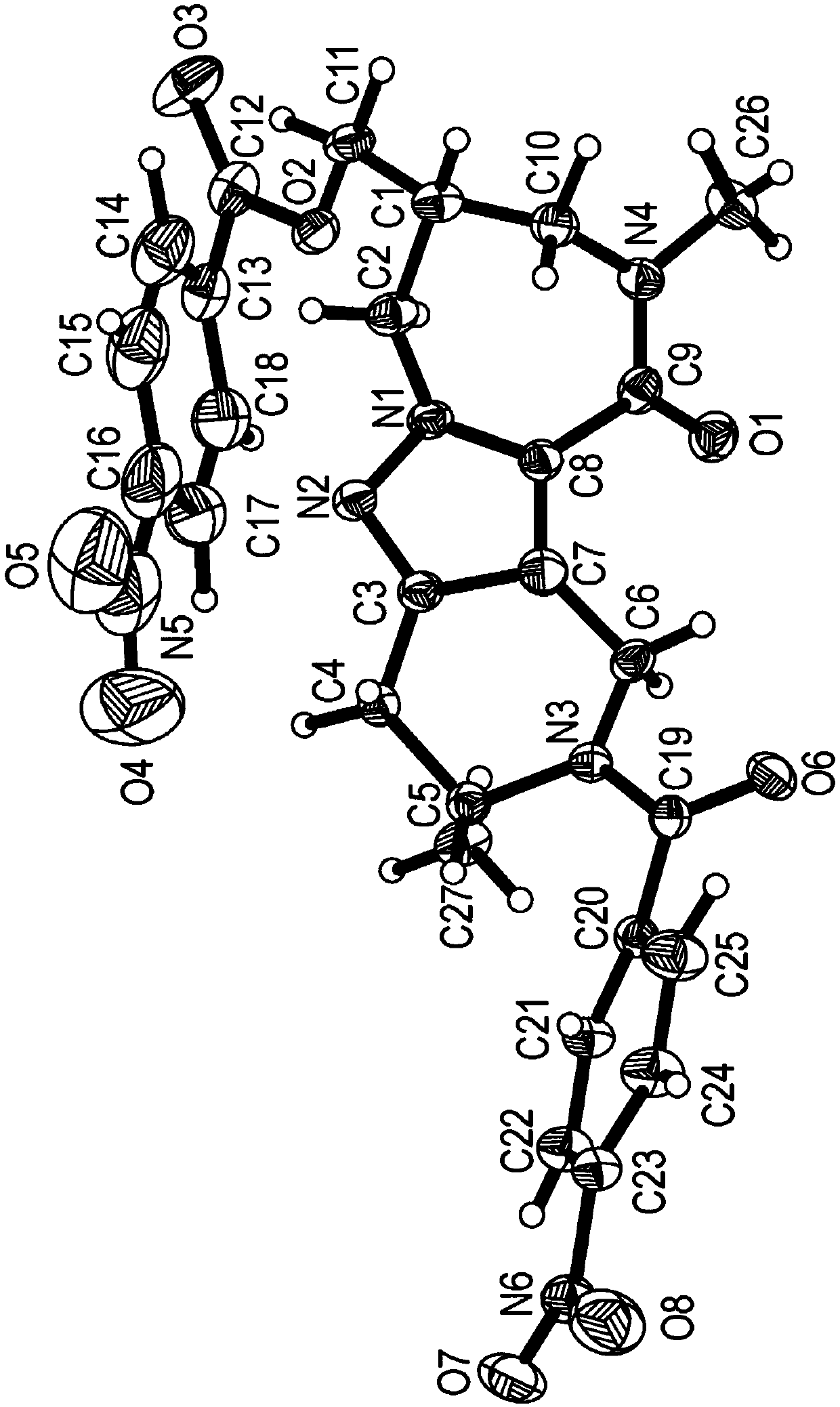
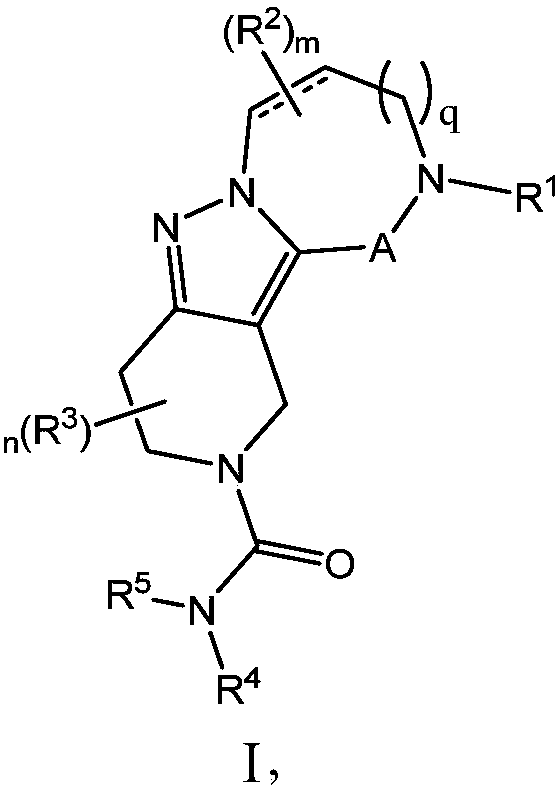
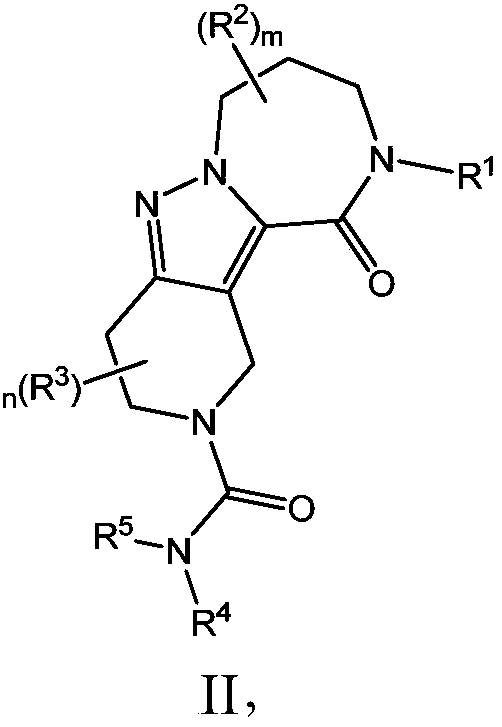
Diazepinone derivatives and their use in the treatment of hepatitis b infections
A compound, heterocycloalkyl technology, applied in the field of diazepinone derivatives and its use in the treatment of hepatitis B infection, can solve the problems of inability to provide cure, difficulty in complete inhibition, low cure rate, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0547] Preparation of the optically active form is accomplished in any suitable manner, including, by way of non-limiting examples, resolution of the racemic form by recrystallization techniques, synthesis from optically active starting materials, chiral synthesis, or use of a chiral stationary phase Perform chromatographic separation. In one embodiment, a mixture of one or more isomers is used as the disclosed compounds described herein. In another embodiment, the compounds described herein contain one or more chiral centers. The compounds are prepared by any means including stereoselective synthesis, enantioselective synthesis or separation of enantiomeric or diastereomeric mixtures. Resolution of compounds and isomers thereof is achieved by any means including, by way of non-limiting examples, chemical methods, enzymatic methods, fractional crystallization, distillation and chromatography.
[0548] It is also to be understood that those compounds which have the same molec...
Embodiment
[0631] Exemplary compounds useful in the methods of the invention will now be described with reference to exemplary synthetic schemes for their general preparation below and the specific examples that follow. Those skilled in the art will recognize that to obtain the various compounds herein, starting materials can be appropriately selected so that, protected or unprotected as required, throughout the reaction schemes will carry the ultimately desired substituents to provide desired product. Alternatively, it may be necessary or desirable to replace the final desired substituent with a suitable group which can be run through the reaction scheme and replaced with the desired substituent where appropriate. Unless otherwise indicated, variables are as defined above for formula (I). The reaction can be carried out between the melting point of the solvent and the reflux temperature, and preferably between 0°C and the reflux temperature of the solvent. The reaction can be heated u...
preparation example
[0640] Exemplary compounds useful in the methods of the invention will now be described with reference to exemplary synthetic schemes for their general preparation below and for the specific illustrations that follow.
[0641] plan 1
[0642]
[0643] According to Scheme 1, a commercially available or synthetically obtained compound of formula (Xa) is synthesized with an alkylating agent such as ethyl prop-2-enoate, ethyl 2-(bromomethyl)prop-2-enoate, etc. (where R a for C 1-6 Alkyl or C 1-6 Haloalkyl, and PG is a suitable nitrogen protecting group, such as BOC, Bn, etc.) Alkylation to provide compounds of formula (XI) (wherein R b is optionally co- 2 C replaced by Me 2-6 alkylene). The compound of formula (Xa) (wherein R a for CH 3 or CH 2 CHF 2 , and PG is BOC) alkylation, for example, with or without such as NaH, Cs 2 CO 3 、K 2 CO 3 base, etc., in a suitable solvent such as THF, DMF, etc., with an alkylating agent such as ethyl 2-(bromomethyl)prop-2-enoat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com