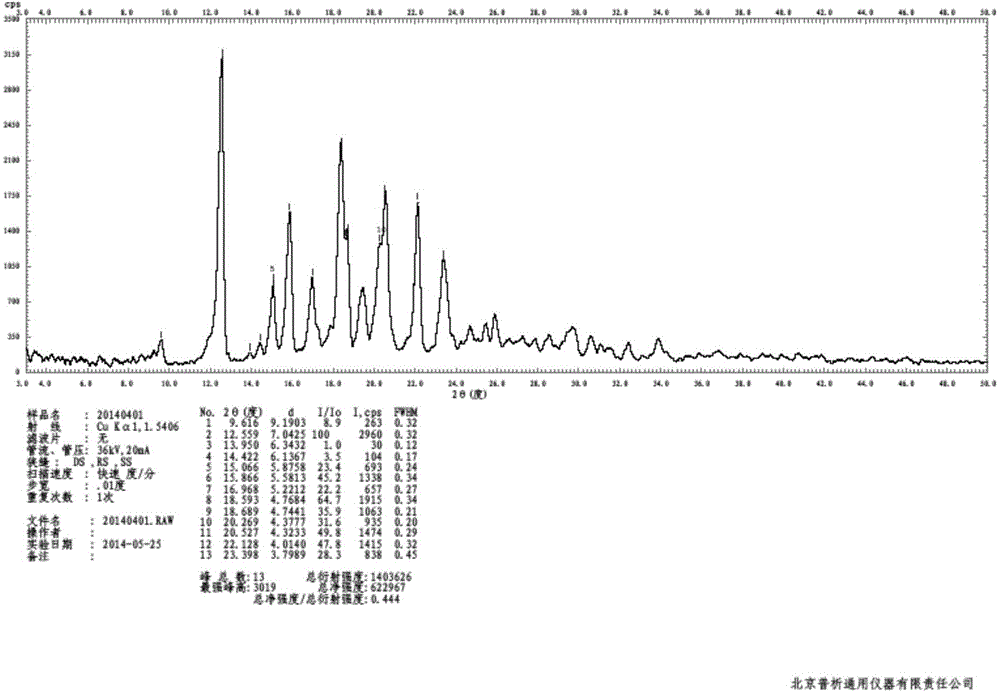
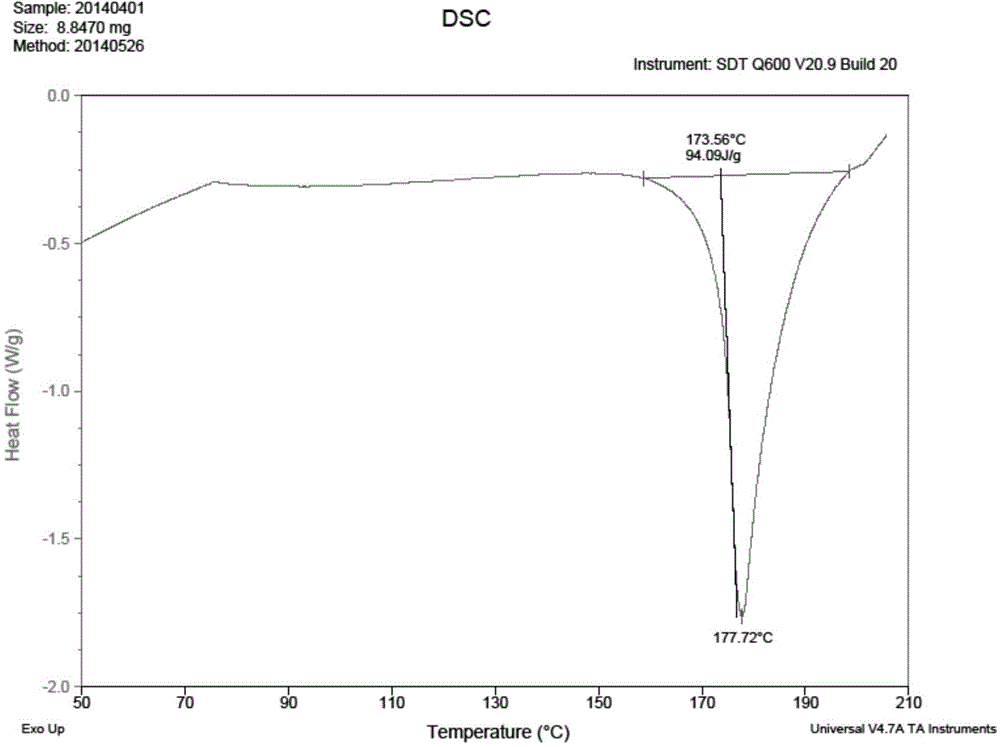
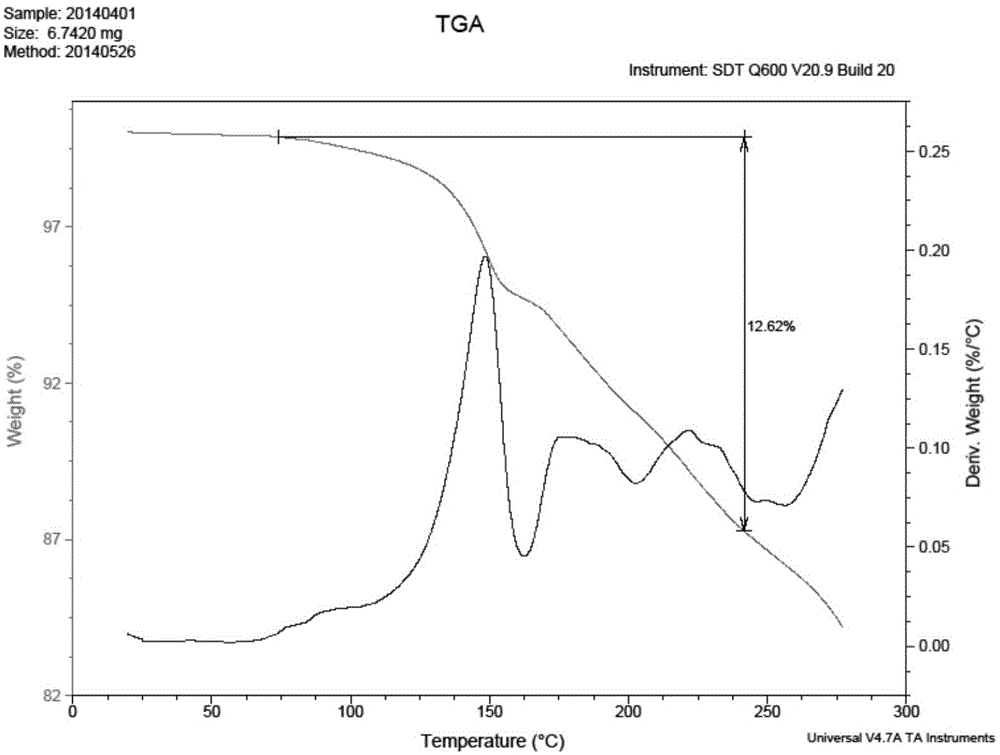
Preparation method of bazedoxifene acetate crystal form A
A technology of xifene crystal form and acetic acid, applied in the field of preparation of bazedoxifene acetate crystal form A, can solve the problems of easy conversion to crystal form B, no industrialized production scale, etc., and achieves low cost, convenient operation, heavy weight and the like. Strong effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0083] The preparation of embodiment 1 bazedoxifene acetate crystal form A
[0084]
[0085] Weigh hexamethyleneimine benzyloxyindole (2kg), ammonium formate (0.58kg), 10% palladium carbon (0.2kg, moisture content 50%) and add in the reactor containing tetrahydrofuran (12L) , heated to 45-50°C and reacted for about 2-3 hours. After the reaction was complete, the palladium carbon was filtered off, the solution was cooled to 10-15°C, and then acetic acid (0.37kg) and methyl tert-butyl ether (10L ), the solution continued to stir for 12 hours at 10‐15 °C. Suction filtration and vacuum drying of the solid at 50° C. for 10 hours gave 1.12 kg of white powdery solid with a yield of 68.7%.
[0086] Weigh the crude form A (1kg) obtained above into a reactor containing tetrahydrofuran (8L), raise the temperature to 45-50°C under the protection of an inert gas, filter the solution after the solid is completely dissolved, and continue cooling the filtrate to 10-15°C , and then add me...
Embodiment 2
[0087] The preparation of embodiment 2 bazedoxifene acetate crystal form A
[0088] Weigh hexamethyleneimine benzyloxyindole (2kg), ammonium formate (0.58kg), 10% palladium carbon (0.1kg, water content 50%) and add in the reactor containing tetrahydrofuran (12L) , heated to 45-50°C and reacted for about 2-3 hours. After the reaction was complete, the palladium carbon was filtered off, the solution was cooled to 10-15°C, and then acetic acid (0.185kg) and methyl tert-butyl ether (5L ), the solution continued to stir for 12 hours at 10‐15 °C. Suction filtration, the solid was vacuum dried at 50°C for 10 hours to obtain the crude crystal form A.
[0089] Weigh the crude form A (1kg) obtained above into a reactor containing tetrahydrofuran (8L), raise the temperature to 45-50°C under the protection of an inert gas, filter the solution after the solid is completely dissolved, and continue cooling the filtrate to 10-15°C , and then add methyl tert-butyl ether (5L) in batches, and ...
Embodiment 3
[0090] The preparation of embodiment 3 bazedoxifene acetate crystal form A
[0091] Weigh hexamethyleneimine benzyloxyindole (2kg), ammonium formate (0.58kg), 10% palladium carbon (0.4kg, water content 50%) and add in the reactor containing tetrahydrofuran (12L) , heated to 45-50°C and reacted for about 2-3 hours. After the reaction was complete, the palladium carbon was filtered off, the solution was cooled to 10-15°C, and then acetic acid (0.185kg) and methyl tert-butyl ether (5L ), the solution continued to stir for 12 hours at 10‐15 °C. Suction filtration, the solid was vacuum dried at 50°C for 10 hours to obtain the crude crystal form A.
[0092] Weigh the crude form A (1kg) obtained above into a reactor containing tetrahydrofuran (8L), raise the temperature to 45-50°C under the protection of an inert gas, filter the solution after the solid is completely dissolved, and continue cooling the filtrate to 10-15°C , and then add methyl tert-butyl ether (5L) in batches, and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com