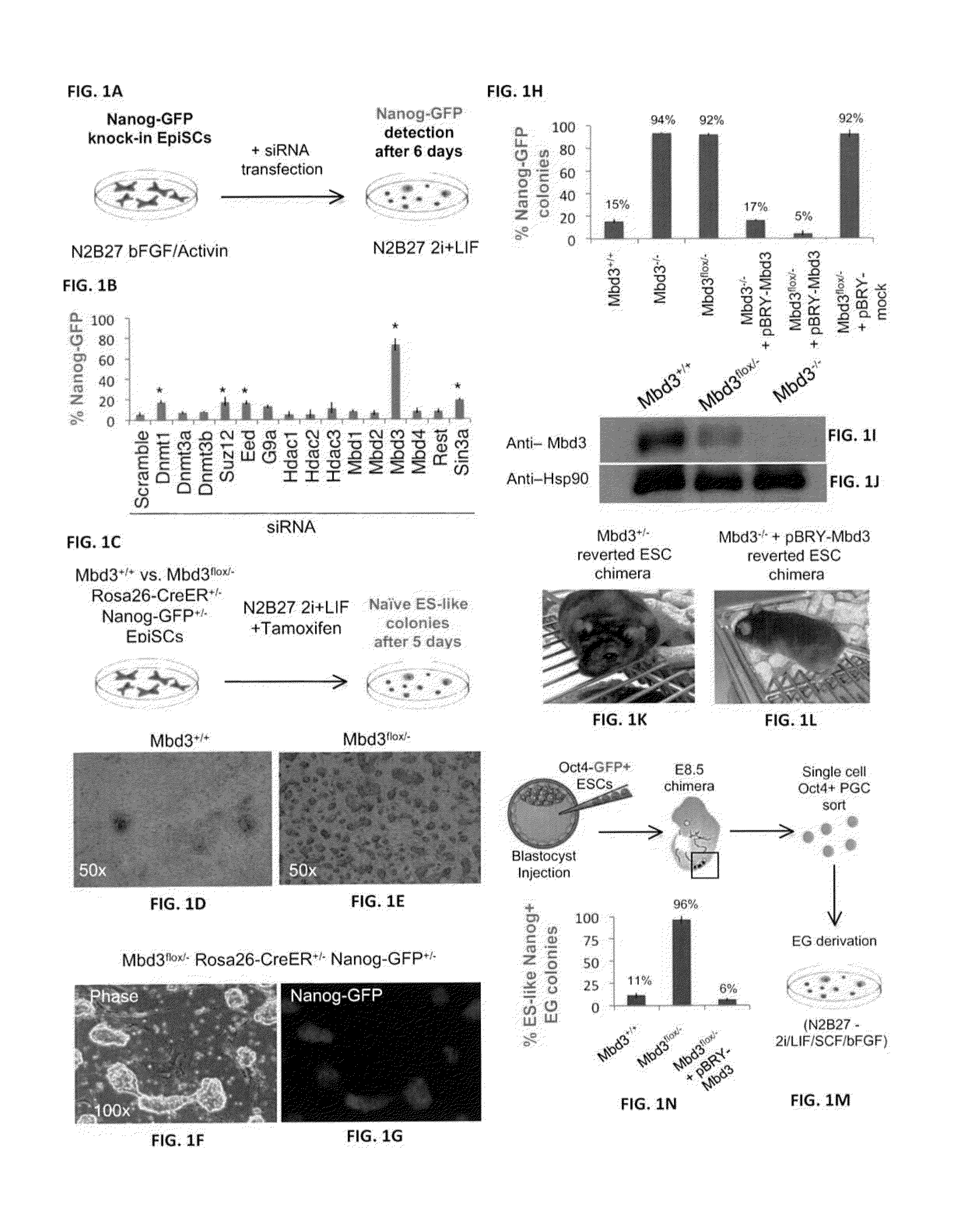
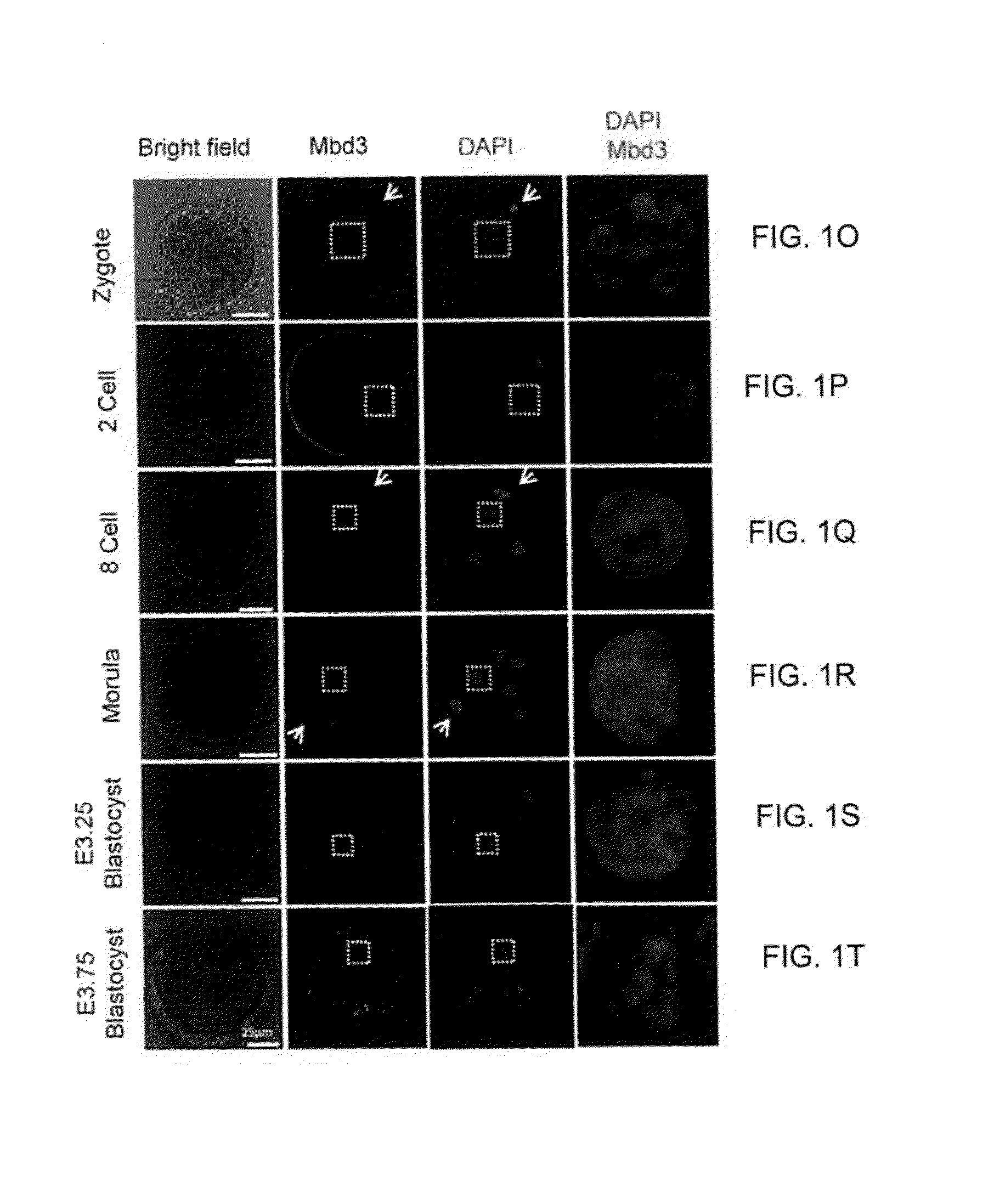
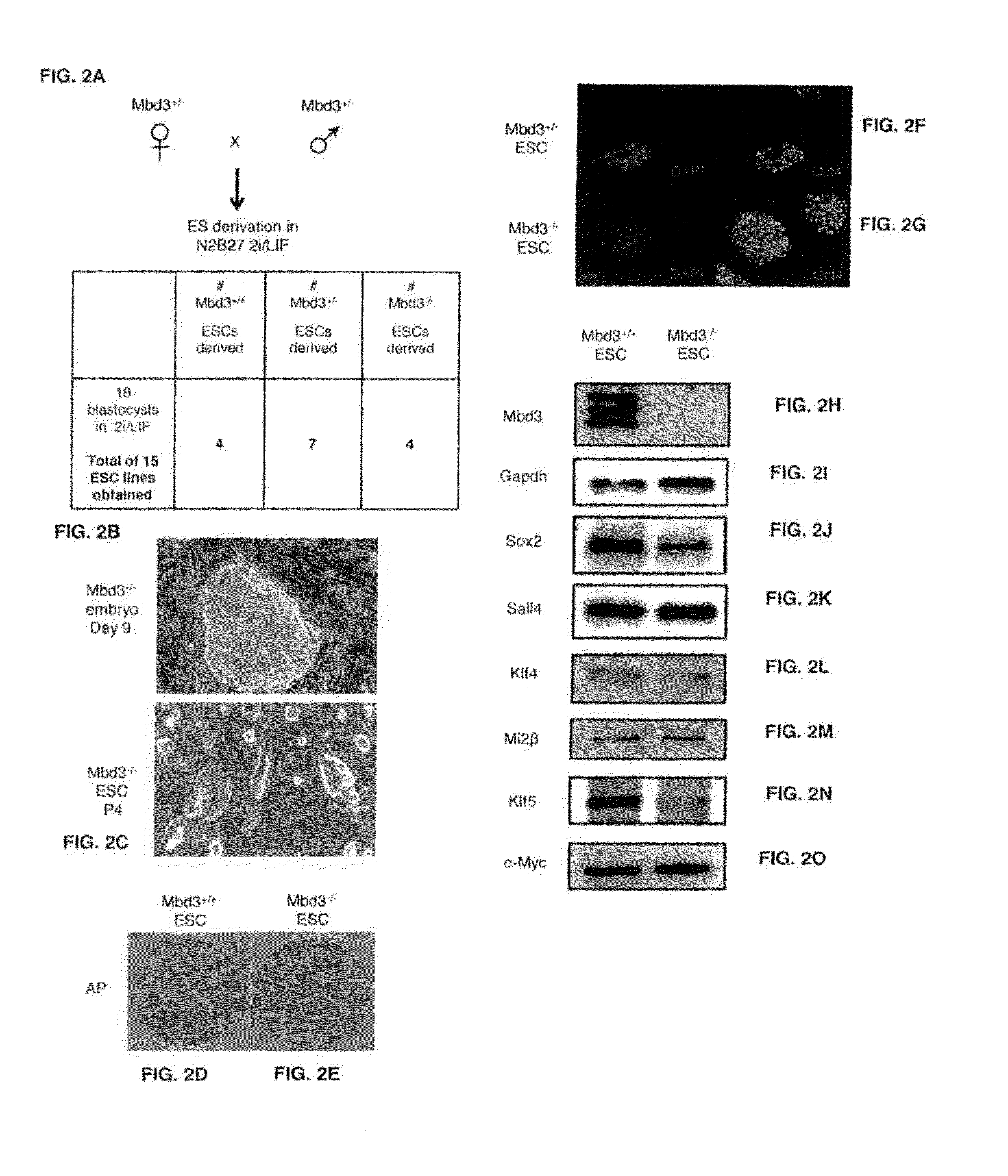
Patents
Literature
58 results about "Jnk inhibitor" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
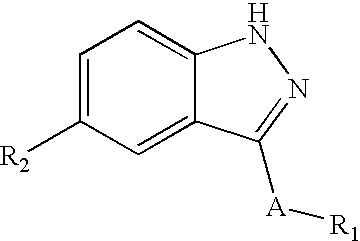
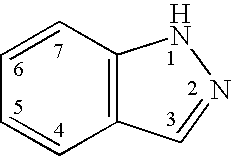
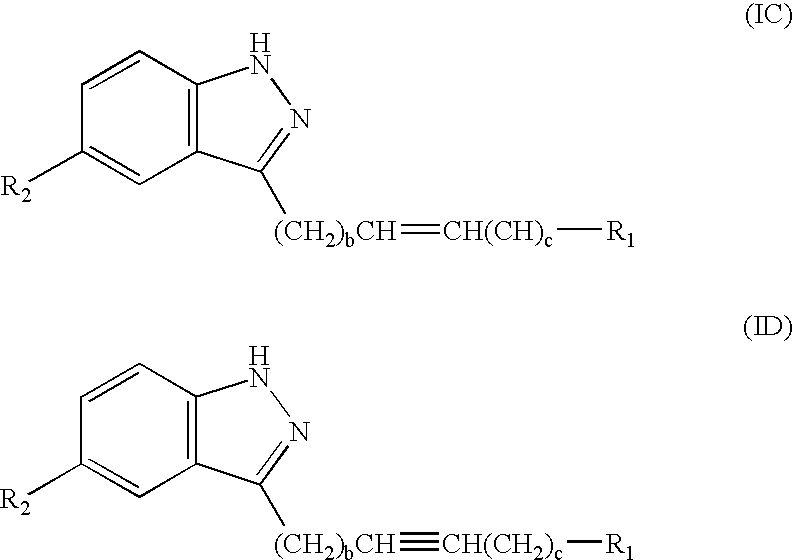
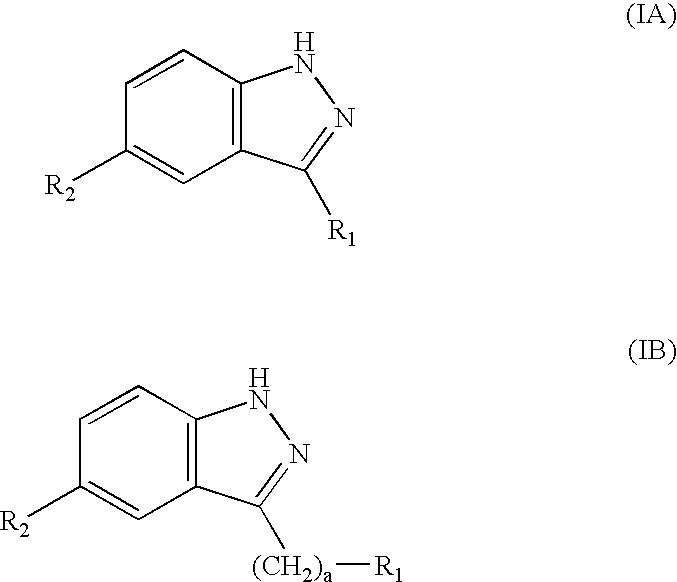
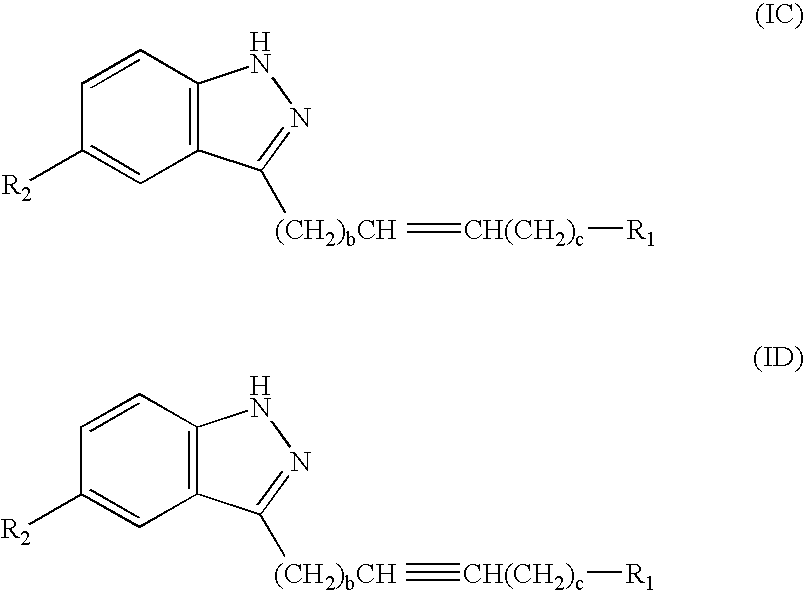
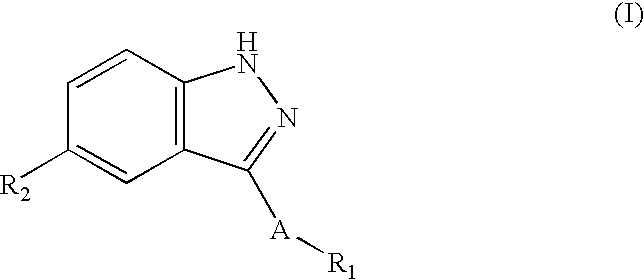
Indazole derivatives as JNK inhibitors and compositions and methods related thereto
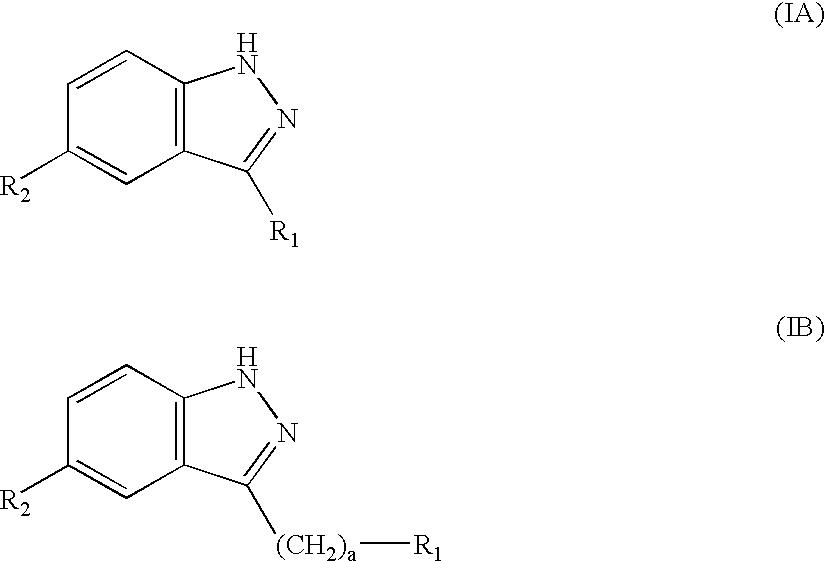
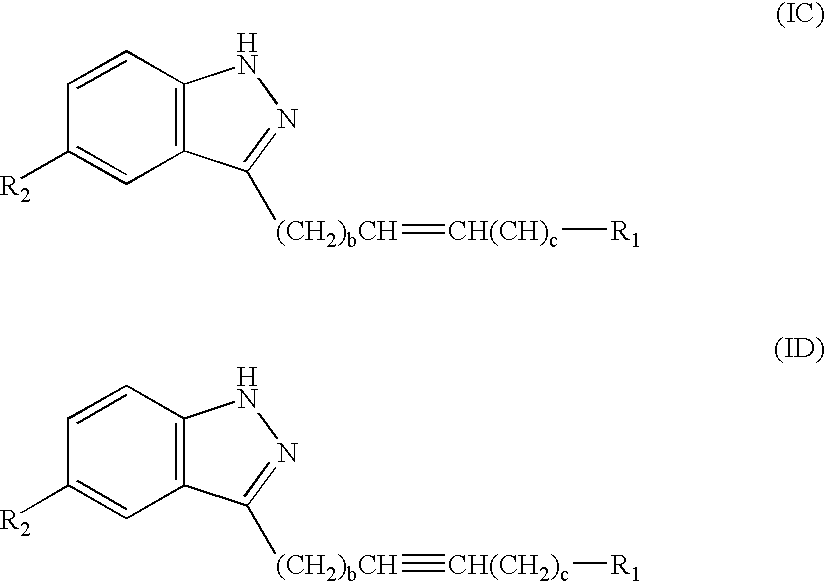
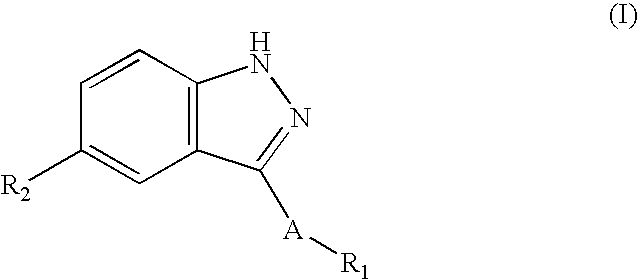
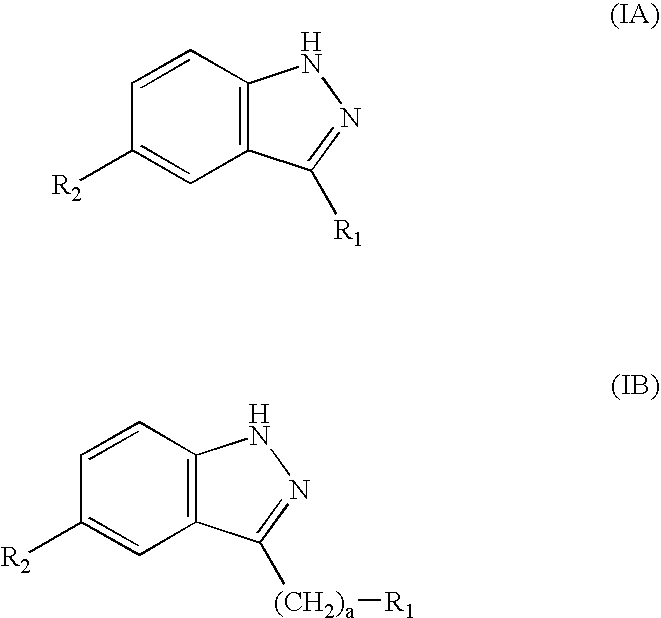
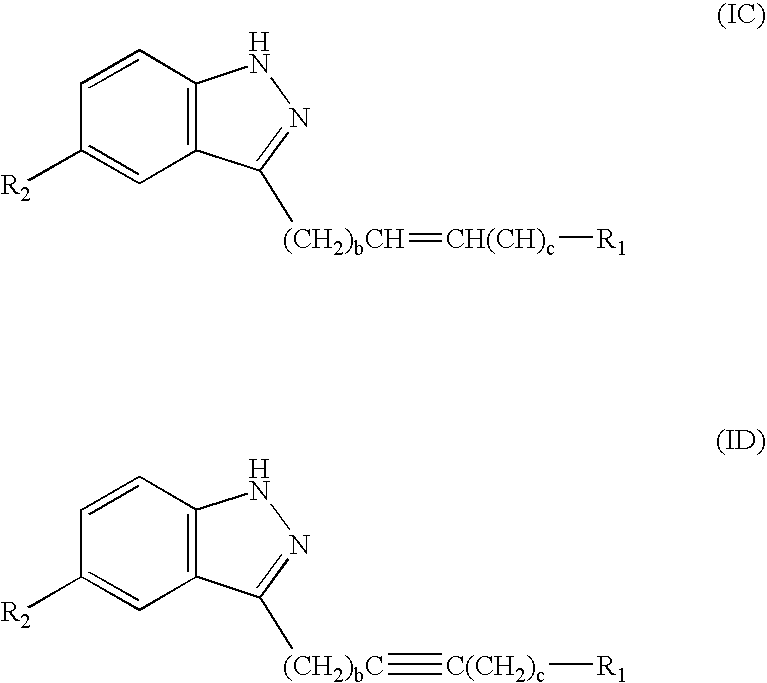
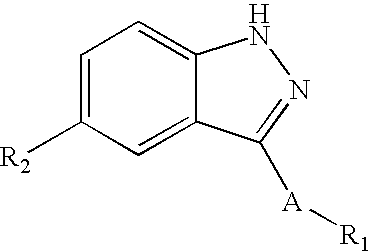
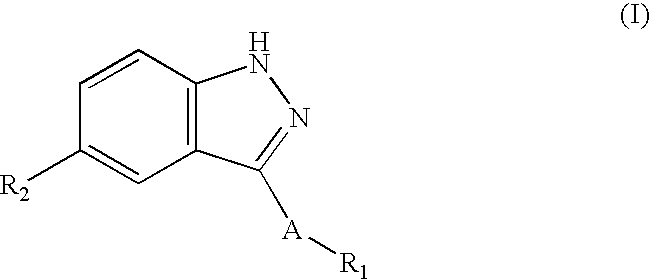
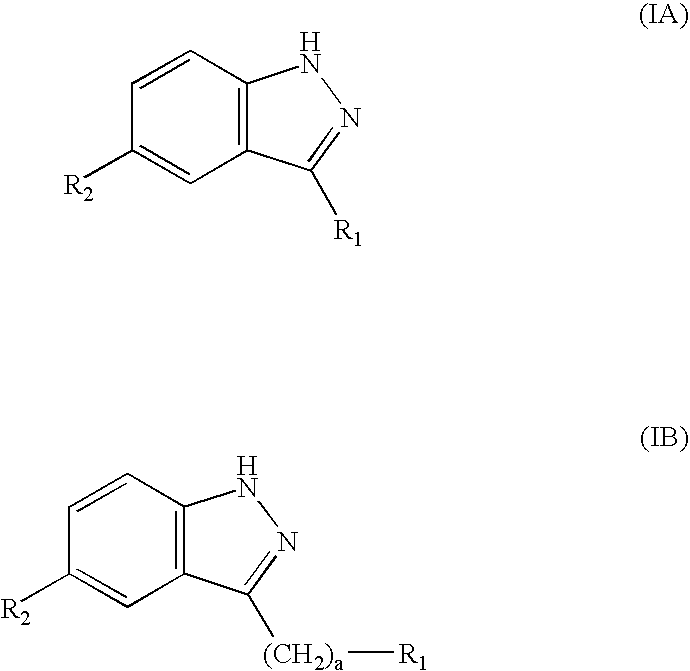
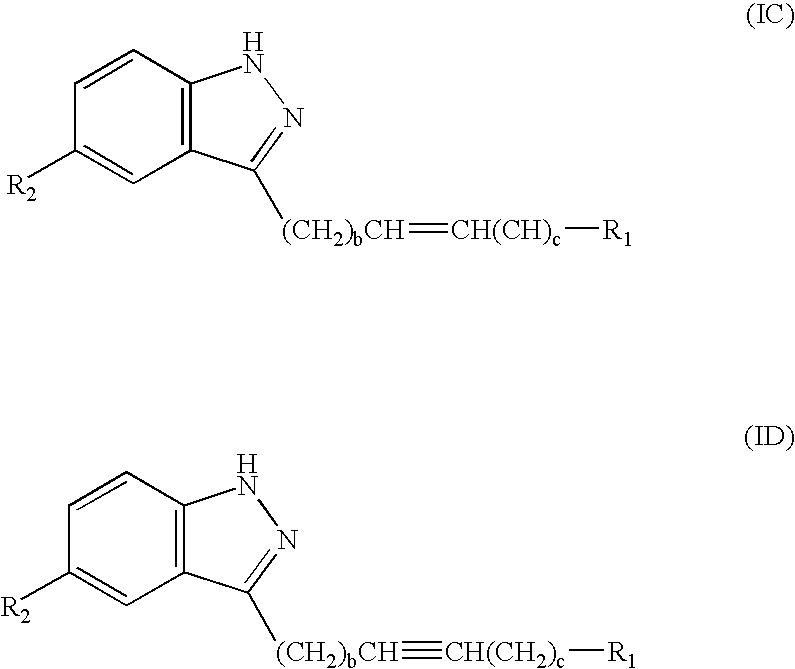
Compounds having activity as selective inhibitors of JNK are disclosed. The compounds of this invention are indazole derivatives having the following structure: wherein R1, R2 and A are as defined herein. Such compounds have utility in the treatment of a wide range of conditions that are responsive to JNK inhibition. Thus, methods of treating such conditions are also disclosed, as are pharmaceutical compositions containing one or more compounds of the above compounds.
Owner:SIGNAL PHARMA LLC
Combination therapy for treating, preventing or managing proliferative disorders and cancers
InactiveUS20040067953A1Improve efficiencyImprove toleranceBiocideHeavy metal active ingredientsCancer preventionBiologically-Based Therapy
The present invention relates to methods and compositions designed for the treatment, management or prevention of cancer. The methods of the invention comprise the administration of an effective amount of one or more inhibitors of JNK in combination with the administration of an effective amount of one or more other agents useful for cancer therapy. The invention also provides pharmaceutical compositions comprising one or more inhibitors of JNK in combination with one or more other agents useful for cancer therapy. In particular, the invention is directed to methods of treatment and prevention of cancer by the administration of an effective amount of one or more inhibitors of JNK in combination with standard and experimental chemotherapies, hormonal therapies, bone marrow transplants, stem cell replacement therapies, biological therapies / immunotherapies and / or radiation therapies for treatment or prevention of cancer. Also included are methods of treatment of cancer by the administration of one or more inhibitors of JNK in combination with surgery, alone or in further combination with standard and experimental chemotherapies, hormonal therapies, bone marrow transplants, stem cell replacement therapies, biological therapies / immunotherapies and / or radiation therapies.
Owner:SIGNAL PHARMA LLC
Methods for preserving tissue
The invention generally relates to methods for preserving tissue, preventing reperfusion injury to implanted tissue, preventing transplant rejection or preserving a cell to be transplanted, comprising contacting tissue or a cell with an effective amount of a JNK Inhibitor. The invention further relates to compositions useful for the preservation of tissue, the compositions comprising an effective amount of a JNK Inhibitor.
Owner:CELGENE CORP
Solid forms of a JNK inhibitor
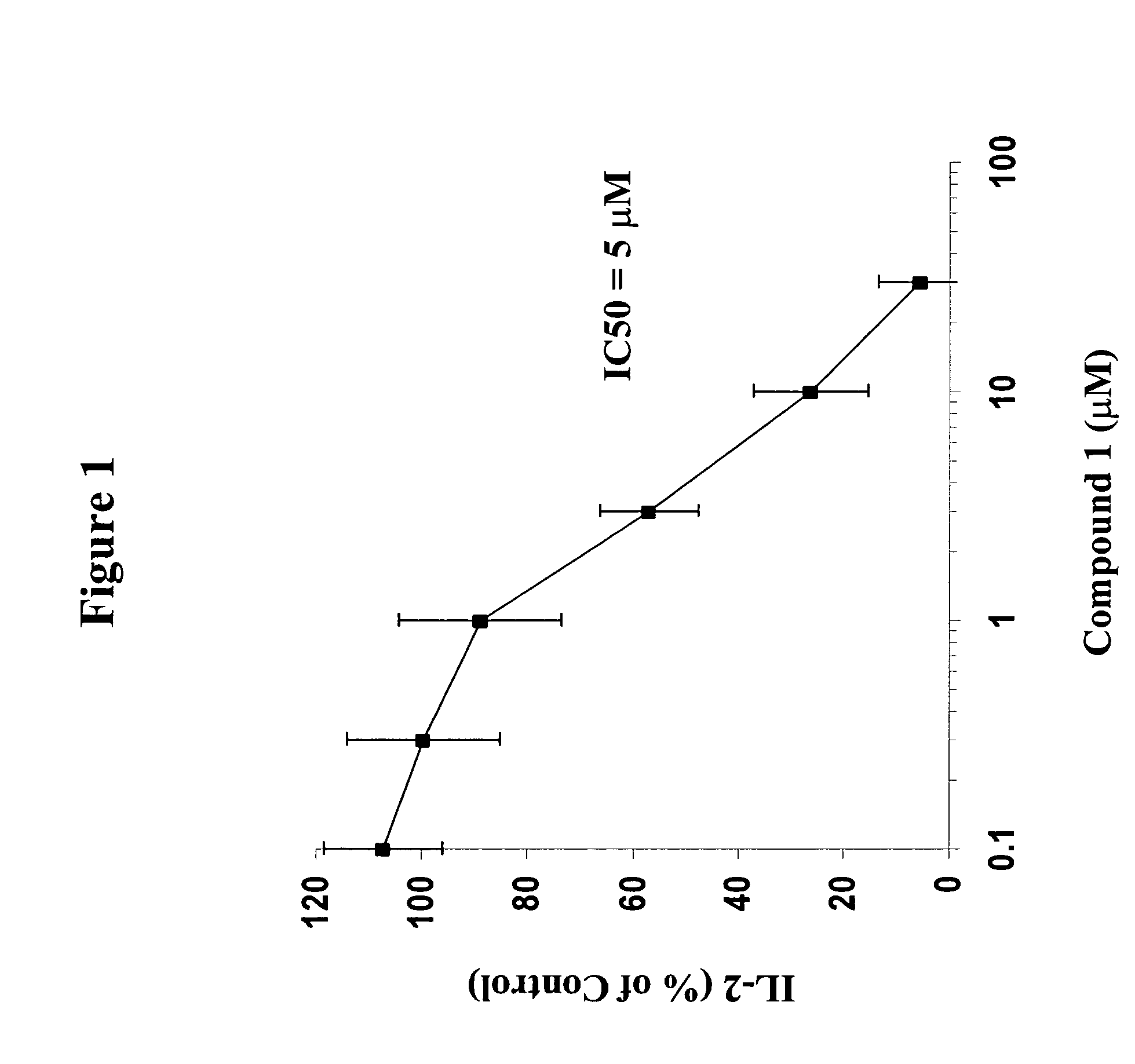
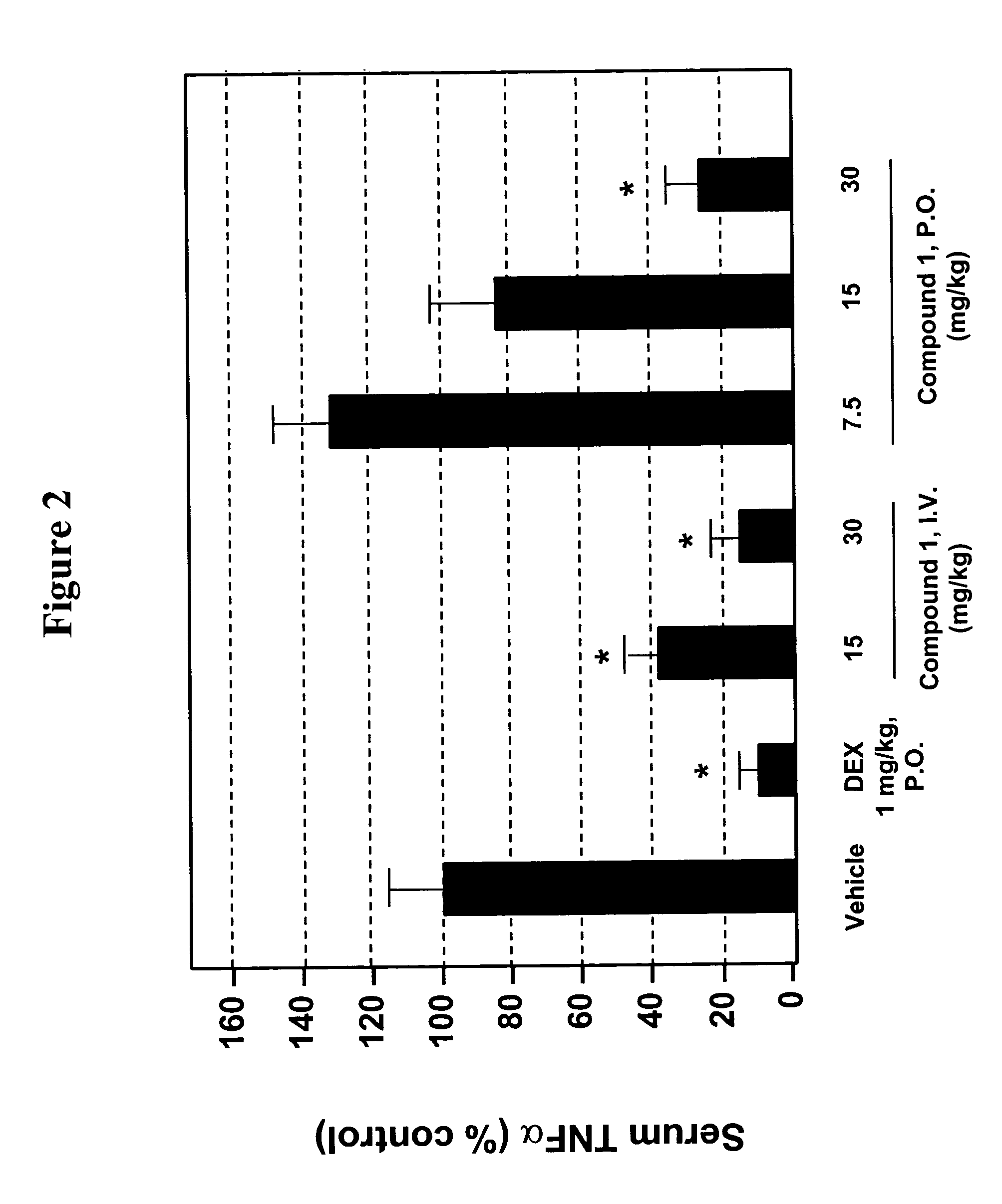
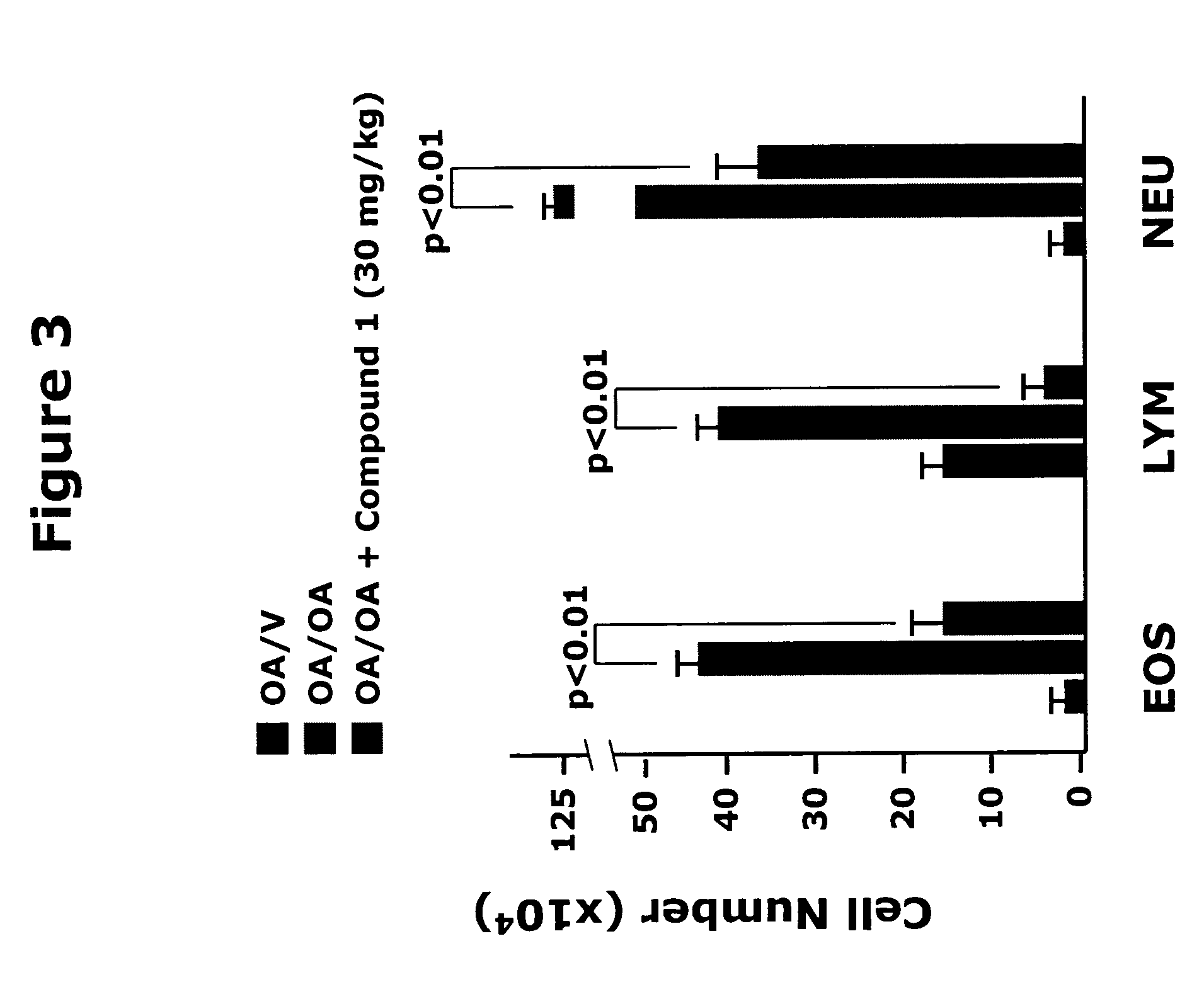
The present invention provides solid forms of Compound (I), pharmaceutical compositions thereof, and methods for the treatment or prevention of diseases including, but not limited to a liver disease, cancer, a cardiovascular disease, a metabolic disease, a renal disease, an autoimmune condition, an inflammatory condition, macular degeneration, pain and related syndromes, disease-related wasting, an asbestos-related condition, pulmonary hypertension, ischemia / reperfusion injury, central nervous system (CNS) injury / damage or a disease treatable or preventable by the inhibition of JNK. In particular, the invention relates to certain novel crystal forms of the compound 1-(5-(1H-1,2,4-triazol-5-yl)(1H-indazol-3-yl))-3-(2-piperidylethoxy)benzene.
Owner:CELGENE CORP
Isolated naive pluripotent stem cells and methods of generating same
ActiveUS20140315301A1Increase generationArtificial cell constructsCell culture active agentsPhysiologyAllele
Provided is an isolated human naive pluripotent stem cell (PSC), wherein: (i) when the naive PSC is a female PSC, then said naive female PSC has two unmethylated alleles of an X-inactive specific transcript (XIST) gene; and (ii) when said naive PSC is a male PSC, then said naive male PSC has an unmethylated allele of said XIST gene. Also provided is a culture medium which comprises an ERK1 / 2 inhibitor, a GSK3beta inhibitor, a p38 inhibitor, a JNK inhibitor, a STAT3 activator and at least one agent selected from the group consisting of: bFGF, TGFbeta 1, a PKC inhibitor, a ROCK inhibitor and a NOTCH inhibitor; or at least agent selected from the group consisting of: a TGFR inhibitor, a FGFR inhibitor, a PKC inhibitor, a ROCK inhibitor and a NOTCH inhibitor.
Owner:YEDA RES & DEV CO LTD
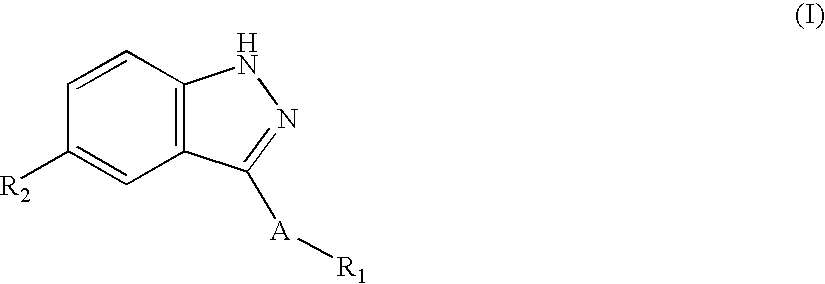
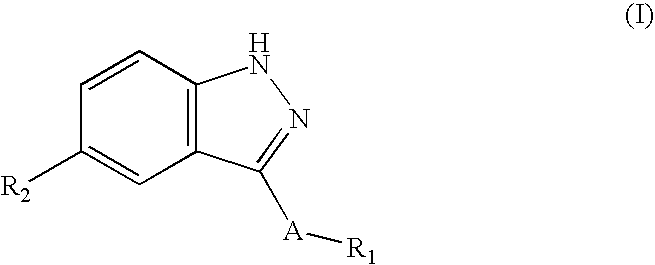
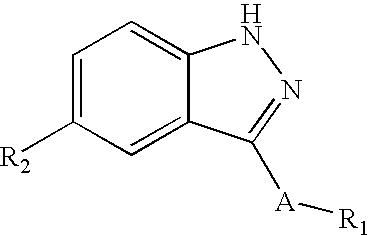
Indazole compounds and compositions thereof as JNK inhibitors and for the treatment of diseases associated therewith
This invention is generally directed to Indazole Derivatives having the following structure:or pharmaceutically acceptable salt thereof, wherein R1, R2 and A are as defined herein. Such compounds have utility in the treatment of a wide range of diseases and disorders that are responsive to JNK inhibition, such as an inflammatory disease or disorder. Thus, methods of treating such diseases and disorders are also disclosed, as are pharmaceutical compositions containing one or more compounds of the above compounds.
Owner:SIGNAL PHARMA LLC
Methods of using and compositions comprising a JNK inhibitor for the treatment, prevention, management and/or modification of pain
InactiveUS20040087642A1Conveniently formedExtension of timeBiocideOrganic active ingredientsActive agentDepressant
The present invention relates to methods for treating, preventing, managing and / or modifying pain, comprising administering an effective amount of a JNK Inhibitor to a patient in need thereof. Specific embodiments encompass the administration of a JNK Inhibitor, alone or in combination with a second active agent and / or surgery or physical therapy. Pharmaceutical compositions, single unit dosage forms, and kits suitable for use in methods of the invention are also disclosed.
Owner:CELGENE CORP
Indazole derivatives as JNK inhibitors and compositions and methods related thereto
Compounds having activity as selective inhibitors of JNK are disclosed. The compounds of this invention are indazole derivatives having the following structure: wherein R1, R2 and A are as defined herein. Such compounds have utility in the treatment of a wide range of conditions that are responsive to JNK inhibition. Thus, methods of treating such conditions are also disclosed, as are pharmaceutical compositions containing one or more compounds of the above compounds.
Owner:SIGNAL PHARMA LLC
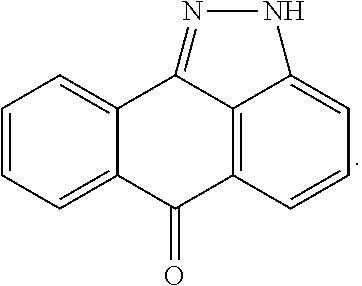
Pyrazoloanthrone and derivatives thereof as JNK inhibitors and compositions and methods related thereto
Owner:SIGNAL PHARMA LLC
Methods and compositions using JNK inhibitors for treatment and management of central nervous system injury
InactiveUS20060122179A1Extension of timeAvoid injuryBiocideSenses disorderActive agentPharmaceutical drug
Methods of treating, preventing and / or managing a central nervous system injury / damage and related syndromes are disclosed. Specific methods encompass the administration of a JNK inhibitor alone or in combination with a second active agent. Pharmaceutical compositions, single unit dosage forms, and kits suitable for use in methods of the invention are also disclosed.
Owner:CELGENE CORP
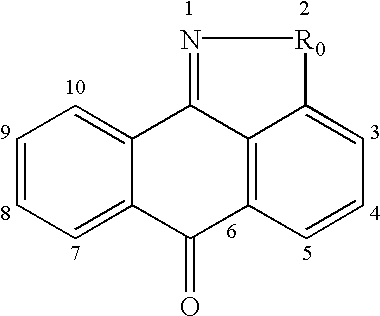
Isothiazoloanthrones, isoxazoloanthrones, isoindolanthrones and derivatives thereof as JNK inhibitors and compositions and methods related thereto
Isothiazoloanthrones, isooxazoloanthrones, isoindolanthrones, and derivatives thereof having the general formula: and pharmaceutically acceptable salts thereof, wherein R0 is -CH2-, -SO-, -O-, -SO2-, or -S-; compositions comprising the isothiazoloanthrones, isooxazoloanthrones, isoindolanthrones, and derivatives thereof; and methods for treating or preventing a disorder alleviated by inhibiting Jun N-terminal kinase (JNK) by administering the isothiazoloanthrones, isooxazoloanthrones, isoindolanthrones, and derivatives thereof are described herein.
Owner:SIGNAL PHARMA LLC
Small-molecular compound combination for differentiated cell reprogramming, reagent kit and application of small-molecular compound combination
ActiveCN108060120AGood multiple germ layer multilineage differentiation potentialStable passageSkeletal/connective tissue cellsCell culture active agentsBeta-cateninReprogramming
The invention discloses a small-molecular compound combination for differentiated cell reprogramming, and a reagent kit and an application of the small-molecular compound combination. The small-molecular compound combination includes the following components: a TGF-beta inhibitor, a WNT / beta-catenin agonist, a cAMP agonist and a PKC inhibitor; furthermore, the small-molecular compound combinationalso includes at least one of an RAR agonist, a DNMT inhibitor, an HMT inhibitor, a histone demethylase inhibitor, ascorbic acid, a JNK inhibitor, an ROCK inhibitor and a lysine deacetylase inhibitor.Differentiated cells can be reprogrammed into mesenchymal stem cells by phased induction with the small-molecular compound combination, all steps can be subjected to precise quality control, and standardization operation and large-scale production are convenient. The method provided by the invention has wide donor sources, a patient himself can be used as a donor, and the mesenchymal stem cells needed for basic research, clinical treatment or tissue engineering production can be obtained in relatively short time.
Owner:YUNNAN JICI INSITUTE FOR REGENERATIVE MEDICINE CO LTD
Adamantyl compounds
Owner:F HOFFMANN LA ROCHE & CO AG
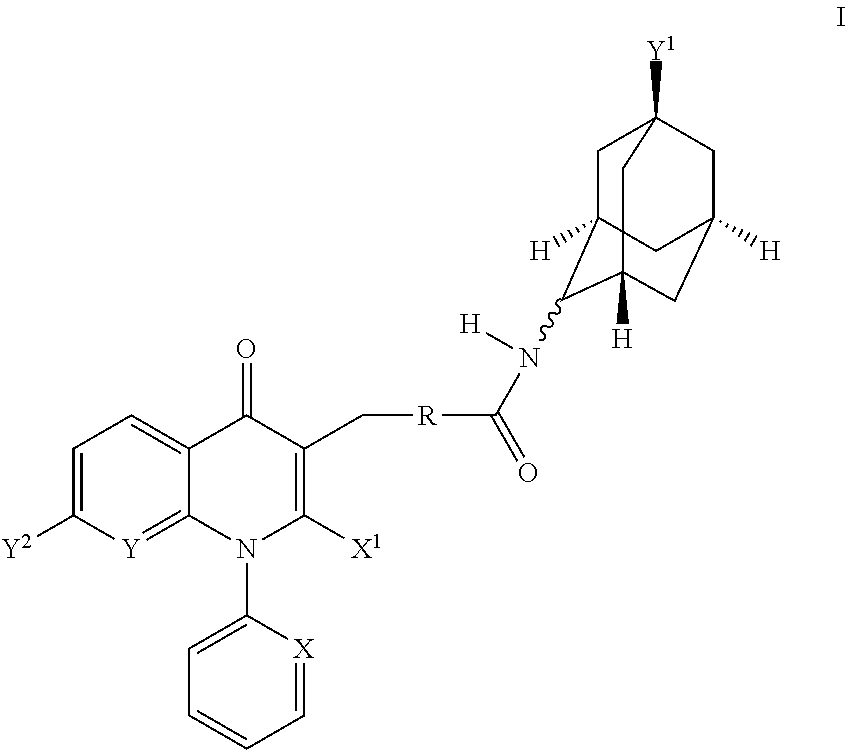
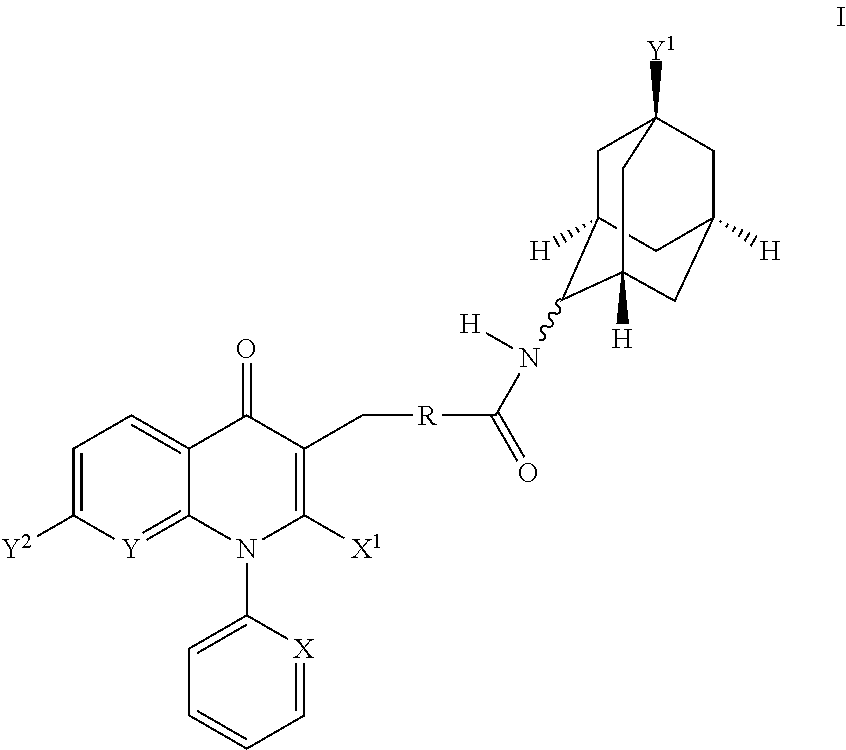
Jnk inhibitor
InactiveUS20050148624A1Improve stabilityLow toxicBiocideNervous disorderHydrogen atomCarboxyl radical
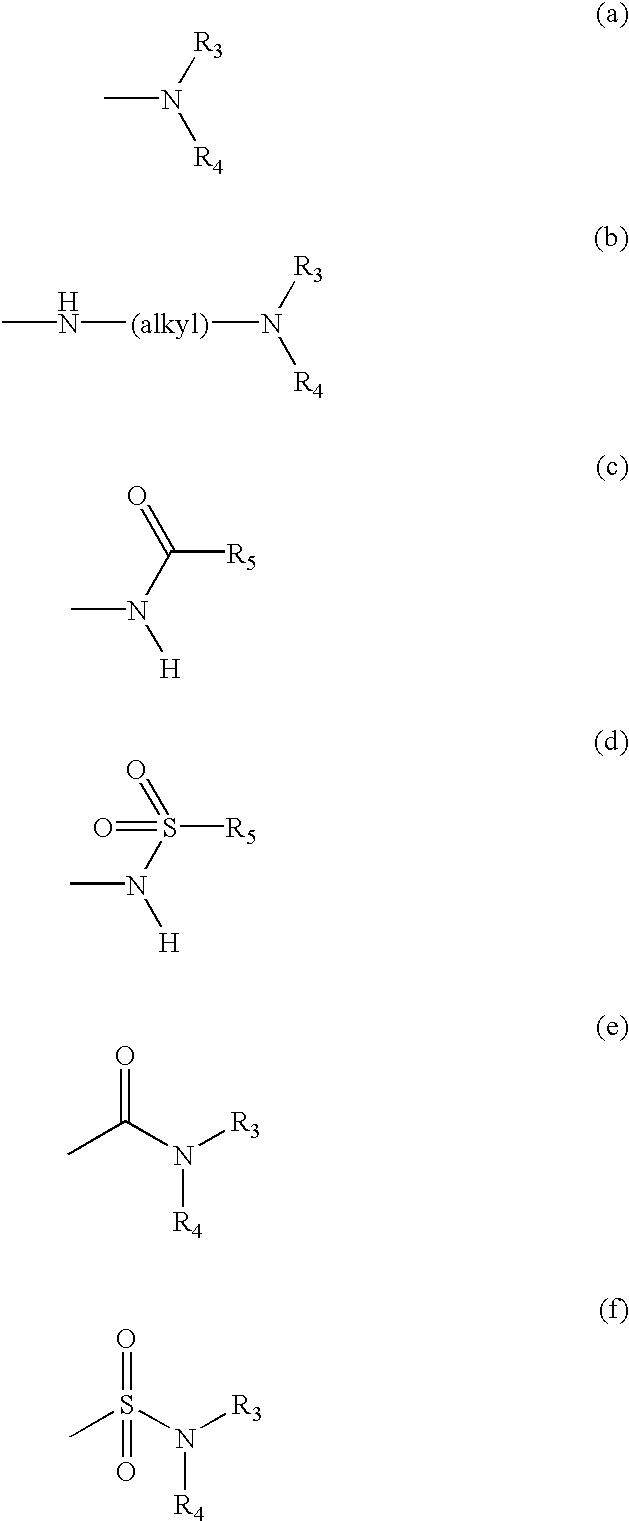
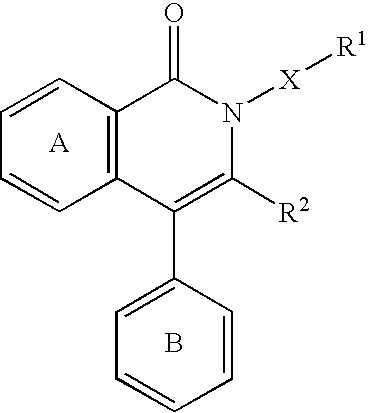
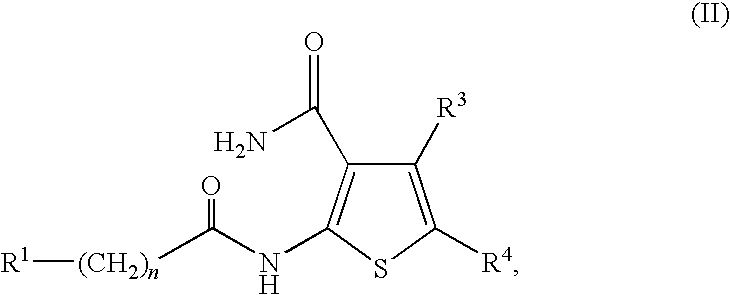
A JNK inhibitor containing a compound having an isoquinolinone skeleton or a salt thereof, such as a compound represented by the formula wherein ring A and ring B are each an optionally substituted benzene ring, X is —O—, —N═, —NR3— or —CHR3—, R2 is an acyl group, an optionally esterified or thioesterified carboxyl group, an optionally substituted carbamoyl group or an optionally substituted amino group and the like, a broken line shows a single bond or a double bond, and R1 is a hydrogen atom, an optionally substituted hydrocarbon group, an optionally substituted heterocyclic group and the like, and the like.
Owner:TAKEDA PHARMA CO LTD
Allosteric jnk inhibitors
Owner:BURNHAM INST FOR MEDICAL RES
Cell-permeable peptide inhibitors of the JNK signal transduction pathway
The present invention refers to protein kinase inhibitors and more specifically to inhibitors of the protein kinase c-Jun amino terminal kinase. Additionally, the present invention provides JNK inhibitor sequences, chimeric peptides, nucleic acids encoding same as well as pharmaceutical compositions for treating pathophysiologies associated with JNK signaling.
Owner:希根因弗兰曼斯有限公司
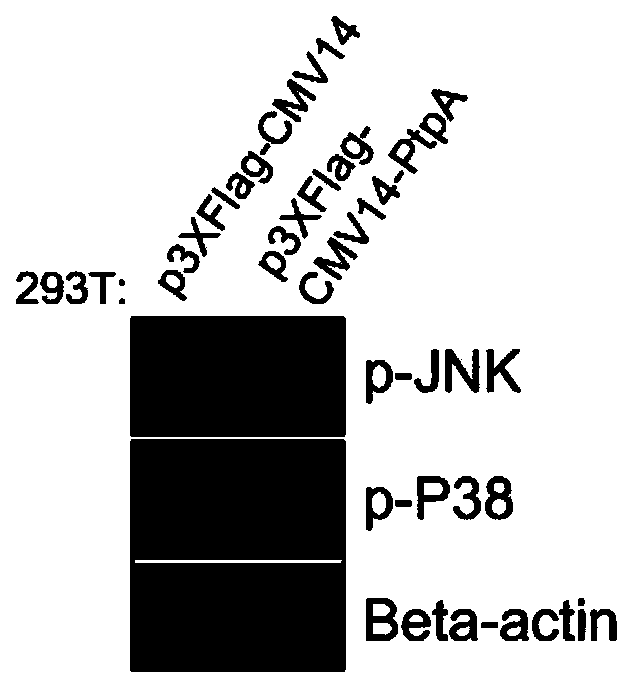
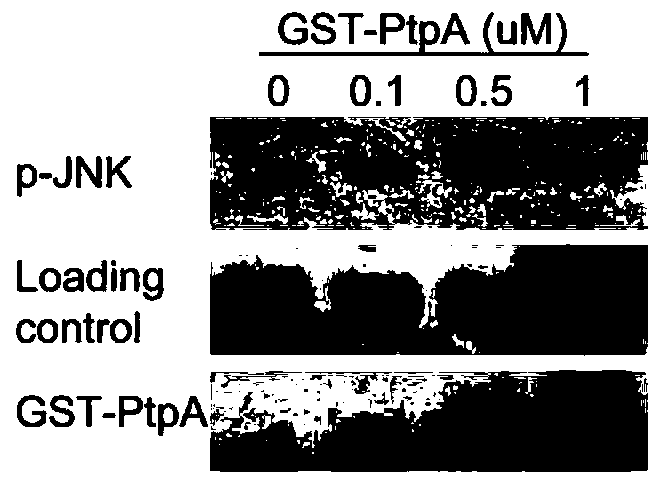
Mycobacterium tuberculosis secretory protein PtpA capable of dephosphorylating p-JNK and p-P38
The invention discloses a mycobacterium tuberculosis secretory protein PtpA capable of dephosphorylating p-JNK and p-P38, and belongs to the cytobiology field. The invention ascertains the mycobacterium tuberculosis secretory protein PtpA capable of inhibiting two signal pathways of p-JNK and p-P38, and provides a protein, namely ubiquitin, capable of increasing PtpA activity for the first time. The secretory protein PtpA and the ubiquitin can provide novel tools and ideas for treatment of a plurality of clinical diseases, particularly have a broad prospect in aspects of development and clinical applications of a plurality of medicines such as antineoplastic medicines, and other aspects, and can be directly used for the scientific research field or for guiding development of JNK inhibitors and P38 inhibitors. In addition, the secretory protein PtpA and the ubiquitin have important theory meaning for searching new medicine targets and screening new medicines.
Owner:INST OF MICROBIOLOGY - CHINESE ACAD OF SCI
Method for monitoring metastasis of cancer cells using cells cultured in three dimensional collagen environment
InactiveUS20160109450A1Increase valueCompound screeningApoptosis detectionLymphatic SpreadCell-Extracellular Matrix
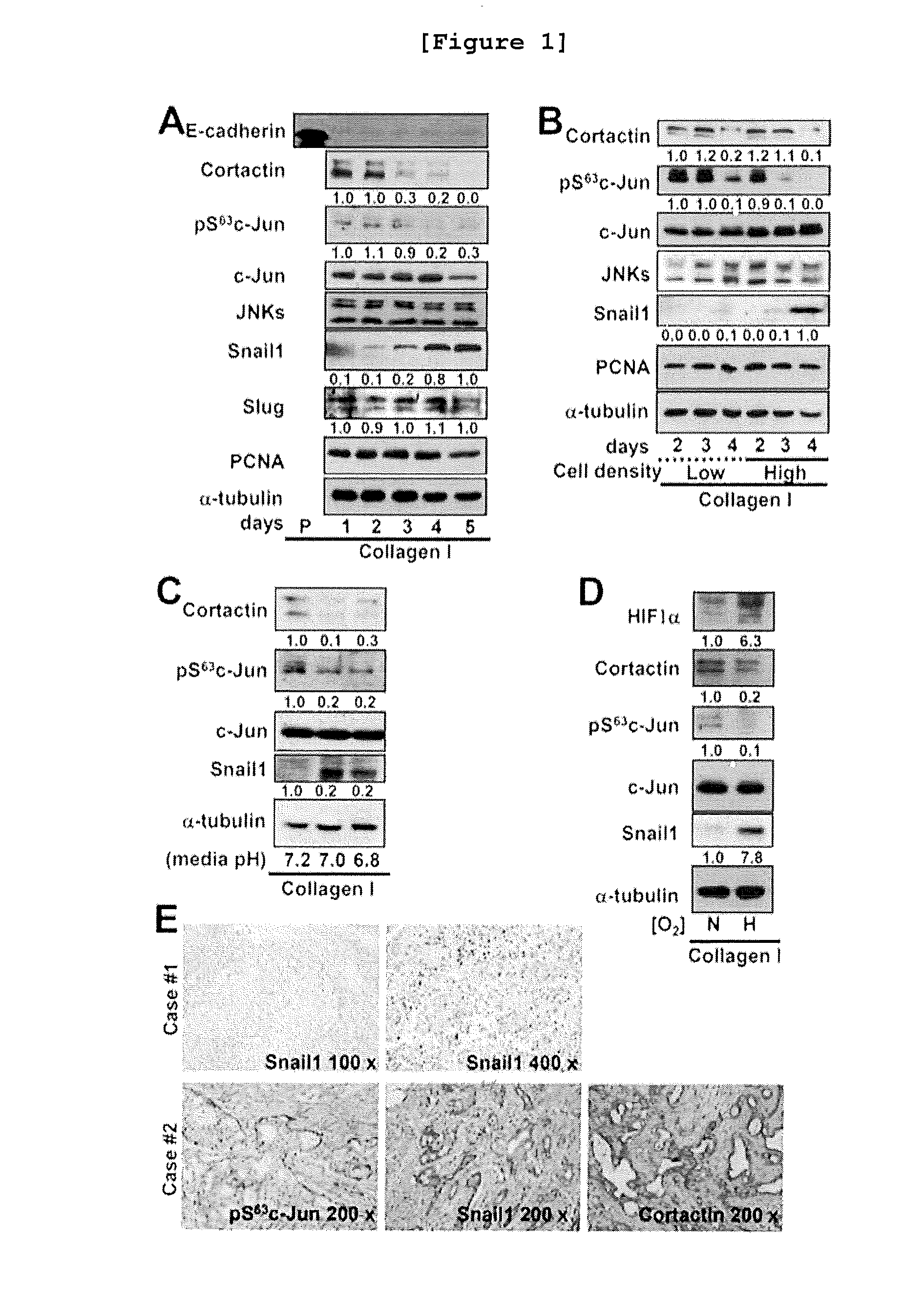
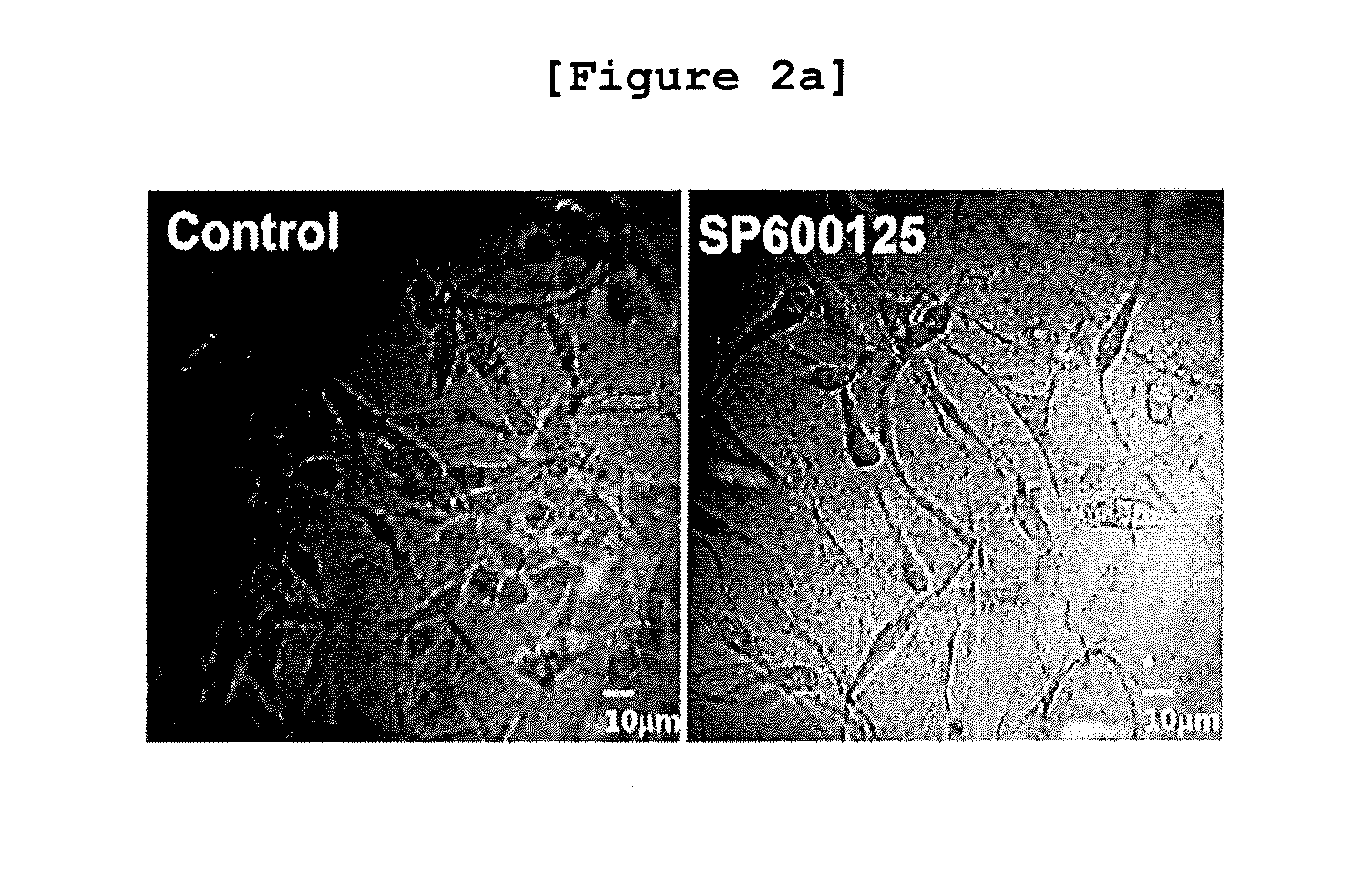
The present invention relates to a method for monitoring migration, invasion, and metastasis of cancer cells by observing the shape of cancer cells cultured in a three-dimensional environment and measuring the activity, expression, and changes in expression sites of proteins associated with invadopodia formation and metastasis, and the degradation of an extracellular matrix; and to a method for screening a cancer metastasis inhibitor. More specifically, it was verified that the reduction in c-Jun phosphorylation induced the increase in snail1 and the decrease in cortactin expression in the cells cultured in a three-dimensional environment and the expression regulation relations between the proteins were identical to those in breast cancer tissues obtained from patients. In addition, it was verified that, when breast cancer cells in a three-dimensional collagen gel environment were treated with a JNK inhibitor, the shape of the cells became longer; the contact region of the cells and the extracellular matrix became flattened and thinner; the migration of cancer cells was decreased; and the changes in protein expression was observed, such as the increase in TGFβ1 expression, the increases in smad2 and smad3 expression and activity, the increase in snail1 expression, the decrease in cortactin expression, and the resulting decrease in invadopodia formation. In addition, in a three-dimensional collagen gel environment, MT1-MMP besides the cortactin can be used as a marker of invadopodia, and it was verified that the inhibition of JNK led to the decrease in cortactin expression and the increase in snail1 expression, badly influenced the site and role of MT1-MMP to inhibit the formation of invadopodia, and inhibited the degrading activity of a collagen gel substrate. Thus, the present invention can be used as a method for monitoring migration, invasion, metastasis, and the degree of metastasis of cancer cells and a method for screening a cancer metastasis inhibitor, and will be useful as one of screening methods capable of creating low-cost, high-efficient added value at the time of pre-clinical tests required for drug development.
Owner:MEDICINAL BIOCONVERGENCE RES CENT
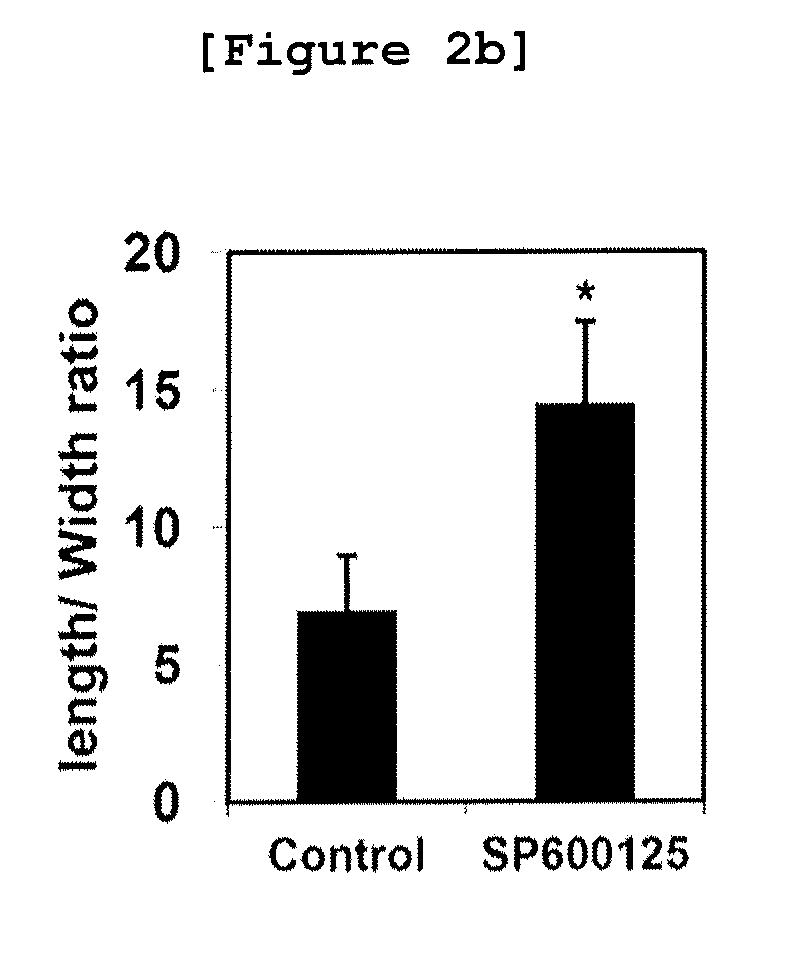
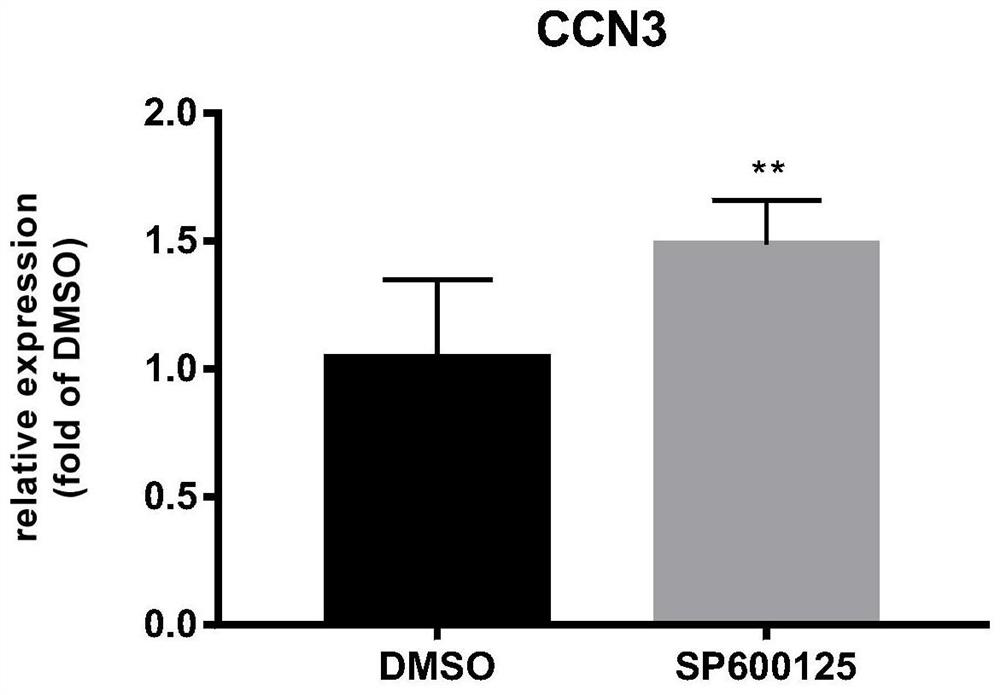
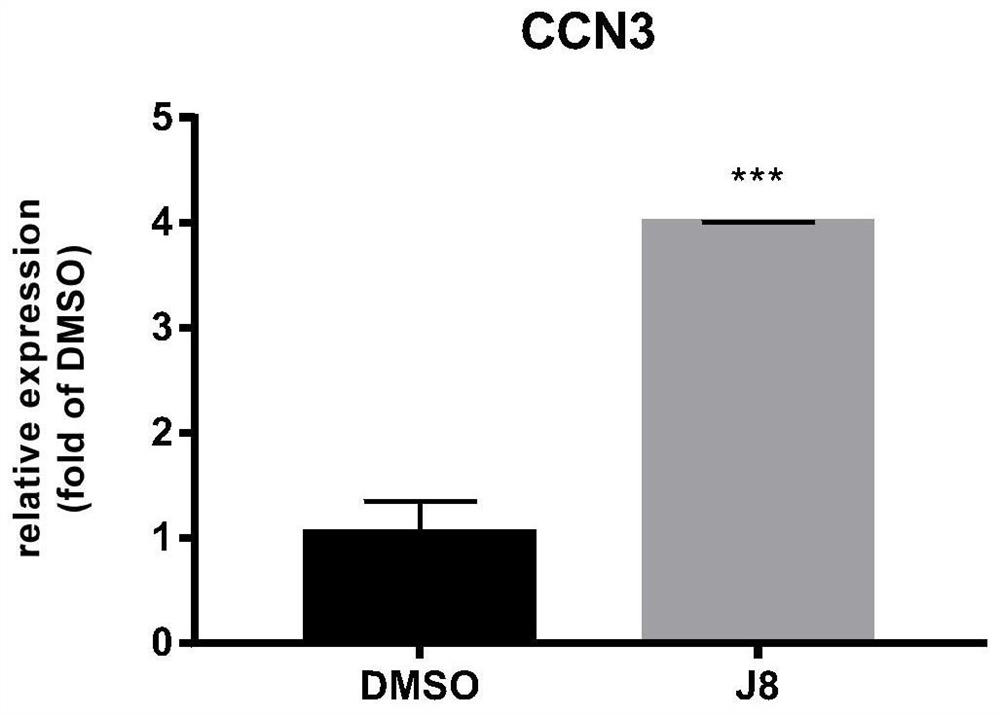
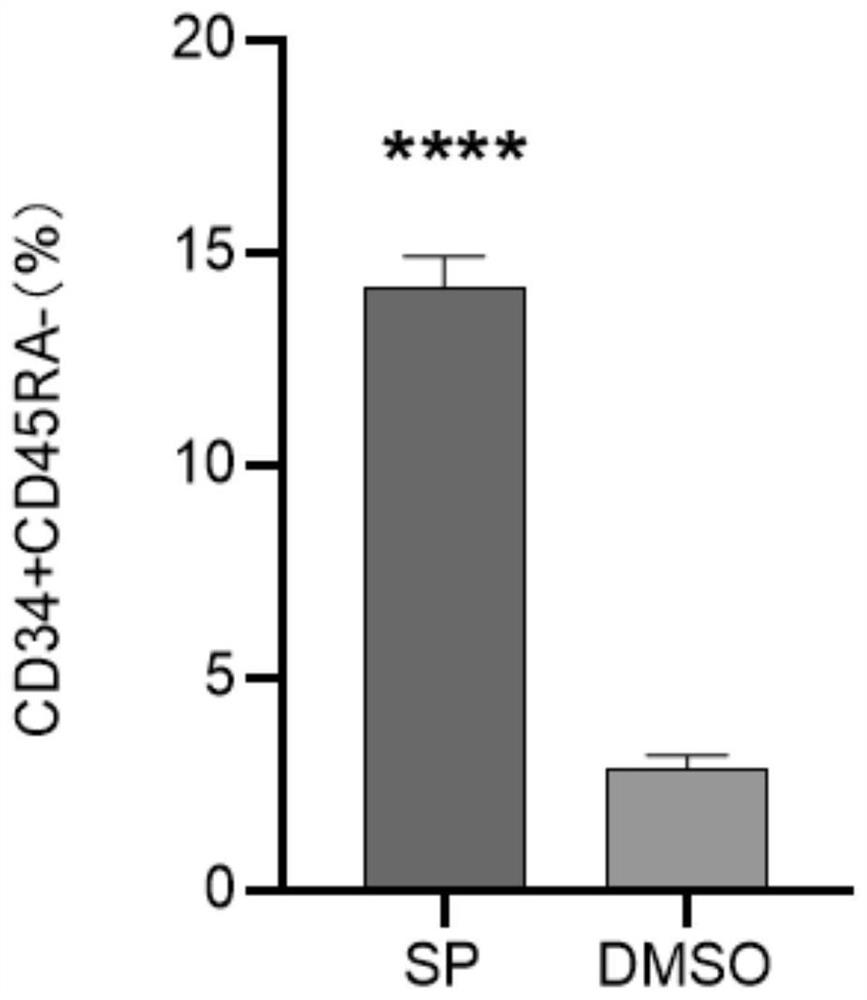
Method for enhancing hematopoietic stem cell transplantation capacity
ActiveCN112048474AHigh transplantabilityReduce accumulationBlood/immune system cellsCell culture active agentsHematopoietic stem cell transplantationHematopoietic stem cell
The invention discloses a method for enhancing hematopoietic stem cell transplantation capacity. The hematopoietic stem cell transplantation capacity is enhanced by up-regulating expression of a hematopoietic stem cell CCN3 gene by adopting a JNK inhibitor. The method is clear in action mechanism, and the hematopoietic stem cells with high transplantation capacity can be effectively obtained, so that the problem that the number of the hematopoietic stem cells derived from the graft is insufficient is effectively solved.
Owner:SUSHENG BIOTECH (HAINAN) CO LTD
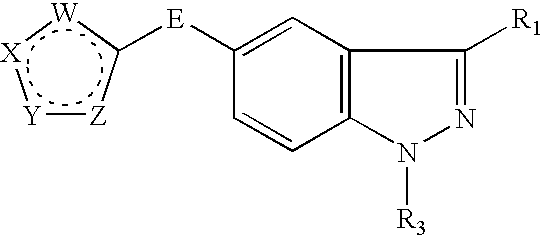
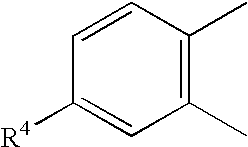
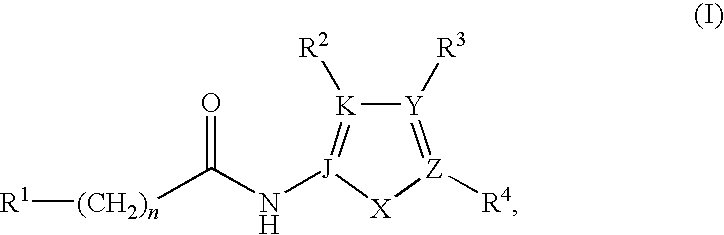
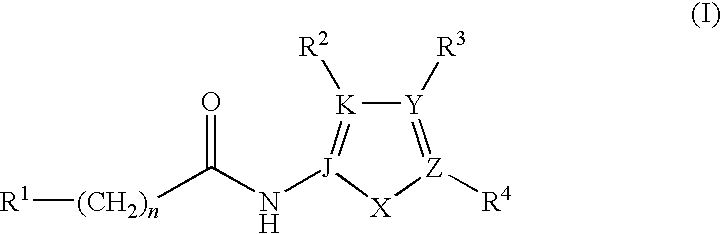
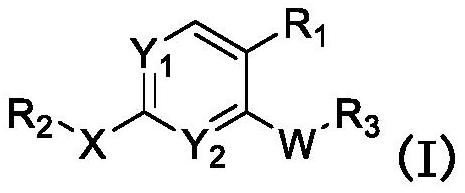
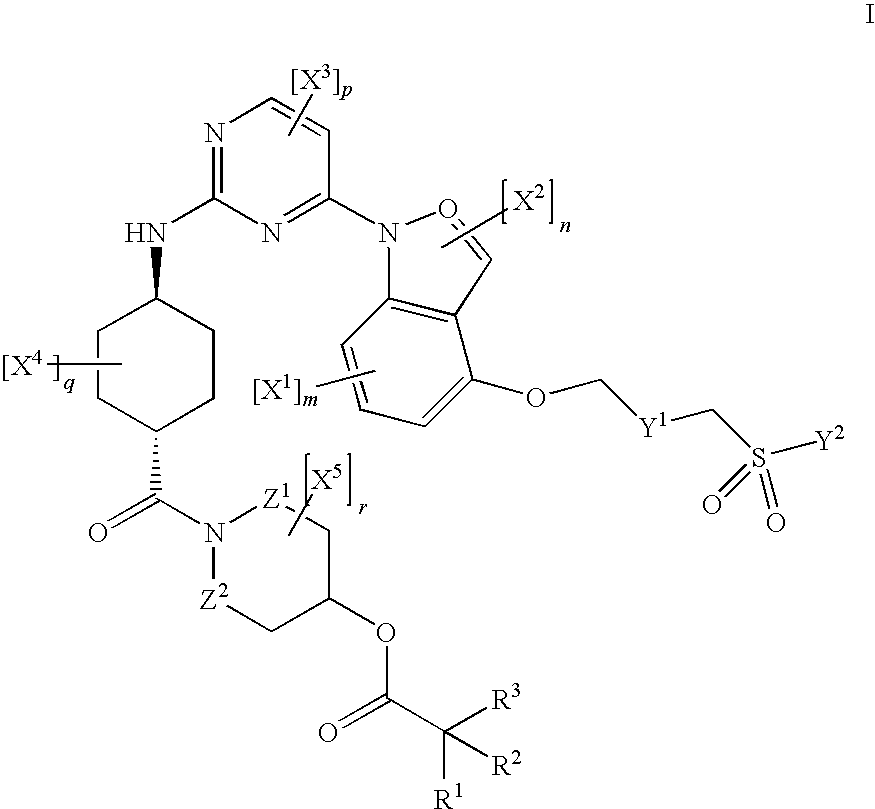
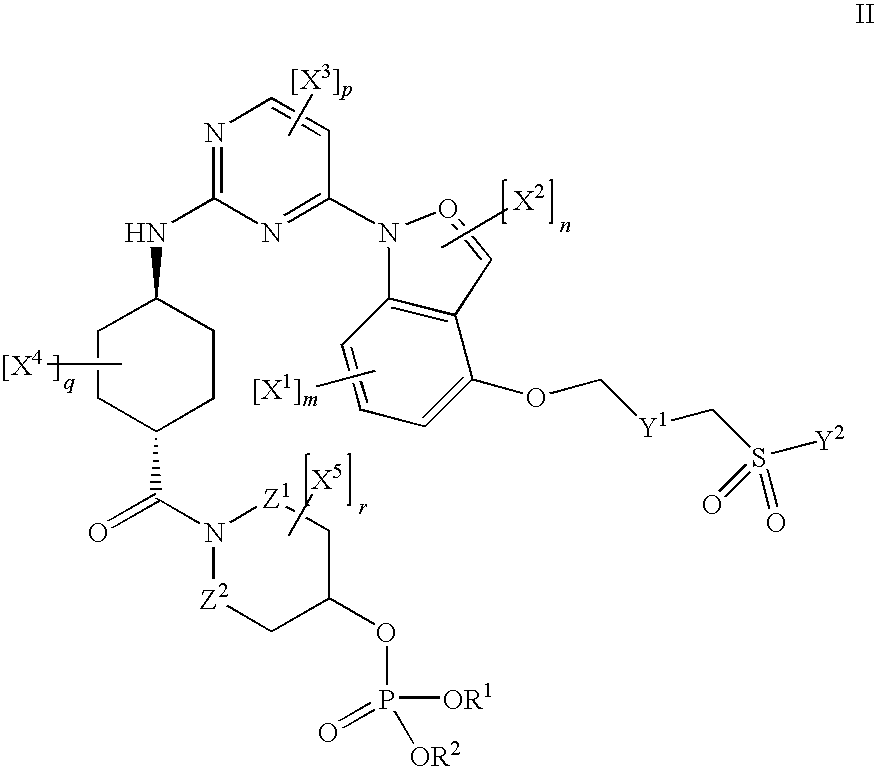
JNK inhibitor as well as pharmaceutical composition and application thereof
PendingCN112574123AExcellent JNK inhibitory activityImprove securityAntibacterial agentsOrganic active ingredientsDiseaseNitrogen oxides
The invention provides a compound represented by a formula (I), racemates, stereoisomers, tautomers, isotope markers, solvates, polymorphic substances, nitrogen oxides, or pharmaceutically acceptablesalts thereof, and application as a JNK inhibitor. The invention also provides a preparation method of the compound shown in the formula (I), a pharmaceutical composition containing the compound shownin the formula (I), and application of the compound shown in the formula (I) to preparation of a medicine, and the medicine is used for treating diseases which can be treated by inhibiting the activity of JNK.
Owner:WUHAN LL SCI & TECH DEV
Methods of Using and Compositions Comprising a Jnk Inhibitor for the Treatment and Management of Asbestos-Related Diseases and Disorders
InactiveUS20070270448A1Conveniently formedAntibacterial agentsBiocideActive agentAsbestos-related diseases
Methods for treating, preventing and / or managing an asbestos-related disease or disorder are disclosed. Specific embodiments encompass the administration of a JNK Inhibitor, or a pharmaceutically acceptable salt, solvate, hydrate, stereoisomer, clathrate, or prodrug thereof, alone or in combination with a second active agent and / or chemotherapy, surgery, or radiation therapy. Pharmaceutical compositions, single unit dosage forms, and kits suitable for use in the methods of the invention are also disclosed.
Owner:CELGENE CORP
Composition Comprising a Jnk Inhibitor and Cyclosporin
The present invention is related to a composition comprising a JNK inhibitor and a cyclosporin, in particular for the treatment of neuronal disorders, autoimmune diseases, cancer and cardiovascular diseases.
Owner:MERCK SERONO SA
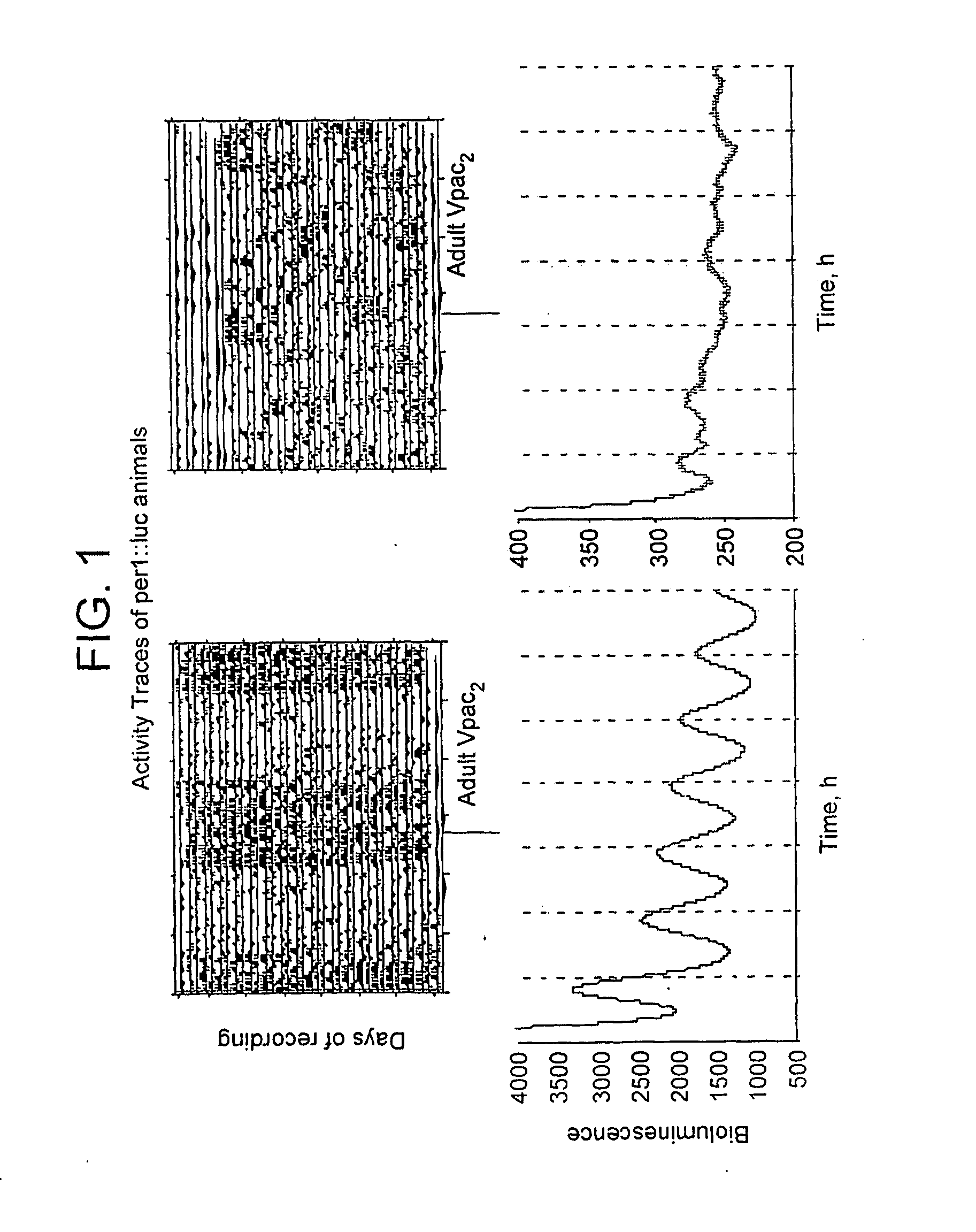
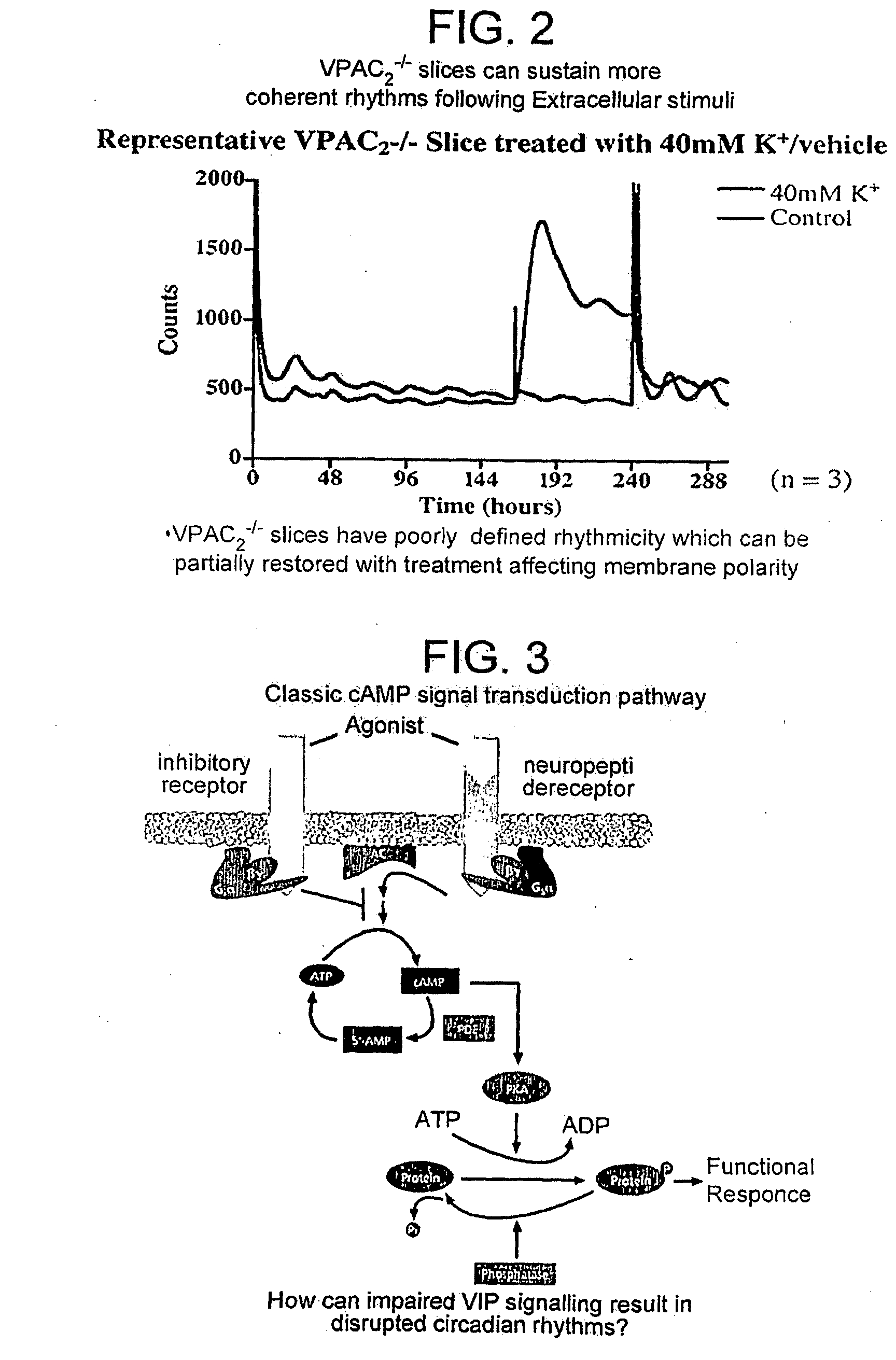
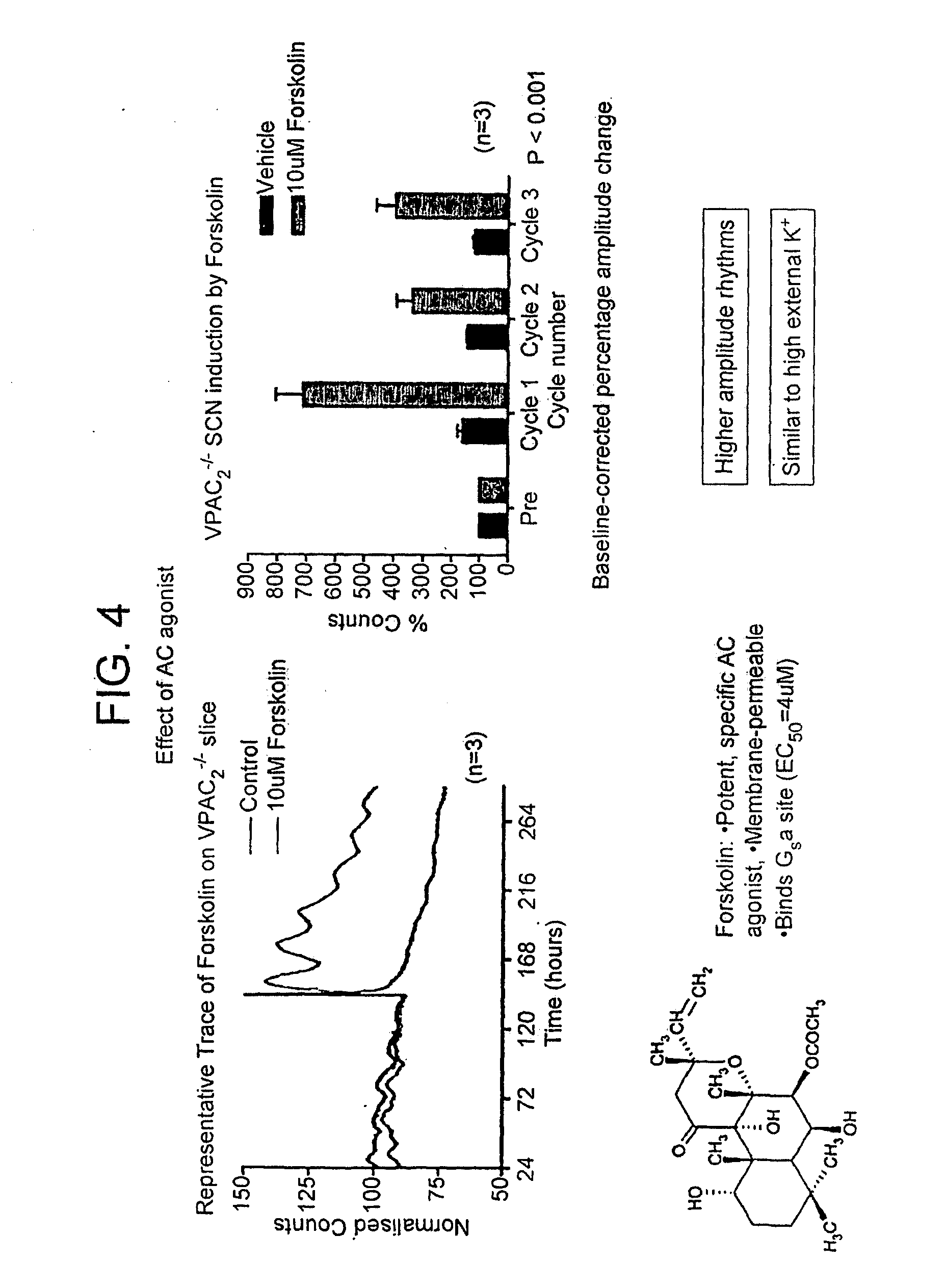
Inhibitor of Adenylyl Cyclase for Treating a Disorder of the Circadian Rhythm
InactiveUS20090264383A1Reduce brain damageBiocideNervous disorderAdenylyl cyclaseAdenylyl Cyclase Inhibitors
The invention relates to use of a composition comprising an inhibitor of adenylyl cyclase in the elongation of circadian rhythm, a method of extending the period of circadian rhythm in a subject, said method comprising administering to said subject an inhibitor of adenylyl cyclase, and to adenylyl cyclase inhibitor for use in the treatment of a disorder of the circadian rhythm. Preferably the inhibitor is a P-site inhibitor, preferably 9-(tetrahydrofuryl)-adenine. The composition may further comprise a JNK inhibitor.
Owner:MEDICAL RESEARCH COUNCIL
Jnk inhibitors for use in treating spinal muscular atrophy
InactiveUS20120077753A1Reduce degradationInhibiting and reducing degenerationOrganic active ingredientsBiocideMedicinePrimary motor neuron
The brain specific isoform (JNK3) of c-Jun NH2-terminal kinase (JNK) has been found to mediate the degeneration of spinal motor neurons caused by SMN deficiency in spinal muscular atrophy (SMA). Moreover, the ability of JNK inhibitors to reduce degeneration of neurons lacking SMN is also disclosed. The JNK signaling pathway can therefore mediate neurode-generation in SMA and represents a therapeutic target for treatment of SMA.
Owner:TEXAS TECH UNIV SYST
JNK Inhibitors for Treatment of Skin Diseases
InactiveUS20090176762A1Beneficial effect treatmentBeneficial effect preventionBiocideOrganic chemistryDiseaseParapsoriasis
This invention relates to the use of a JNK inhibitor for treating and / or preventing skin diseases selected from psoriasis and dermatitis.
Owner:MERCK SERONO SA
Aminomethyl quinolone compounds
Owner:F HOFFMANN LA ROCHE & CO AG
Inhibitors of JNK
Owner:ROCHE PALO ALTO LLC
Application of JNK inhibitor in preparing drug
ActiveCN106580999AInhibition of activationGood preventionOrganic active ingredientsAntimycoticsAnti fungalGene targeting
The invention discloses an application of a JNK inhibitor in preparing a drug. The medicines are used for preventing and treating fungal infection. The JNK inhibitor, as a gene target drug, is capable of conducting targeted therapy on the fungal infection, and the drug is strong in application and is capable of effectively improving the capacity of a body's immune system to resist the fungal infection, so as to prevent and treat the fungal infection. The JNK inhibitor can enhance the accuracy of fungus treatment from body self, and the JNK inhibitor can effectively prevent the occurrence of drug-resistance bacteria, so that a curative effect of preventing and treating the fungal infection is obviously improved. In addition, the JNK inhibitor, in comparison with existing broad-spectrum anti-fungal drugs, is low in cost and cheap in price, and the JNK inhibitor is relatively high in economic significance and clinical application value.
Owner:TSINGHUA UNIV
Inhibitors of JNK
Owner:ROCHE PALO ALTO LLC
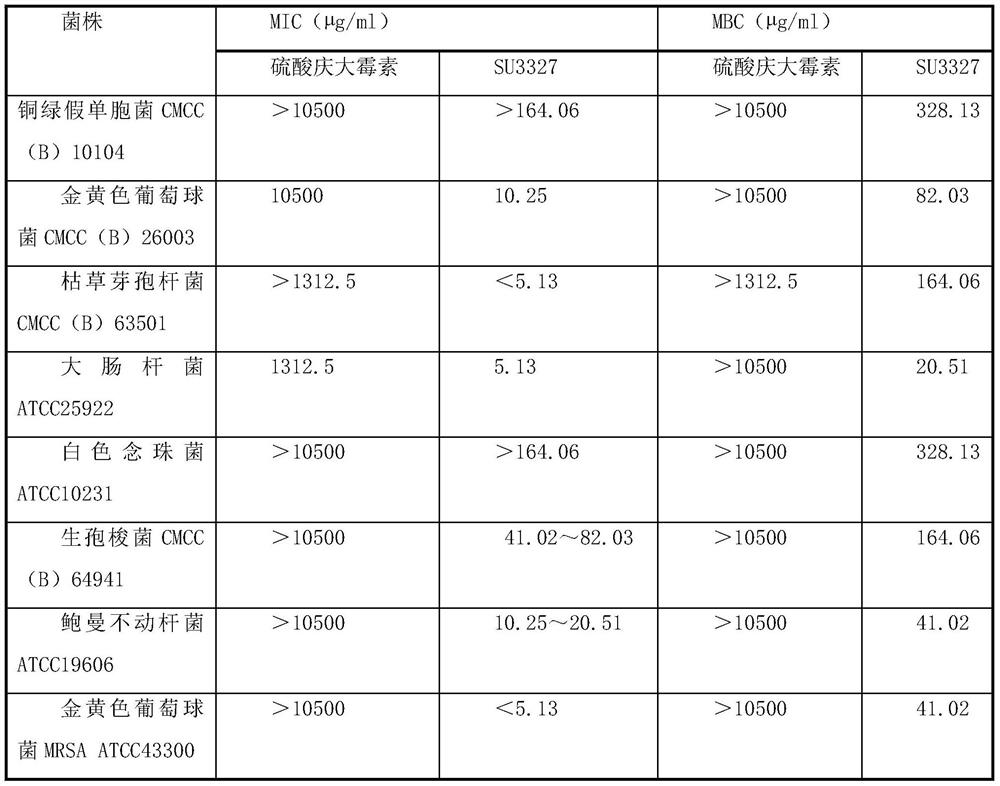
Application of C-JNK (JUN N terminal kinase) inhibitor SU3327
The invention relates to an application of a C-JNK (JUN N terminal kinase) inhibitor SU3327. The C-JNK inhibitor SU3327 is used for bacteriostasis of different animal and human species. The C-JNK inhibitor SU3327 acts on strains isolated from humans or / and animals or clinically isolated from infected persons and then cultured to kill and inhibit bacteria.
Owner:陈洪亮
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com