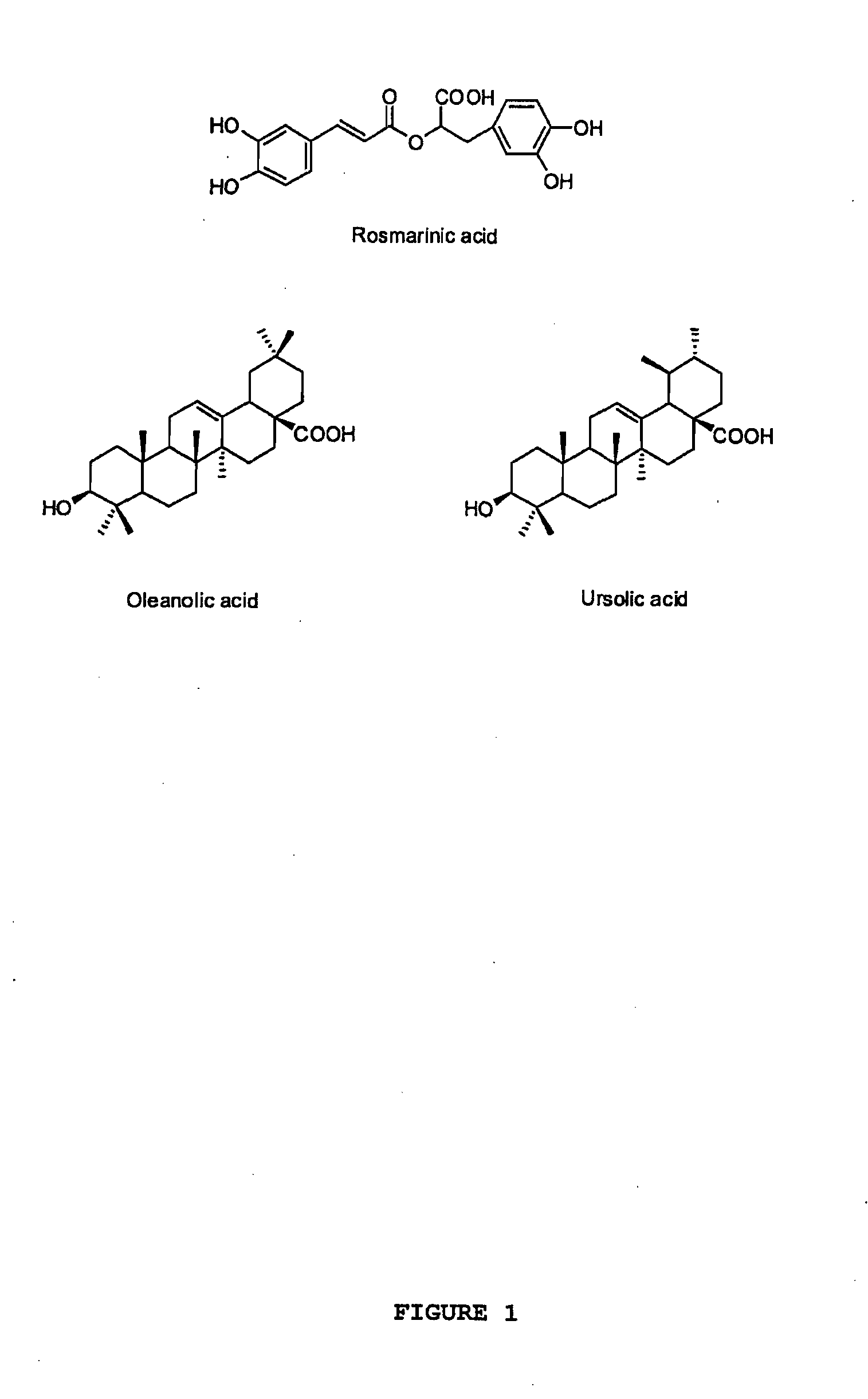
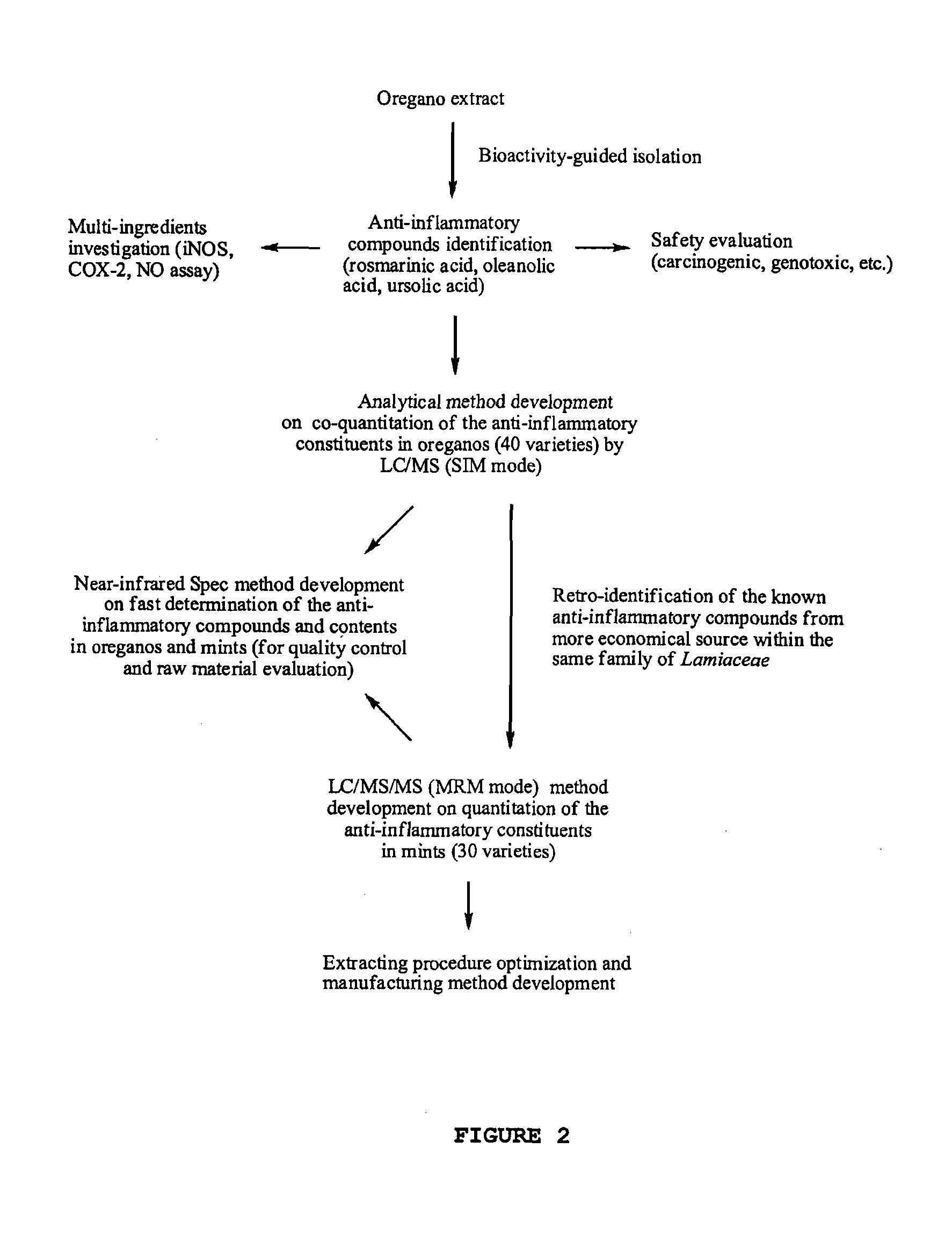
The present invention relates to bioactivity-guided isolation and identification of bioactive compounds from oregano and mint plants, in particular, rosmarinic acid, oleanolic acid and ursolic acid, and use of these compounds or combinations thereof as anti-inflammatory agents for the treatment of conditions related to pain and inflammation and / or as ingredients of dietary supplements. The invention also relates to optimization of the methods for qualitative and quantitative analysis of the bioactive compounds in oregano and mint plants. In particular, this invention introduces an LC / MS (SIM mode) method to achieve co-quantitation of the three organic acids using a unique tandem column system. In addition, the invention also relates to the methods for recovering various water-soluble polyphenols and triterpenes from aromatic plants.
Owner:RUTGERS THE STATE UNIV
Method for enriching clenbuterol, ractopamine and salbutamol in urine sample of breeding livestock
InactiveCN103076416AEfficient enrichmentThe pre-processing process is simpleComponent separationPretreatment methodPolystyrene
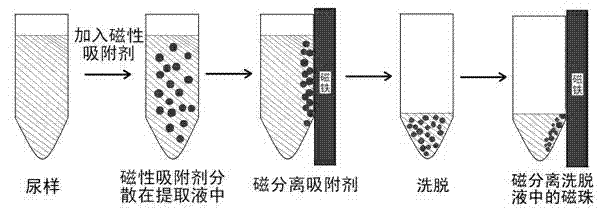
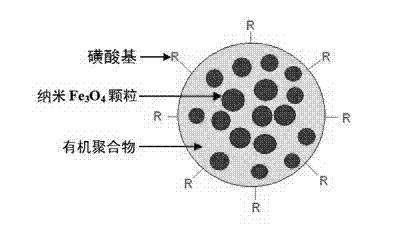
The present invention discloses a method for enriching clenbuterol, ractopamine and salbutamol in a urine sample of breeding livestock, and relates to the field of analytical chemistry, particularly to a sample pretreatment method. The method comprises: carrying out a sample pretreatment, adding sulfonic group surface-modified polystyrene superparamagnetic nanometer beads or sub-micron beads to adsorb, carrying out elution, and collecting the eluent, wherein the elution solution can be directly used for liquid chromatography-mass spectrometry to quantitatively analyze contents of clenbuterol, ractopamine and salbutamol. The method has characteristics of simple operation process, high extraction recovery rate, less elution solvent use amount, and no requirement of rotary evaporation.
Owner:NANCHANG UNIV
Method for detecting tobacco specific nitrosamine by liquid phase chromatography and series mass spectrometry
ActiveCN1616962AEfficient separationEliminate the effects ofComponent separationLiquid chromatography mass spectroscopyPhosphate
The present invention relates to liquid phase chromatography-tandem mass spectrometry process for detecting specific tobacco fume nitrosamine content. The detection process includes: trapping tobacco fume with filtering sheet, adding internal standard substances, vibration extracting the filtering sheet with citric acid-phosphate buffering solution and cyclohexane, concentrating the organic phase extracted liquid in N2 protection, acidifying with hydrochloric acid, phase separating, neutralizing water phase with alkali solution, transferring the water phase solution into reverse chemical bonded solid phase extracting column, leaching with polar organic solvent aqua, eluting with polar organic solvent aqua, concentrating elutent, and analyzing in LC / MS / MS instrument quantitatively based on the area ratio between the component peak and the internal standard substance peak. The present invention has high repeatability, high recovering rate and low detection limit.
Owner:CHINA TOBACCO HUNAN INDAL CORP
Determination of corticosteroids in human plasma using micromass LC/MS/MS
InactiveUS6541263B2Component separationBiological testingInternal standardLiquid chromatography mass spectroscopy
The present invention is directed to a method of detecting a corticosteroid in a sample by adding an internal standard to a sample suspected of containing a corticosteroid; removing interfering compounds from the sample; placing the sample on an HPLC column equilibrated with a NH4OAc:MeOH solution and collecting an eluent; and analyzing the eluent of the HPLC column with a MS, wherein if contained in the sample, the corticosteroid forms an adduct that is detected by the MS.
Owner:PURDUE PHARMA LP
Detection of short-chain fatty acids in biological samples
Described herein is a method of detecting and / or quantifying analytes, such as short-chain fatty acids. Analysis for the presence and / or quantity of the small molecule can be performed on a biological sample from a subject. In some embodiments, a liquid chromatography / mass spectrometry (LC-MS / MS) instrumentation is combined with a solid-phase extraction (SPE). Methods of derivatization can also be incorporated with LC-MS / MS and SPE instrumentation to detect and quantify target analytes. In addition to derivation, methods of reconstituting derivatized molecules can also be incorporated with LC-MS / MS and SPE instrumentation to detect and quantify target analytes.
Owner:HEMAQUEST PHARMA INC
Method for detecting antibiotic residues in milk, and application thereof
InactiveCN104990996AAvoid Analyte LossImprove detection efficiency and throughputComponent separationMass spectrum analysisQuinolone
The invention relates to a method for detecting antibiotic residues in milk, and an application thereof, and belongs to the field of food safety. The invention discloses an online solid phase extraction-high performance liquid chromatography-mass spectrometry detection method in order to solve the detection problem of antibiotic residues in milk. The method allows five types of 33 veterinary drug residues comprising sulfanilamides, quinolones, beta-lactams, tetracyclines and chloramphenicols in the milk to be simultaneously detected. Influences of various factors on the reservation and selectivity of various antibiotics are optimized, so the method has the characteristics of high sensitivity, high recovery rate, high stability and high precision. The detected antibiotics can be qualitatively and quantitatively determined according to the reservation time and accurate mass numbers. The amounts of samples and reagents required and used in the method are small, the detection speed is fast, all operation processes are completed within 15.5min, and cost reduction and operating risk mitigation of dairy product processing enterprises are guaranteed.
Owner:ANIMAL AND PLANT & FOOD DETECTION CENTER JIANGSU ENTRY EXIT INSPECTION AND QUARANTINE BUREAU
Liquid chromatography-mass spectrometry screening method of unknown toxic substances in blood
ActiveCN107478747AEnsure highly sensitive and effective testingQuick checkComponent separationToxicantScreening method
The invention discloses a liquid chromatography-mass spectrometry screening method of unknown toxic substances in blood. The liquid chromatography-mass spectrometry screening method comprises the following steps: taking a blood sample to be detected, adding acetonitrile precipitated protein, oscillating, centrifuging, taking a supernatant, filtering by using a 0.22-micron microporous organic filter membrane, then performing LC-MS / MS analysis, and judging whether the blood sample to be detected contains toxic substance components or not according to an analysis result; the used instrument is an ultraLC 100-XL-4000Q TRAP liquid chromatograph / mass spectrometer or an Agilent 1100 liquid chromatography-AB 3200 QTRAP triple serial quadrupole mass spectrometer from an AB company. By the liquid chromatography-mass spectrometry screening method, detection methods of different types of toxic substances are established, detection is fast and accurate, the detection limits of common toxic substances are given, high-sensitivity and effective detection of all target objects are guaranteed, a scientific data support is provided for a negative detection result, and thus a strong scientific basis is provided for relevant clinical diagnosis and rescue as well as criminal investigation and litigation.
Owner:山东省公安厅
Method for studying metabolic difference of transgenic rice and non-transgenic rice
InactiveCN102478563AThe pre-processing process is simpleImprove reliabilityComponent separationBiotechnologyGenetically modified rice
The invention discloses a method for studying metabolic difference of transgenic rice and non-transgenic rice. The method analyzes rice seed extract with liquid chromatography-mass spectrometry (LC-MS) technology to obtain metabolic profiling of rice, studies integral difference of metabolic profiling data between transgenic rice and non-transgenic rice by multivariate statistics method, and finds out compound with significant contribution to metabolic phenotype difference, to provide a basis for evaluation of unintended effect and safety of transgenic rice. The simple and rapid analysis method with good repeatability is suitable for batch analysis of practical samples.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Extraction and detection method for ketamine, norketamine and amphetamin-type substances in hairs
ActiveCN103575831AEfficient removalEffective separation and extractionComponent separationFluid phaseGas liquid chromatographic
The invention provides an extraction method for ketamine, norketamine and amphetamin-type substances in hairs. The extraction method comprises the steps of extraction and purification of the hairs. The extraction method also comprises a step of derivatization. The invention also provides a method for detecting the ketamine, the norketamine and the amphetamin-type substances in the hairs, and adopts a gas chromatographic mass spectrometer or a liquid-chromatographic mass spectrometer. Compared with the traditional method for detecting poison residues of the hairs, the method provided by the invention is mild, has the advantages that drugs in the hairs are effectively extracted, multiple impurity interfering substances in hair detection materials are effectively removed and the sensitivity and the accuracy are high, and the extraction method can be used by law enforcement departments, detection departments and drug inspection institutions in the government.
Owner:广州正孚检测技术有限公司
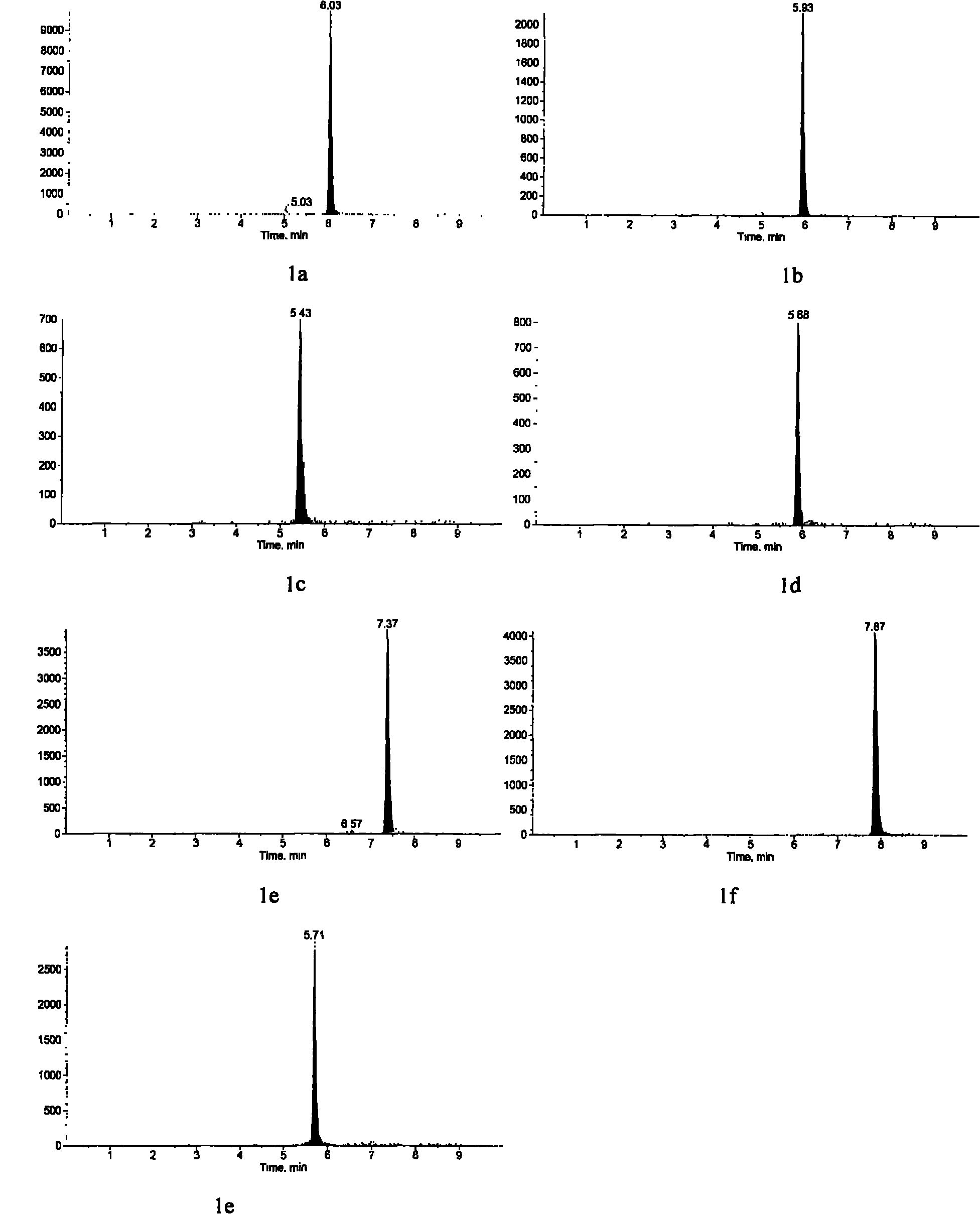
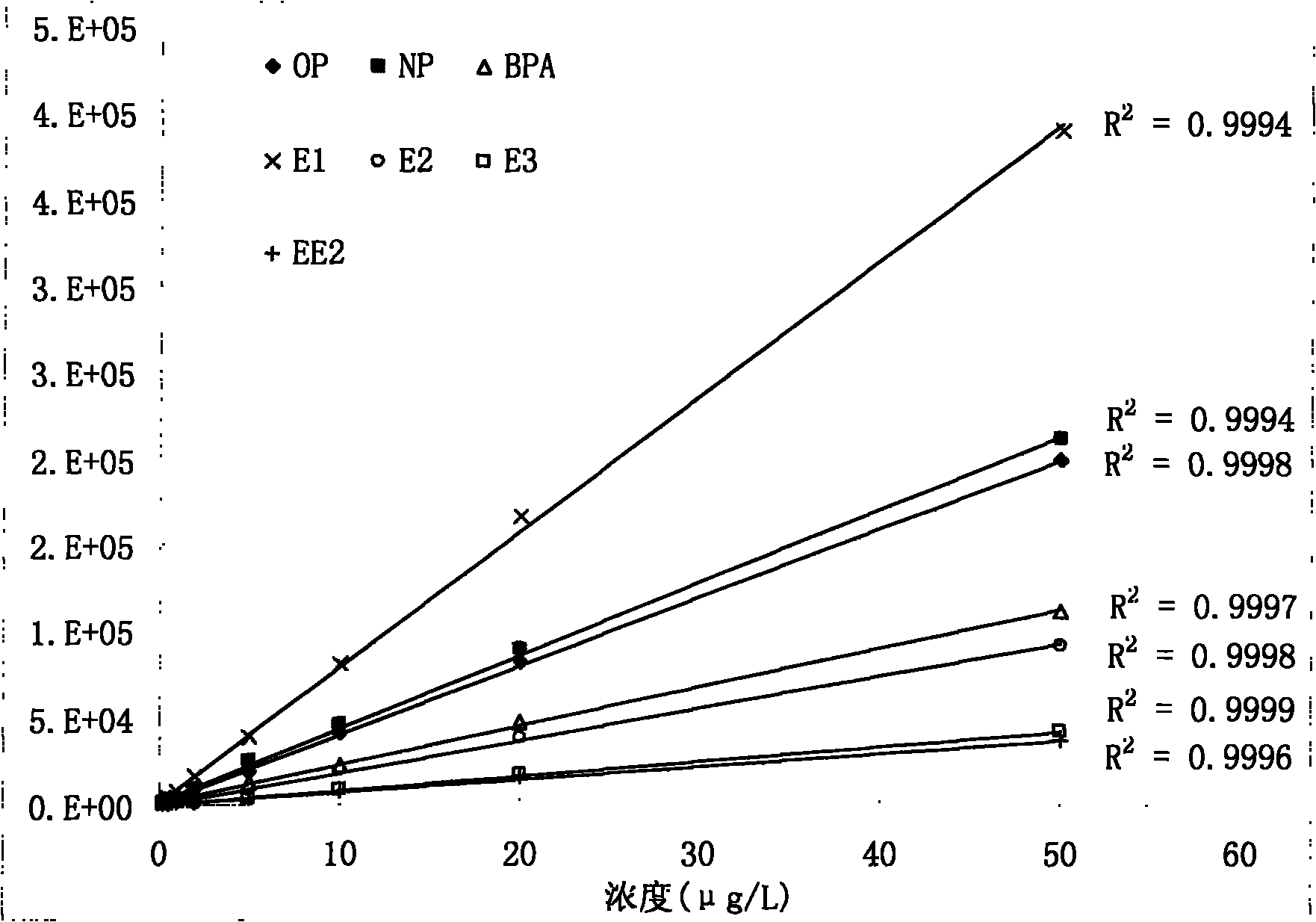
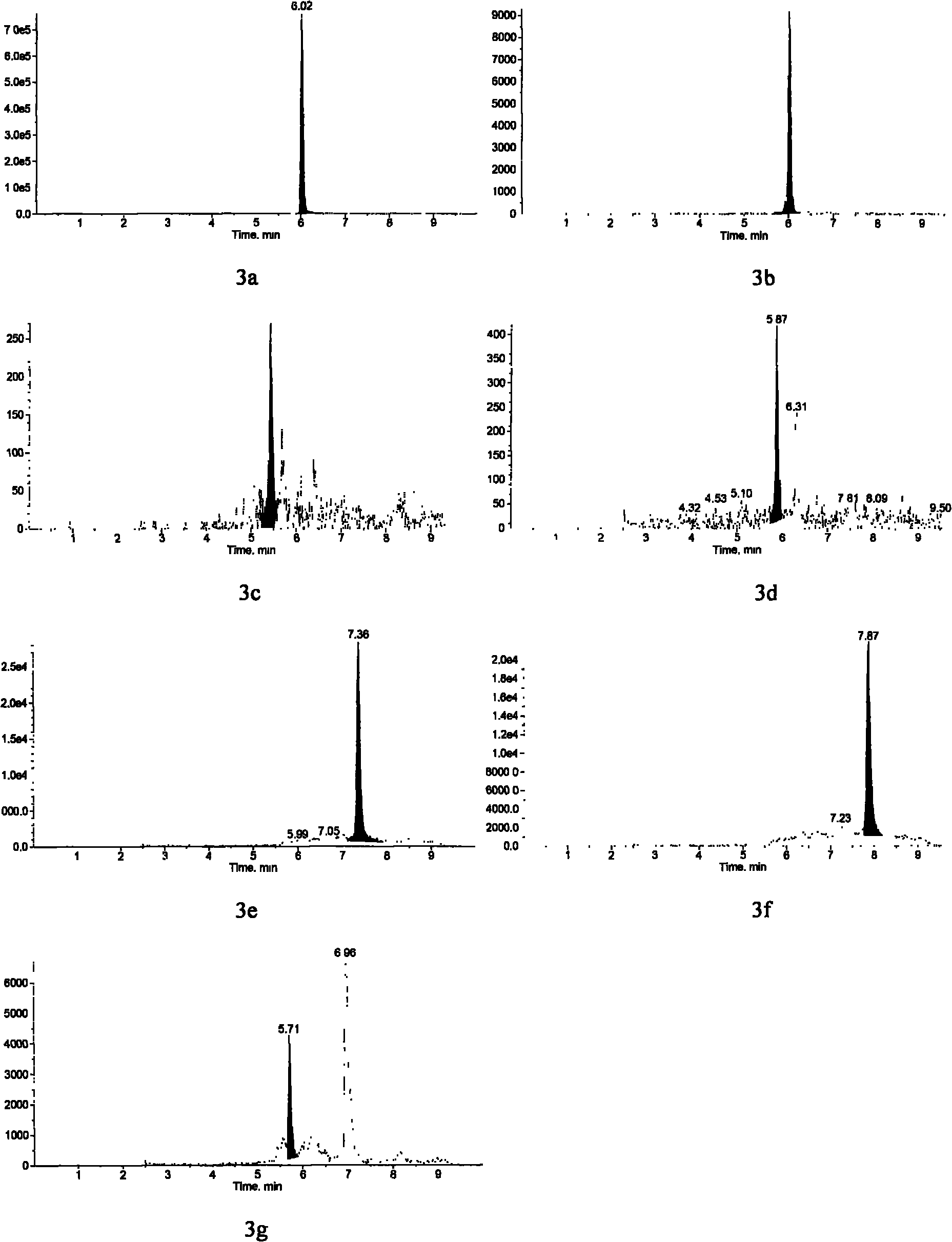
Method for detecting estrogen, nonyl phenol, octyl phenol and bisphenol A in water body sediment together
InactiveCN102183606AEasy to analyzeFast extractionComponent separationAutosamplerLiquid chromatography mass spectroscopy
The invention discloses a method for detecting estrogen, nonyl phenol, octyl phenol and bisphenol A in water body sediment based on an accelerated solvent extraction (ASE), liquid-liquid extraction (LLE) and liquid chromatography / tandem mass spectrum (LC / MS / MS) technology. The method comprises the following steps of: performing accelerated solvent extraction by using acetone as an extracting agent to extract the estrogen, the nonyl phenol, the octyl phenol and the bisphenol A in the water body sediment; dissolving residues generated after extract is blown by nitrogen by using 1mol / L sodium hydroxide solution, performing centrifugal collection on the supernate, and performing liquid-liquid extraction by using ethyl acetate under the condition that the pH is equal to 2; and performing concentration detection by using a 3200QTRAP type liquid chromatography / tandem mass spectrometer and an Agilent 1100 high performance liquid chromatography system of American Applied Biosystem Company and an automatic sample injector of American Agilent Company. The recovery rate is 59 to 94 percent, and the detection limit is 0.04ng / g.
Owner:BEIJING NORMAL UNIVERSITY
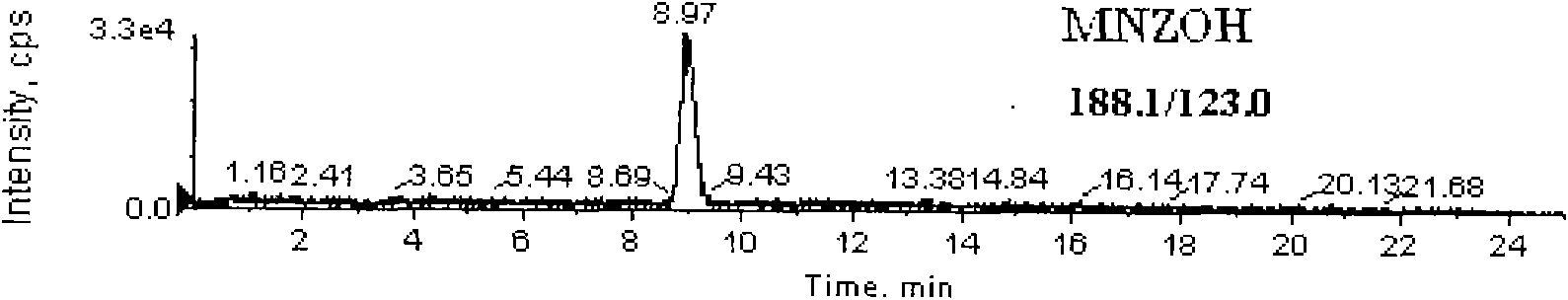
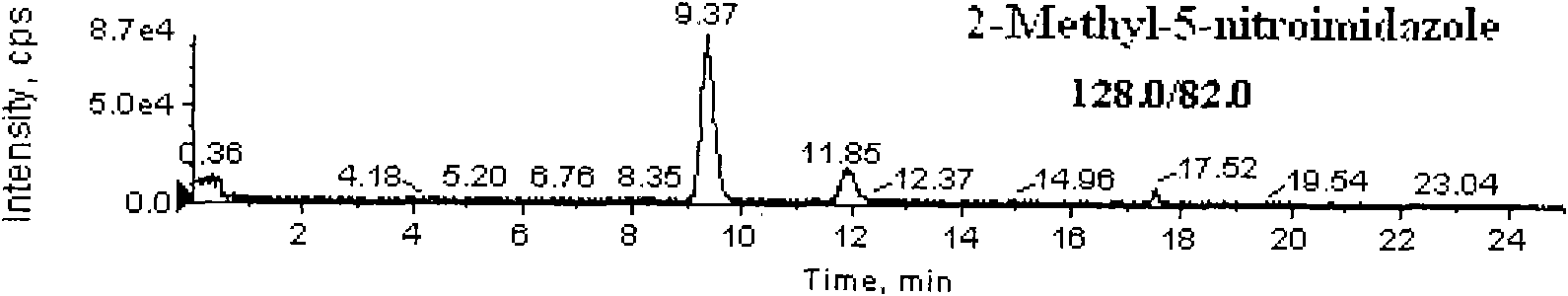
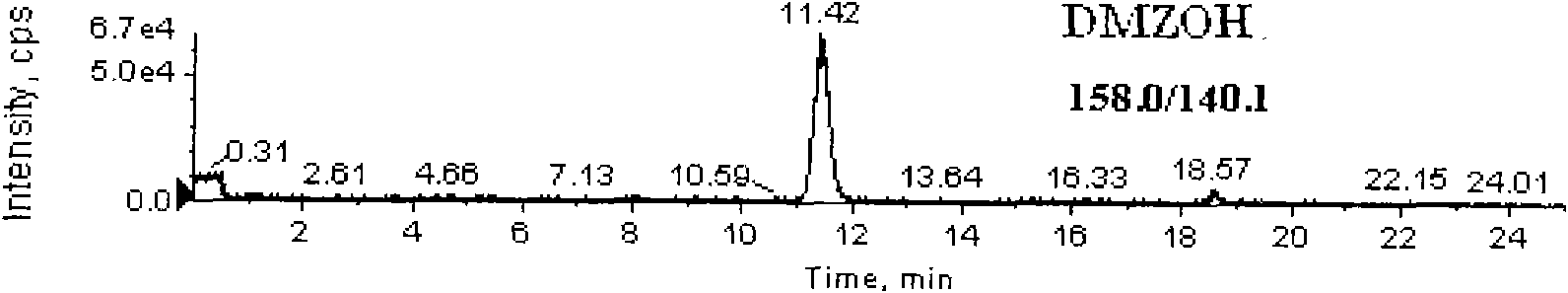
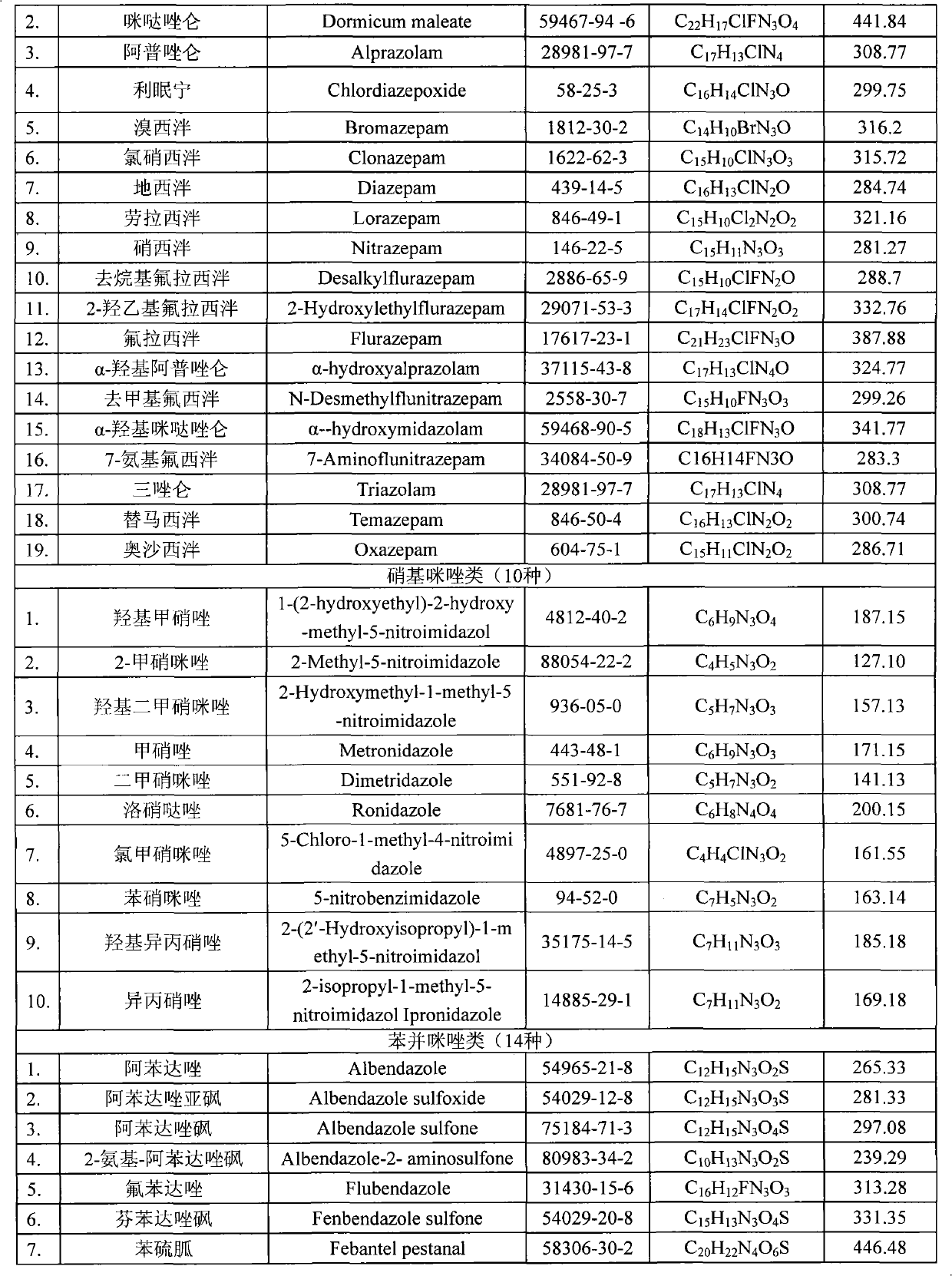
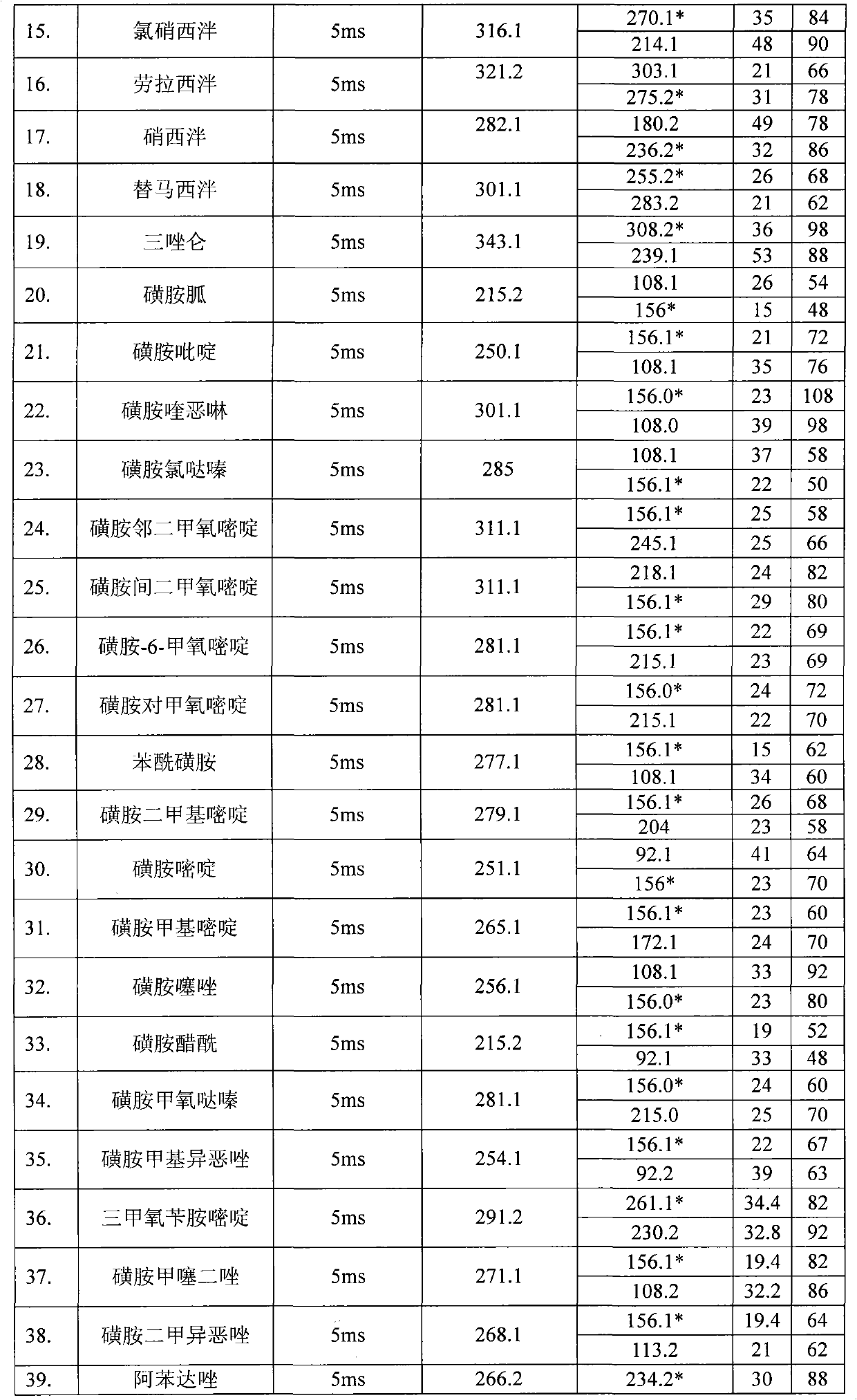
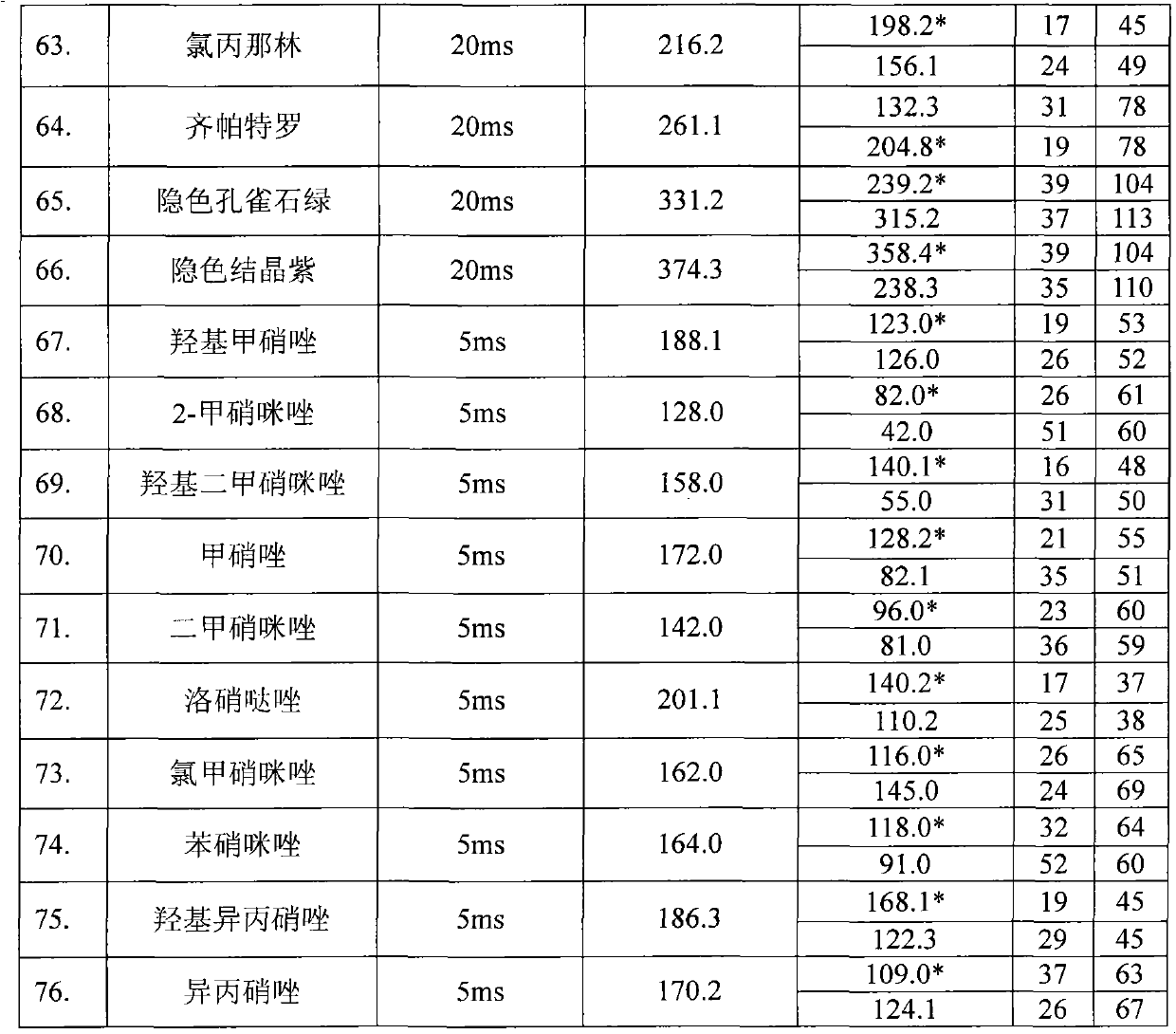
Detection method for simultaneously measuring residue of nitroimidazoles drugs in royal jelly
InactiveCN101571526AStrong specificityHigh sensitivityIon-exchange process apparatusComponent separationMetaboliteRelative standard deviation
The invention relates to a method for measuring the residue of nitroimidazoles drugs in royal jelly, in particular to a method for measuring the residue of nitroimidazoles drugs, such as 1-(2-hydroxyethyl)-2-hydroxy-methyl-5-nitroimidazol (MNZOH), 2-methyl-5-nitroimidazole, 2-hydroxymethyl-1-methyl-5-nitroimidazole (DMZOH / HMMNI), metronidazole (MNZ), dimetridazole (DMZ), ronidazole (RNZ), 5-chloro-1-methyl-4-nitroimidazole, 5-nitrobenzimidazole, 2-(2'-hydroxyisopropyl)-1-methyl-5-nitroimidazol (IPZOH), 2-isopropyl-1-methyl-5-nitroimidazol (Ipronidazole, IPZ) and the like, in the royal jelly by the liquid chromatography-mass spectrometry / mass spectrometer (LC-MS / MS). The method comprises the following steps: precipitating protein by methanol; extracting; further carrying out the purification using HLB (hydrophilic-lipophilic balance) and C18 solid-phase extraction (SPE) columns; and measuring the residue by LC-MS / MS. The invention has the characteristics of high specificity, high sensitivity and accurate results, and allows for the measurement of both the nitroimidazoles original drugs and the metabolites thereof; the lower limit of detection (10 mug / kg) in the method meets the existing requirements for the residue of nitroimidazoles drugs in the royal jelly at home and aboard; the recovery rate ranges from 70.7% to 105.0%; and the relative standard deviation (RSD) is lower than 12.7%.
Owner:THE INSPECTION & QUARANTINE TECH CENT ZHEJIANG ENTRY EXIT INSPECTION & QUARANTINE BUREAU
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
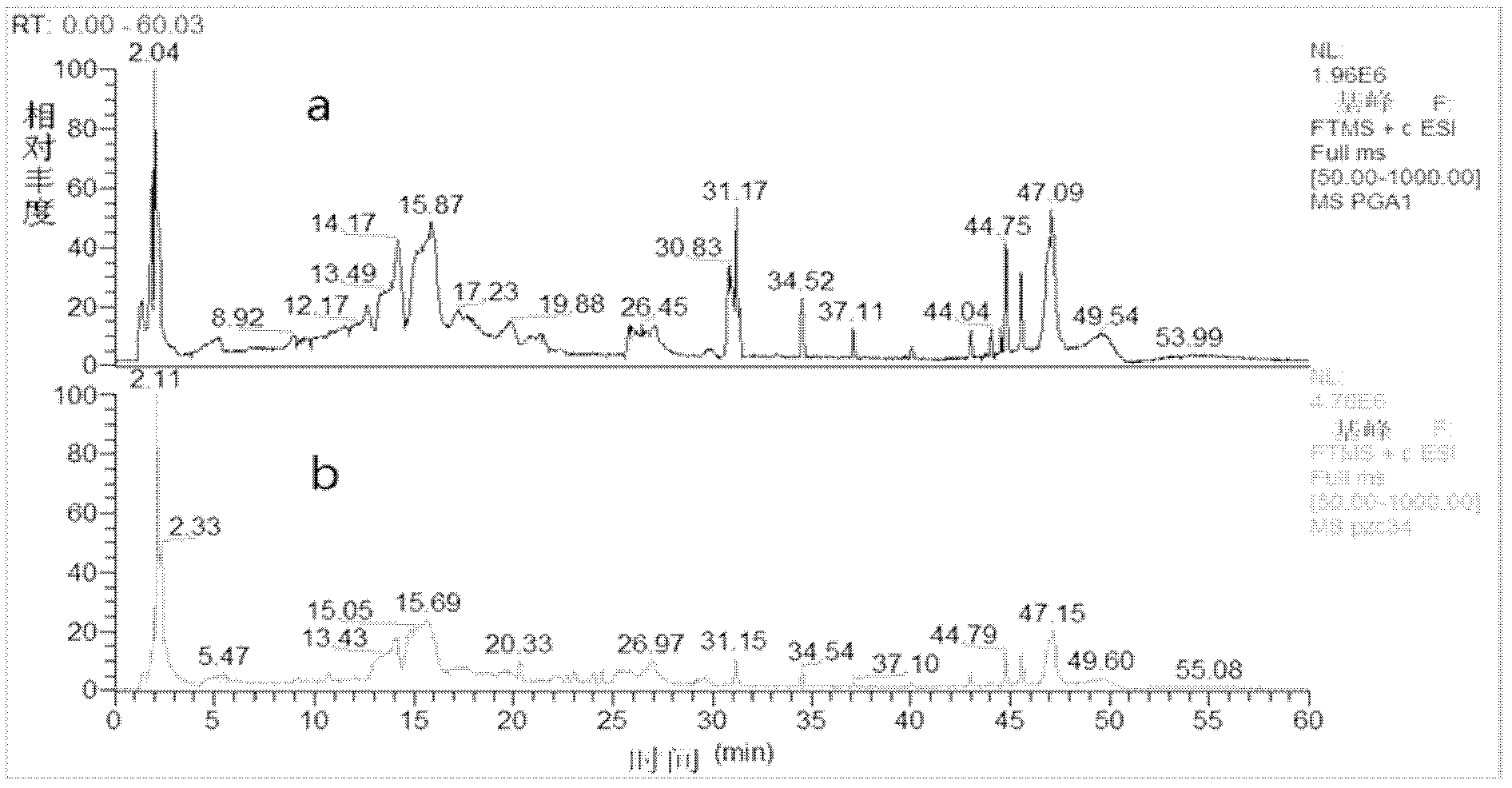
![Orthogonal ion sampling for electrospray [LC/MS] mass spectrometry Orthogonal ion sampling for electrospray [LC/MS] mass spectrometry](https://images-eureka.patsnap.com/patent_img/41ab4661-8056-4ee1-b546-9edddac1efab/00000002_0000.png)
![Orthogonal ion sampling for electrospray [LC/MS] mass spectrometry Orthogonal ion sampling for electrospray [LC/MS] mass spectrometry](https://images-eureka.patsnap.com/patent_img/41ab4661-8056-4ee1-b546-9edddac1efab/00000003_0000.png)
![Orthogonal ion sampling for electrospray [LC/MS] mass spectrometry Orthogonal ion sampling for electrospray [LC/MS] mass spectrometry](https://images-eureka.patsnap.com/patent_img/41ab4661-8056-4ee1-b546-9edddac1efab/00000004_0000.png)