A method for analyzing fingerprints of Jinghuaweikang capsules
A fingerprint, Kang capsule technology, applied in the field of medicine, to achieve good linearity, good repeatability and good stability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
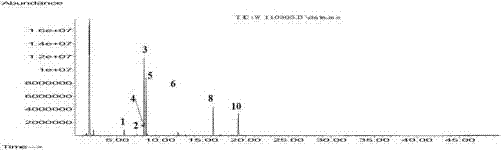
[0102] (1) Preparation of reference substance solution: Take an appropriate amount of p-cymene reference substance, weigh it accurately, add ethyl acetate to make a solution containing about 0.5 mg of p-cymene per 1 mL, and obtain it.
[0103] (2) Preparation of the test solution: take an appropriate amount of the content of Jinghua Weikang capsules, weigh about 30 mg respectively, weigh them accurately, put them in a 5mL volumetric flask, add an appropriate amount of ethyl acetate to dissolve, and adjust the volume to the mark. Instantly.
[0104] (3) Take the test solution and analyze it by gas chromatography to obtain a fingerprint.
[0105] Chromatographic conditions:
[0106] Chromatographic column: the stationary phase is filled with 14% cyanopropyl-phenyl 86% dimethylpolysiloxane; column temperature: programmed temperature rise; inlet temperature: 250 °C; FID detector; temperature: 250 °C; Gas: Nitrogen.
[0107] The temperature programming conditions are:
[0108] ...
Embodiment 2
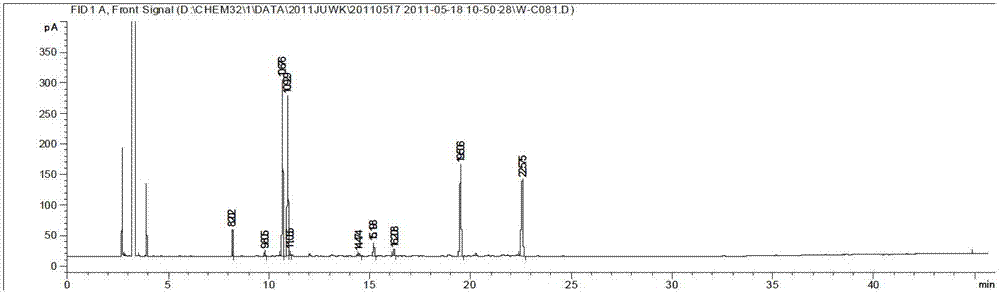
[0110] (1) Preparation of reference substance solution: Take an appropriate amount of p-cymene reference substance, weigh it accurately, add ethyl acetate to make a solution containing about 0.3 mg of p-cymene per 1 mL, and obtain it.
[0111] (2) Preparation of the test solution: take an appropriate amount of the contents of Jinghua Weikang capsules, weigh about 10 mg respectively, weigh them accurately, put them in a 5mL volumetric flask, add an appropriate amount of ethyl acetate to dissolve, and adjust the volume to the mark. Instantly.
[0112] (3) Take the test solution and analyze it by gas chromatography to obtain a fingerprint.
[0113] Chromatographic conditions:
[0114] Chromatographic column: stationary phase with 5% phenyl 95% dimethyl polysiloxane as filler, Agilent HP-5 (30m×0.32mm×0.25μm); column temperature: programmed temperature rise; inlet temperature: 150°C; FID detector; temperature: 300°C; carrier gas: nitrogen.
[0115] The temperature programming c...
Embodiment 3
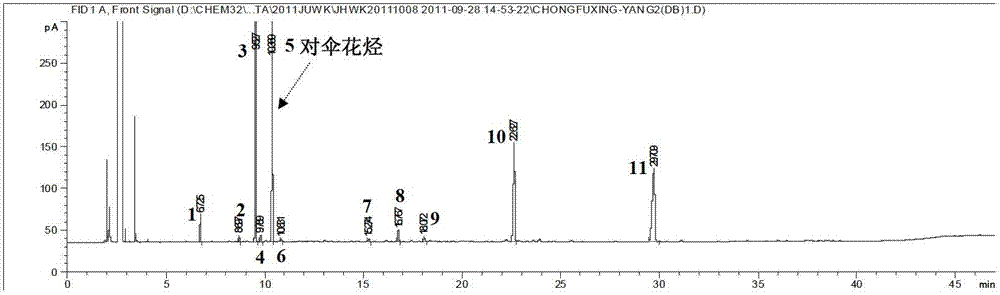
[0118] (1) Preparation of reference substance solution: Take an appropriate amount of p-cymene reference substance, weigh it accurately, add ethyl acetate to make a solution containing about 0.7 mg of p-cymene per 1 mL, and obtain it.
[0119] (2) Preparation of the test solution: Take an appropriate amount of the contents of Jinghua Weikang capsules, weigh about 50 mg respectively, weigh them accurately, put them in a 5mL volumetric flask, add an appropriate amount of ethyl acetate to dissolve, and adjust the volume to the mark. Instantly.
[0120] (3) Take the test solution and analyze it by gas chromatography to obtain a fingerprint.
[0121] Chromatographic conditions:
[0122] Chromatographic column: cross-linked 50% phenyl-methyl polysiloxane as the stationary phase capillary column (column length is 30m, inner diameter is 0.32mm, film thickness is 0.25μm); column temperature: programmed temperature; inlet temperature : 200°C; FID detector; temperature: 300°C; carrier ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com