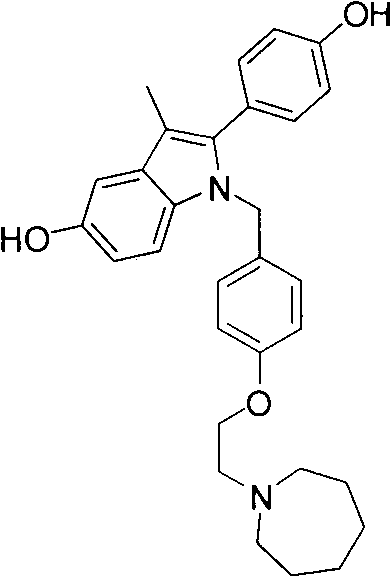
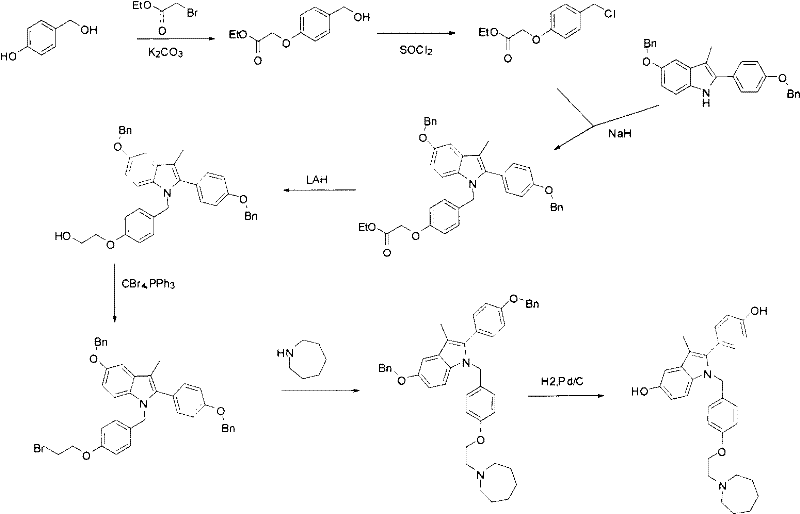
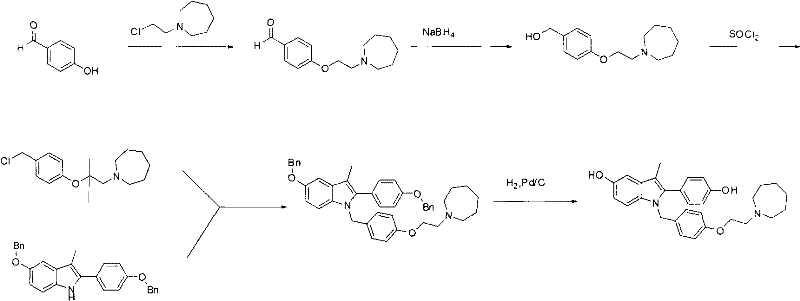
New synthetic method of bazedoxifene
A new synthesis, benzyloxy technology, applied in the direction of organic chemistry, can solve the problems of increasing the difficulty of purification of the final product, increasing the cost of synthesis, and increasing the amount of starting material, so as to improve the overall conversion rate, shorten the reaction steps, The effect of mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1: the synthesis of intermediate 4-(2-(azepan-1-yl)ethoxy)benzaldehyde (4)
[0032] The first step: the synthesis of intermediate 1-(2-bromoethyl)azepine (2)
[0033]
[0034] Add 100g of azepane (1), 246.2g of 1,2-dibromoethane (2), and 208.9g of potassium carbonate into a 2L three-necked flask, and finally add 1.25L of acetonitrile, and heat at 100 degrees to react after everything is mixed evenly. After the reaction was detected by TLC, the reaction solution was cooled to room temperature, potassium carbonate was removed by filtration, the organic solvent was removed from the filtrate under reduced pressure, the residue was evaporated and dissolved in 800 mL of dichloromethane, washed 2-3 times with water, dried over anhydrous sodium sulfate, and then decompressed. Removal of the dichloromethane gave an oil 185.5 g, 89.1% yield.
[0035] The second step: the synthesis of intermediate 4-(2-(azepan-1-yl)ethoxy)benzaldehyde (4)
[0036]
[0037] Add 1...
Embodiment 2
[0038] Embodiment 2: Synthesis of intermediate N-(4-(2-(azepan-1-yl)ethoxy)benzyl)-4-(benzyloxy)aniline (6)
[0039]
[0040] Add 50.0g of 4-(benzyloxy)aniline (5) and 74.4g of 4-(2-(azepan-1-yl)ethoxy)benzaldehyde (4) into a 1L three-necked flask, and dissolve them in 500mL of ethanol , the reaction system was cooled to 0-5 degrees in an ice-water bath, and 106.3 g of sodium triacetoxyborohydride was slowly added in batches. After the addition, the temperature of the reaction solution was raised to 35 degrees, and the reaction was stirred. After the reaction was detected by TLC, the reaction solution was cooled to 0-5 degrees, and 200 mL of water was slowly added to it. After the addition, it was stirred for two small tests, separated, the water phase was discarded, the organic phase was concentrated to dryness, and the product was purified by silica gel column chromatography. 85.5 g, yield 79.2%.
Embodiment 3
[0041] Example 3: 1-(4-(2-(azepan-1-yl)ethoxy)benzyl)-5-(benzyloxy)-2-(4-(benzyloxy)phenyl Synthesis of )-3-methyl-1H-indole (8)
[0042]
[0043] Add N-(4-(2-(azepan-1-yl)ethoxy)benzyl)-4-(benzyloxy)aniline (6) 5.0g in a 25mL single-necked bottle, 1-(4 -(Benzyloxy)phenyl)-2-bromoethane-1-one (7) 9.2g, and triethylamine 2.9g, dissolved in 4mL N,N-dimethylformamide, heated to 120 degrees under stirring to react After the reaction was detected by TLC, the reaction liquid was cooled to 0-5 degrees, poured into 20 mL of water, extracted with ethyl acetate and recrystallized in methanol to obtain 10.3 g of solids, with a yield of 55.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com