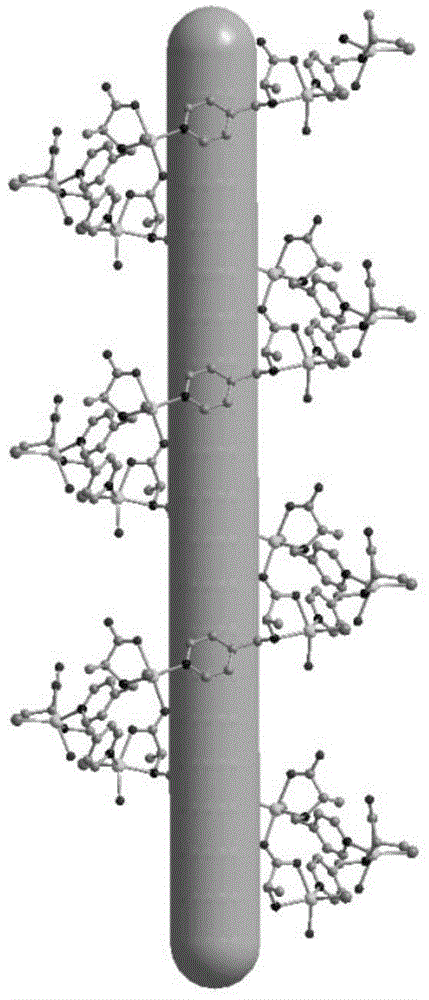
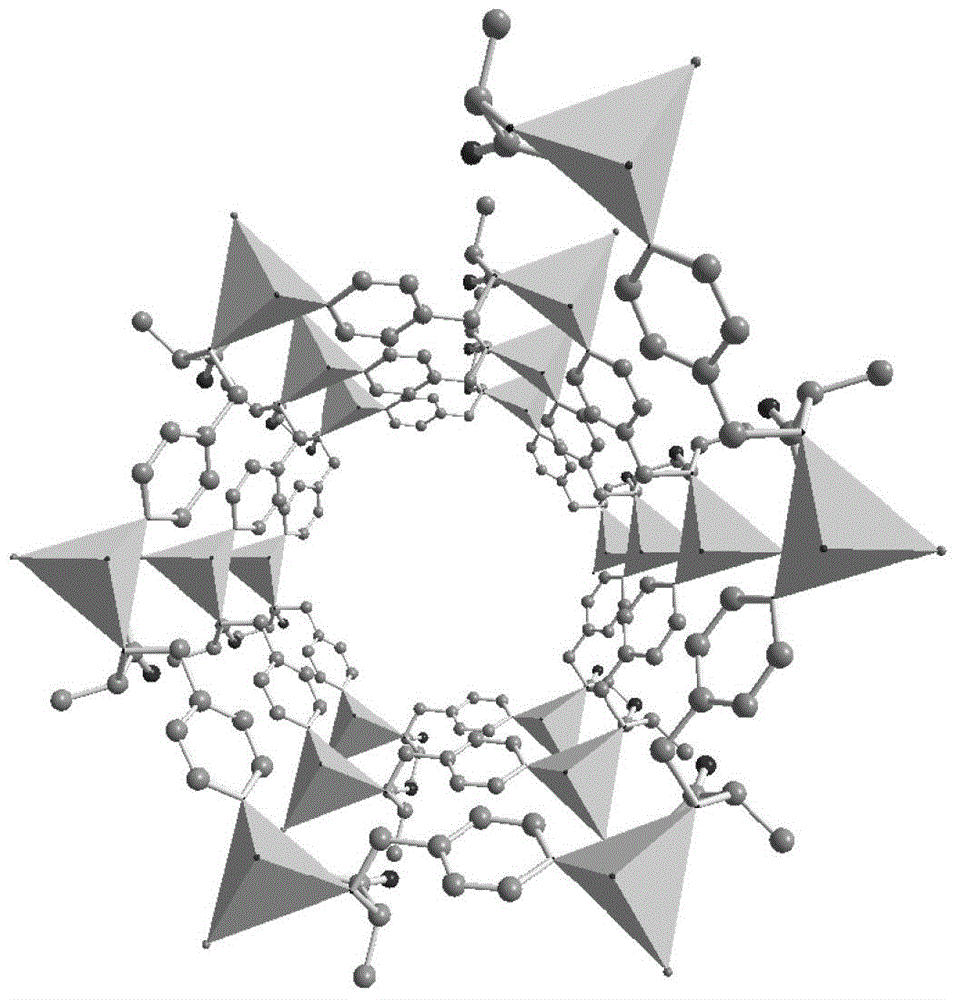
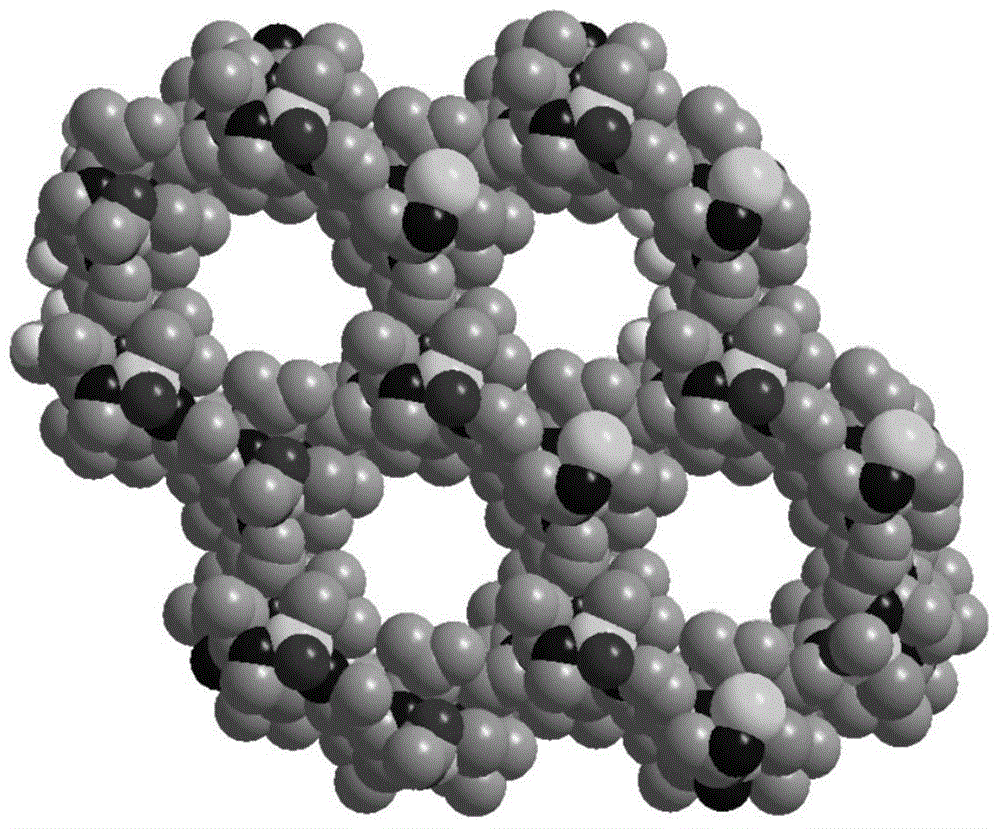
Non-interpenetrating chiral MOF stationary phase, its preparation method and application in enantiomer separation in HPLC
A stationary phase, chiral technology, applied in the fields of metal organic compounds, analytical chemistry, and material synthesis, to achieve the effects of mild conditions, simple synthesis process, and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034]Add 1.0 g of L-leucine, 0.5 g of sodium carbonate, 1.0 g of 4-formylpyridine and 5 mL of methanol to 30 mL of water in sequence, stir at room temperature for 1 h; cool in an ice bath, then slowly add 5 g of sodium borohydride aqueous solution , continue to stir for 0.5h; acidify with hydrobromic acid to pH 3, continue to stir for 1h; then, distill under reduced pressure to a solid state; extract 3 times with 10mL of methanol, filter, and rotate the filtrate to obtain the chiral ligand 1 -HL; the sodium borohydride aqueous solution, the mass fraction of sodium borohydride is 7%.
[0035] Add 0.4g of the synthesized chiral ligand l-HL and 0.25g of zinc acetate to 10mL of secondary ionized water, place it in a small beaker, and put the small beaker into a large beaker containing 50mL of methanol, seal it , diffused at room temperature for 2.5 days, filtered the crystallized mixed solution in a small beaker, and washed the filtered crystals with 7.5 mL of methanol aqueous so...
Embodiment 2
[0037] Add 1.5g of L-leucine, 1.0g of sodium carbonate, 2.0g of 4-formylpyridine and 10mL of methanol to 40mL of water in sequence, stir at room temperature for 2h; cool in an ice bath, then slowly add 10g of sodium borohydride aqueous solution , continue to stir for 1 h; acidify with hydrobromic acid to pH 4.5, continue to stir for 2 h; then, distill under reduced pressure to solid state; extract 3 times with 20 mL of methanol respectively, filter, and rotate the filtrate to obtain the chiral ligand l- HL; the sodium borohydride aqueous solution, the mass fraction of sodium borohydride is 11%.
[0038] Add 0.4g of the synthesized chiral ligand l-HL and 0.25g of zinc acetate to 10mL of secondary ionized water, place it in a small beaker, and put the small beaker into a large beaker containing 50mL of methanol, seal it , diffused at room temperature for 2.5 days, filtered the crystallized mixed solution in a small beaker, and washed the filtered crystals with 7.5 mL of methanol...
Embodiment 3
[0040] Add 3.0g of L-leucine, 1.5g of sodium carbonate, 3.0g of 4-formylpyridine and 15mL of methanol to 50mL of water in sequence, stir at room temperature for 3h; cool in an ice bath, then slowly add 15g of sodium borohydride aqueous solution , continue to stir for 1.5h; acidify with hydrobromic acid to pH 6, continue to stir for 3h; then, distill under reduced pressure to a solid state; extract 3 times with 30mL of methanol, filter, and rotate the filtrate to obtain the chiral ligand 1 -HL; the sodium borohydride aqueous solution, the mass fraction of sodium borohydride is 15%.
[0041] Add 0.4g of the synthesized chiral ligand l-HL and 0.25g of zinc acetate to 10mL of secondary ionized water, place it in a small beaker, and put the small beaker into a large beaker containing 50mL of methanol, seal it , diffused at room temperature for 2.5 days, filtered the crystallized mixed solution in a small beaker, and washed the filtered crystals with 7.5 mL of methanol aqueous solut...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The inside diameter of | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com