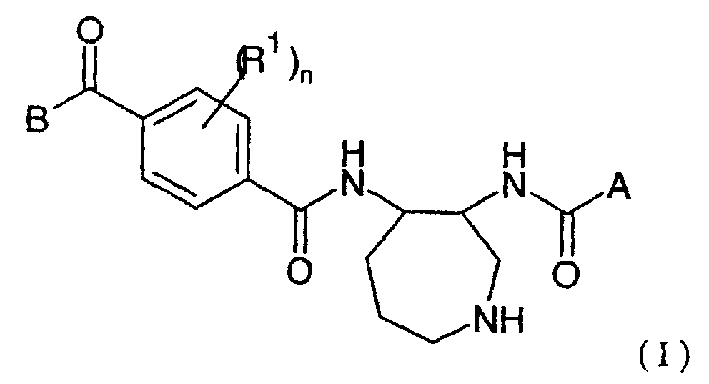
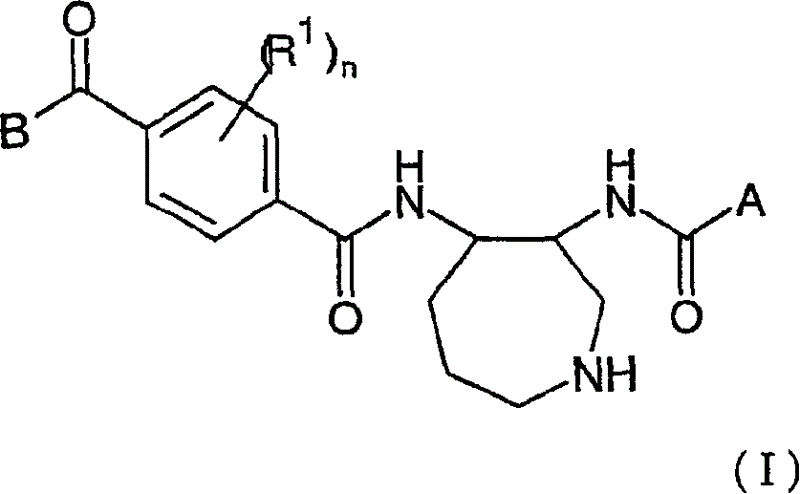
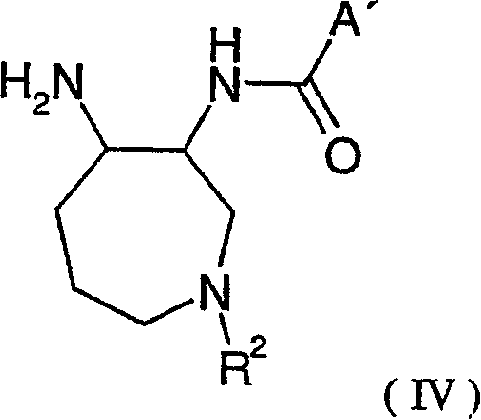
Novel azepane derivatives
A technology of heterocyclic groups and alkyl groups, applied in the field of preparing the azepane derivatives, can solve the problems of unreported small molecule inhibitors of AKT1
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1a
[0096] N-{(3R,4R)-4-[4-(2-fluoro-6-hydroxy-3-methoxy-benzoyl)-benzoylamino]azepan-3-yl}- Isonicotinamide hydrochloride (1a)
[0097] 0.05 g of 1b was dissolved in 2 ml of hydrochloric acid in dioxane (4M) at room temperature and stirred for 24 hours. The solvent was evaporated in vacuo and the residue was redissolved in methanol and evaporated to dryness three times, yielding 0.038 g (82%) of 1a as pale yellow crystals. MS (ESI): m / z (%): 507 (MH + ), 505 ([M-H] + ). Mp.200-222°C.
Embodiment 1b
[0099] (3R, 4R)-4-[4-(2-fluoro-6-methoxymethoxy-3-methoxy-benzoyl)-benzoylamino]-3-[(pyridine-4- Carbonyl)-amino]-azepane-1-carboxylate tert-butyl ester (1b)
[0100] Dissolve 0.167 g of 1c in 5 mL of CH at room temperature 2 Cl 2 middle. 0.167 g of 4-(2-fluoro-3-methoxy-6-methoxymethoxy-benzoyl)-benzoic acid (1d), 0.031 g of 4-dimethylaminopyridine, and 0.113 g of DCC were added. The reaction mixture was stirred at room temperature for 5 hours. The precipitate was filtered off and washed with CH 2 Cl 2 washing. The residue was evaporated in vacuo to yield 0.46 g of crude product. Column chromatography (SiO 2 , pentane / ethyl acetate 1:10) afforded 0.248 g (76%) of 1b as white crystals. M.p.106°C; MS (ESI): m / z (%): 651 (MH + ), 649 ([M-H] + .
Embodiment 1c
[0102] (3R,4R)-4-Amino-3-[(pyridine-4-carbonyl)-amino]-azepane-1-carboxylic acid tert-butyl ester (1c)
[0103] 5.5 g of 1e was dissolved in 135 mL of THF and 15 mL of ethanol, and 1 g of Pd / C (10%) was added. The reaction mixture was hydrogenated at 1 bar for 8 hours. After filtration, the residue was evaporated in vacuo to yield 4.6 g (90%) of 1c as a light brown powder. MS (ESI): m / z (%): 335 (MH + ), 333 ([M-H] + ).
[0104] The synthesis of 1d(4-(2-fluoro-3-methoxy-6-methoxymethoxy-benzoyl)-benzoic acid) is described in EP0663 393A1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com