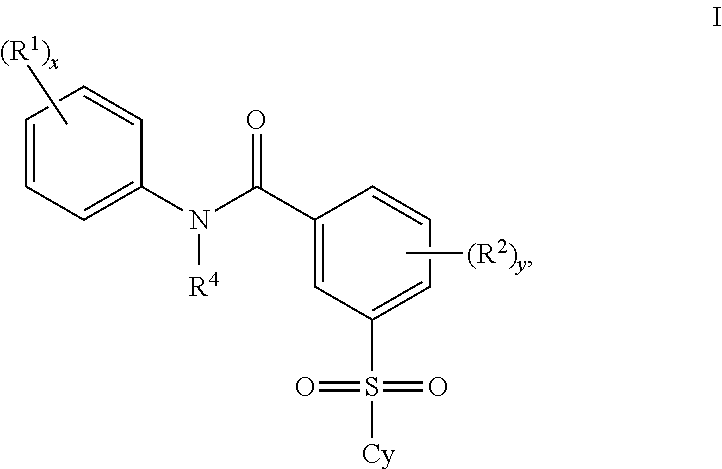
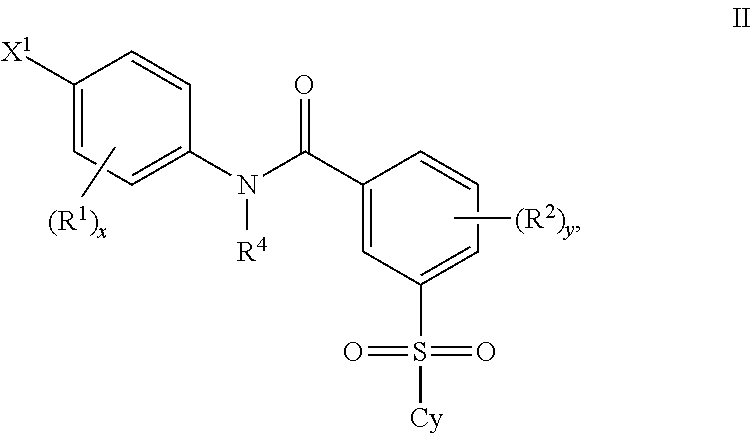
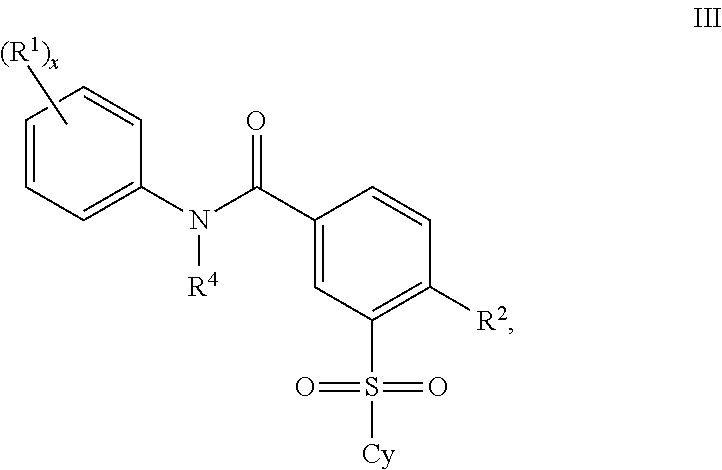
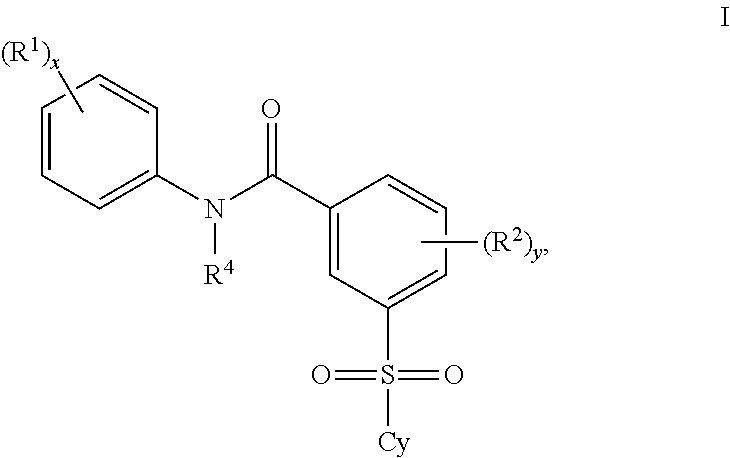
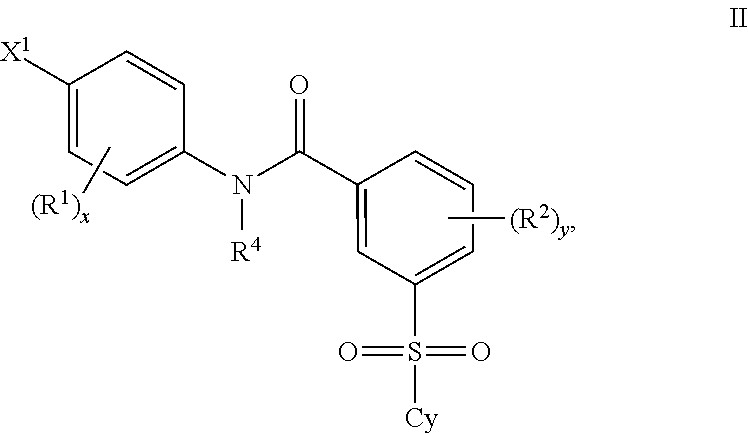
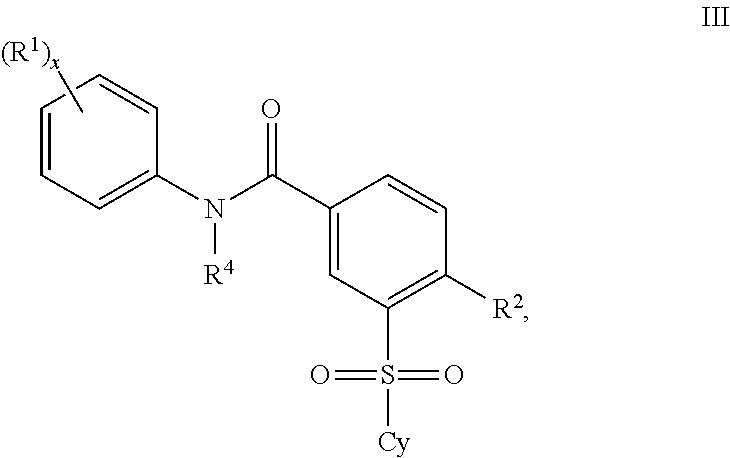
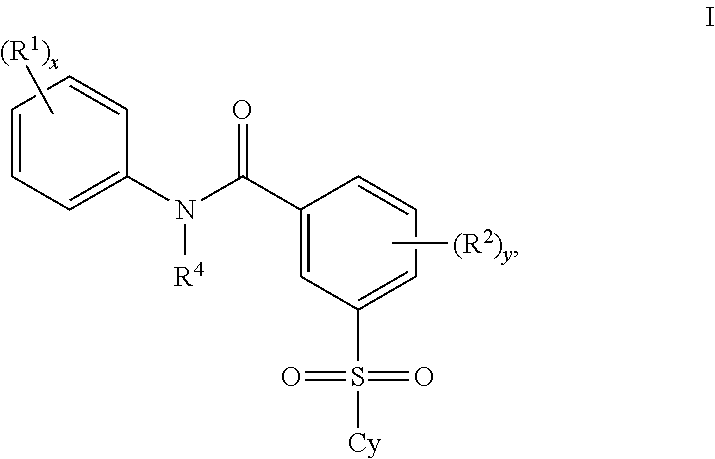
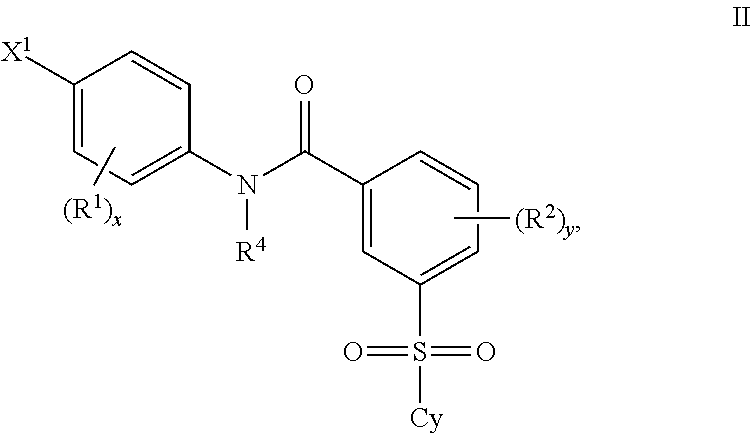
Patents
Literature
87 results about "Hepatitis B infection" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
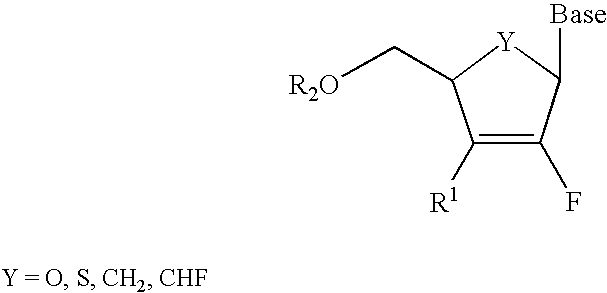
2′-fluoronucleosides
InactiveUS6911424B2Sure easyUseful in treatmentBiocideGroup 5/15 element organic compoundsPhosphoric Acid EstersPurine
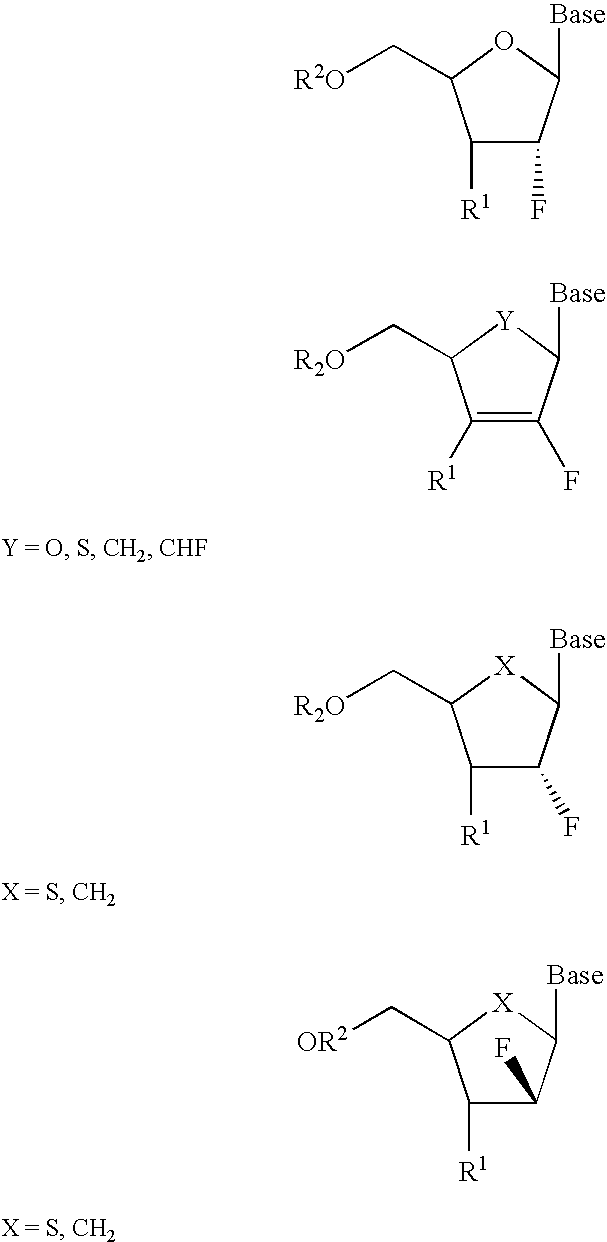
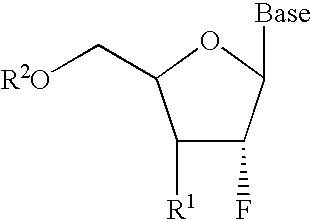
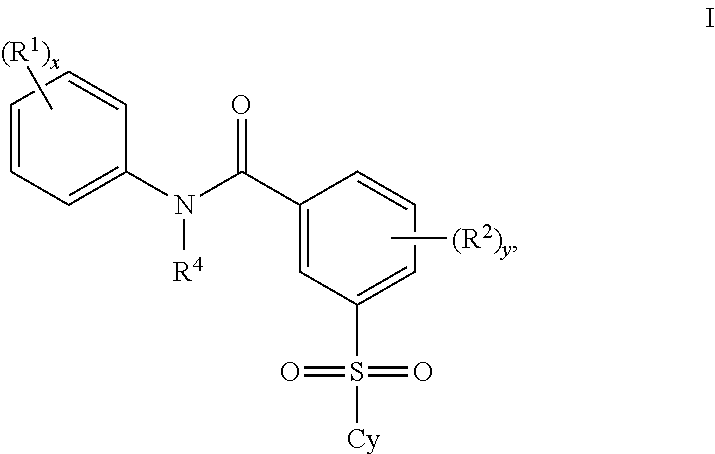
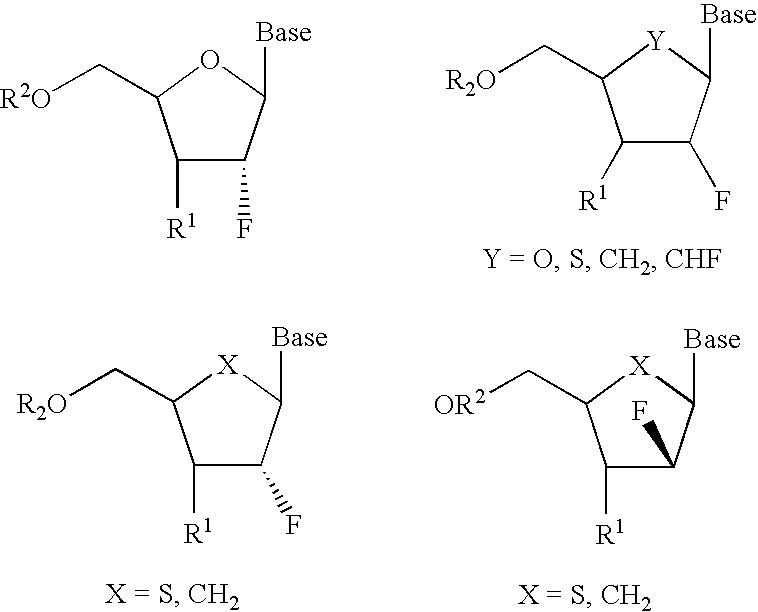
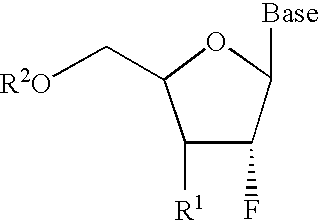
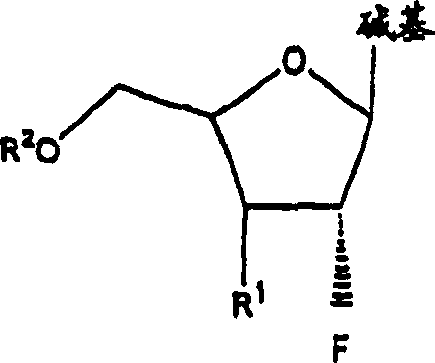
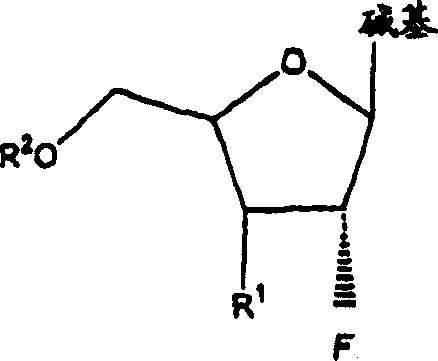
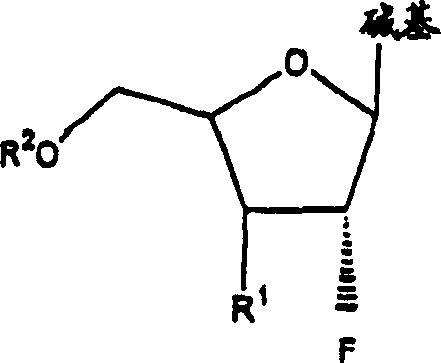
A class of 2′-fluoro-nucleoside compounds are disclosed which are useful in the treatment of hepatitis B infection, hepatitis C infection, HIV and abnormal cellular proliferation, including tumors and cancer. The compounds have the general formulae: wherein[0001]Base is a purine or pyrimidine base;[0002]R1 is OH, H, OR3, N3, CN, halogen, including F, or CF3, lower alkyl, amino, loweralkylamino, di(lower)alkylamino, or alkoxy, and base refers to a purine or pyrimidine base;[0003]R2 is H, phosphate, including monophosphate, diphosphate, triphosphate, or a stabilized phosphate prodrug; acyl, or other pharmaceutically acceptable leaving group which when administered in vivo, is capable of providing a compound wherein R2 is H or phosphate; sulfonate ester including alkyl or arylalkyl sulfonyl including methanesulfonyl, benzyl, wherein the phenyl group is optionally substituted with one or more substituents as described in the definition of aryl given above, a lipid, an amino acid, peptide, or cholesterol; and[0004]R3 is acyl, alkyl, phosphate, or other pharmaceutically acceptable leaving group which when administered in vivo, is capable of being cleaved to the parent compound, or a pharmaceutically acceptable salt thereof.
Owner:EMORY UNIVERSITY
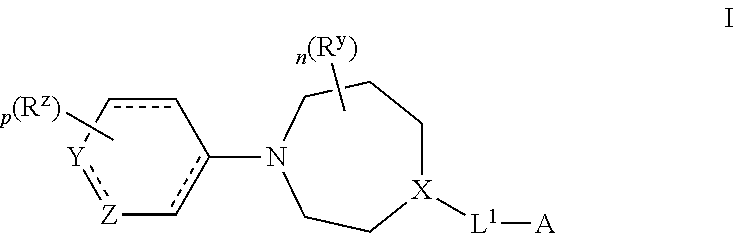
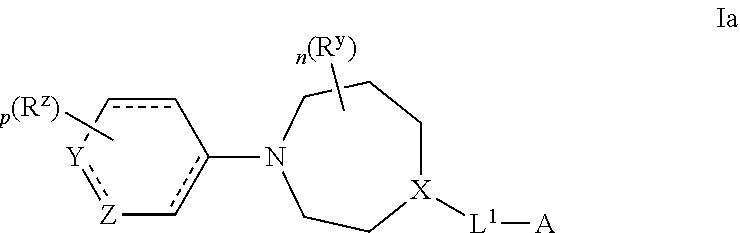
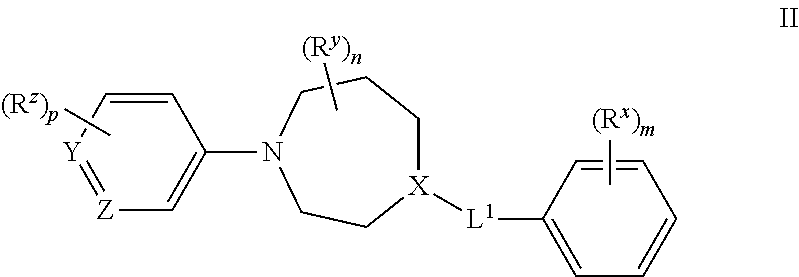
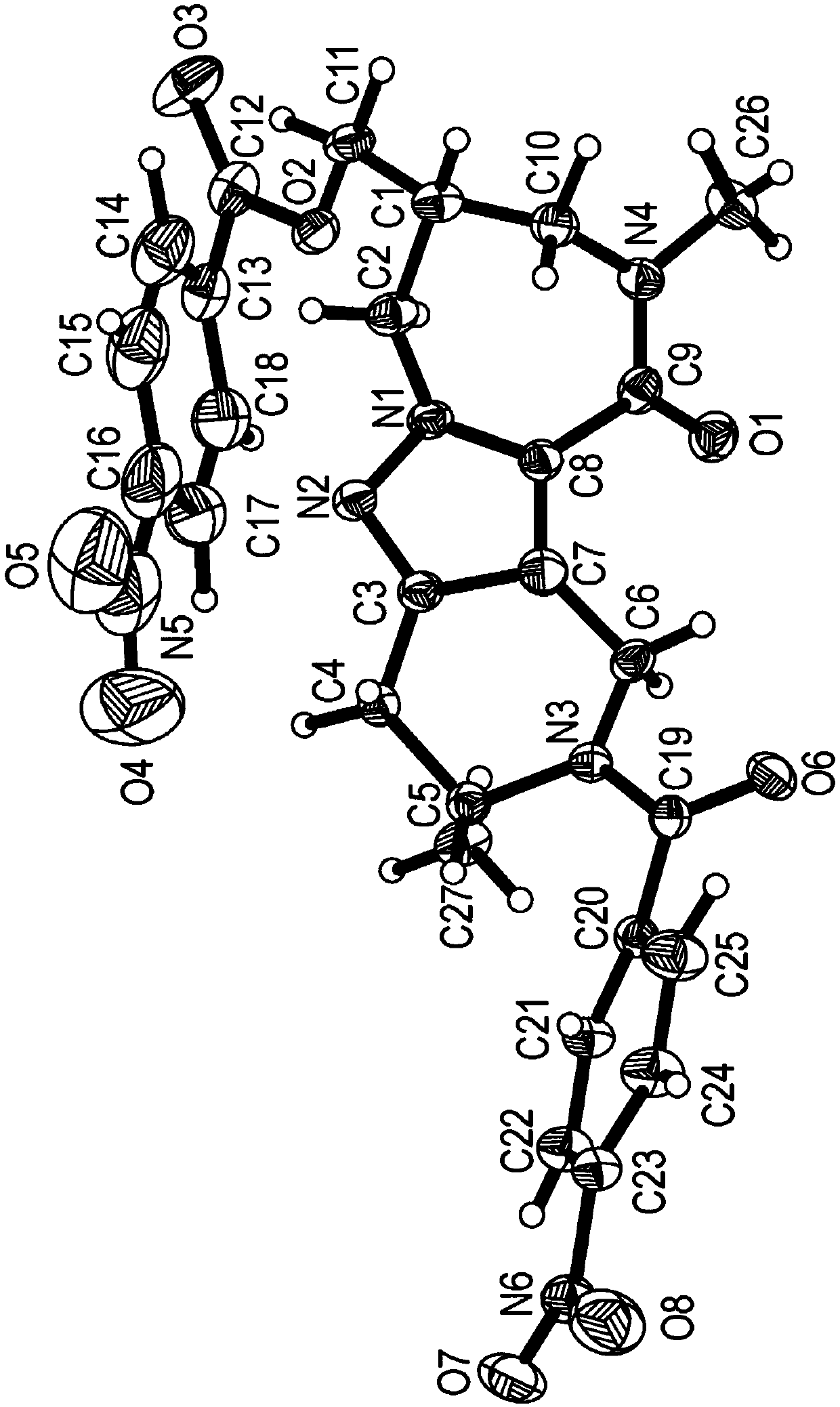
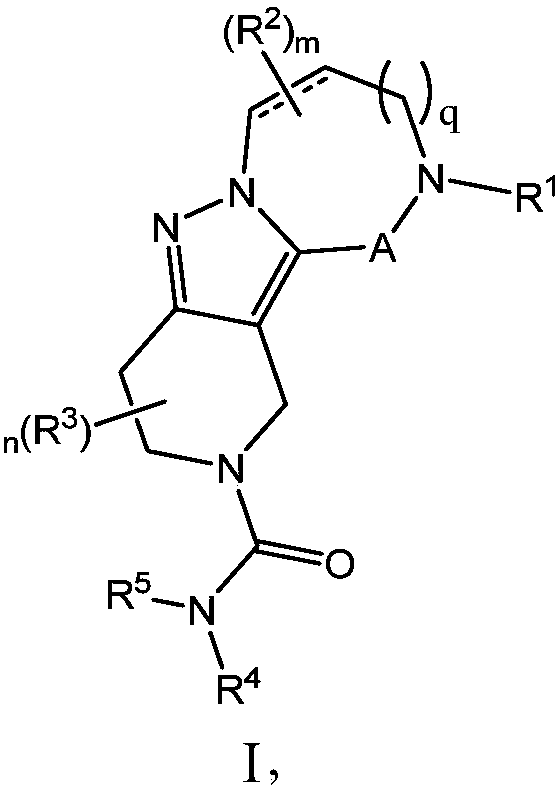
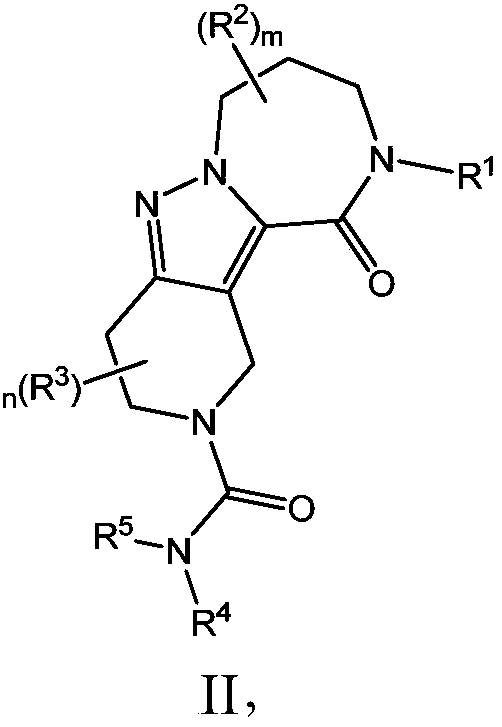
Azepane derivatives and methods of treating hepatitis B infections
ActiveUS9181288B2Reduce doseReduce frequencyPeptide/protein ingredientsGroup 5/15 element organic compoundsMedicineAzepane
Owner:NOVIRA THERAPEUTICS
2′-Fluoronucleosides
InactiveUS7307065B2Sure easyUseful in treatmentBiocidePeptide/protein ingredientsPhosphoric Acid EstersPhosphate
A class of 2′-fluoro-nucleoside compounds are disclosed which are useful in the treatment of hepatitis B infection, hepatitis C infection, HIV and abnormal cellular proliferation, including tumors and cancer. The compounds have the general formulae:whereinBase is a purine or pyrimidine base;R1 is OH, H, OR3, N3, CN, halogen, including F, or CF3, lower alkyl, amino, loweralkylamino, di(lower)alkylamino, or alkoxy, and base refers to a purine or pyrimidine base;R2 is H, phosphate, including monophosphate, diphosphate, triphosphate, or a stabilized phosphate prodrug; acyl, or other pharmaceutically acceptable leaving group which when administered in vivo, is capable of providing a compound wherein R2 is H or phosphate; sulfonate ester including alkyl or arylalkyl sulfonyl including methanesulfonyl, benzyl, wherein the phenyl group is optionally substituted with one or more substituents as described in the definition of aryl given above, a lipid, an amino acid, peptide, or cholesterol; andR3 is acyl, alkyl, phosphate, or other pharmaceutically acceptable leaving group which when administered in vivo, is capable of being cleaved to the parent compound, or a pharmaceutically acceptable salt thereof.
Owner:EMORY UNIVERSITY +1
Azepane derivatives and methods of treating hepatitis b infections
Owner:NOVIRA THERAPEUTICS
Azepane derivatives and methods of treating hepatitis B infections
ActiveUS9339510B2Reduce loadReduce morbidityPeptide/protein ingredientsGroup 5/15 element organic compoundsMedicineAzepane
Owner:NOVIRA THERAPEUTICS
Azepane derivatives and methods of treating hepatitis B infections
ActiveUS9169212B2Reduce doseReduce frequencyOrganic chemistryPeptide/protein ingredientsMedicineAzepane
Owner:NOVIRA THERAPEUTICS
Azepane derivatives and methods of treating hepatitis b infections
ActiveUS20160000812A1Reduce loadReduce morbidityPeptide/protein ingredientsGroup 5/15 element organic compoundsPharmaceutical drugAzepane
Owner:NOVIRA THERAPEUTICS
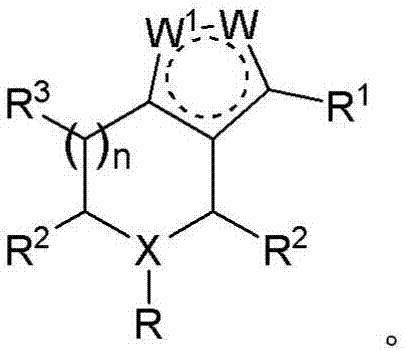
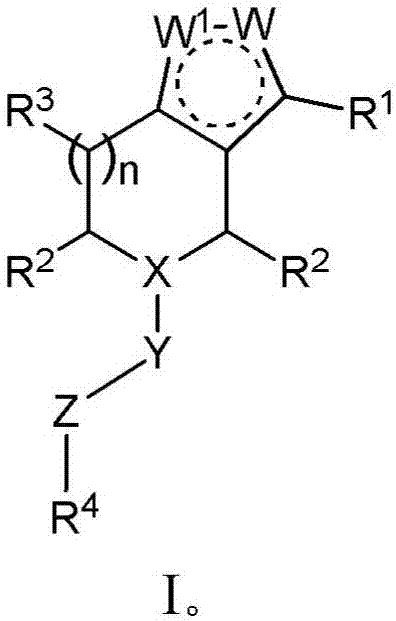
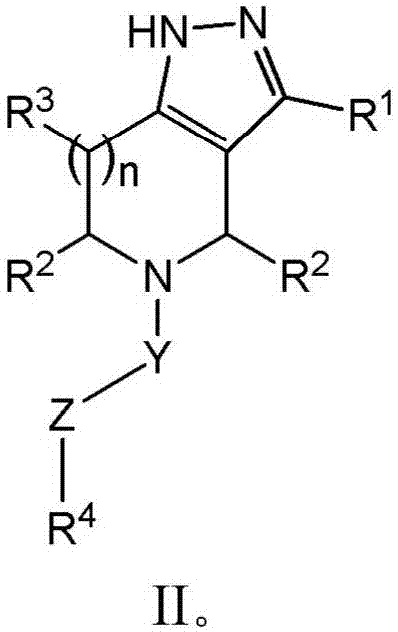
Piperidine derivatives and methods of treating hepatitis B infections
ActiveUS9400280B2Reduce loadReduce incidenceOrganic chemistryHeterocyclic compound active ingredientsMedicineHepatitis B
Owner:NOVIRA THERAPEUTICS
Azepane derivatives and methods of treating hepatitis b infections
ActiveUS20170015629A1Reduce loadReduce morbidityOrganic chemistryPeptide/protein ingredientsMedicineAzepane
Owner:NOVIRA THERAPEUTICS
Surface antibody testing fine particles for hepatitis B virus, and preparation and application thereof
InactiveCN101769929AReduce sensitivityHigh sensitivityChemiluminescene/bioluminescenceBiological testingChronic hepatitisMicroparticle
The invention relates to hepatitis B diagnosis reagent, which discloses surface antibody testing fine particles for hepatitis B, which are luminous fine particles for coating surface antibody of hepatitis B. The invention also discloses a preparation and an application for the surface antibody testing fine particles for hepatitis B. In addition, the invention further discloses an in vitro diagnosis kit for measuring the surface antibody of hepatitis B and simultaneously discloses a method for using the kit. The kit can be used jointly with other blood serum and clinical information to diagnose the condition of acute or chronic hepatitis B infection of an individual, and can also be used for sieving hepatitis B of females in the perinatal period, so as to judge the danger of hepatitis B infection of newly born babies.
Owner:BEYOND DIAGNOSTICS (SHANGHAI) CO LTD
Inhibitors of hepatitis b virus convalently closed circular DNA formation and their method of use
Pharmaceutical compositions of the invention comprise covalently closed circular DNA formation inhibitors having a disease-modifying action in the treatment of diseases associated with the formation of covalently closed circular DNA that include hepatitis B infection, and any disease involving formation of covalently closed circular DNA.
Owner:PHILADELPHIA HEALTH & EDUCATION CORP +2
Hepatitis B virus surface antigen detection particles, preparation thereof and use thereof
InactiveCN101666801AReduce sensitivityHigh sensitivityChemiluminescene/bioluminescenceBiological testingSerum igeAntigen testing
The invention relates to a reagent for the diagnosis of hepatitis B and discloses hepatitis B surface antigen detection particles, which are luminous particles coated by a hepatitis B surface antigen.The invention also discloses the preparation and use of the hepatitis B surface antigen detection particles. In addition, the invention also discloses an in-vitro diagnostic kit for determining the hepatitis B surface antigen in a sample and also discloses the using method of the kit. The kit of the invention can be used in combination with other serums and clinic information to diagnose the acute or chronic hepatitis B infection condition of an individual and can also be used for screening hepatitis B in perinatal females to judge the hepatitis B infection risk of the newborns.
Owner:BEYOND DIAGNOSTICS (SHANGHAI) CO LTD
Hepatitis B microRNA molecular marker composition and application thereof
InactiveCN104232636AEasy to operateStrong specificityMicrobiological testing/measurementDNA/RNA fragmentationInfection diagnosisMicroRNA
The invention provides a hepatitis B microRNA molecular marker composition and application thereof in preparing a hepatitis B infection diagnosis and / or prognosis evaluation kit. The hepatitis B microRNA molecular marker composition comprises more than one microRNA molecule as shown in SEQ NO. 1-18. The invention also provides a diagnostic kit for instructing the infection diagnosis and / or prognosis evaluation of hepatitis B. The hepatitis B microRNA molecular marker and the diagnostic kit which are provided by the invention have the characteristics of easiness for operation, safety, no injury, high specificity, high sensitivity and easiness for large-scale screening in instructing the infection diagnosis and / or prognosis evaluation of a hepatitis B patient.
Owner:北京旷博生物技术股份有限公司
Anti-hepatitis B virus X protein peptide medicament
InactiveCN103992388AObvious pharmacodynamic effectInhibit biological activityFungiBacteriaFunctional activityMolecular level
The invention relates to the field of polypeptide medicament, and particularly relates to an anti-hepatitis B virus X protein peptide and polypeptide coding the peptide, and application thereof. Specifically, the invention relates to a polypeptide with functional activity to inhibit hepatitis B virus X protein (HBx) at the molecular level, cellular level and animal level, and therefore can inhibit hepatitis caused by hepatitis B virus infection of, cirrhosis caused by repeated attack of hepatitis, and the liver cancer occurred on the basis of cirrhosis. The polypeptide and peptide analogs thereof including their functional fragments and functional variants, and the genes encoding these peptides, peptide analogs or their functional fragments and functional variants can be widely used for the prevention and control of hepatopathy after hepatitis B infection including hepatitis, cirrhosis and liver cancer.
Owner:TIANJIN TOPTECH BIO SCI & TECH
Vaccine composition useful for HPV and hepatitis B infections and a method for preparing the same
InactiveCN102112152AAntibody mimetics/scaffoldsViral antigen ingredientsHPV AntigenHepatitis B virus
The invention describes a vaccine compositions comprising chimeric fusions of the HPV antigens with viral or bacterial proteins conferring enhanced immunogenicity useful for Hepatitis B virus as well as human papillomavirus (HPV) infections.
Owner:BHARAT BIOTECH INTERNATIONAL
Viral immunotherapy drug compound and purpose thereof
InactiveCN104338132AEnhance immune responseAvoid unresponsiveness andOrganic active ingredientsPeptide/protein ingredientsDiseaseAntiendomysial antibodies
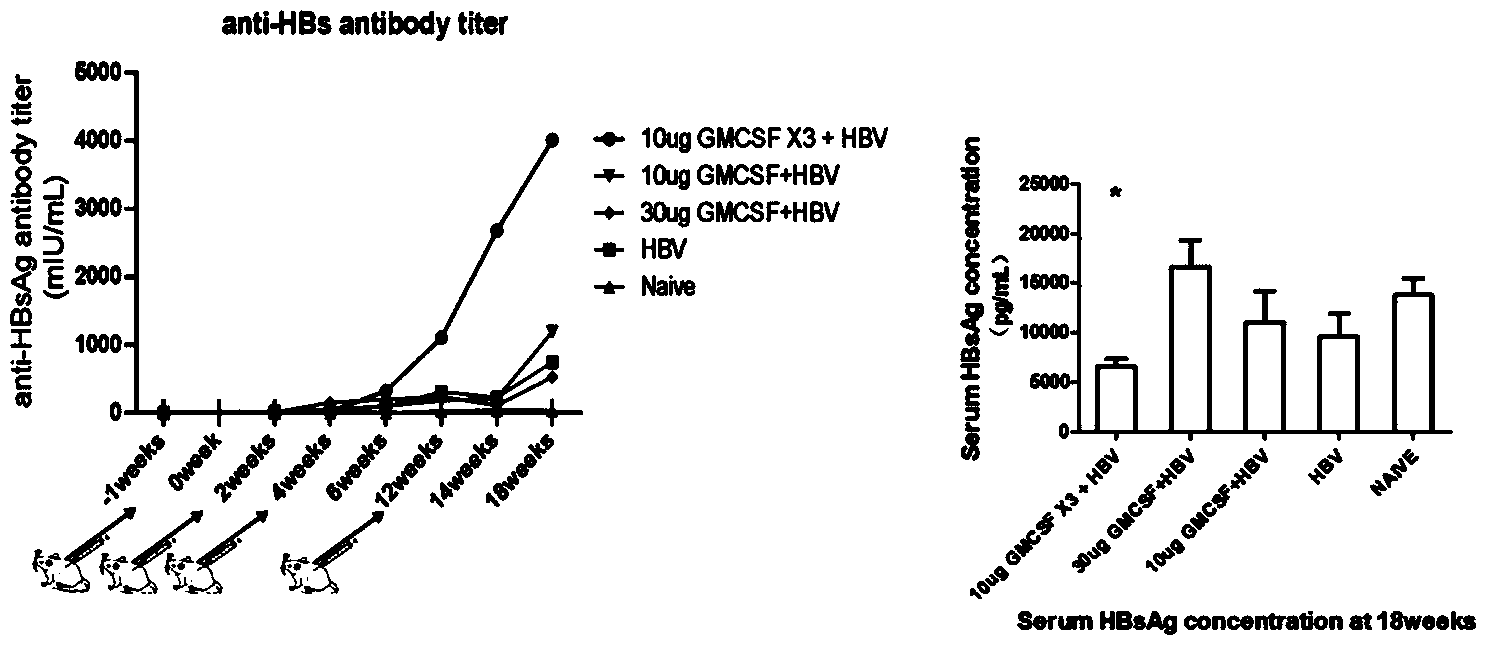
The present invention involves a new viral immunotherapy drug complex, and in particular, a viral immunotherapy drug complex for persistent hepatitis B infection. The present drug consists of antiviral drugs, immunoregulating drugs and a recombinant hepatitis B vaccine, for use in treating hepatitis B and in particular chronic hepatitis B infections. Antiviral drugs of the described drug complex are selected among α-IFN and nucleosides; the immunoregulating drugs are selected from among GM-CSF and similar.
Owner:FUDAN UNIV
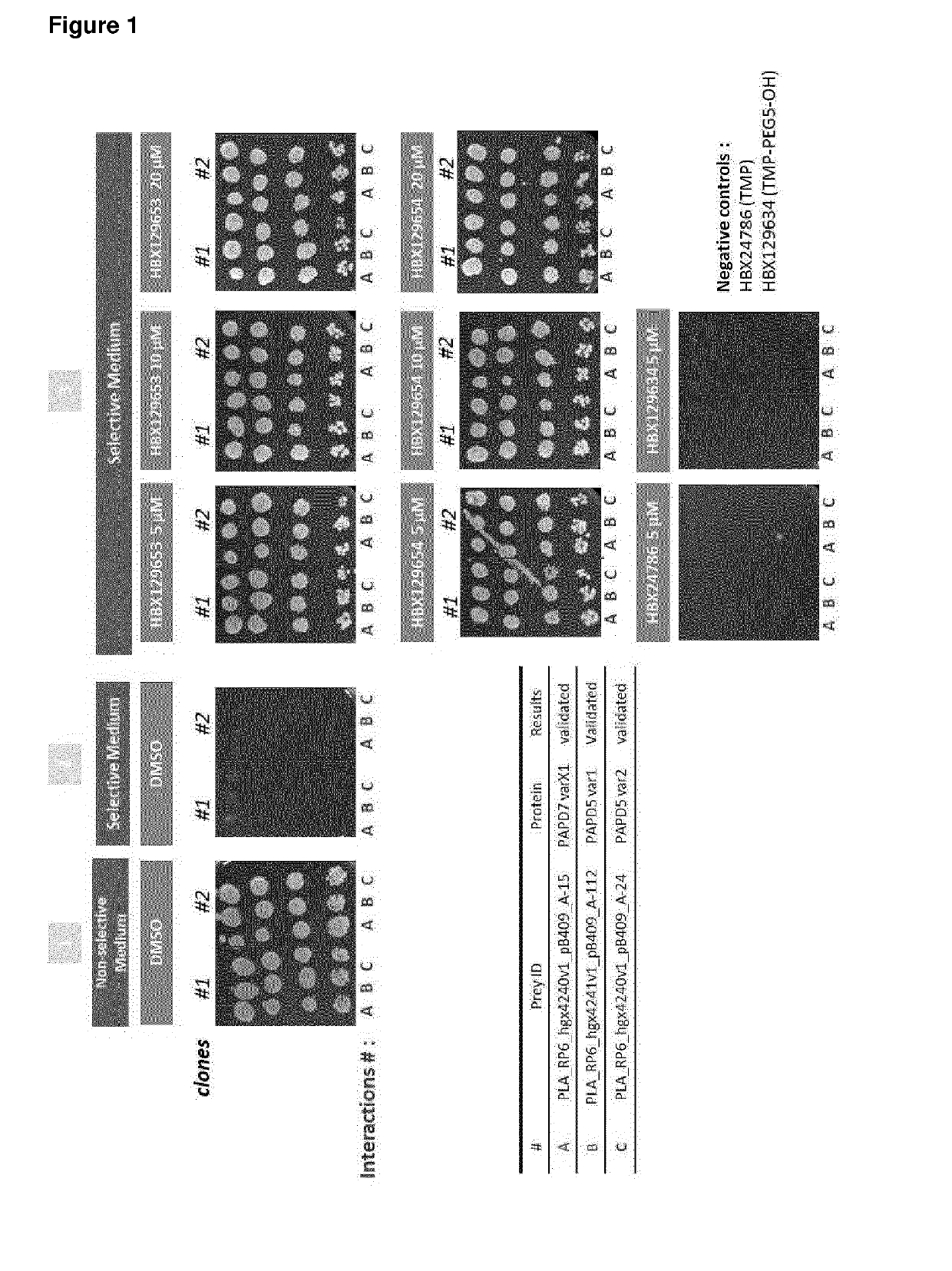
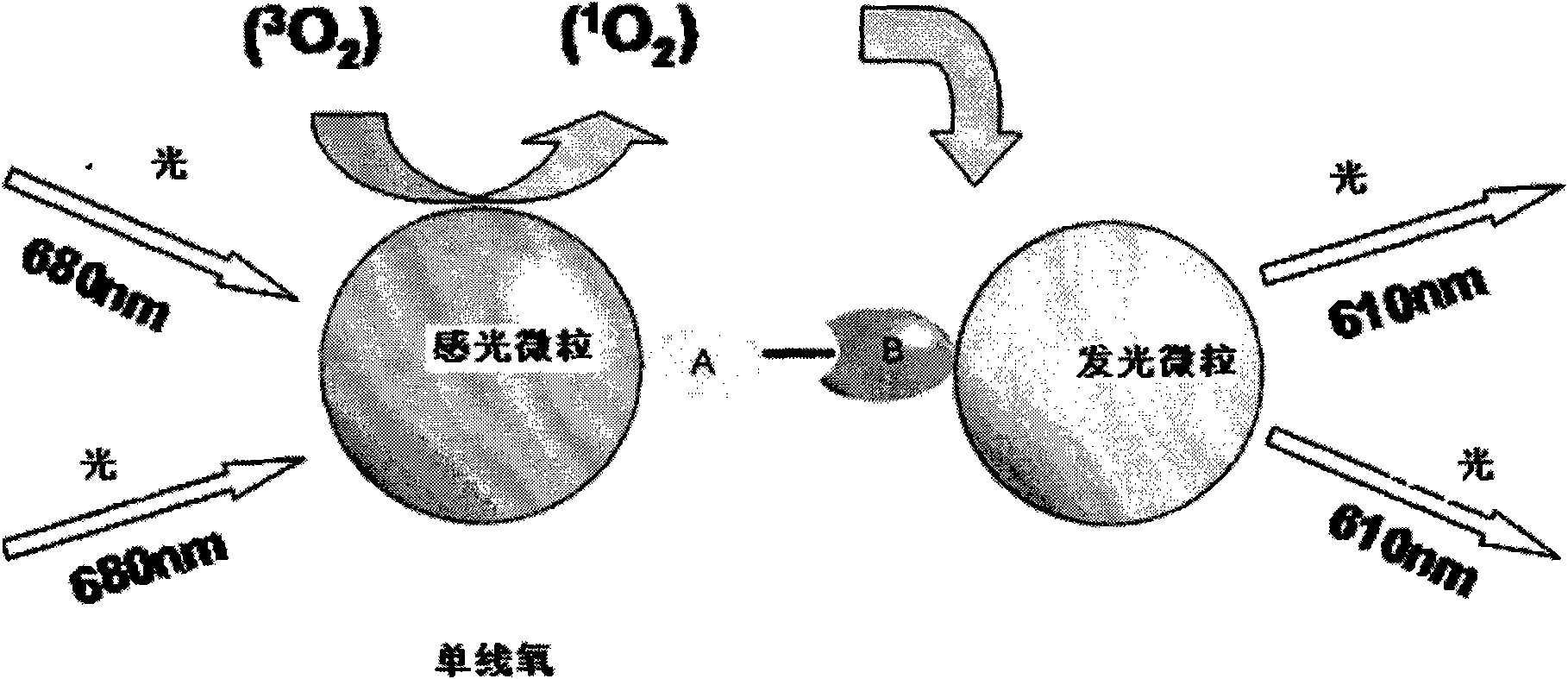
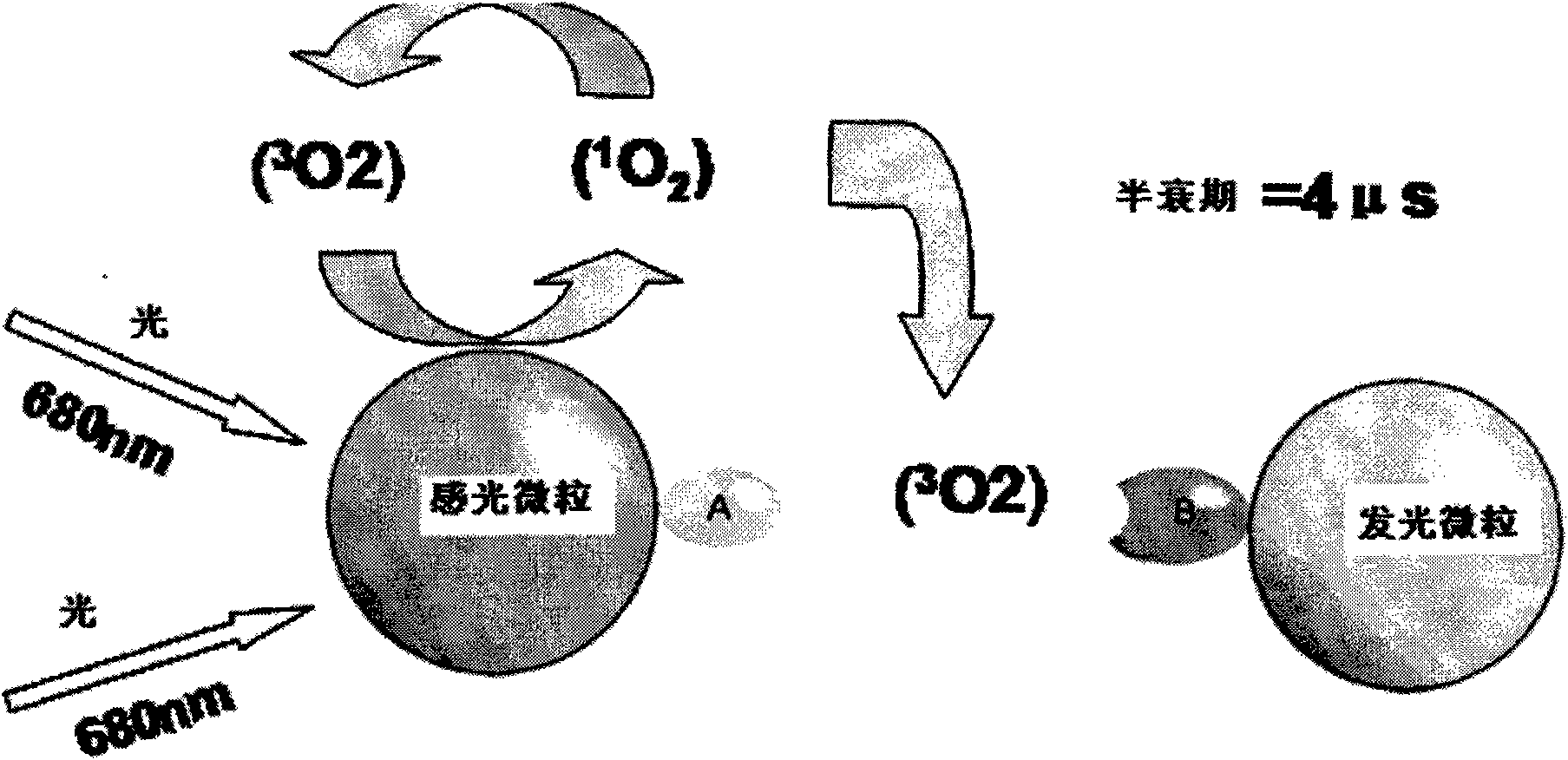
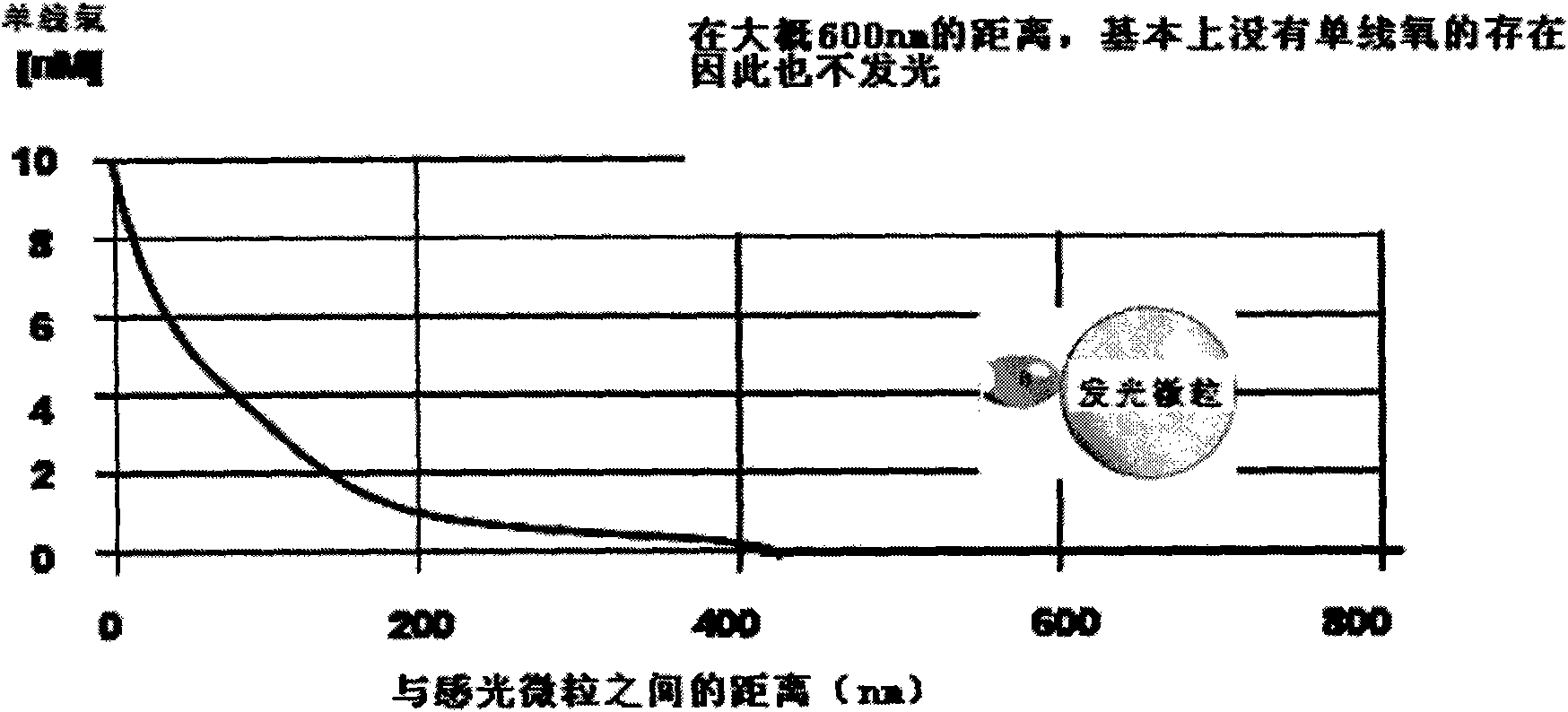
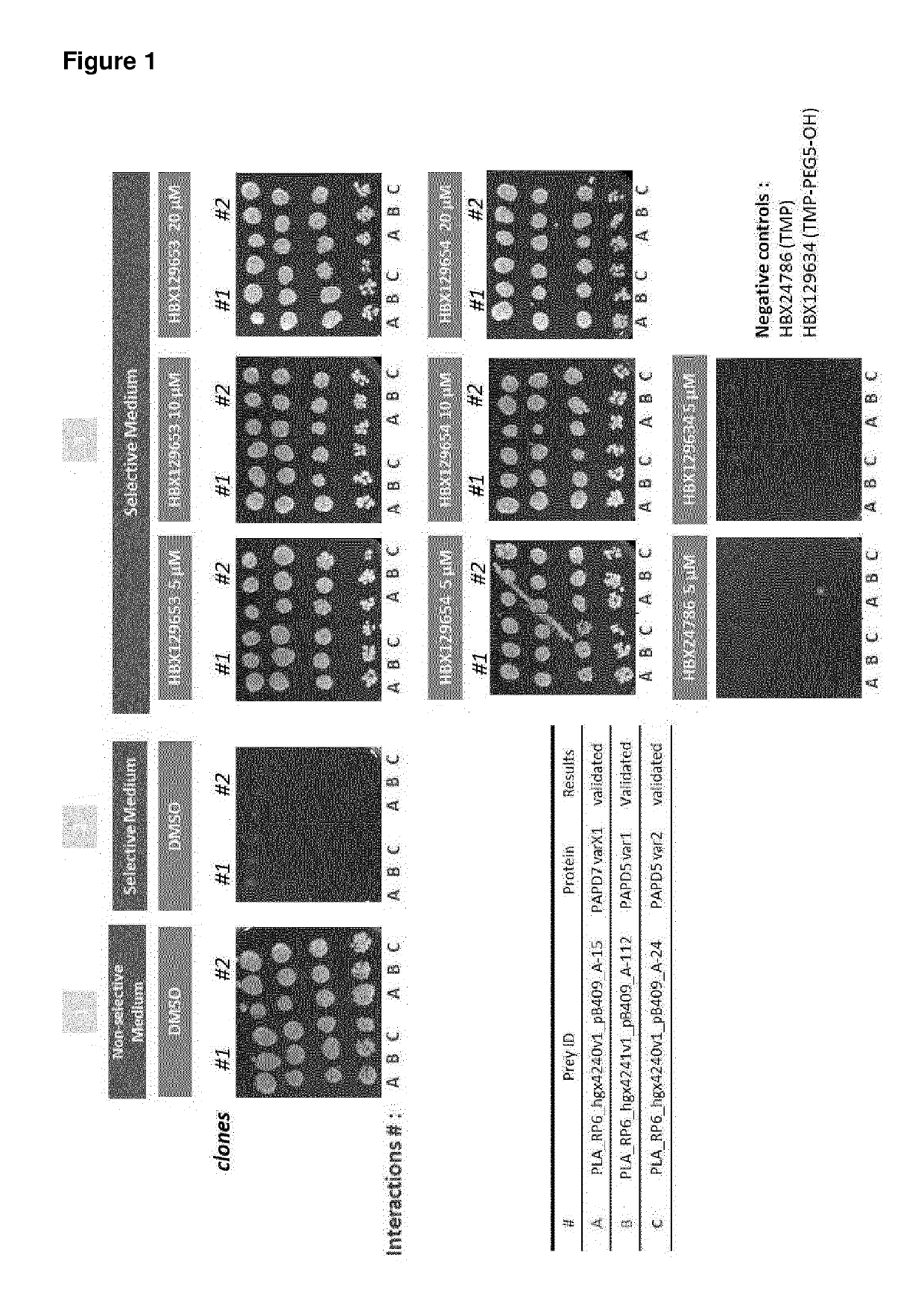
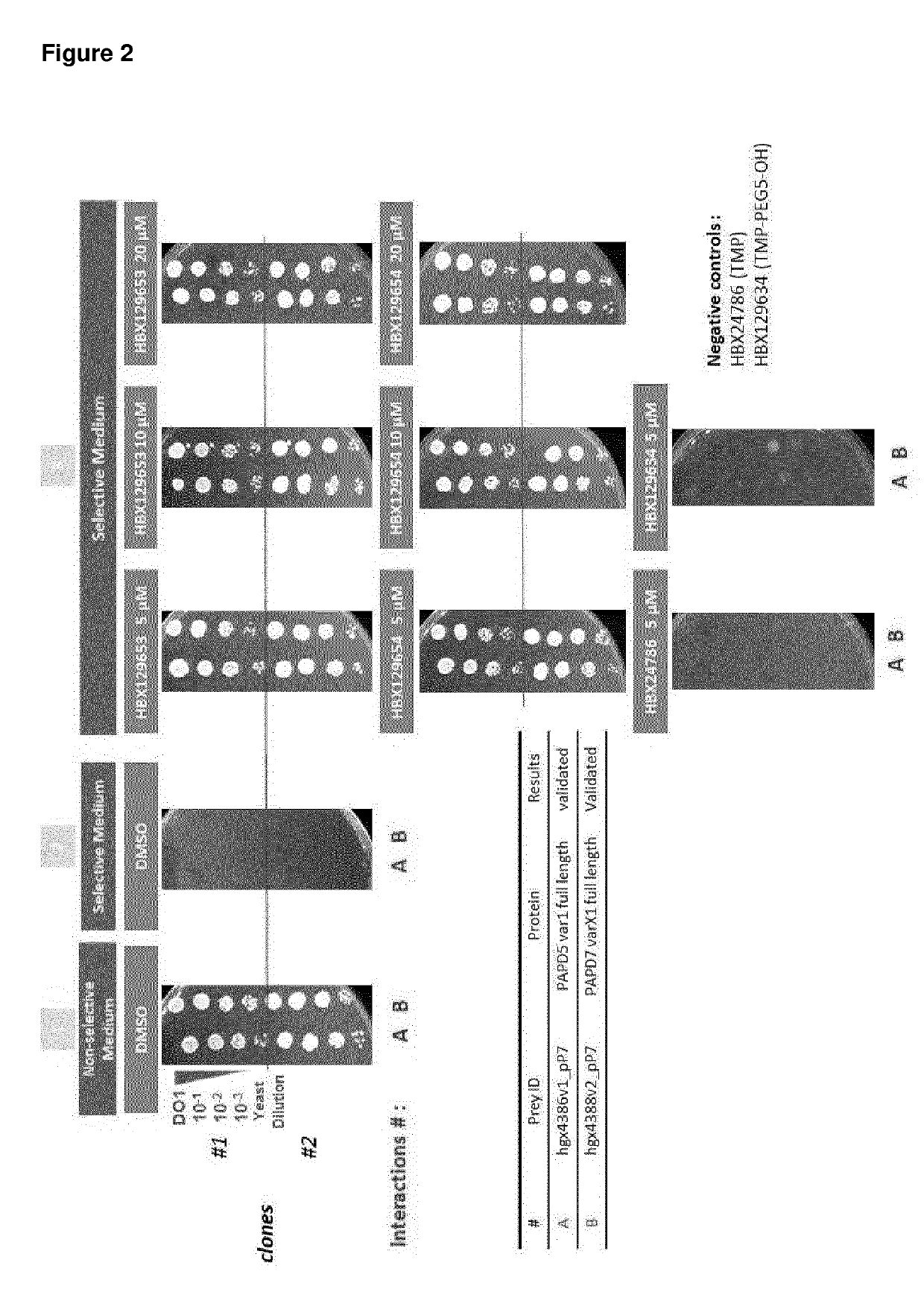
Nucleic acid molecules for reduction of papd5 or papd7 mRNA for treating hepatitis b infection
ActiveUS20190216846A1Reduces expression and activityAvoid infectionOrganic active ingredientsMicrobiological testing/measurementTreatment successHepatitis B virus
The present invention relates to a method for identifying a compound that prevents, ameliorates and / or inhibits a hepatitis B virus (HBV) infection, wherein a compound that reduces the expression and / or activity of PAP associated domain containing 5 (PAPD5) and / or PAP associated domain containing 7 (PAPD7) is identified as a compound that prevents, ameliorates and / or inhibits a HBV infection. The invention also provides for inhibitors of PAPD5 or PAPD7 for use in treating and / or preventing a HBV infection; as well as a combined preparation comprising an inhibitor of PAPD5 and an inhibitor of PAPD7 for simultaneous or sequential use in the treatment or prevention of a HBV infection. Also comprised in the present invention is a pharmaceutical composition for use in the treatment and / or prevention of a HBV infection, and a method for monitoring the therapeutic success during the treatment of a HBV infection.
Owner:F HOFFMANN LA ROCHE & CO AG
Core antibody testing fine particles for hepatitis b virus, and preparation and application thereof
ActiveCN101769928AReduce sensitivityHigh sensitivityChemiluminescene/bioluminescenceBiological testingChronic hepatitisMicroparticle
The invention relates to hepatitis B diagnosis reagent, which discloses core antibody testing fine particles for hepatitis B, which are luminous fine particles for coating core antibody of hepatitis B. The invention also discloses a preparation and an application for the core antibody testing fine particles for hepatitis B. In addition, the invention further discloses an in vitro diagnosis kit for measuring the core antibody of hepatitis B and simultaneously discloses a method for using the kit. The kit can be used jointly with other blood serum and clinical information to diagnose the condition of acute or chronic hepatitis B infection of an individual, and can also be used for sieving hepatitis B of females in the perinatal period, so as to judge the danger of hepatitis B infection of newly born babies.
Owner:BEYOND DIAGNOSTICS (SHANGHAI) CO LTD
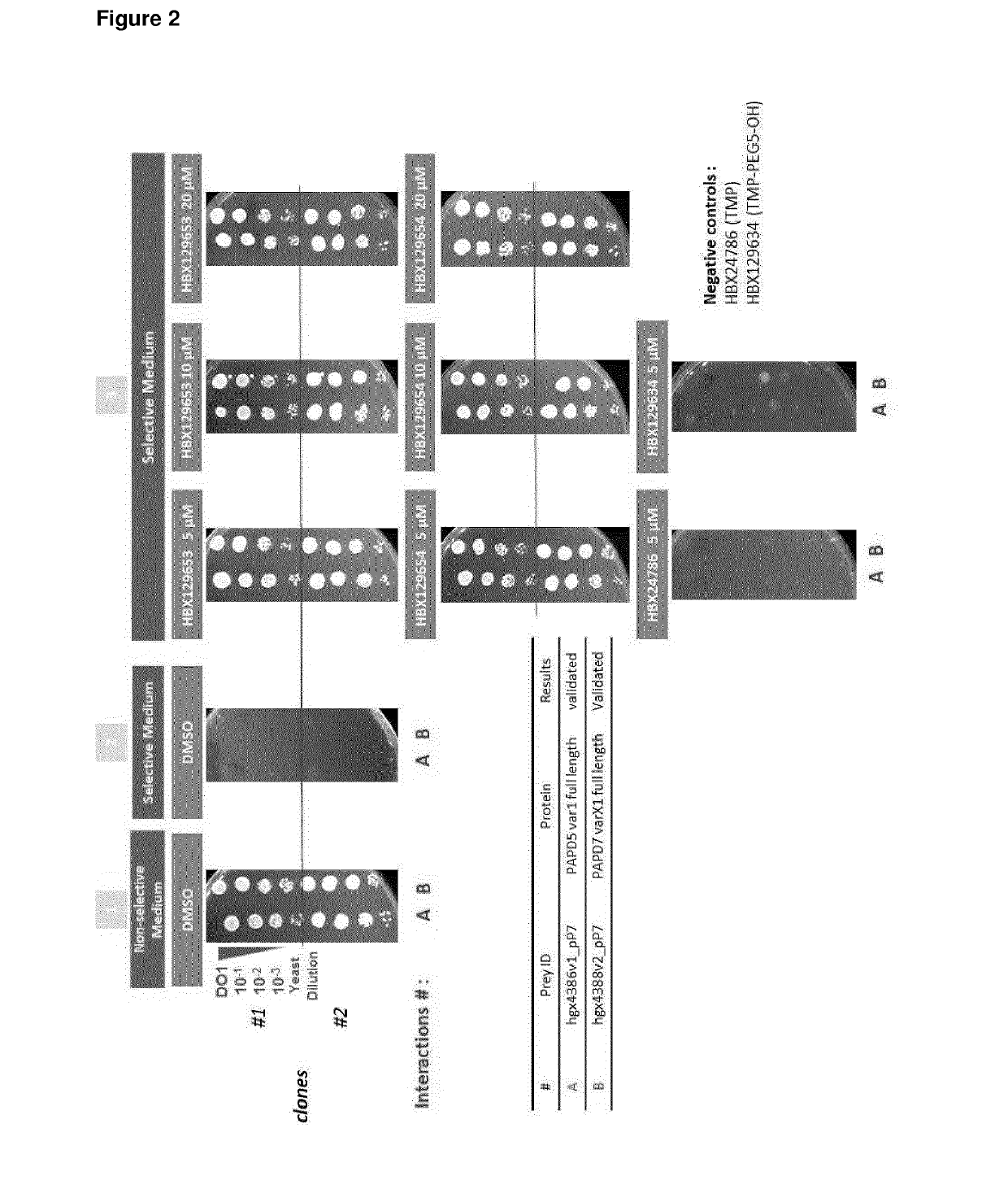
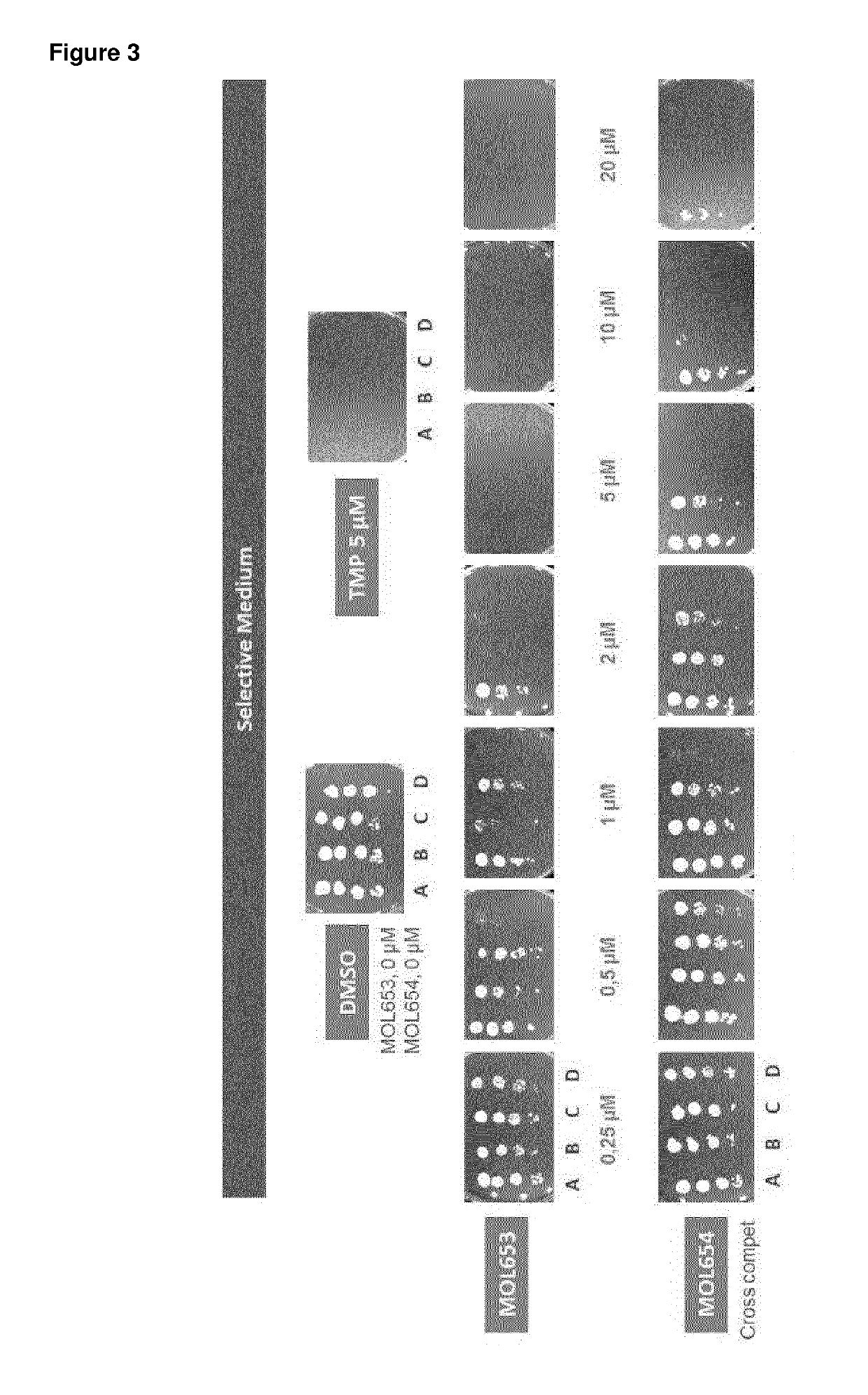
Papd5 and papd7 inhibitors for treating a hepatitis b infection
ActiveUS20190194768A1Reduced activityReduce expressionOrganic active ingredientsMicrobiological testing/measurementHepatitis B virusHepacivirus
The present invention relates to a method for identifying a compound that prevents, ameliorates and / or inhibits a hepatitis B virus (HBV) infection, wherein a compound that (i) reduces the expression and / or activity of PAP associated domain containing 5 (PAPD5) and / or PAP associated domain containing 7 (PAPD7); and / or (ii) binds to PAPD5 and / or PAPD7 and inhibits 5 propagation of HBV; is identified as a compound that prevents, ameliorates and / or inhibits a HBV infection. The invention also provides for an inhibitor of PAPD5 and / or PAPD7 for use in treating and / or preventing a HBV infection; as well as a combined preparation comprising an inhibitor of PAPD5 and an inhibitor of PAPD7 for simultaneous or sequential use in the treatment or prevention of a HBV infection. Also comprised in the present invention is a 10 pharmaceutical composition for use in the treatment and / or prevention of a HBV infection, and a method for monitoring the therapeutic success during the treatment of a HBV infection.
Owner:F HOFFMANN LA ROCHE INC
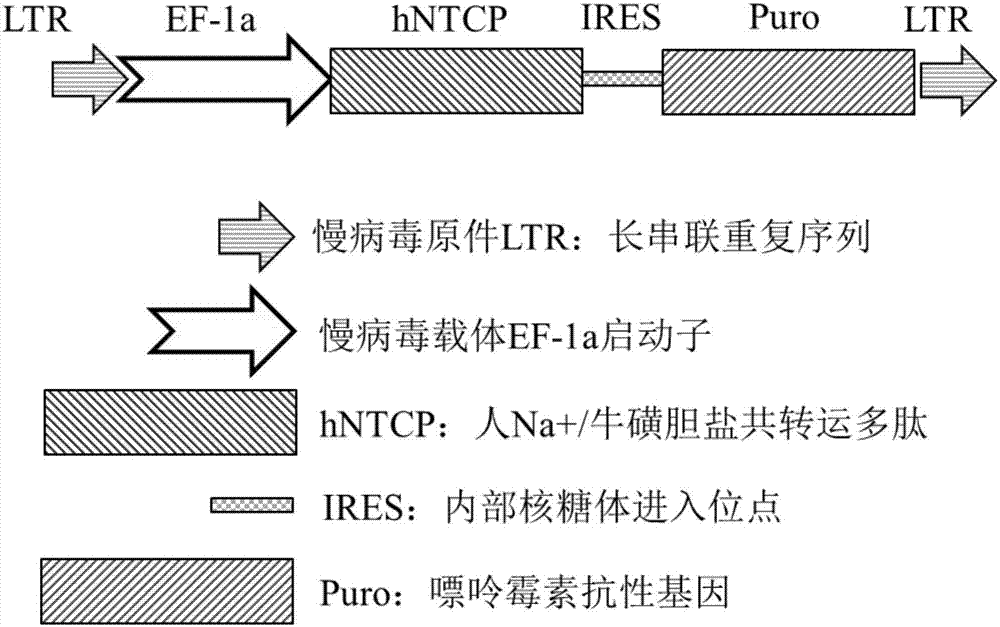
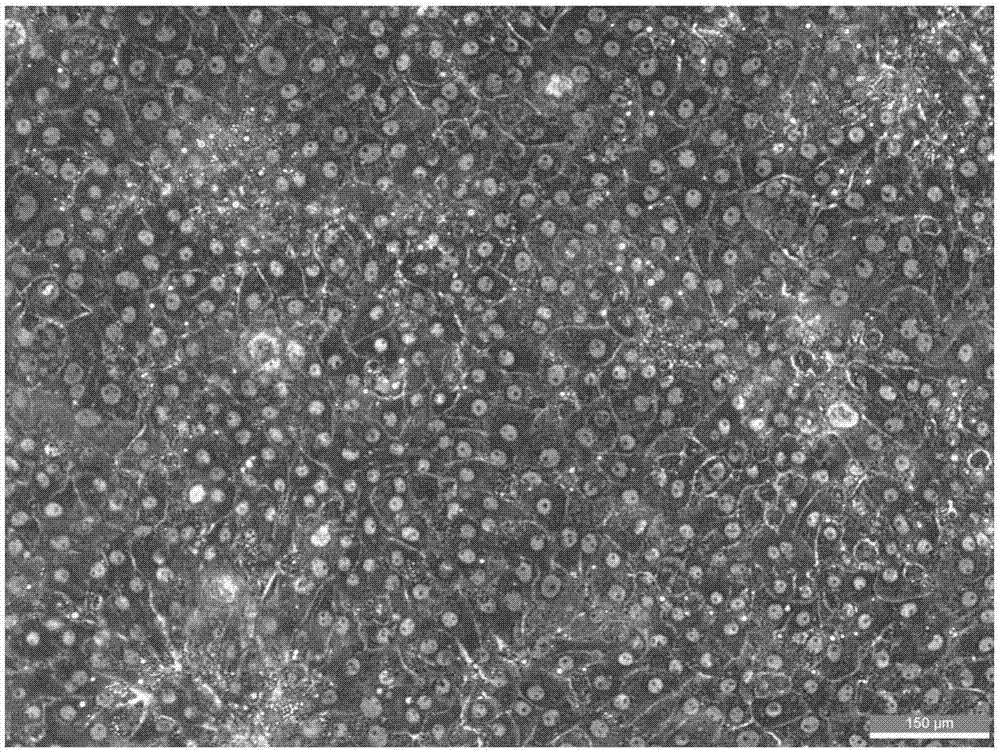
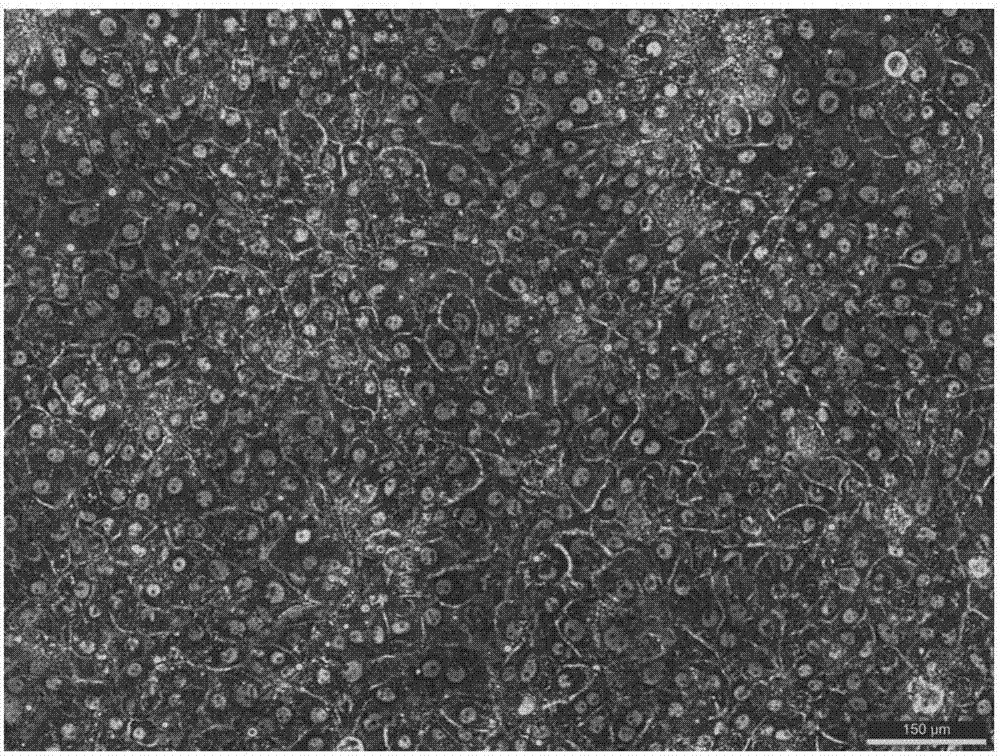
Method of establishing hepatitis B virus infection cell model by using porcine primary hepatocyte and hNTCP recombinant lentivirus
ActiveCN107988260AIncreased susceptibilityUnrestricted sourceCell dissociation methodsArtificial cell constructsLiver tissueSingle cell suspension
The invention discloses a method of establishing a hepatitis B virus infection cell model by using porcine primary hepatocyte and hNTCP recombinant lentivirus and belongs to the technical field of cell modification. The method comprises the following steps: S1, preparing an hNTCP recombinant lentivirus concentrated liquid; S2, preparing liver tissues which are fully digested; S3, preparing the porcine primary hepatocyte; S4, preparing a porcine primary hepatocyte single cell suspension; S5, preparing a cell suspension for paving a plate; S6, paving cell plate and culturing; S7, carrying out hNTCP recombinant lentivirus infection on the porcine primary hepatocyte; and S8, establishing the hepatitis B virus infection cell model. According to the invention, the recombinant lentivirus containing an hNTCP gene is constructed in vitro, the hNTCP gene is integrated into the primary hepatocyte genome through the lentivirus with high efficiency of infection to achieve stable overexpression of hNTCP, so that the primary hepatocyte supports hepatitis B virus infection.
Owner:立沃生物科技(深圳)有限公司
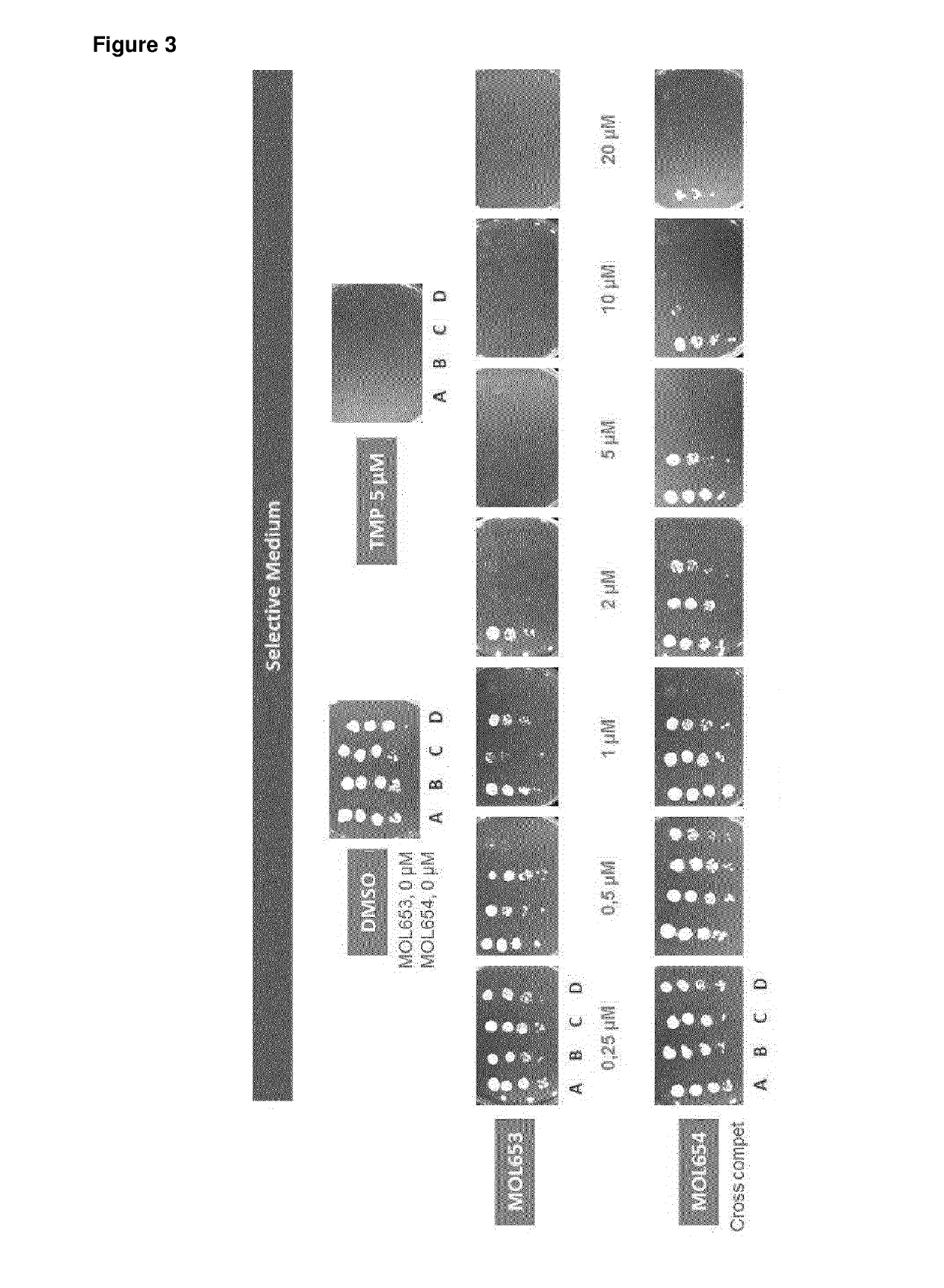
NUCLEIC ACID MOLECULE FOR REDUCTION OF PAPD5 AND PAPD7 mRNA FOR TREATING HEPATITIS B INFECTION
ActiveUS20200147123A1Inhibit expressionSimplicity of delivery to the target cellOrganic active ingredientsSugar derivativesPharmaceutical drugHepatitis B infection
The present invention relates to nucleic acid molecules that are complementary to both PAP associated domain containing 5 (PAPD5) and PAP associated domain containing 7 (PAPD7), leading to inhibition of the expression of both PAPD5 and PAPD7 when using a single nucleic acid molecule. The invention also provides for PAPD5 and PAPD7 specific nucleic acid molecules for use in treating and / or preventing a HBV infection, in particular a chronic HBV infection. Also comprised in the present invention is a pharmaceutical composition for use in the treatment and / or prevention of a HBV infection.
Owner:F HOFFMANN LA ROCHE INC
Derivatives and methods of treating hepatitis B infections
Owner:NOVIRA THERAPEUTICS
Hbv treatment
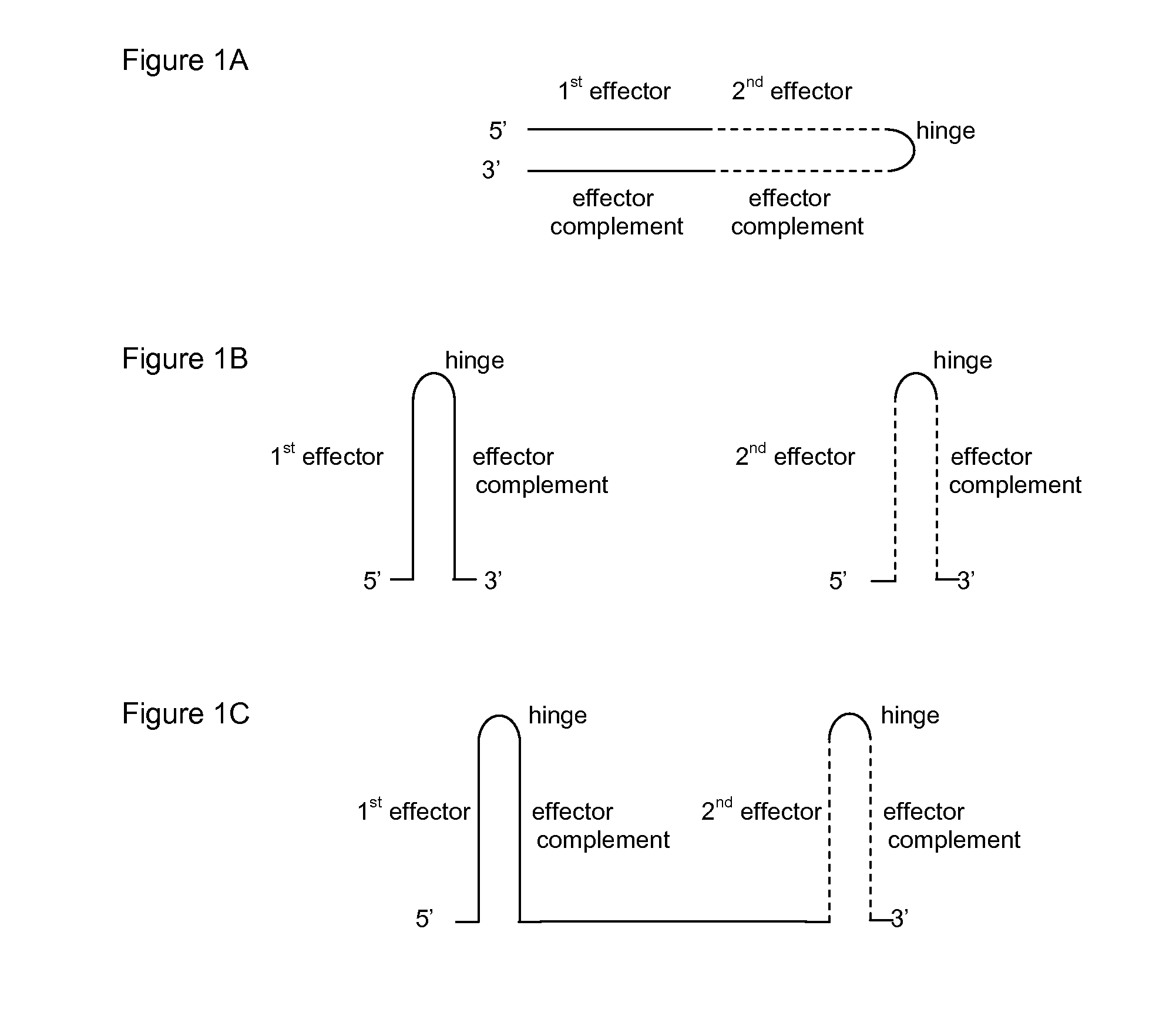
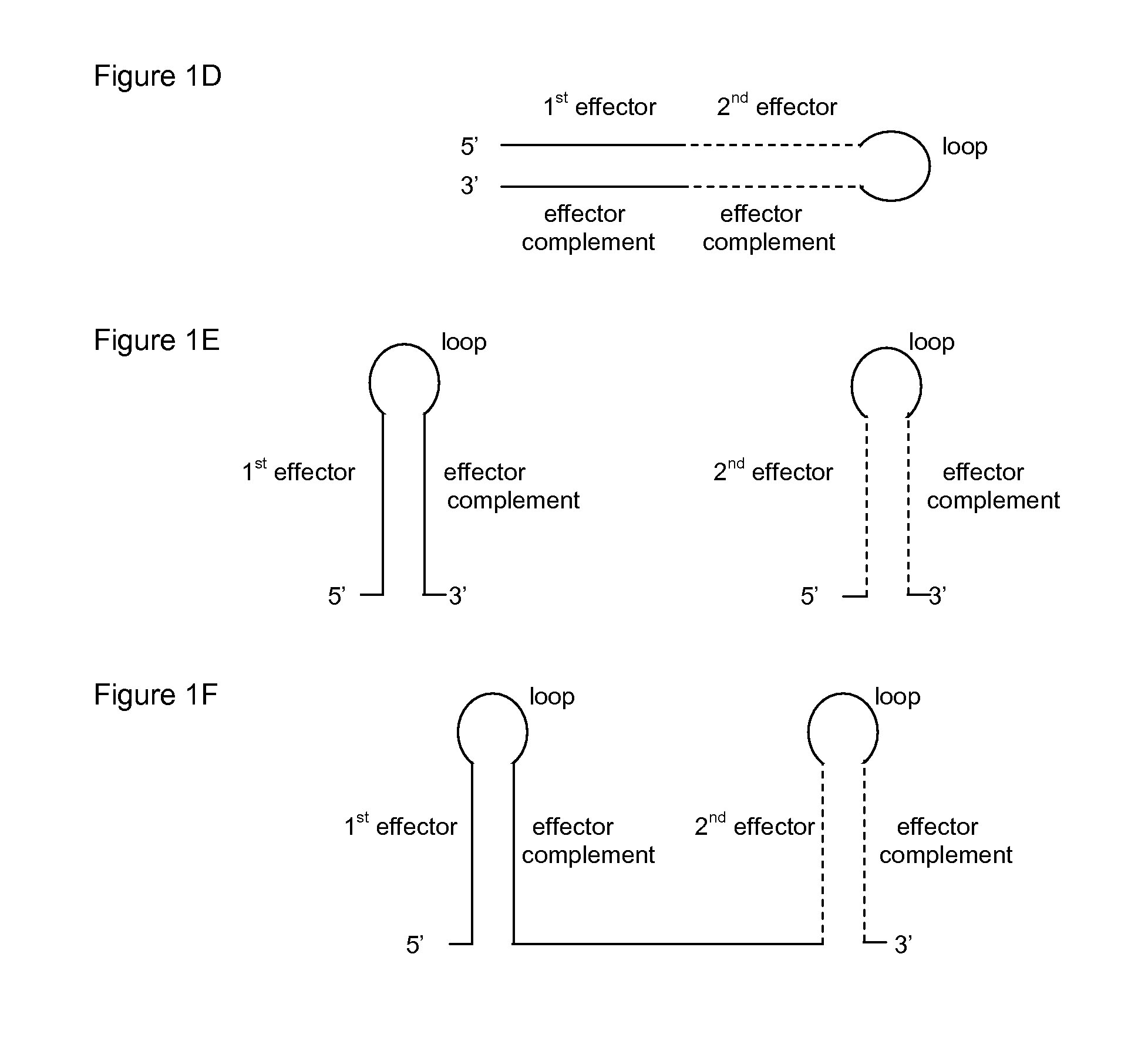
ActiveUS20130296401A1Effectively “ silencing ”Inhibit expressionSugar derivativesGenetic material ingredientsHepatitis B virusHepatitis B infection
RNA interference (RNAi) agents and the use of the RNAi agents for treating hepatitis B infection in individuals, as well as pharmaceutical compositions containing the RNAi agents are provided. The RNAi agents, or constructs for expressing them are utilized to inhibit expression of at least one Hepatitis B virus (HBV) gene, wherein each agent comprises an effector sequence complementary to or substantially complementary to a predicted sequence transcribed from a target region. In some embodiments of the present invention, the agents have more than one effector sequence; wherein the multiple effectors may target the same region of an HBV gene, different (possibly overlapping) regions of the same gene and / or different HBV genes.
Owner:BENITEC IP HLDG INC
Treatment of hepatitis B infection with thymosin alpha 1 and lamivudine
InactiveCN1320041AEffective treatmentCombined securityPeptide/protein ingredientsPharmaceutical delivery mechanismDrug regimenFamciclovir
A method of treatment of hepatitis B virus (HBV) infection in a patient by administering to the patient a drug regimen including an antiviral-effective amount of thymosin alpha 1 (Talpha1), an antiviral-effective amount of lamivudine, and optionally an antiviral-effective amount of famciclovir is disclosed.
Owner:SCICLONE PHARMACEUTICAL INC
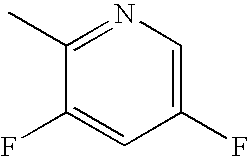
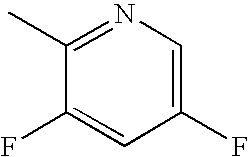
Bromo-phenyl substituted thiazolyl dihydropyrimidines
This invention relates to a bromo-phenyl substituted thiazolyl dihydropyrimidine, its preparation method and use as a medicament for treating and preventing hepatitis B infections. The invention also relates to a composition comprising the dihydropyrimidine, one or more antiviral agents and, optionally, an immunomodulator for treating and preventing HBV infections.
Owner:SUNSHINE LAKE PHARM CO LTD
Continuous subcutaneous administration of interferon-alpha to hepatitis b infected patients
Methods and systems for treating Hepatitis B infections are provided. Typically the method comprises administering interferon-a to the patient subcutaneously using a continuous infusion apparatus, wherein this therapeutic regimen is sufficient to maintain circulating levels of interferon-a in the serum of the patient above a target concentration for a certain period of time.
Owner:MEDTRONIC INC
Diazepinone derivatives and their use in the treatment of hepatitis b infections
InactiveCN109641896AOrganic active ingredientsOrganic chemistryPharmaceutical SubstancesHepatitis B infection
Owner:NOVIRA THERAPEUTICS
Pharmacy use of laetiporus sulphureus extract
PendingCN110179831AAvoid infectionPrevent relapseOrganic active ingredientsBacteria material medical ingredientsSolventHepatitis B virus
The invention provides an application of a laetiporus sulphureus extract to preparation of a medicine for resisting hepatitis b virus. The laetiporus sulphureus extract is an extract obtained throughperforming refluxing on a laetiporus sulphureus fruiting body or mycelium with an alcohol solvent, wherein the alcohol solvent comprises ethanol, and the concentration of the ethanol is 95% and above.The laetiporus sulphureus and components thereof can prevent hepatitis b infection, treat hepatitis b infectious hepatitis, prevent liver cancer, treat hepatitis b relevance liver cancer, and preventrecurrence. The laetiporus sulphureus extract has positive effects in the overall process of pathological changes. The laetiporus sulphureus extract has important significance on solving the difficult problem of clinical treatment of hepatitis b infection and particularly solving the difficult problem of a hepatitis b relevance liver cancer technique.
Owner:CHINA THREE GORGES UNIV
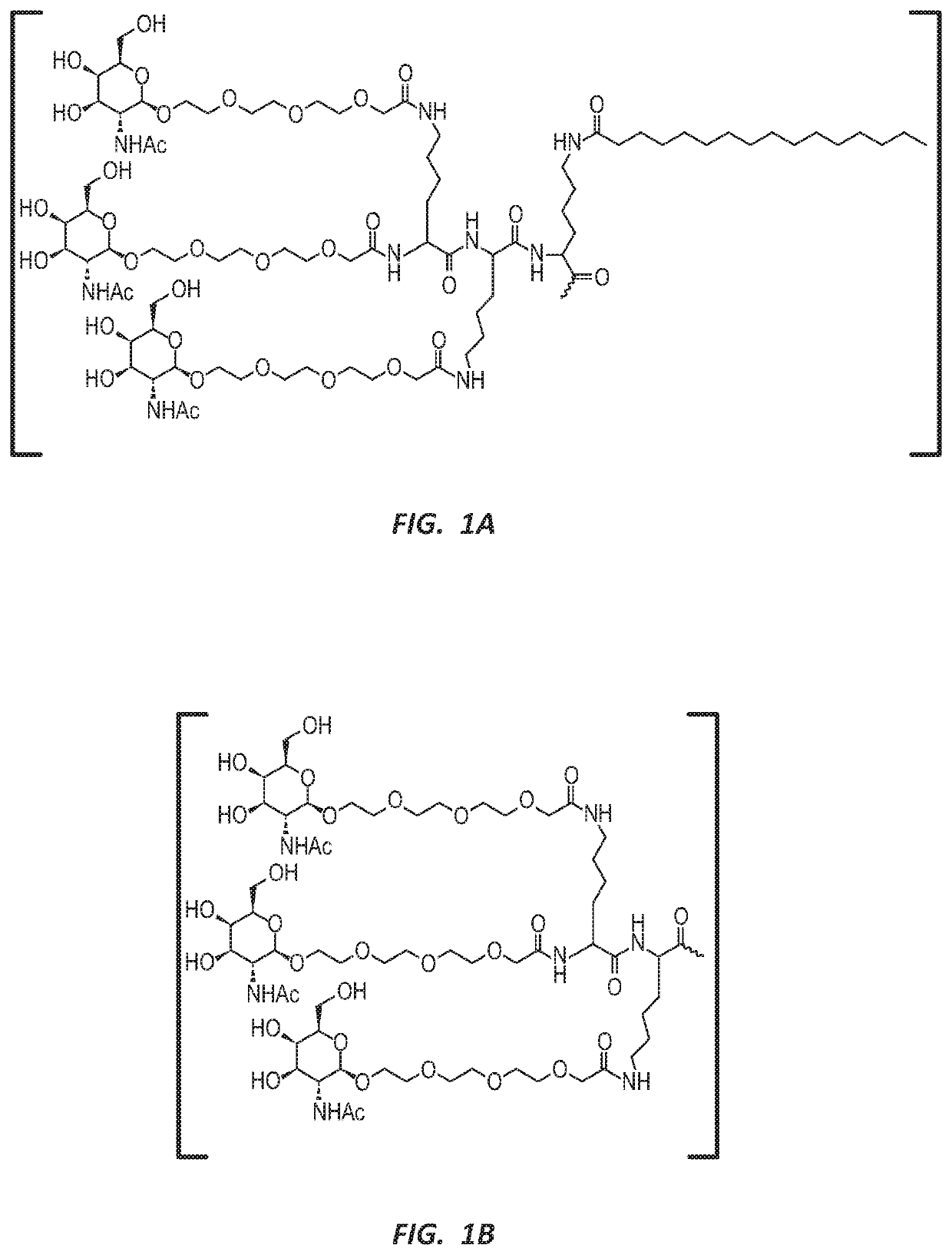
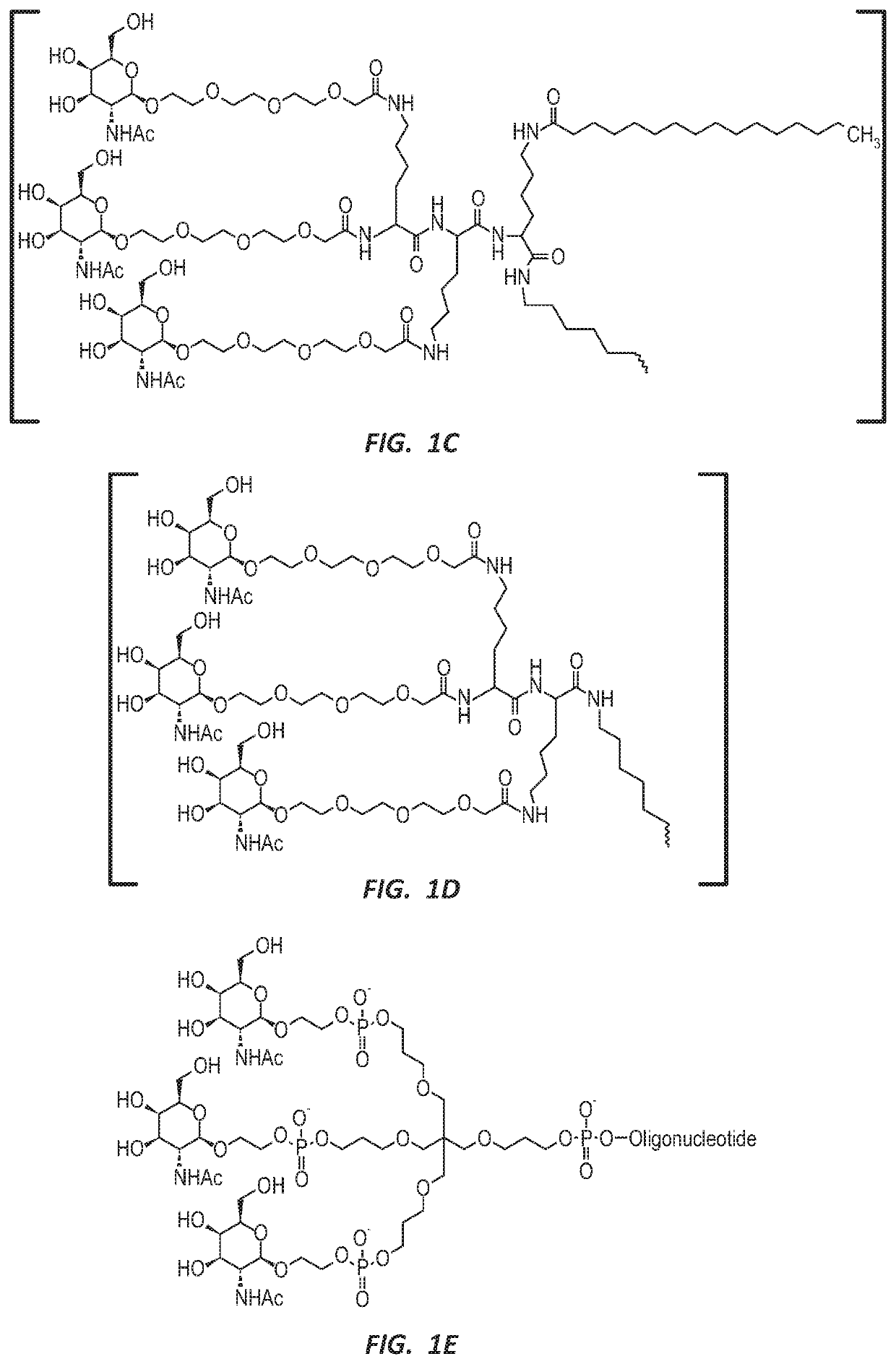
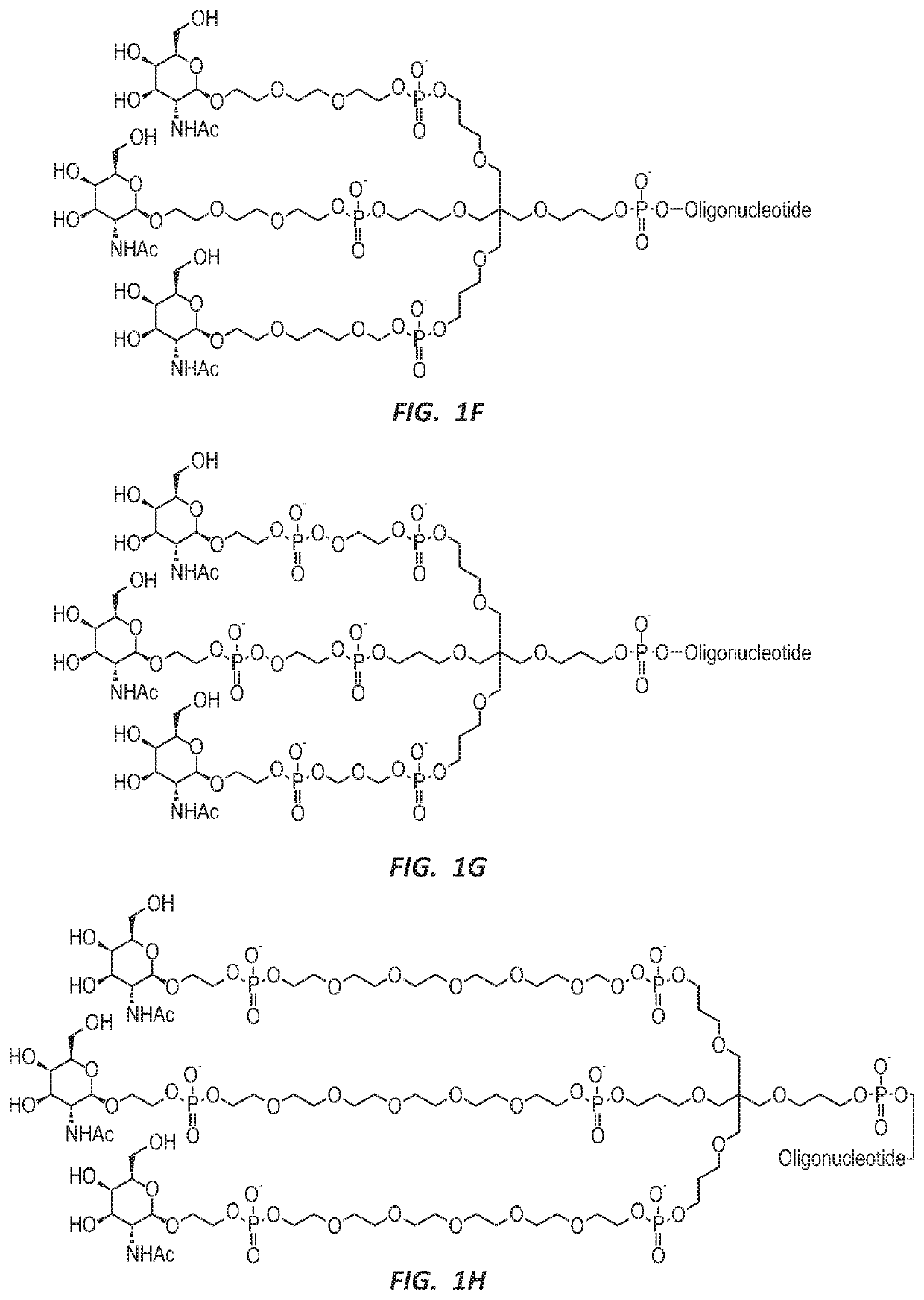
Methods for treating hepatitis b infection
ActiveUS20200171069A1Easy to optimizeStable reductionOrganic active ingredientsGenetic material ingredientsHepatitis B immunizationOligonucleotide
This application relates to potent oligonucleotides useful for reducing HBsAg expression and treating HBV infections.
Owner:DICERNA PHARM INC
2'-fluoroncucleosides
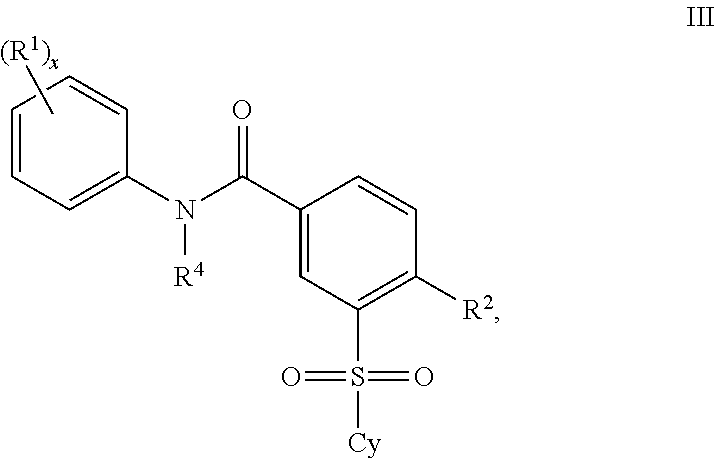
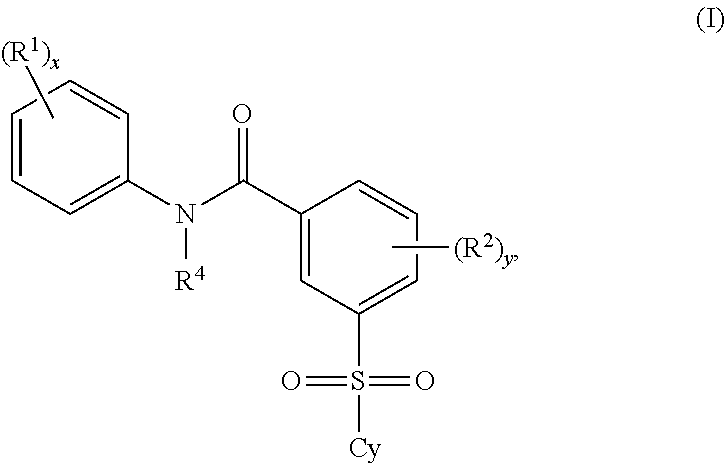
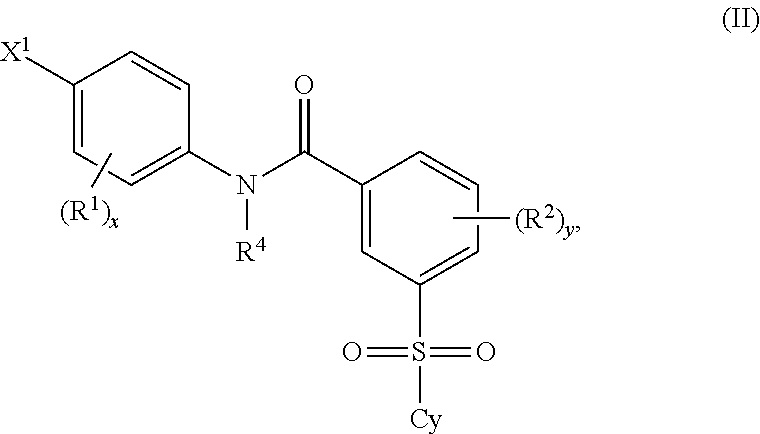
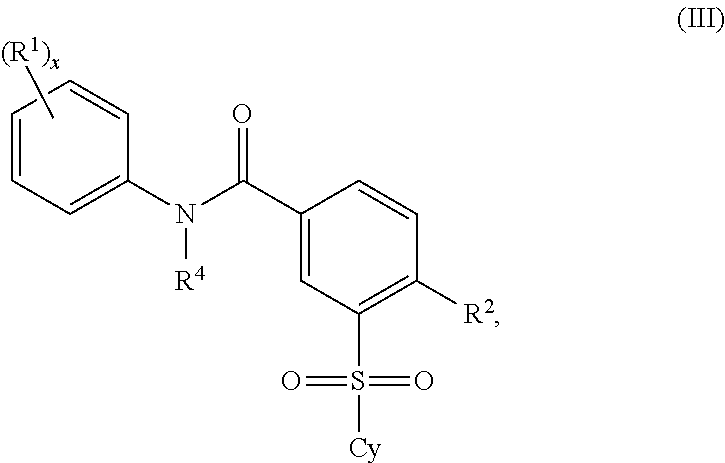
2'-fluoronucleoside compounds are disclosed for use in the treatment of hepatitis B infection, hepatitis C infection, HIV and abnormal cell proliferation including tumors and cancers. These compounds have the general formula (I), (II), (III), (IV) or pharmaceutically acceptable salts thereof, wherein the base is a purine or pyrimidine base; R 1 is OH, H, OR 3 , N 3 , CN, halogen including F, or CF 3 , lower alkyl, amino, lower alkylamino, two lower alkylamino, or alkoxy; R 2 is H, phosphate, including monophosphate, diphosphate, triphosphate, or a stable phosphate prodrug; acyl, or other pharmaceutically acceptable leaving group that provides R when used in vivo 2 Compounds that are H or phosphate; sulfonates include alkyl or aralkylsulfonyl, including methylsulfonyl, benzyl, wherein phenyl is optionally substituted by one or more of the substituents described above in the definition of aryl substitution, lipid, amino acid, peptide or cholesterol; and R 3 is an acyl, alkyl, phosphate or other pharmaceutically acceptable leaving group, ie, a group that cleaves to the parent compound when used in vivo.
Owner:EMORY UNIVERSITY +9
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com