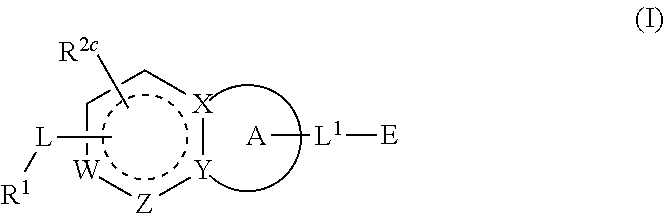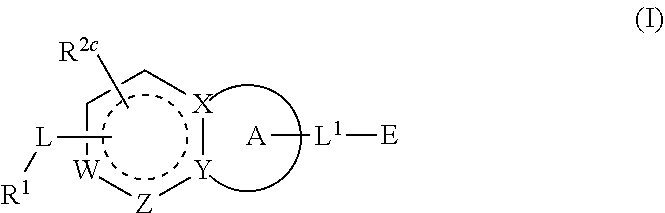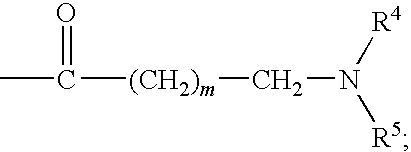Patents
Literature
115 results about "Azepane" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Azepane is the organic compound with the formula (CH₂)₆NH. It is a colorless liquid. A cyclic secondary amine, it is a precursor to several drugs and pesticides. It is produced by partial hydrogenolysis of hexamethylene diamine.
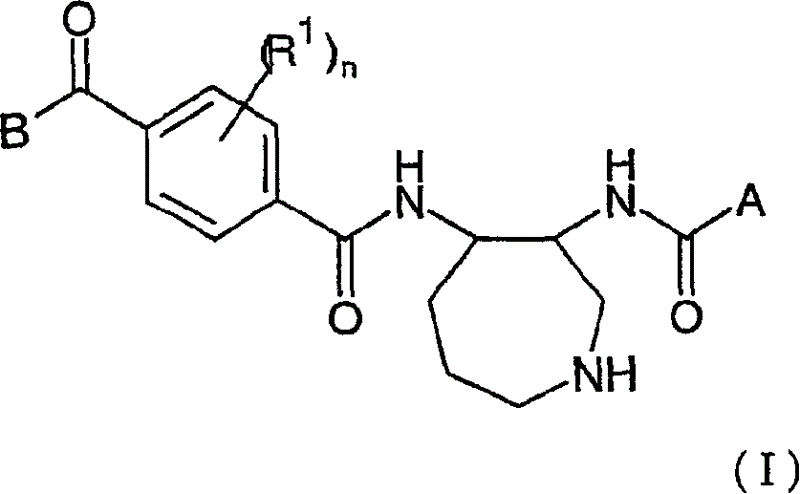
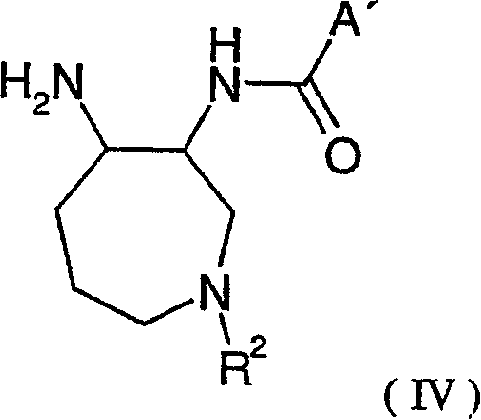
Azepane derivatives and methods of treating hepatitis B infections
ActiveUS9181288B2Reduce doseReduce frequencyPeptide/protein ingredientsGroup 5/15 element organic compoundsMedicineAzepane
Owner:NOVIRA THERAPEUTICS
Azepane derivatives and methods of treating hepatitis b infections
Owner:NOVIRA THERAPEUTICS
Production of monocycloalkyl aromatic compounds
There is described a process for the transalkylation of a polycycloalkyl aromatic compound, particularly the transalkylation of dicyclohexylbenzene to produce monocyclohexylbenzene. The process comprises contacting the polycycloalkyl aromatic compound with benzene in the presence of a catalyst selected from the group consisting of an acidic solid comprising a Group IVB metal oxide modified with an oxyanion of a Group VIBA metal oxide, TEA-mordenite, zeolite beta and a porous crystalline material having an X-ray diffraction pattern including d-spacing maxima at 12.4±0.25, 6.9±0.15, 3.57±0.07 and 3.42±0.07 Angstrom. Preferably the catalyst is a WOx / ZrO2 material.
Owner:EXXONMOBIL CORP (US)
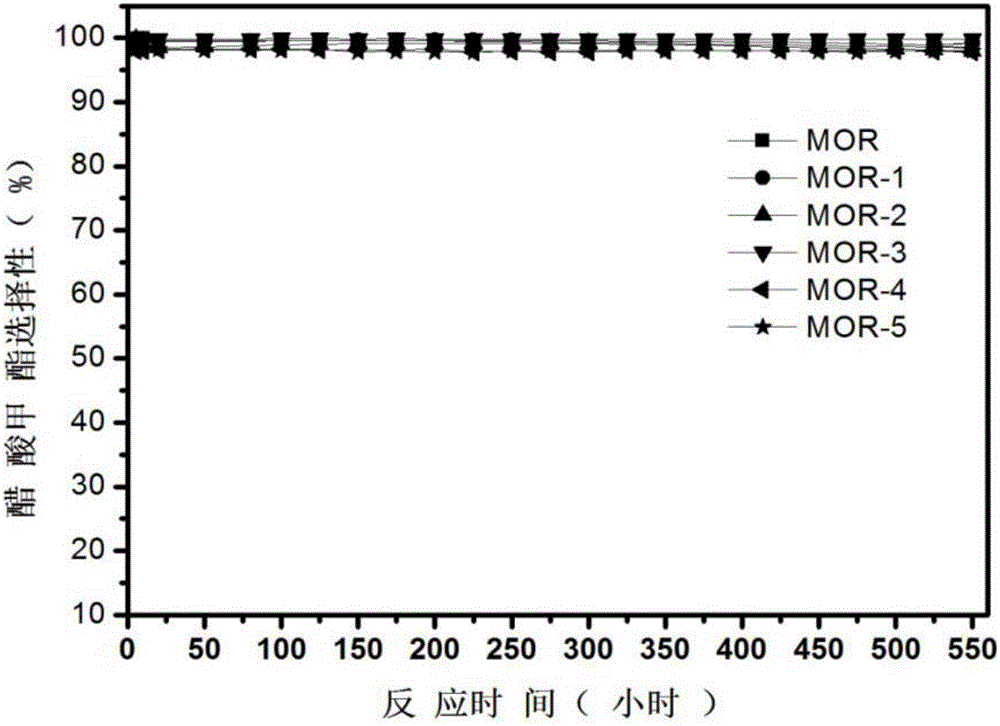
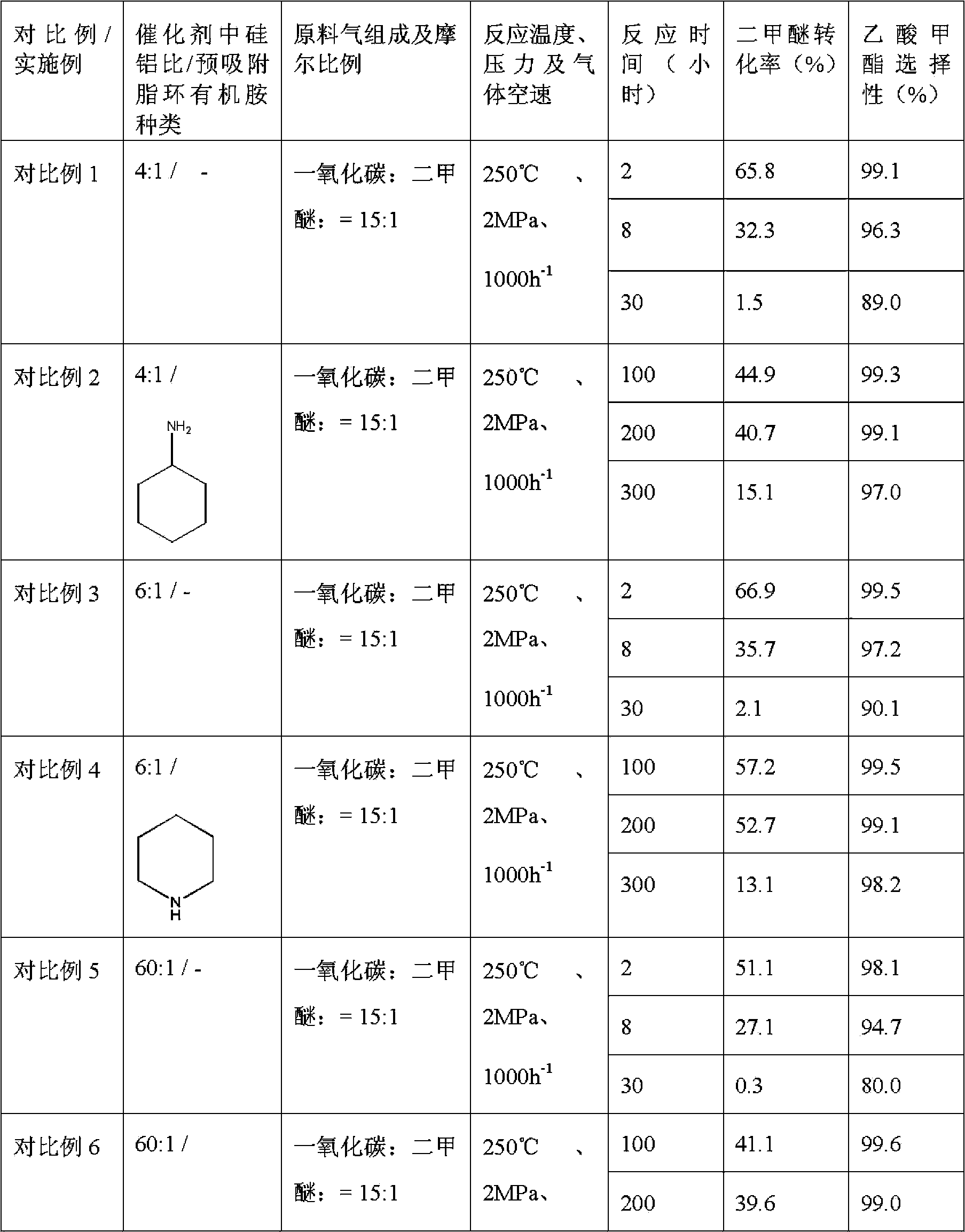
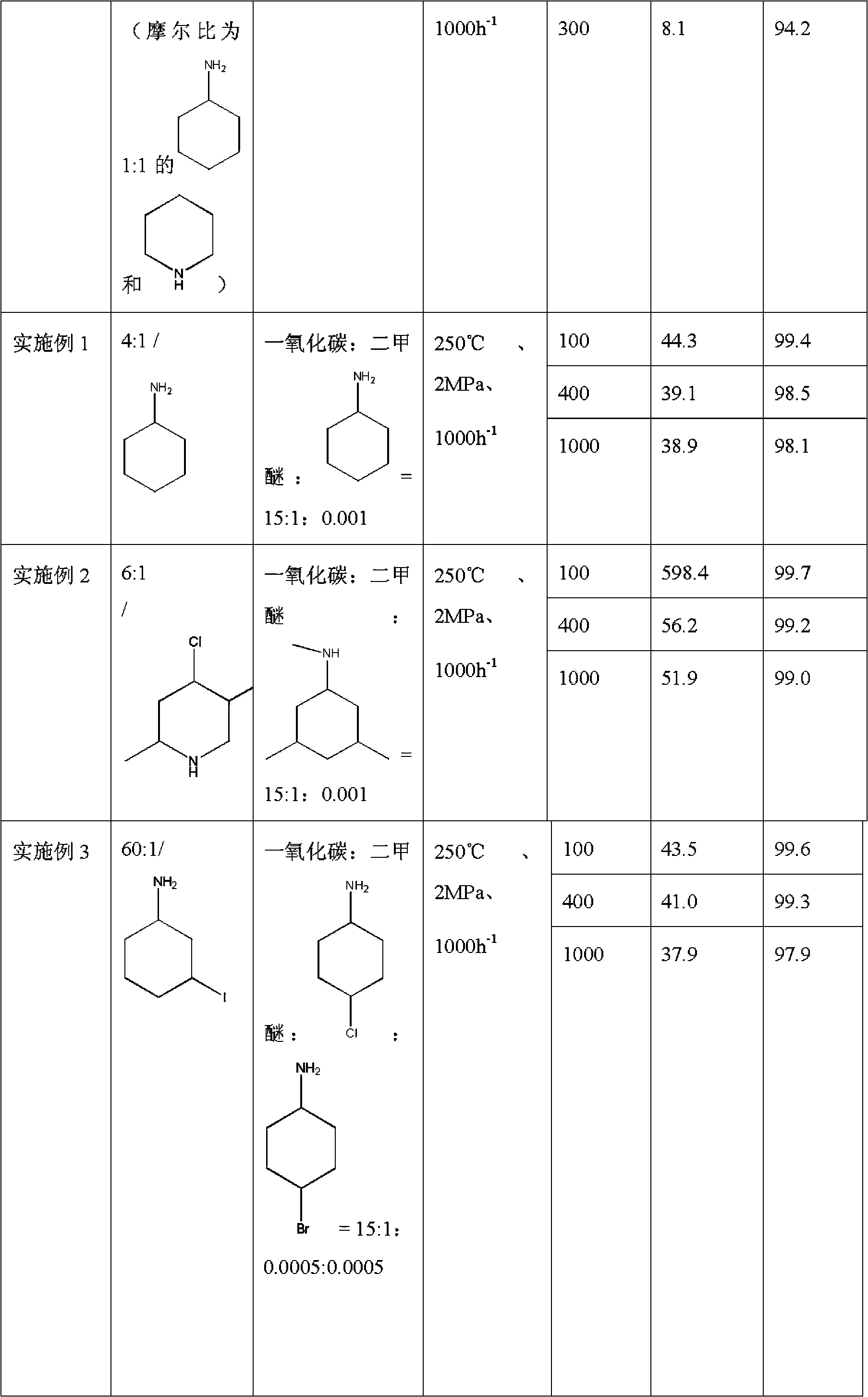
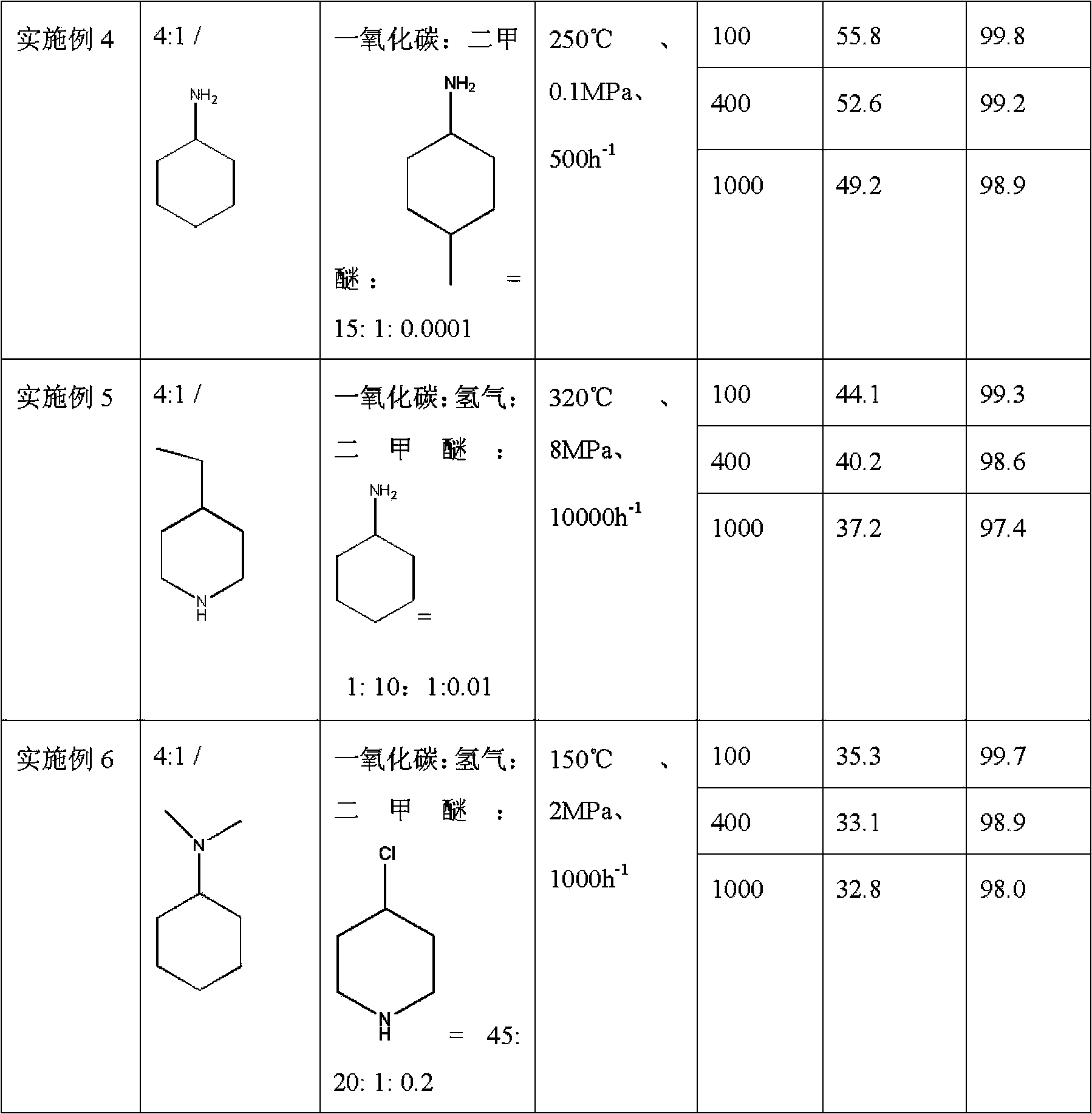
Method used for producing methyl acetate
ActiveCN103896766AStable compensation/inhibition of desorptionCompensate/inhibit desorptionPreparation by carbon monoxide or formate reactionMolecular sieveHydrogen
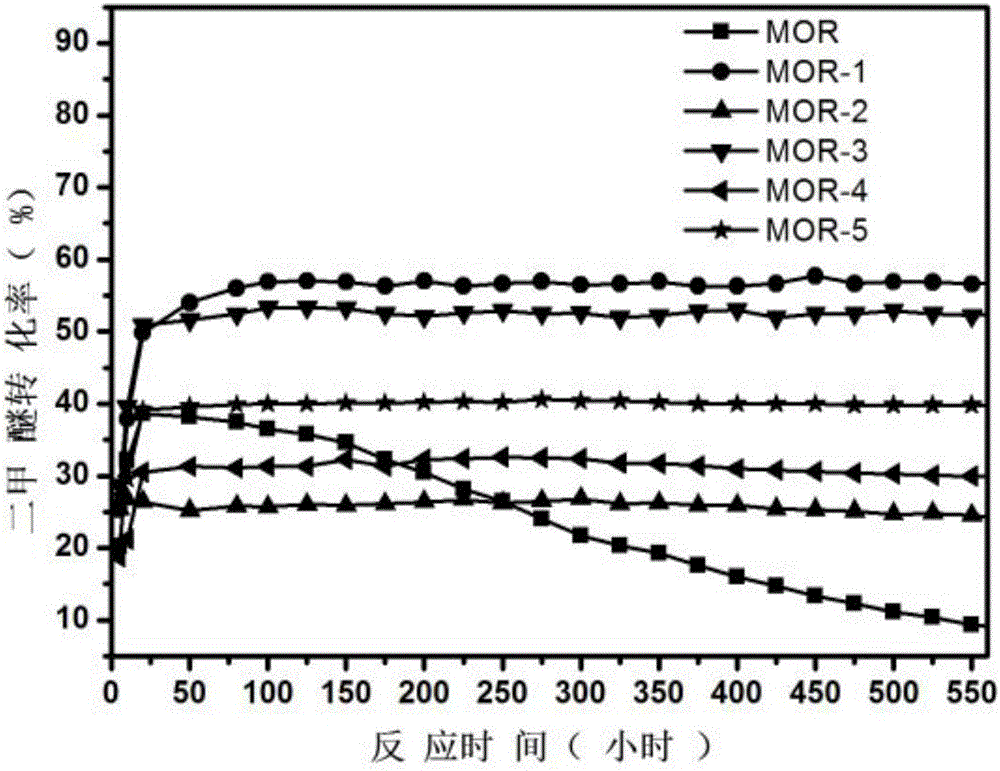
The invention provides a method used for producing methyl acetate. According to the method, raw material gas containing alicyclic organic amine, dimethyl ether, carbonic oxide, and optionally selected hydrogen passes through a reaction reactor filled with a hydrogen-type mordenite molecular sieve catalyst so as to prepare methyl acetate, wherein the hydrogen-type mordenite molecular sieve catalyst is alicyclic organic amine absorbed hydrogen-type mordenite molecular sieve catalyst. According to the method, alicyclic organic amine absorbed hydrogen-type mordenite molecular sieve is taken as the catalyst, and alicyclic organic amine is added into the raw material gas, so that desorption of alicyclic organic amine in reaction processes can be made up stably, stability of the catalyst is increased, and service life of the catalyst is prolonged.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Azepane derivatives and methods of treating hepatitis B infections
ActiveUS9339510B2Reduce loadReduce morbidityPeptide/protein ingredientsGroup 5/15 element organic compoundsMedicineAzepane
Owner:NOVIRA THERAPEUTICS
Azepane derivatives and methods of treating hepatitis B infections
ActiveUS9169212B2Reduce doseReduce frequencyOrganic chemistryPeptide/protein ingredientsMedicineAzepane
Owner:NOVIRA THERAPEUTICS
Azepane derivatives and methods of treating hepatitis b infections
ActiveUS20160000812A1Reduce loadReduce morbidityPeptide/protein ingredientsGroup 5/15 element organic compoundsPharmaceutical drugAzepane
Owner:NOVIRA THERAPEUTICS
Azepane derivatives and methods of treating hepatitis b infections
ActiveUS20170015629A1Reduce loadReduce morbidityOrganic chemistryPeptide/protein ingredientsMedicineAzepane
Owner:NOVIRA THERAPEUTICS
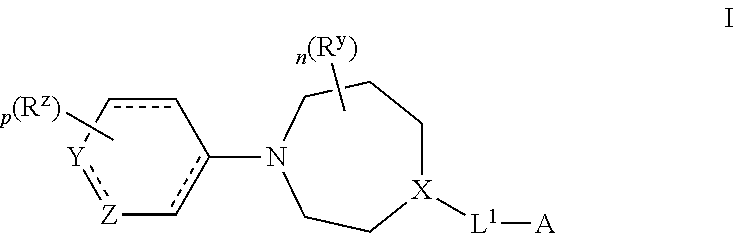
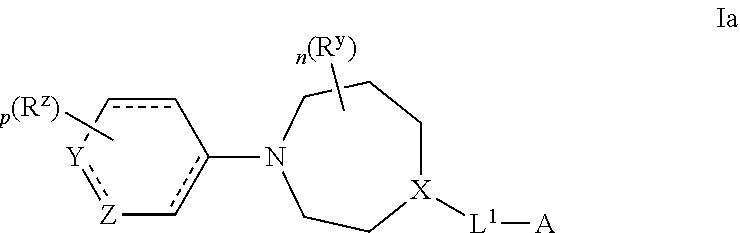
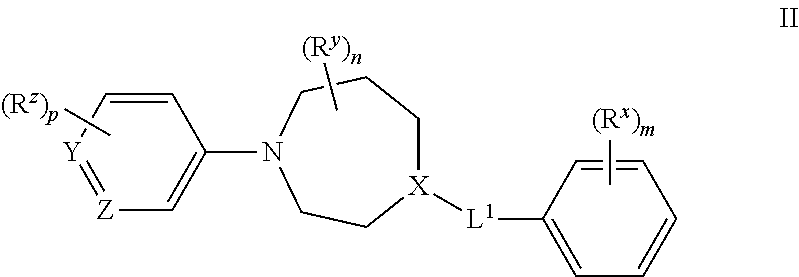
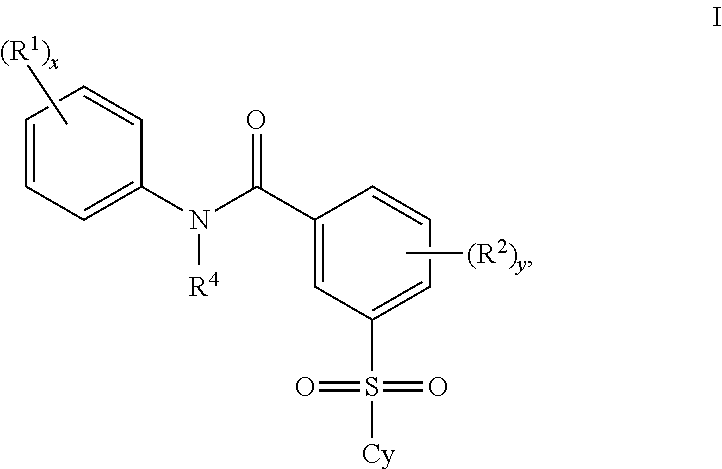
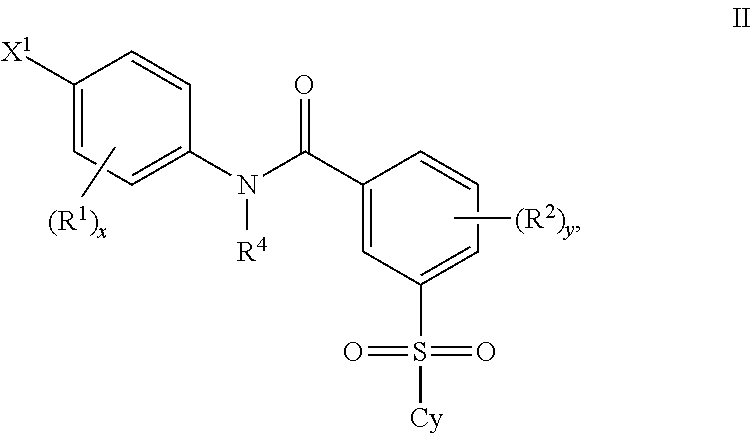
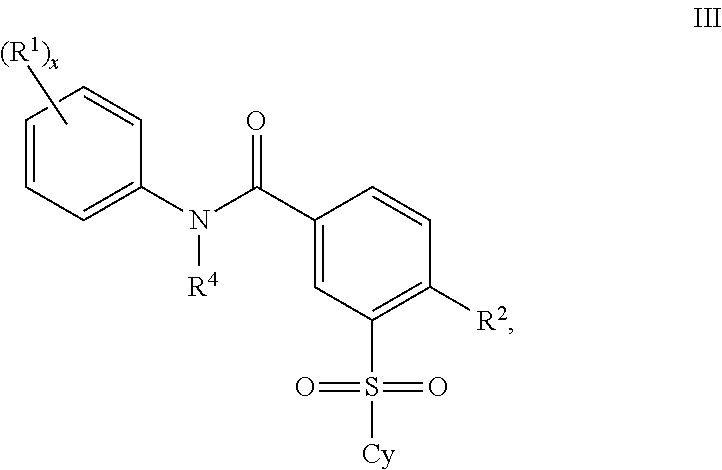
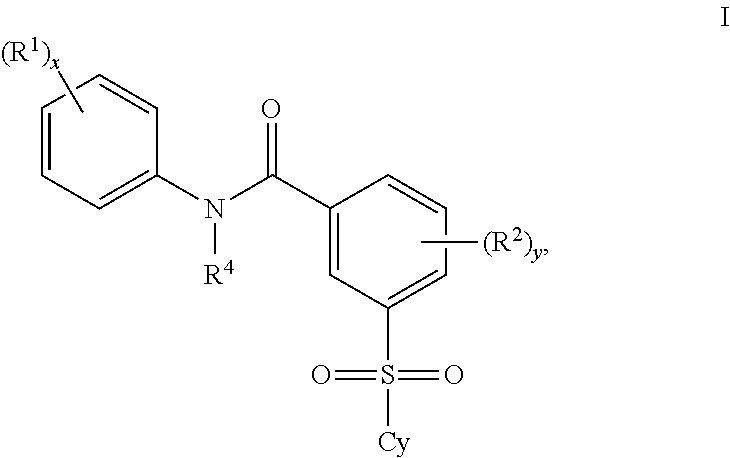
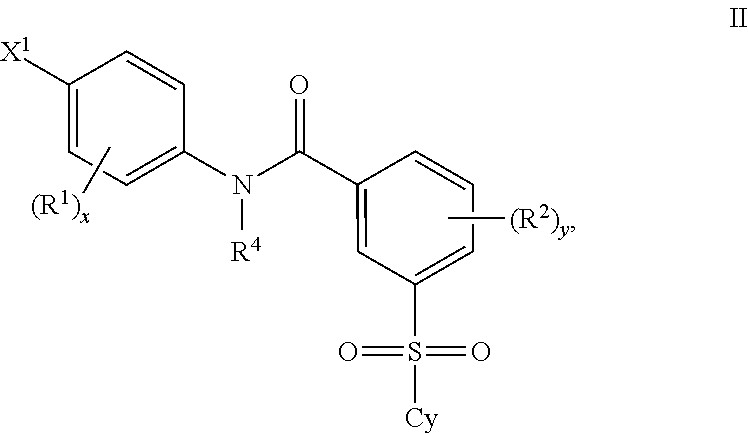
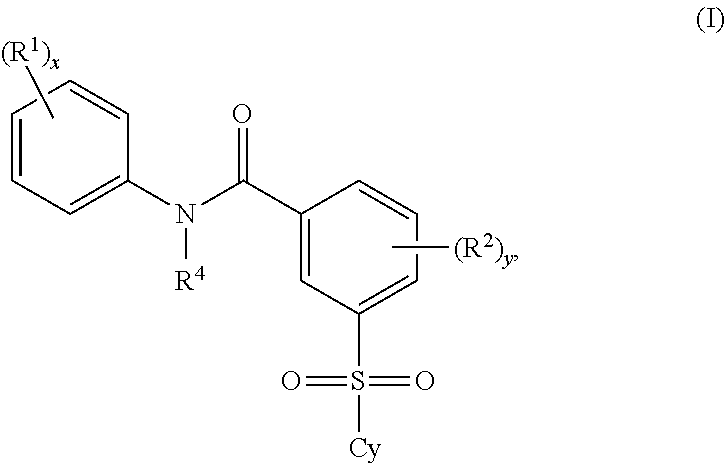
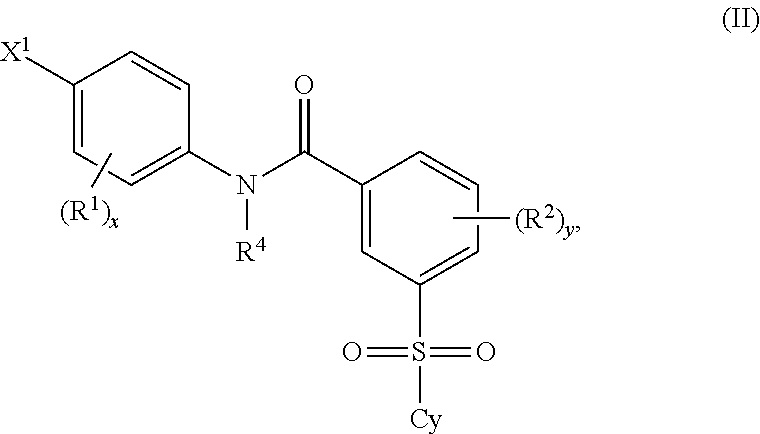
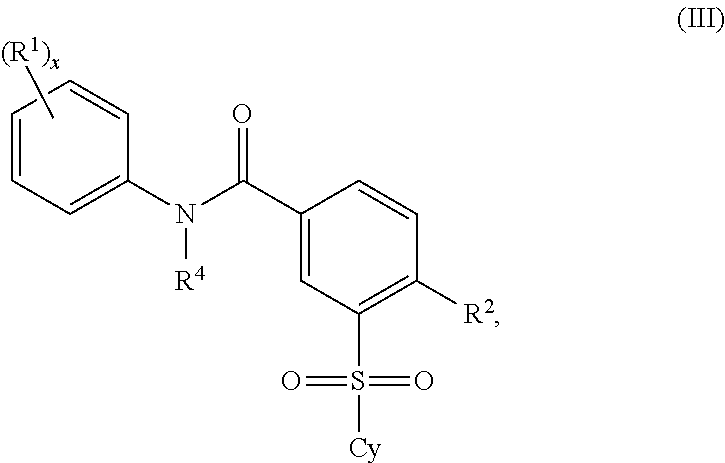
Certain (2S)-n-[(1S)-1-cyano-2-phenylethyl]-1,4-oxazepane-2-carboxamides as dipeptidyl peptidase 1 inhibitors for treating bronchiectasis
InactiveUS20180028541A1Increase the length of timeReduce probabilityAntibacterial agentsOrganic active ingredientsBenzoxazoleDipeptidyl peptidase
The present disclosure relates to methods for treating bronchiectasis, for example, non-cystic fibrosis bronchiectasis with compositions comprising an effective amount of certain (2S)—N-[(1S)-1-cyano-2-phenylethyl]-1,4-oxazepane-2-carboxamide compounds of Formula (I), including pharmaceutically acceptable salts thereof,that inhibit dipeptidyl peptidase 1 (DPP1) activity. Methods provided herein are useful for prophylaxis, increasing the lung function in a patient, and / or and / or decreasing the rate of pulmonary exacerbation in a patient. In one embodiment, the compound of Formula (I) is (2S)—N-{(1S)-1-cyano-2-[4-(3-methyl-2-oxo-2,3-dihydro-1,3-benzoxazol-5-yl)phenyl]ethyl}-1,4-oxazepane-2-carboxamide.
Owner:ASTRAZENECA AB +1
Non-interpenetrating chiral MOF stationary phase, its preparation method and application in enantiomer separation in HPLC
InactiveCN103331151ARaw materials are cheap and easy to getEasy to operateAmino compound purification/separationOther chemical processesEnantiomerStructural formula
The invention relates to a non-interpenetrating chiral MOF (metal organic framework) stationary phase, its preparation method and application in enantiomer separation in HPLC (high-performance liquid chromatography). The stationary phase is a non-interpenetrating chiral three-dimensional porous framework complex with a structural formula as {[ZnL].H2O}n. An asymmetric structural unit {[ZnL].H2O} of the complex is composed of a Zn<2+>, an L ligand and a guest water molecule. The L ligand is -NH- containing chiral pyridine carboxylic acid, its chemical composition is [(N-(4-pyridylmethyl)-L-leucine.HBr)], and its molecular formula is C12H19BrN2O2. Chiral amino acid and 4-pyridylaldehyde are selected as raw materials to synthesize the-NH- containing pyridine carboxylic acid chiral ligand by a one-step process. The ligand and zinc acetate are adopted as raw materials to undergo room temperature diffusion so as to obtain the MOF stationary phase. The material provided in the invention has uniform chiral helical channel, uniform aperture and orifice, and can be used for separation of chiral drugs and other enantiomers. The separation is selectively dependent on the size of a separated enantiomer molecular size, but is not dependent on the functional group of the separated enantiomer. Thus, the non-interpenetrating chiral MOF stationary phase has the characteristics of traditional zeolite molecular sieve separation.
Owner:SHANDONG NORMAL UNIV
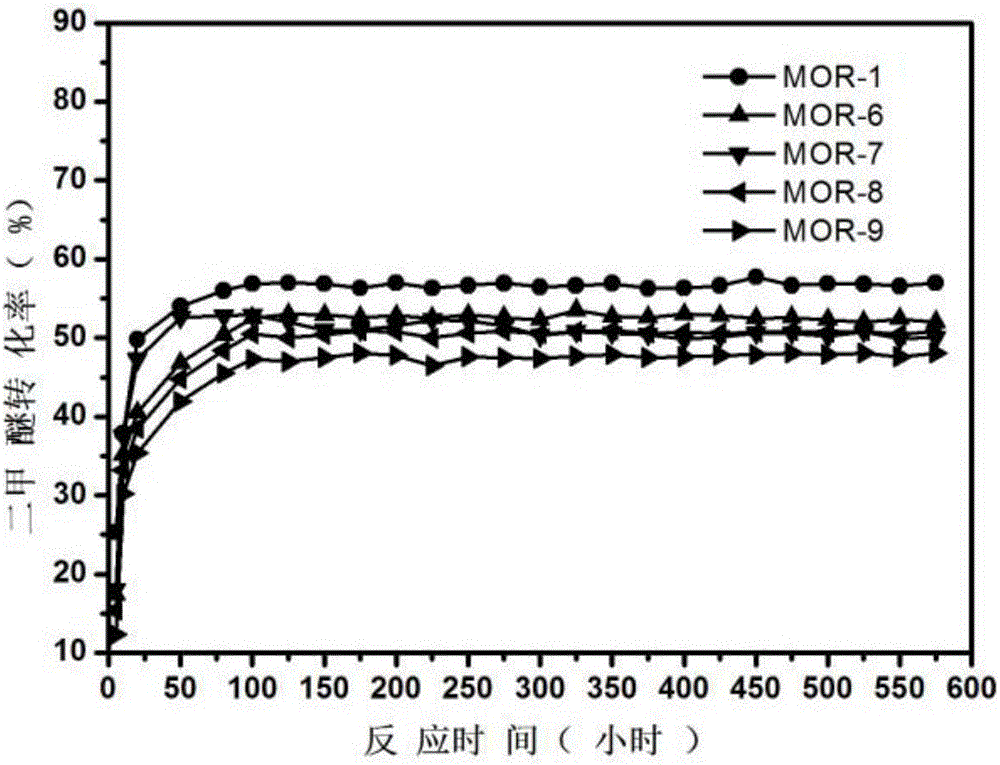
Method for making methyl acetate through carbonylation of dimethyl ether and the modified molecular sieve catalyst and modification method thereof
ActiveCN106311336AExtend your lifeImprove conversion rateCatalyst protectionMolecular sieve catalystsMolecular sieveHydrogen
The invention discloses a method for making methyl acetate through carbonylation of dimethyl ether and the modified molecular sieve catalyst and modification method thereof. The modification method is to pass the modified substance that is diluted by the diluent gas through the reactor filled with hydrogen type zeolite molecular sieve catalyst at an adsorbent temperature of 100-400 oC under normal pressure condition, so that the modified substance can have chemisorption on the hydrogen type zeolite molecular sieve catalyst to obtain modified molecular sieve catalyst; the modified substance can be liquid modified substance and / or gaseous modified substance. The modified molecular sieve catalyst can be obtained by using the modification method in which the modified molecular sieve catalyst for making methyl acetate through carbonylation of dimethyl ether can be obtained. The method for making methyl acetate through carbonylation of dimethyl ether is to pass the raw gas consisting of dimethyl ether, carbon monoxide and hydrogen through the reactor filled with the modified molecular sieve catalyst for reaction to obtain the methyl acetate. Through the modification of hydrogen type zeolite molecular sieve catalyst by the modified substance, the method can effectively inhibit the side-reaction of carbon deposit and greatly prolong the service life.
Owner:SOUTHWEST RES & DESIGN INST OF CHEM IND
1-(3-(6-(3-hydroxynaphthalen-1-yl)benzofuran-2-yl)azetidin-1 yl)prop-2-en-1-one derivatives and similar compounds as kras g12c modulators for treating cancer
ActiveUS20190382377A1Prevent proliferationOrganic chemistryPharmaceutical delivery mechanismDiseaseBenzoxazole
Compounds having activity as inhibitors of G12C mutant KRAS protein are provided. The compounds have the following structure (I): or a pharmaceutically acceptable salt, isotopic form or stereoisomer thereof, wherein A is a five-membered heteroaryl comprising 1 or 2 non-adjacent heteroatoms, inclusive of X and Y; W, X, Y, Z, L, L1, E, R1, R2bR2c and the dotted circle are as defined herein. Methods associated with preparation and use of such compounds, pharmaceutical compositions comprising such compounds and compounds for use in methods to modulate the activity of G12C mutant KRAS protein for treatment of disorders, such as cancer, are also provided. Preferred compounds are e.g. 1-(3-(6-(3-hydroxynaphthalen-1-yl)benzofuran-2-yl)azetidin-1yl)prop-2-en-1-one derivatives and related compounds such as e.g. the corresponding derivatives with e.g. a benzoimidazole, indole, benzooxazole, imidazopyridine or imidazole core structure, substituted on ring A by e.g. azetidine, pyrrolidine, azepane or bicyclopentane-amine (L1) each substituted by e.g. propenone (E), and the core structure substituted on the six-membered ring with e.g. 3-hydroxynaphthalene or indazole or hydroxy-, alkoxy- and / or fluoro-substituted phenyl (R1).
Owner:ARAXES PHARMA LLC
Dual zeolite catalyst comprising a group VIII metal and a group IIIA metal, and its use in isomerization of aromatic C8 compounds
ActiveUS8183172B2Improve catalytic performanceReduce lossesHydrocarbon by isomerisationMolecular sieve catalystsCompound aIsomerization
Owner:INST FR DU PETROLE
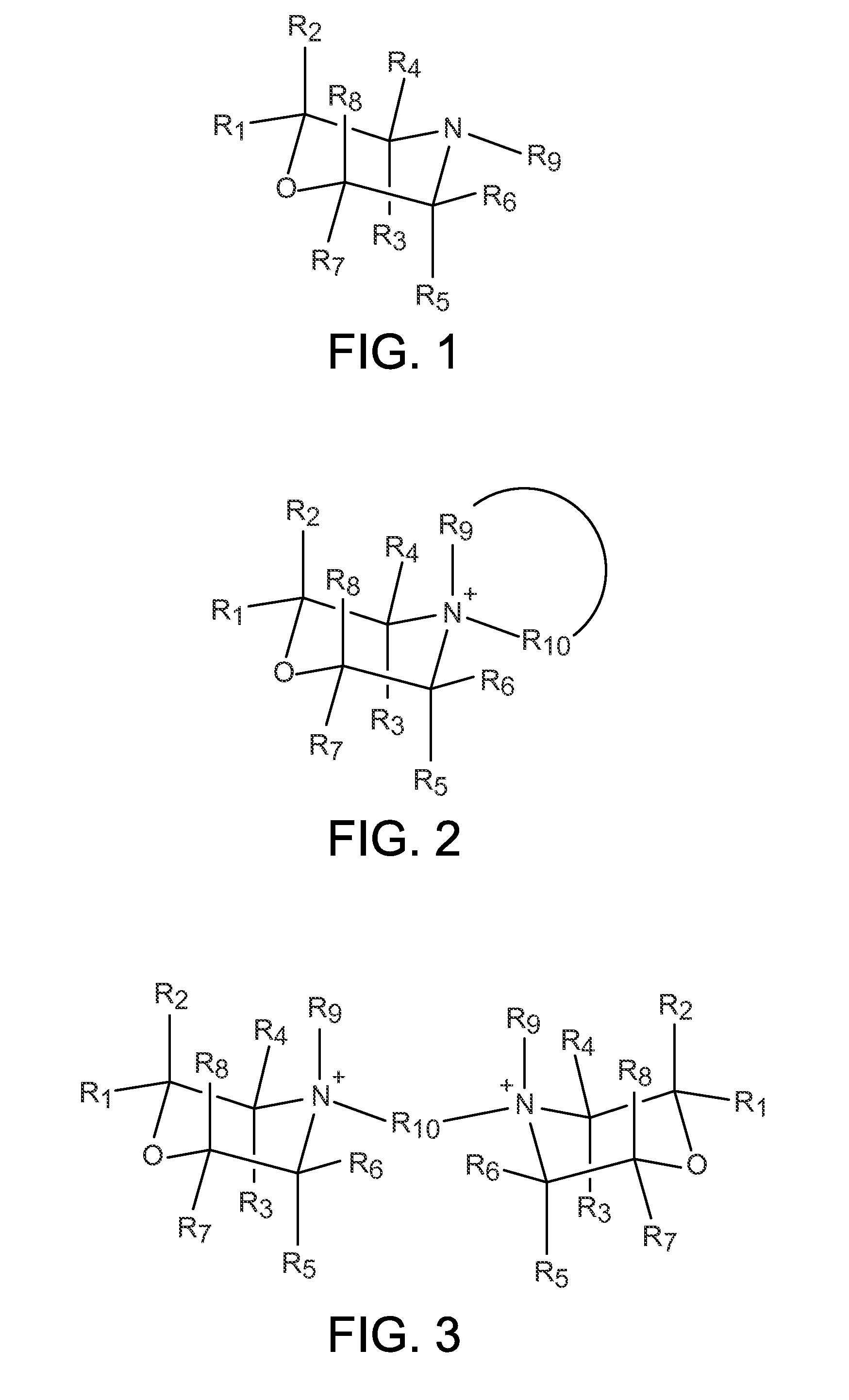
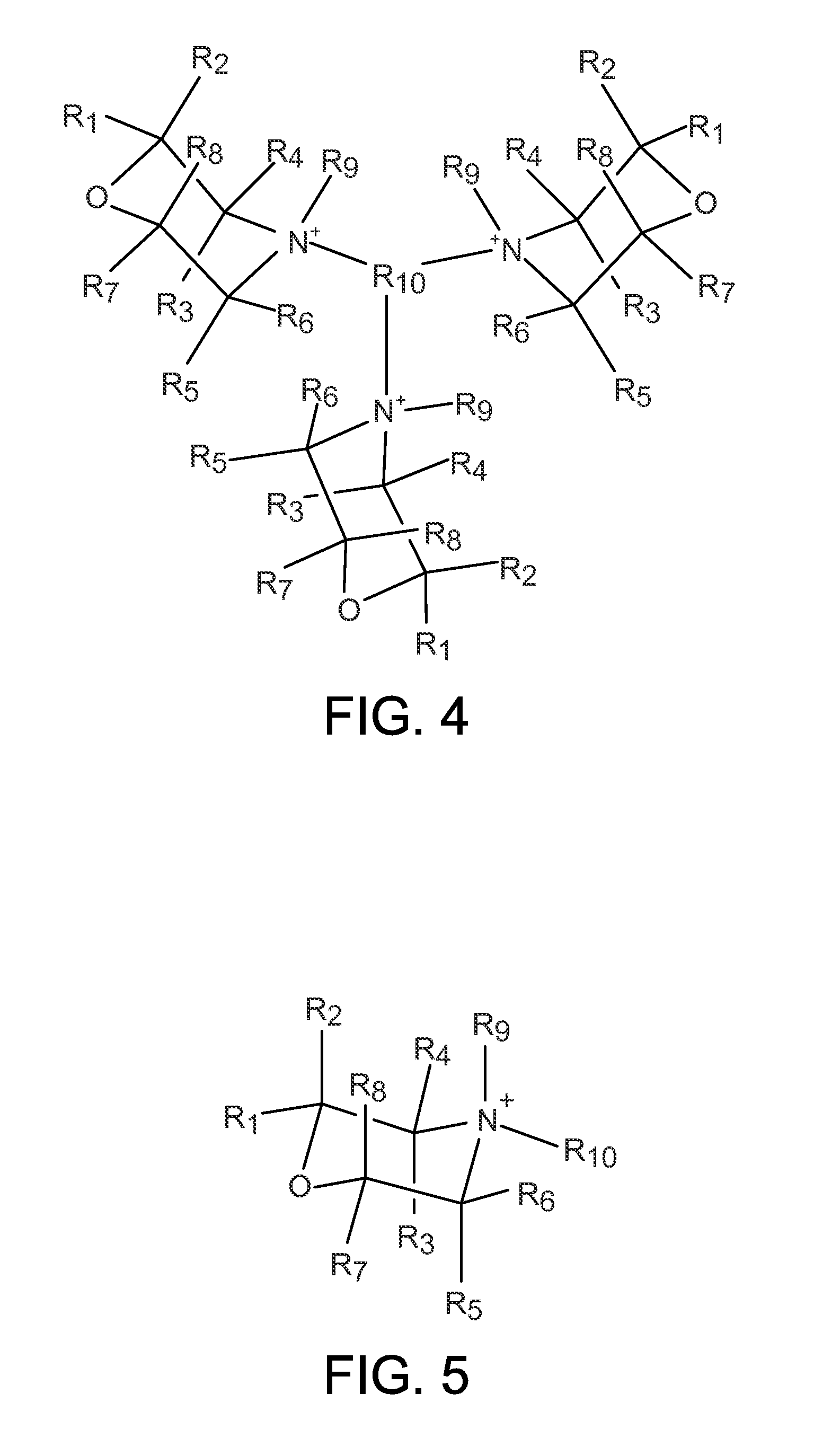
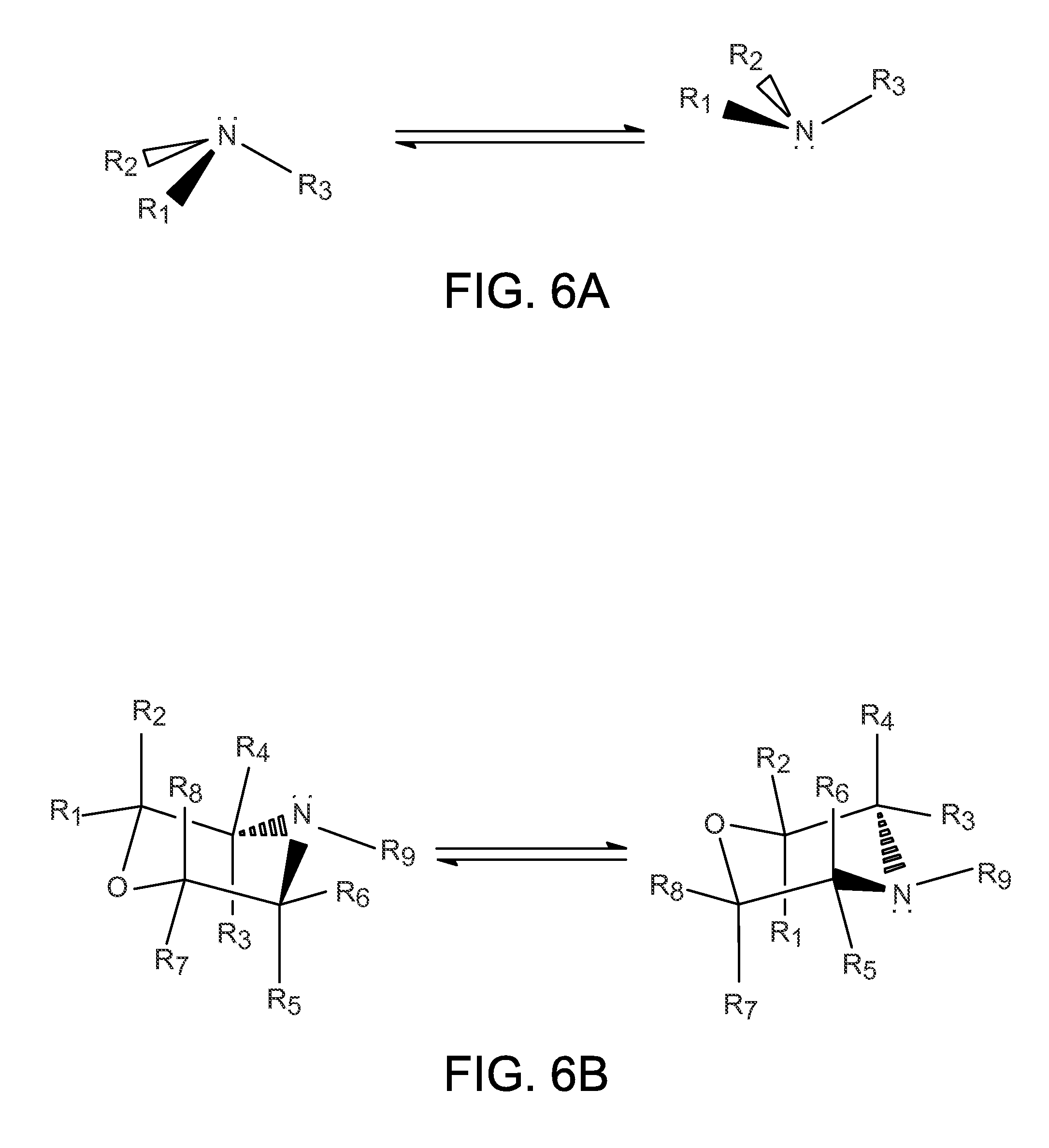
Organo-1-oxa-4-azonium cyclohexane compounds
Novel 1-oxa-4-azonium cyclohexane salts are described. These compounds can be used as structure directing agents, and they overcome many of the typical problems associated with OSDA synthesis and subsequent zeolite synthesis. Methods for synthesis of the 1-oxa-4-azonium cyclohexane salts from a variety of starting materials are also described. A substituted hydrocarbon is added to water to form a mixture, and a 1-oxa-4-azacyclohexane derivative is then added. The reaction mixture stirred until a solution containing the 1-oxa-4-azonium cyclohexane salt is obtained.
Owner:UOP LLC
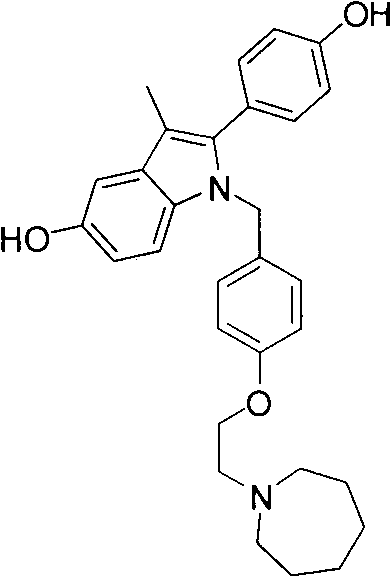
Novel azepane derivatives
The present invention provides novel azepane derivatives or pharmaceutically acceptable salts thereof according to the general formula (I) and their preparation methods, wherein the remaining symbols have the meanings given in the description. The compounds according to the invention have anti-proliferative activity and show increased plasma stability.
Owner:F HOFFMANN LA ROCHE & CO AG
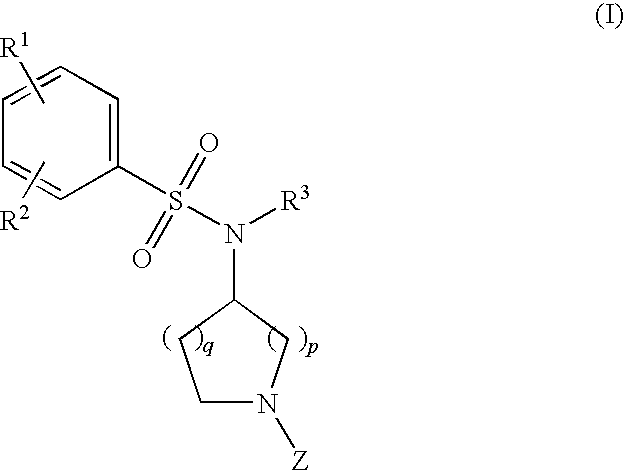
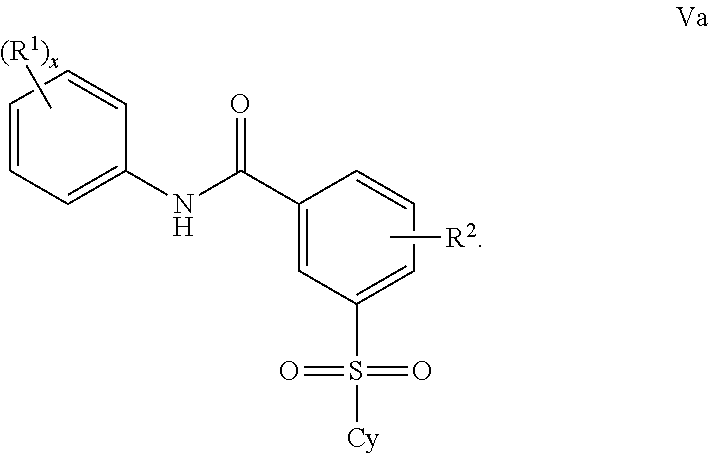
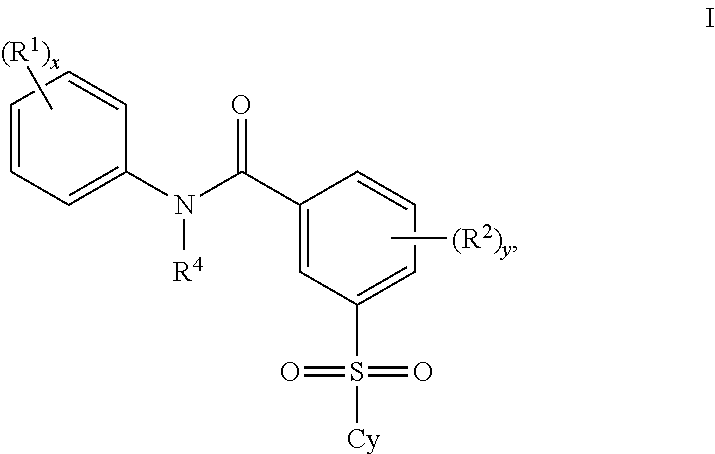
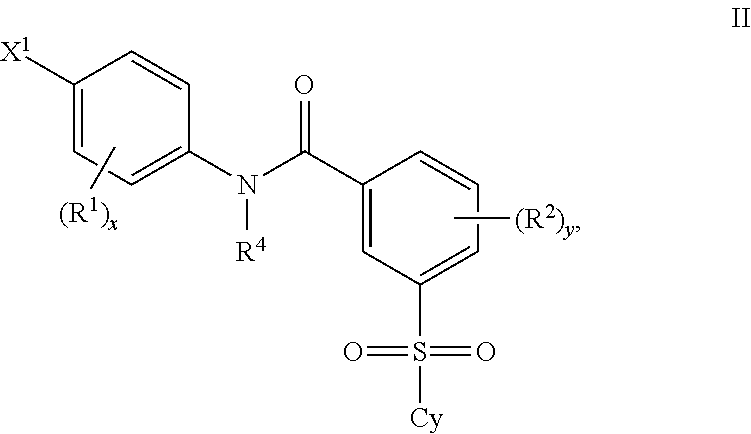
Benzenesulfonamide Compounds and the Use Thereof
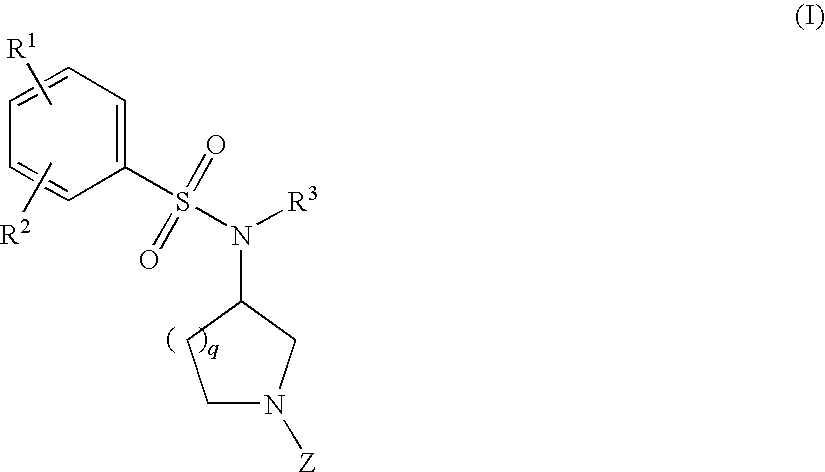
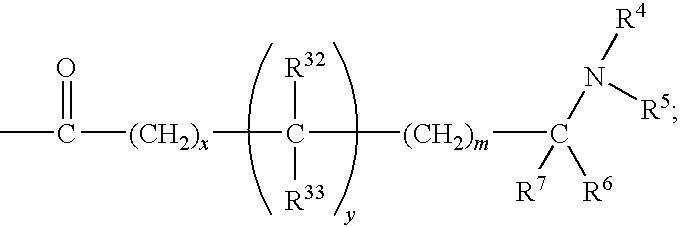
The invention relates to azetidinyl, pyrrolidinyl, piperidinyl, and hexahydroazepinyl compounds of Formula (I): and pharmaceutically acceptable salts, prodrugs, or solvates thereof, wherein R1-R3, Z, p and q are defined as set forth in the specification. The invention is also directed to the use compounds of Formula (I) to treat, prevent or ameliorate a disorder responsive to the blockade of calcium channels, and particularly N-type calcium channels. Compounds of the present invention are especially useful for treating pain.
Owner:PURDUE PHARMA LP
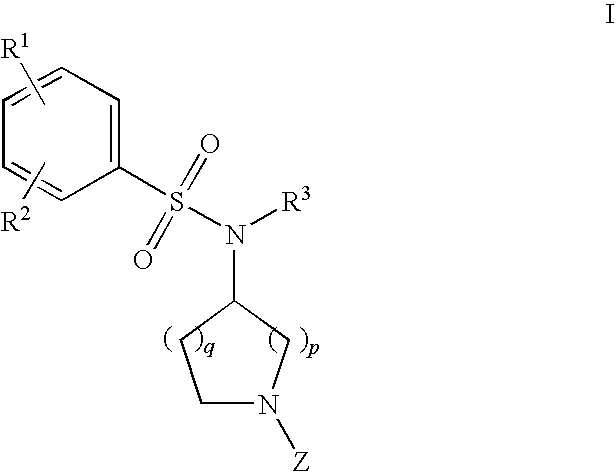
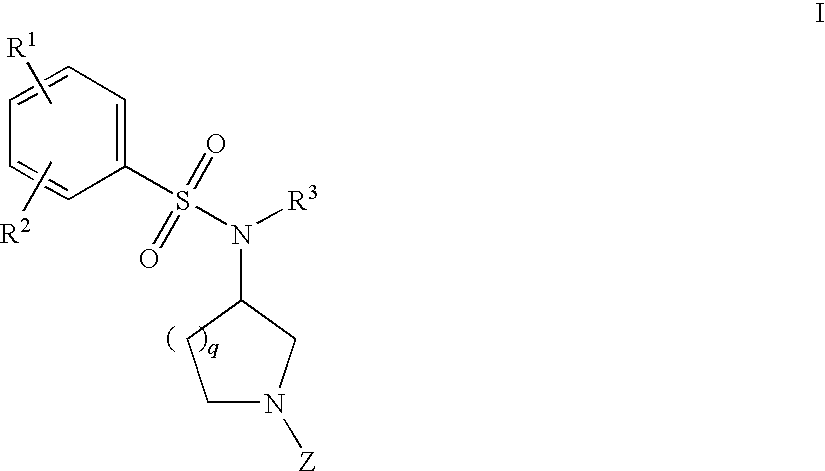
Benzenesulfonamide Compounds and Their Use
The invention relates to azetidinyl, pyrrolidinyl, piperidinyl, and hexahydroazepinyl compounds of Formula I and pharmaceutically acceptable salts, prodrugs, or solvates thereof, wherein R1-R3 and Z are defined as set forth in the specification. The invention is also directed to the use compounds of Formula I to treat, prevent or ameliorate a disorder responsive to the blockade of calcium channels, and particularly N-type calcium channels. Compounds of the present invention are especially useful for treating pain.
Owner:PURDUE PHARMA LP
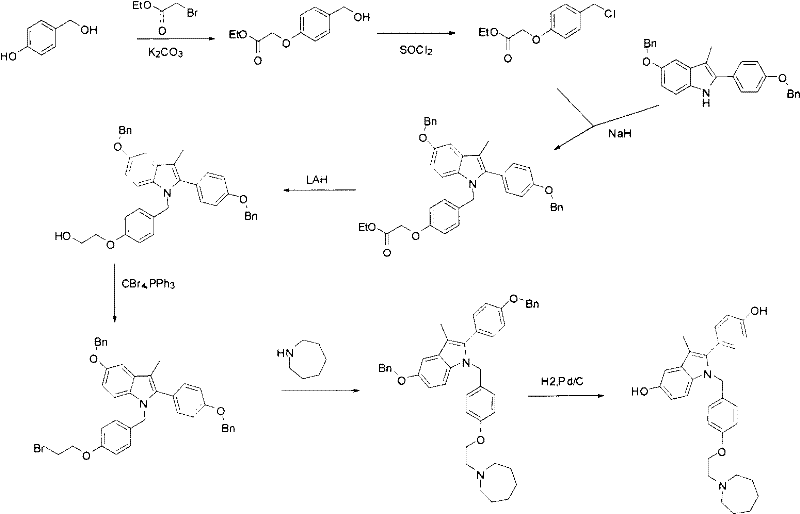
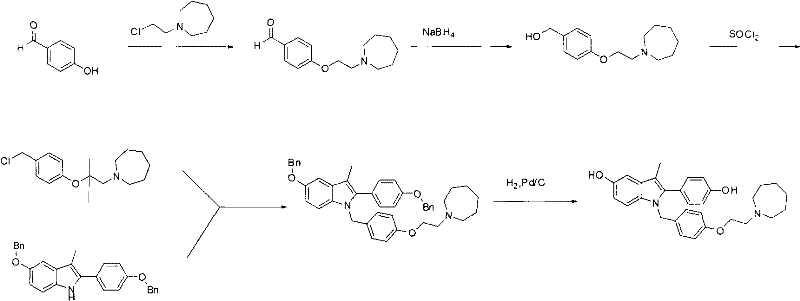
New synthetic method of bazedoxifene
The invention discloses a new synthetic method of bazedoxifene. Bazedoxifene is prepared by the steps of bromination, substitution, reductive amination, substitution, cyclization, deprotection, and the like of azepane which is selected as a raw material. The synthetic method of the invention has the advantages of mild reaction conditions, simple and convenient operating process, easily obtained reagent, and low cost.
Owner:南京正济医药销售有限公司
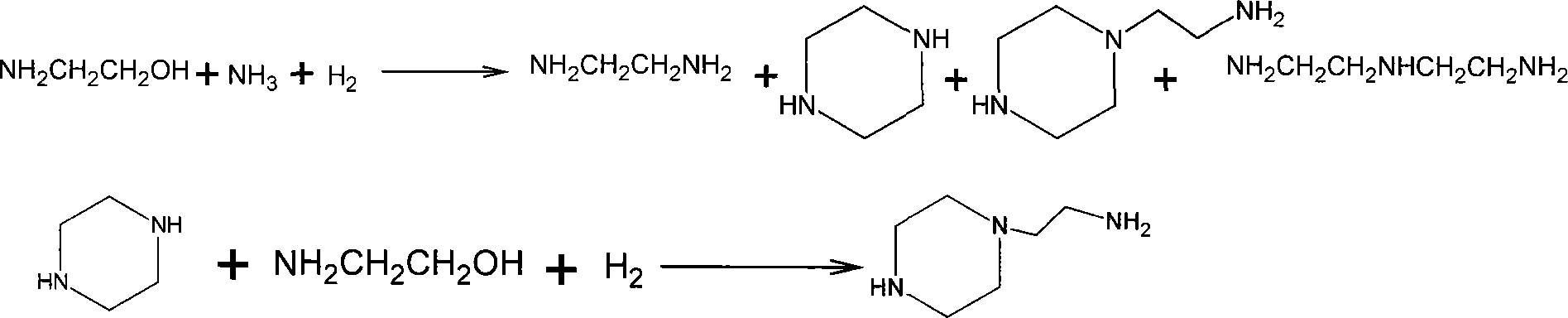
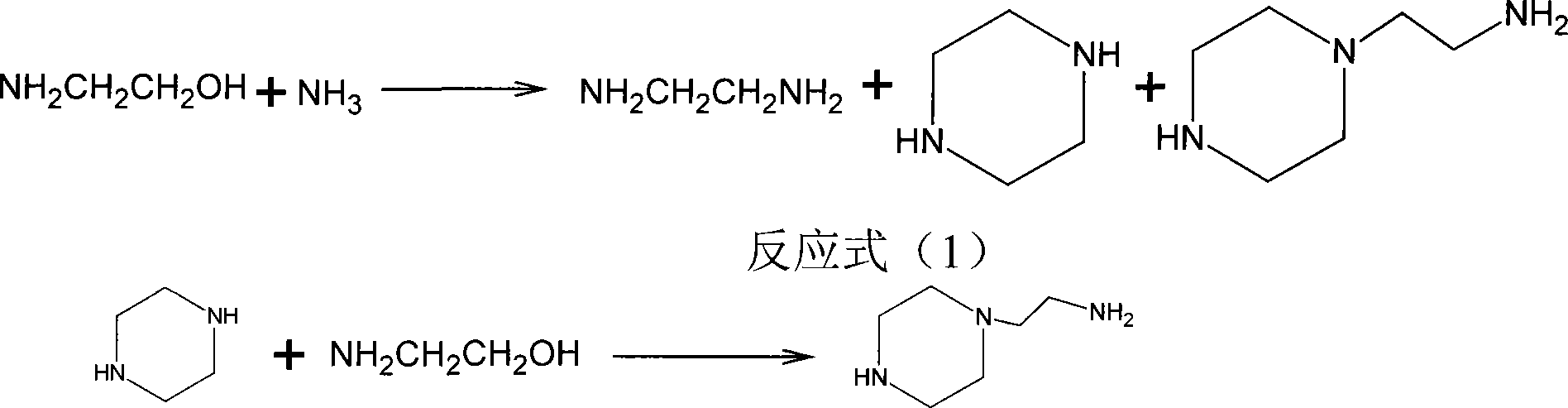
Combined preparation method for ethylene diamine and aminoethylpiperazine
InactiveCN101215239BHigh yieldMolecular sieve catalystsAmino compound preparation by condensation/addition reactionsEthylenediamineContact time
The invention discloses a combined preparation process of ethane diamine and aminoethyl piperazine, aiming to solve the problem low yield when prepared associated ethane diamine and aminoethyl piperazine. The invention comprises the steps as flowed that firstly mixing ethane diamine and ammonia to react under the conditions of the exist of mordenite of condensed amination catalyst phosphorous modification, 2.0MPa-5.0MPa pressure, 300-350 DEG C temperature, 6-12:1 of ammonia and aminoethyl alcohol weight ratio, 20-30 seconds of reaction contact time, distilling and separating product liquid toobtain aminoethyl alcohol product, secondly reacting piperazine which is obtained in first step and aminoethyl alcohol under the conditions of the exist of ZSM-5 zeolite of condensed amination catalyst silicon modification, 4.0MPa-8.0MPa pressure, 350-400 DEG C temperature, 0.5-1.5:1 of piperazine and aminoethyl alcohol weight ratio, and 10-20 seconds of reaction contact time, distilling and separating product liquid to obtain aminoethyl piperazine product. The invention is mainly combined to be used to prepare ethane diamine and aminoethyl piperazine.
Owner:山西玉龙化工有限公司
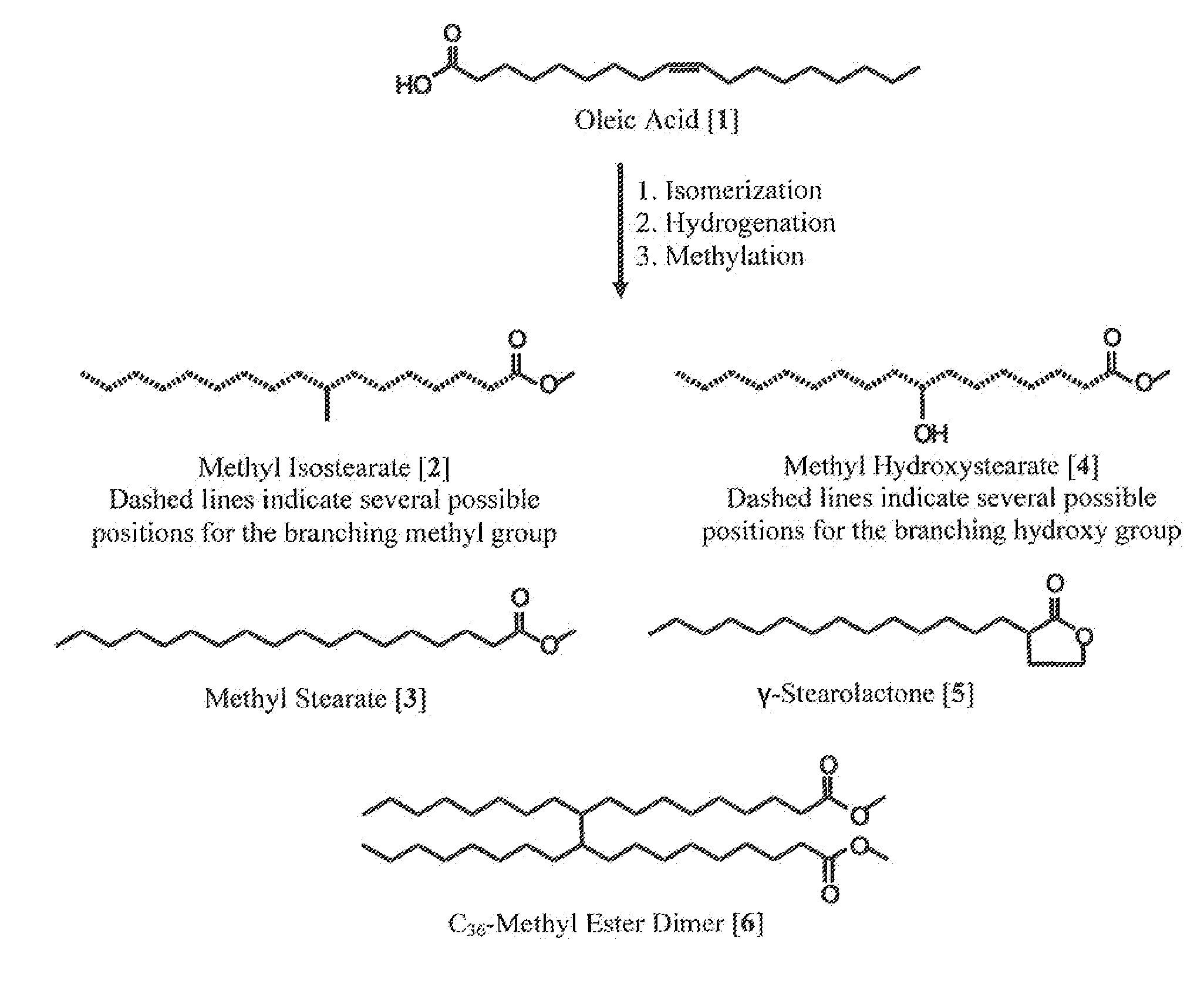
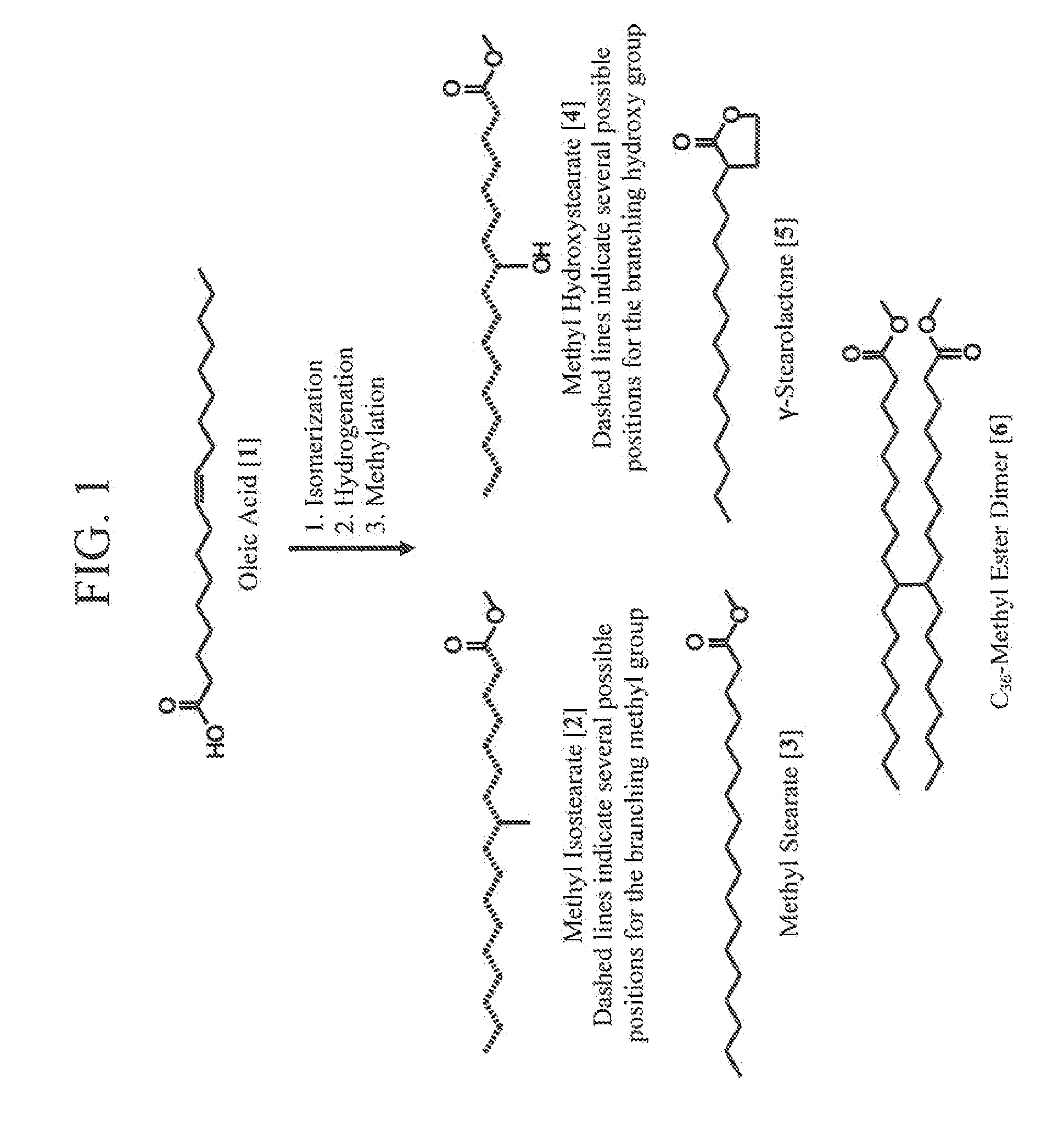
Process for Preparing Saturated Branched Chain Fatty Acids
ActiveUS20110275844A1Organic compound preparationCarboxylic preparation by ozone oxidationBranched chain fatty acidsIsomerization
A process for preparing saturated branched chain fatty acids or alkyl esters thereof involving subjecting unsaturated fatty acids having 10 to 25 carbon atoms, alkyl esters thereof or mixtures thereof to a skeletal isomerization reaction in the presence of water or a lower alcohol at a temperature of about 240° C. to about 280° C. using a combination of a stericly hindered Lewis base and zeolite as a Brönsted or Lewis acid catalyst, and isolating saturated branched chain fatty acids or alkyl esters thereof or mixtures thereof from the reaction mixture obtained by the skeletal-isomerization reaction. The yield of said saturated branched chain fatty acids is ≧70 wt %. The stericly hindered Lewis base is a tertiary amine or phosphine with linear or branched C1 to C6 alkyl or phenyl groups attached thereto.
Owner:US SEC AGRI
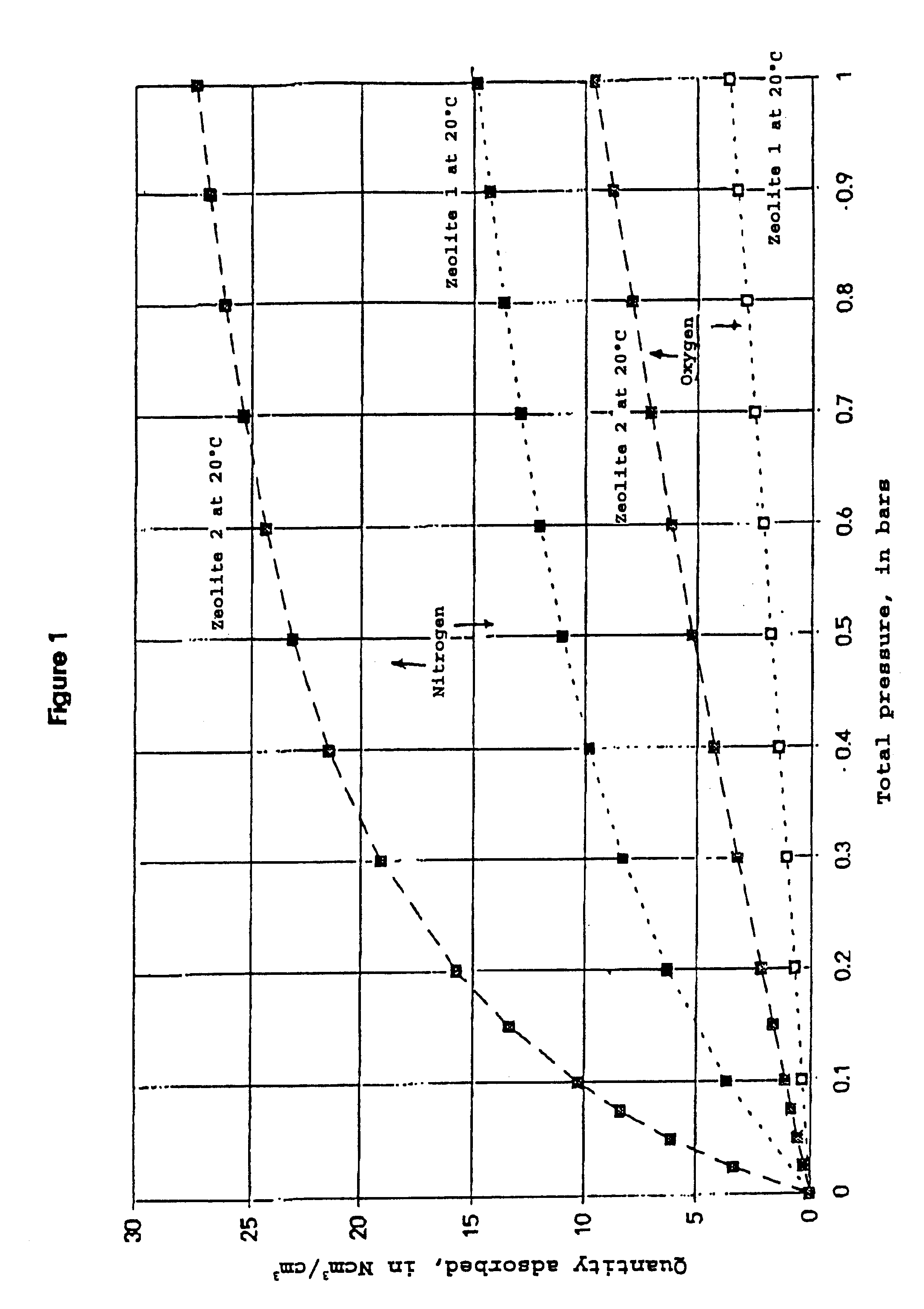
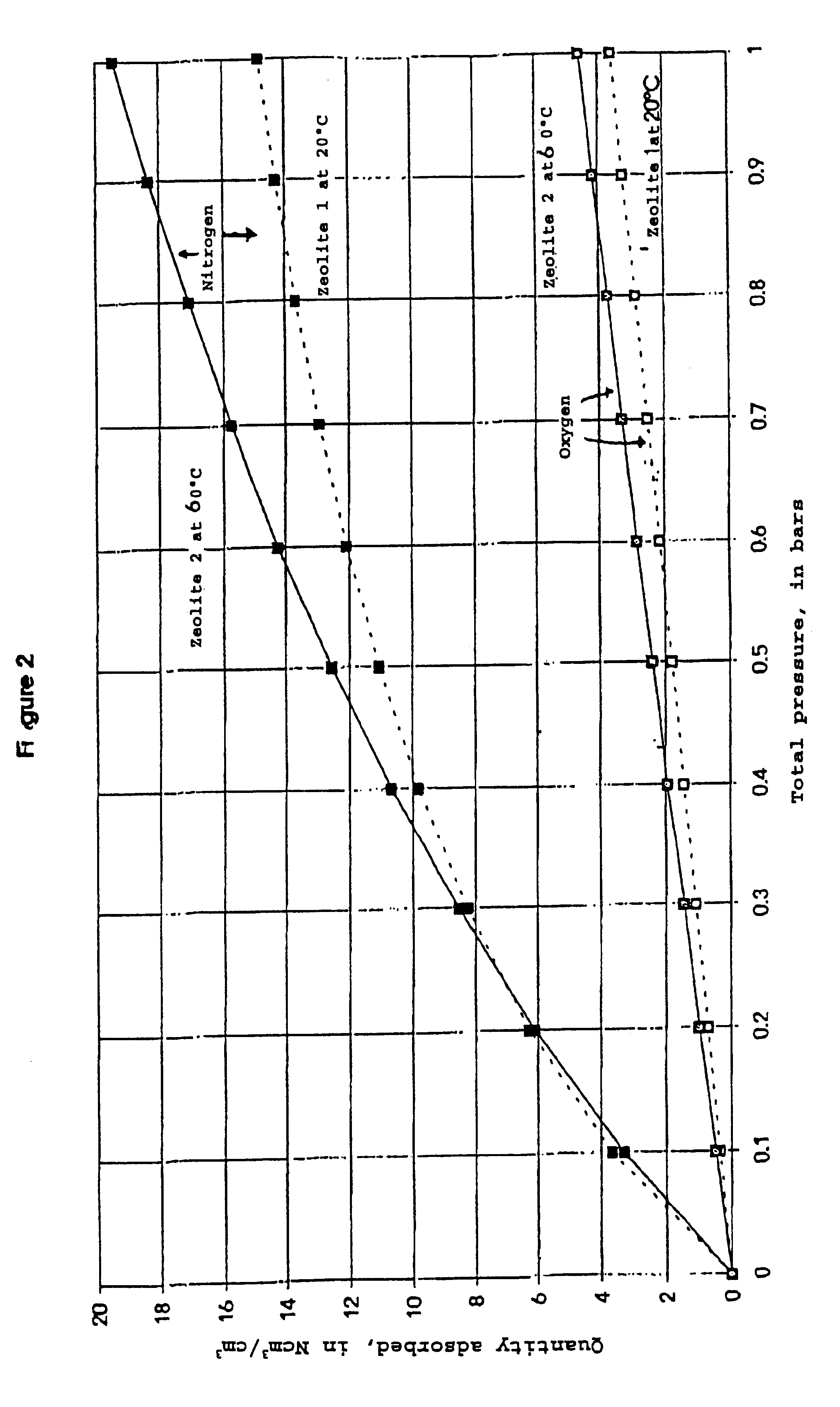
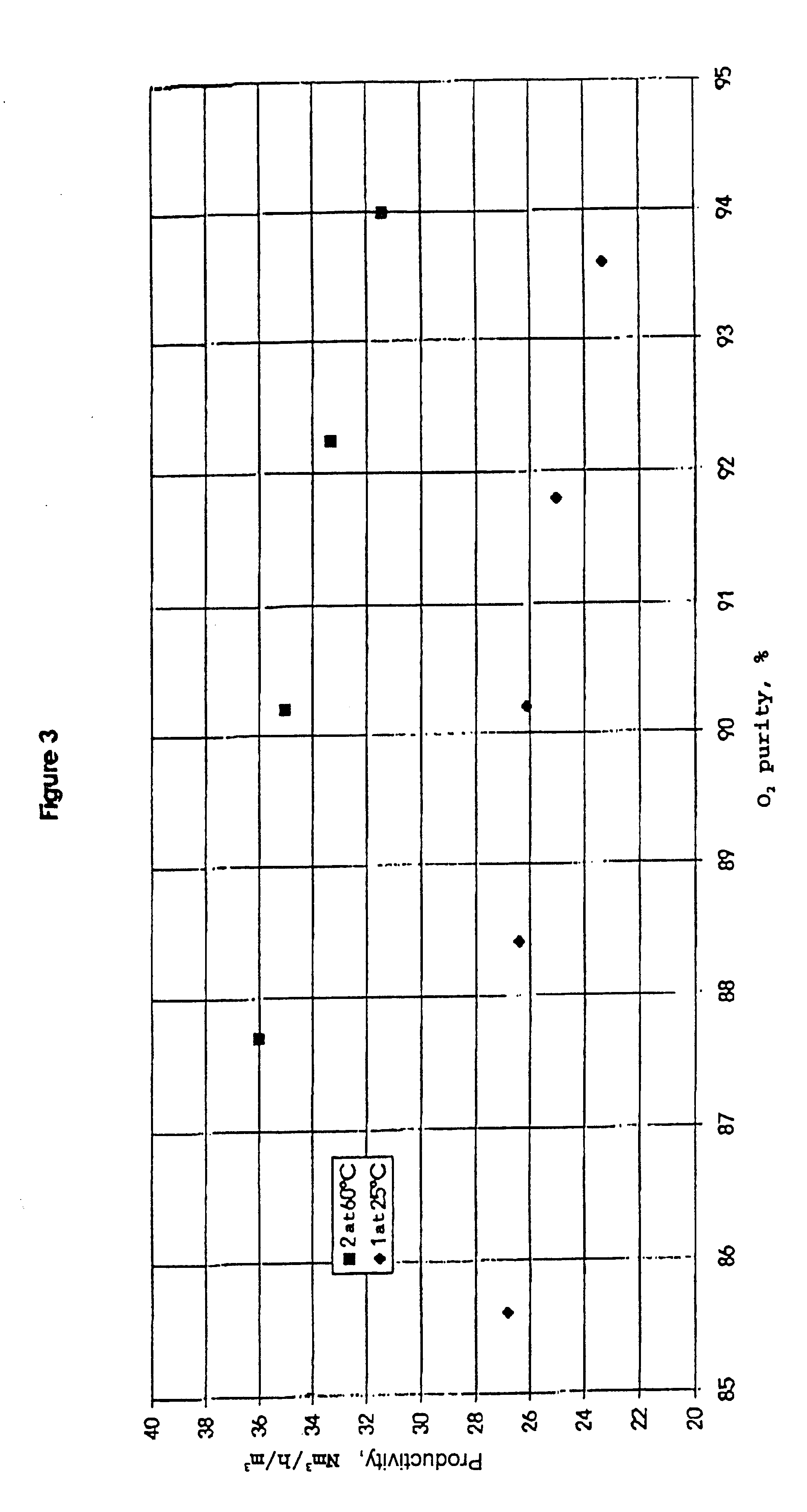
Process for separating nitrogen from less polar compounds
Process for separating nitrogen from a gas mixture containing nitrogen and at least one gas which is less polar than nitrogen, and employing a differential gas adsorption technique, called PSA process, using an adsorbent of zeolite type, according to which the PSA process is used at a temperature of at least 40° C. by employing as adsorbent a zeolite whose nitrogen adsorption isotherm at 20° C. exhibits a curvature characterized by a parameter C defined by the formula:where q(P1) denotes the quantity of nitrogen adsorbed at pressure P1 andq(P2) that adsorbed at pressure P2, andthe pressures P1 and P2 are defined respectively from the high and low pressures of the PSA cycle in question,C being equal to at least 2.5.
Owner:LAIR LIQUIDE SA POUR LETUDE & LEXPLOITATION DES PROCEDES GEORGES CLAUDE
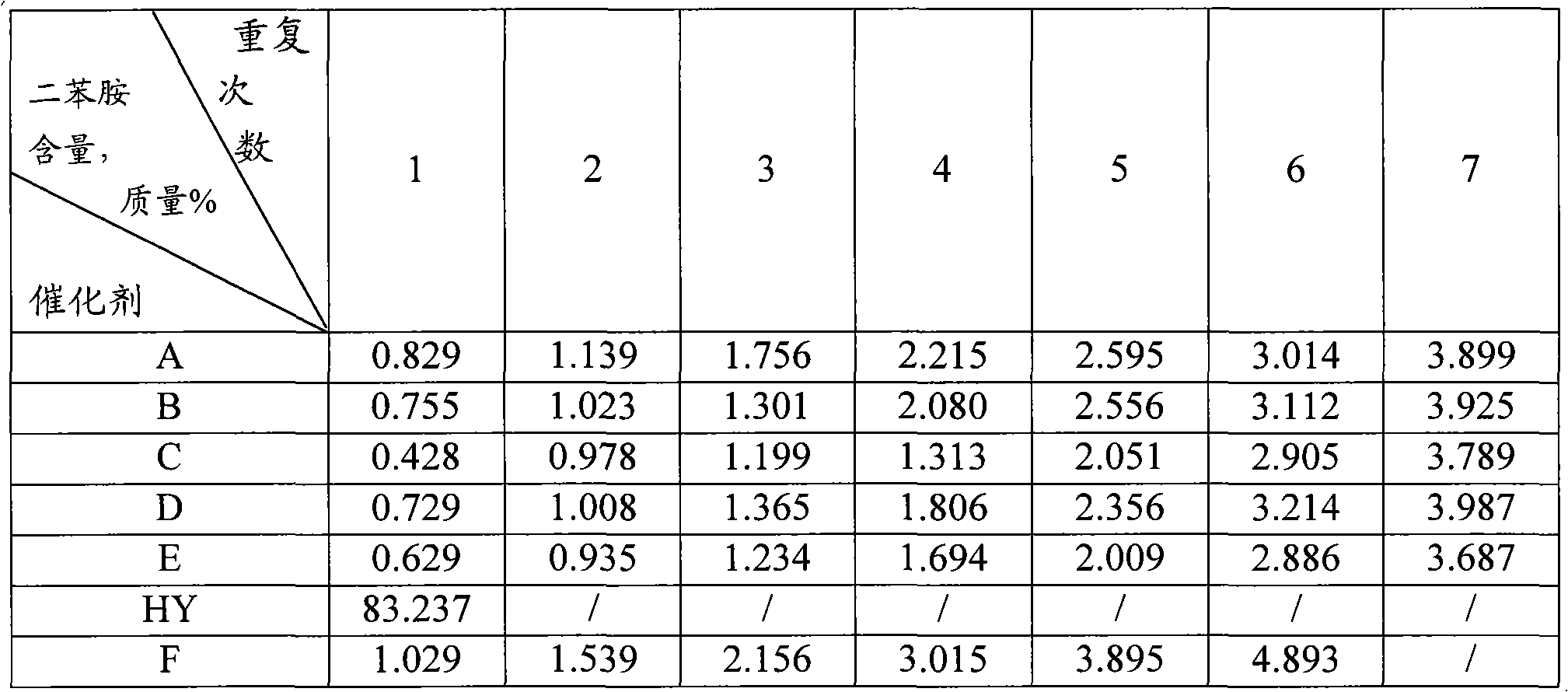
Diphenylamine alkylation catalyst and preparation method thereof
ActiveCN102371174AEffectively regulate acidity distributionReduce dosageMolecular sieve catalystsAmino preparation from aminesMolecular sieveHeteropoly acid
The invention relates to a diphenylamine alkylation catalyst. The catalyst comprises, by mass, 85-99% of a molecular sieve and 1-15% of an acid, wherein the molecular sieve comprises faujasite and / or a MFI-type molecular sieve, the acid comprises an inorganic acid containing oxygen or a heteropoly acid. The catalyst is prepared by adopting an acid-containing solution to carry out saturated immersion for the molecular sieve, and carrying out treatments of dehydration and drying. The catalyst is used for the diphenylamine alkylation reaction, and has high reaction activity and reusability.
Owner:CHINA PETROLEUM & CHEM CORP +1
Purification method of, 1, 1-difluoroethane
InactiveCN1878738AEfficient productionHalogenated hydrocarbon preparationHydrogen fluoridePurification methods
Crude 1,1-difluoroethane containing at least one compound selected from the group consisting of unsaturated compounds each having two carbon atoms within the molecule and saturated chlorine-containing compounds each having two carbon atoms within the molecule is brought into contact with a zeolite and / or a carbonaceous adsorbent, or crude 1,1-difluoroethane containing hydrogen fluoride and, as impurities, at least one compound selected from the group consisting of unsaturated compounds each having two carbon atoms within the molecule is brought into contact with a fluorination catalyst in a gas phase state. High-purity 1,1-difluoroethane usable as a cryogenic refrigerant, or as an etching gas, can be produced in an industrially advantageous manner.
Owner:SHOWA DENKO KK
Methods for preparing piperazine and catalyst by gas-solid-phase catalytic synthesis of ethylene glycol
InactiveCN106749099ALow priceSimple process routeMolecular sieve catalystsOrganic compound preparationCobaltAzepane
The invention provides methods for preparing piperazine and a catalyst by gas-solid-phase catalytic synthesis of ethylene glycol. The raw material (ethylene glycol) is mixed with certain quantities of ammonia and nitrogen to react via an amination catalyst to produce piperazine and derivatives of piperazine; and nickel, iron, copper, zinc, cobalt and the like serve as active components or additives in the amination process, mordenite serves as a carrier, and the mixed metal oxide amination catalyst is prepared by the impregnation method. The conversion rate of ethylene glycol can reach 42%, and the selectivity of piperazine reaches 75%.
Owner:SHAANXI YANCHANG PETROLEUM GRP
Method of Preparation of Benzofuran-2-Carboxylic Acid -Amide
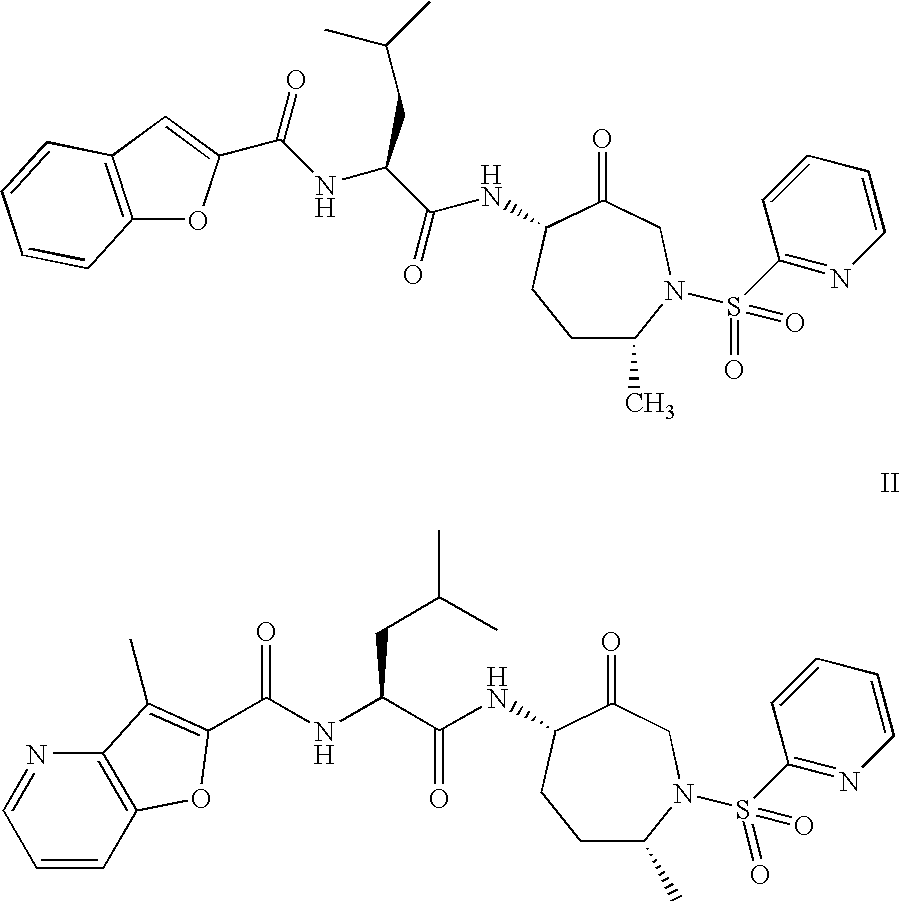
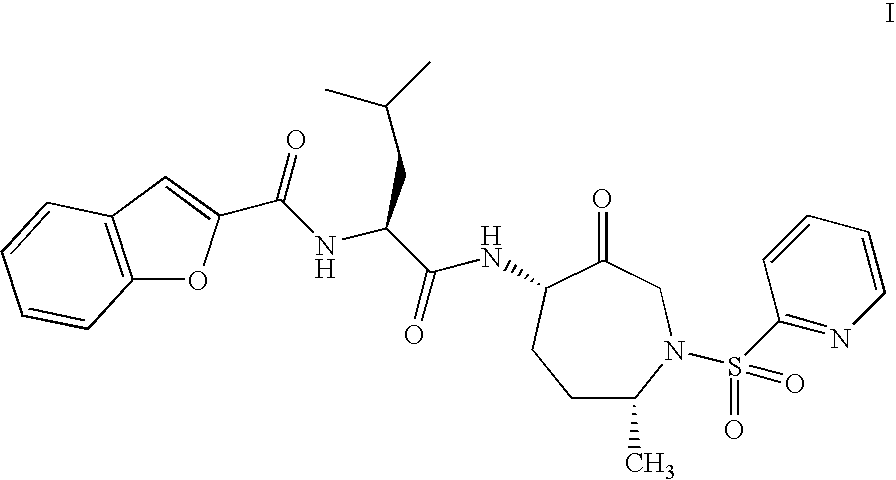
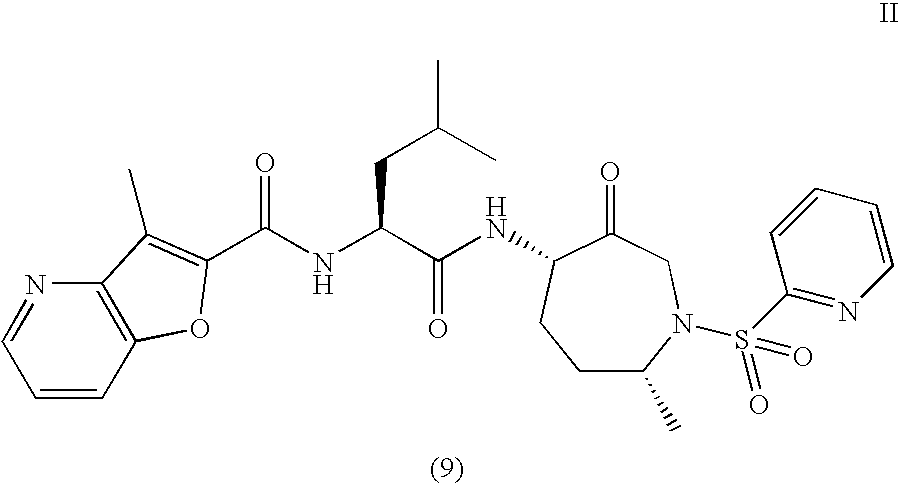
This invention relates to a method of preparation of benzofuran-2-carboxylic acid {(S)-3-methyl-1-[(4S,7R)-7-methyl-3-oxo-1-(pyridine-2-sulfonyl)-azepan-4-ylcarbamoyl]-butyl}-amide.
Owner:SMITHKLINE BECKMAN CORP
Liquid crystal composition containing nitrogen-containing cyclic compound and liquid crystal display device
ActiveUS20160208172A1Avoid photolysisImprove solubilityLiquid crystal compositionsOrganic chemistryCrystallographyDielectric anisotropy
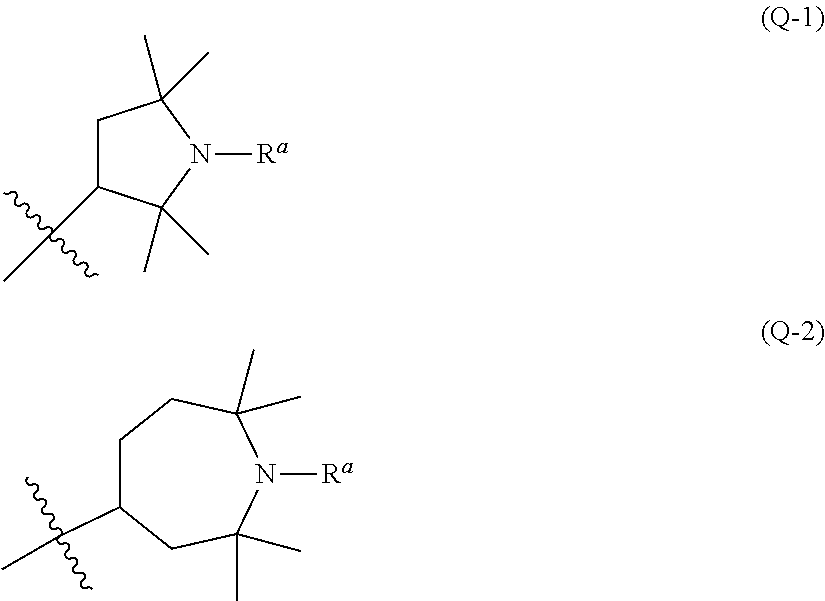
The disclosure shows a liquid crystal composition that contains a compound having an effect of preventing photolysis of the liquid crystal composition and having high solubility in the liquid crystal composition, and that satisfies at least one of characteristics such as high maximum temperature, low minimum temperature, small viscosity, suitable optical anisotropy, large positive or negative dielectric anisotropy, large specific resistance, high stability to ultraviolet light or heat, and a suitable elastic constant, etc. and so on.The disclosure shows a liquid crystal composition that contains a compound having the following azolidine ring (Q-1) or azepane ring (Q-2), a liquid crystal display device and so on.
Owner:JNC CORP +1
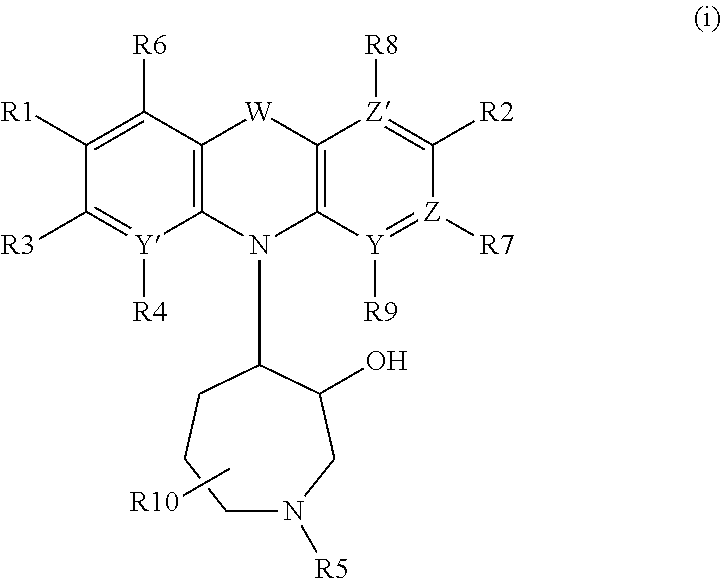
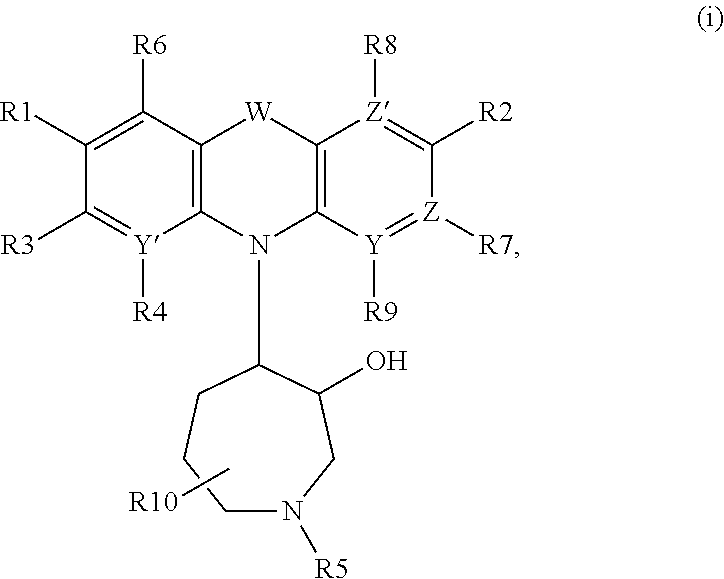
Azepanyl-derivatives and pharmaceutical compositions comprising the same with antiparasitic activity
The present invention provides compounds of Formula (i). Furthermore, pharmaceutical compositions are provided comprising at least one compound of Formula (i), for the treatment of parasitic diseases including malaria, as well as neurodegenerative diseases.
Owner:MERCK PATENT GMBH
Herbicide containing fluroxypyr, nicosulfuron and atrazine and preparation method thereof
InactiveCN102972422AImprove permeabilityGood stretchabilityBiocideAnimal repellantsVegetable oilFluroxypyr
The invention provides a herbicide containing fluroxypyr, nicosulfuron and atrazine. According to the invention, fluroxypyr, nicosulfuron and atrazine are used as main components, urea is used as a stabilizing agent, and 1-dodecyl azacycloheptane-2-one is used as a synergist; addition of 1-dodecyl azacycloheptane-2-one as the synergist substantially improves the effect of the herbicide. Turpentine-based vegetable oil used as a filling material is extracted from the timber of Chinese pine; the raw material for the filling material is natural and environment-friendly, resources are renewable, and the problem that usage of considerable organic solvents in pesticide preparations poses severe pollution to the environment is thoroughly overcome. Nicosulfuron, atrazine and fluroxypyr have obvious synergistic interaction, and integral drug effect of the herbicide is obviously better than a mixture of individual preparations of nicosulfuron, atrazine and fluroxypyr. Low dosage of the herbicide provided by the invention has a minimum control effect of 80.1% on grassy weeds, a minimum control effect of 86.9% on broad-leaf weeds and a comprehensive control effect of 83.5 to 99.9%; substantial synergy and reduction in drug cost are realized.
Owner:辽宁海佳农化有限公司
Method for separating straight-chain conjugated diene
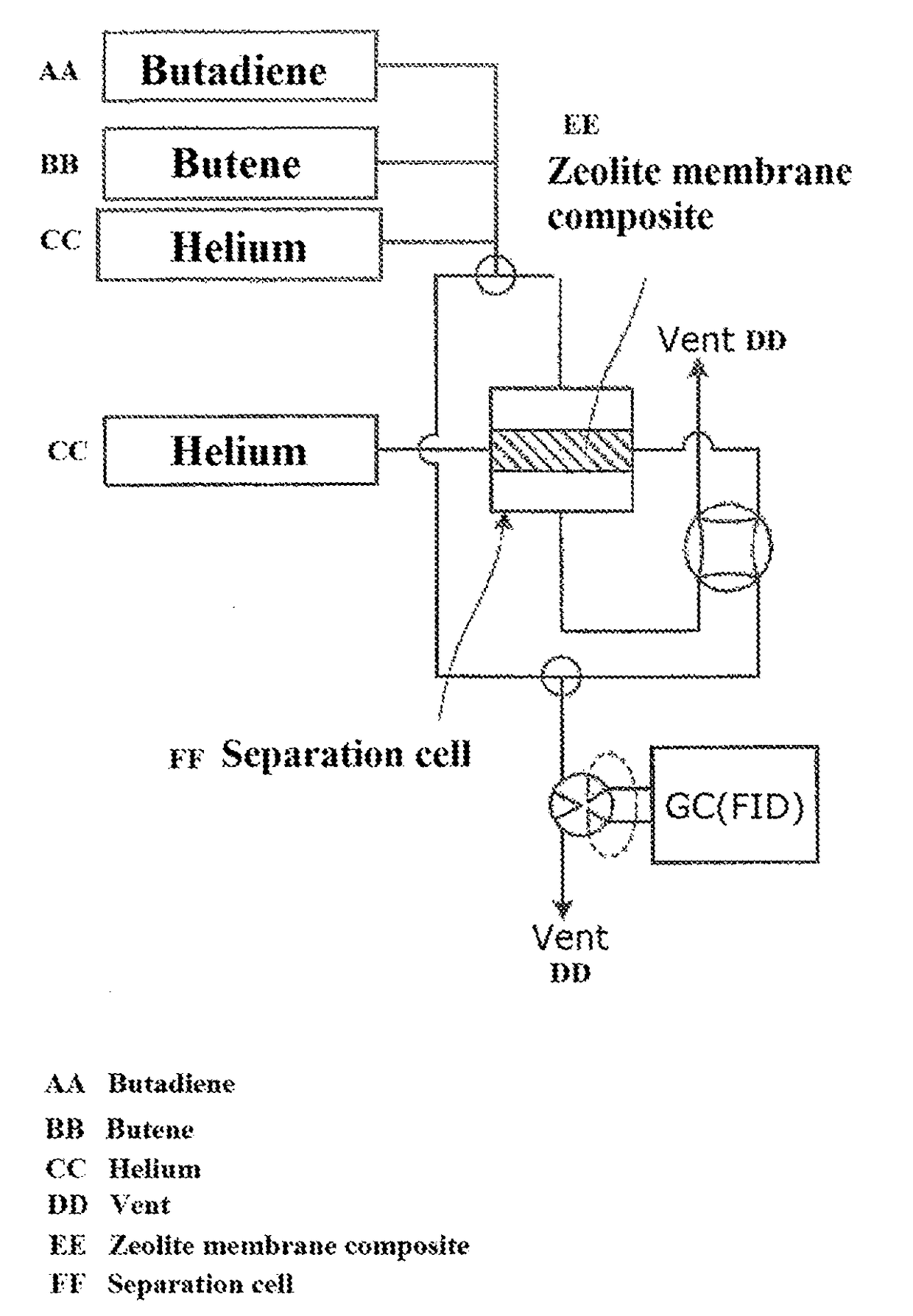
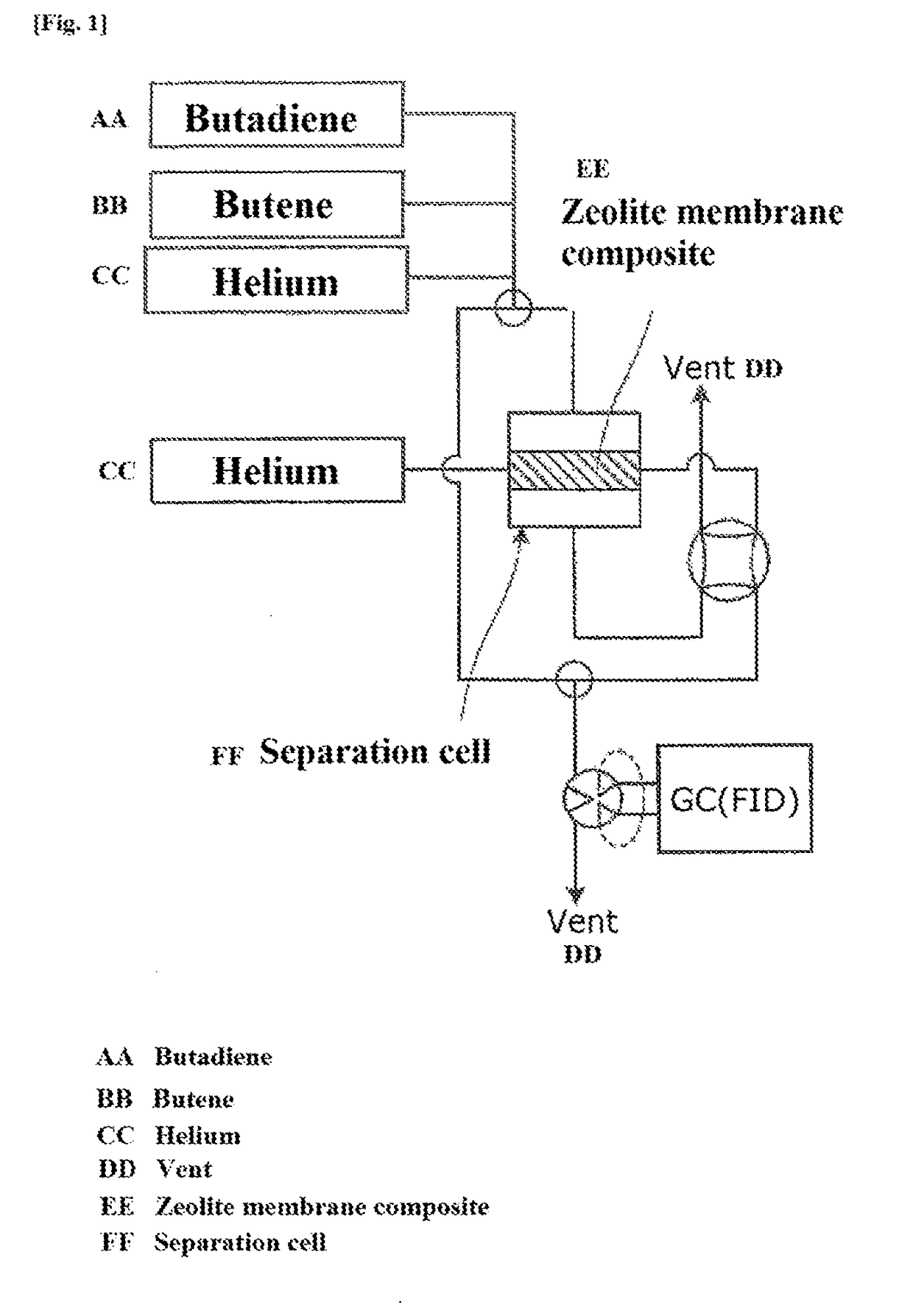
ActiveUS20170349510A1High purityImprove efficiencySemi-permeable membranesGas treatmentAzepaneAlkali metal
The present invention provides a method for selectively separating a straight-chain conjugated diene with high purity from a mixture containing the straight-chain conjugated diene and at least one type of straight-chain olefin. The method involves separating the straight-chain conjugated diene from the mixture containing the straight-chain conjugated diene and the straight-chain olefin using a zeolite membrane composite. The composite contains a porous support and a zeolite layer formed on the surface and in the fine pores of the support, and the zeolite contains an alkali metal cation.
Owner:ENEOS CORP +1
Method used for preparing 2,4-dichloro-5-fluorobenzoyl chloride
ActiveCN105669435AImpede the formation of diffusionHinder carbon depositionMolecular sieve catalystsHalogenated hydrocarbon preparationChlorideMedicinal chemistry
The invention relates to a method used for synthesis of 2,4-dichloro-5-fluorobenzoyl chloride and suitable for industrialized production; the method comprises the steps: firstly, with 2,4-dichlorofluorobenzene as a raw material, and in the presence of a composite zeolite solid super acidic catalyst, carrying out a reaction with carbon tetrachloride to obtain 2,4-dichloro-5-fluorotrichlorotoluene, and hydrolyzing to obtain 2,4-dichloro-5-fluorobenzoyl chloride.
Owner:ZHEJIANG YONGTAI TECH CO LTD
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Certain (2S)-n-[(1S)-1-cyano-2-phenylethyl]-1,4-oxazepane-2-carboxamides as dipeptidyl peptidase 1 inhibitors for treating bronchiectasis Certain (2S)-n-[(1S)-1-cyano-2-phenylethyl]-1,4-oxazepane-2-carboxamides as dipeptidyl peptidase 1 inhibitors for treating bronchiectasis](https://images-eureka.patsnap.com/patent_img/4b914f3c-69a9-4bcc-8c7e-af7950ba3cba/US20180028541A1-20180201-C00001.png)
![Certain (2S)-n-[(1S)-1-cyano-2-phenylethyl]-1,4-oxazepane-2-carboxamides as dipeptidyl peptidase 1 inhibitors for treating bronchiectasis Certain (2S)-n-[(1S)-1-cyano-2-phenylethyl]-1,4-oxazepane-2-carboxamides as dipeptidyl peptidase 1 inhibitors for treating bronchiectasis](https://images-eureka.patsnap.com/patent_img/4b914f3c-69a9-4bcc-8c7e-af7950ba3cba/US20180028541A1-20180201-C00002.png)
![Certain (2S)-n-[(1S)-1-cyano-2-phenylethyl]-1,4-oxazepane-2-carboxamides as dipeptidyl peptidase 1 inhibitors for treating bronchiectasis Certain (2S)-n-[(1S)-1-cyano-2-phenylethyl]-1,4-oxazepane-2-carboxamides as dipeptidyl peptidase 1 inhibitors for treating bronchiectasis](https://images-eureka.patsnap.com/patent_img/4b914f3c-69a9-4bcc-8c7e-af7950ba3cba/US20180028541A1-20180201-C00003.png)