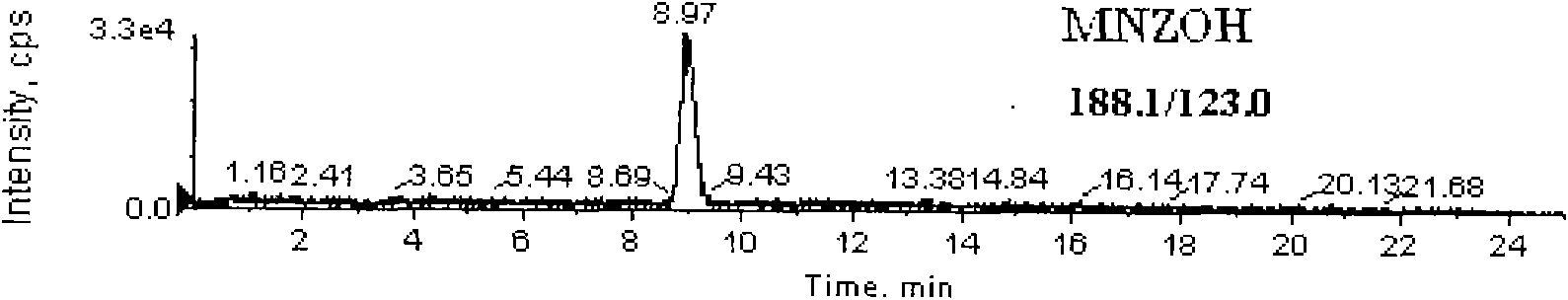
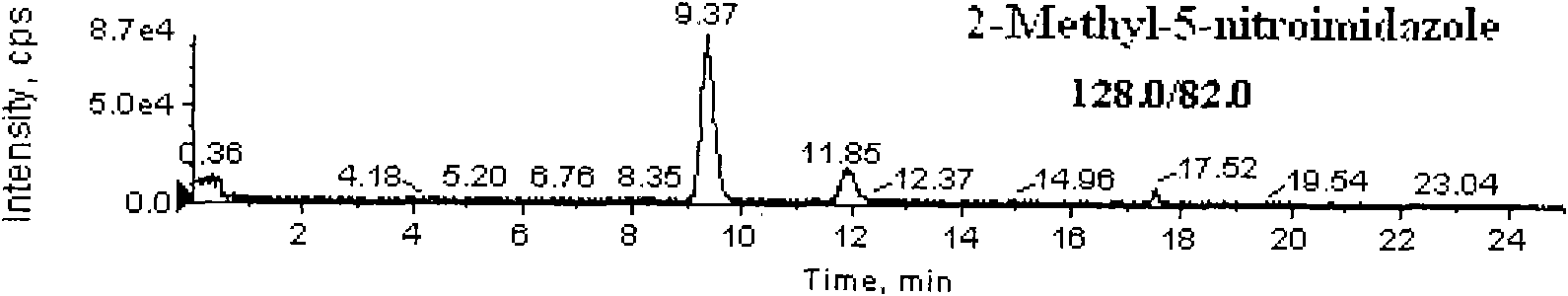
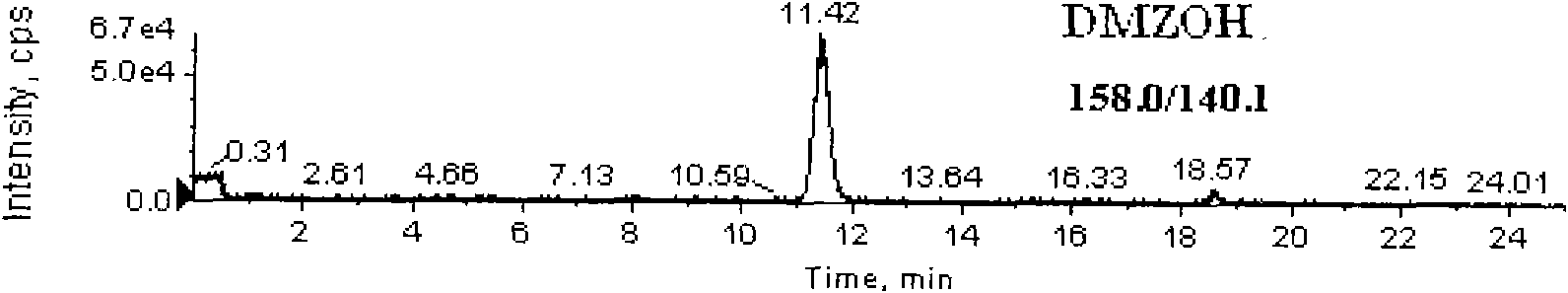
Detection method for simultaneously measuring residue of nitroimidazoles drugs in royal jelly
A technology for nitroimidazoles and a detection method is applied in the detection field of simultaneous determination of the residues of multiple nitroimidazoles in royal jelly, and achieves the effects of high sensitivity, accurate results and elimination of sample matrix effects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0037] A detailed description will be given below of specific embodiments of the present invention.
[0038]The detection method for simultaneously measuring multiple nitroimidazoles drug residues in royal jelly of the present invention comprises the following steps:
[0039] 1. Extraction
[0040] Weigh 2g of the sample (accurate to 0.01g) and place it in a 50mL centrifuge tube with stopper, add 0.2mL dimetidazole-d3, ronidazole-d3 isotope internal standard solution (200ng / mL), hydroxydimethylnitrate Imidazole-d3, Hydroxypronidazole-d3, Pronidazole-d3 isotope internal standard solution (100ng / mL), add 10mL water, mix well, let stand for 2min, then add methanol to 20mL, put on a vortex mixer Mix at 2000r / min for 1min, centrifuge at 6000r / min for 10min, filter, pipette 10.0mL supernatant, add 10mL phosphate buffer solution (dissolve 13.8g sodium dihydrogen phosphate in 950mL water, oxidize with 0.1mol / L hydroxide Adjust the pH value of the solution to 8.0 with sodium solution...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com