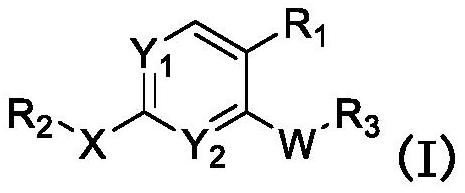
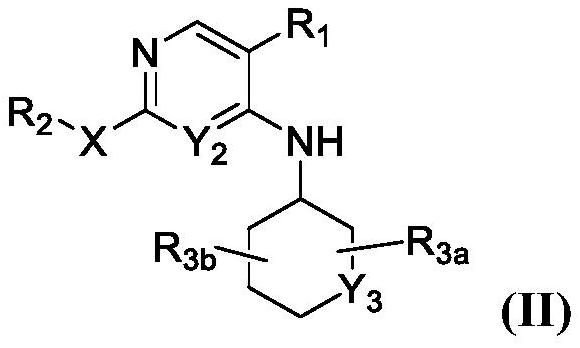
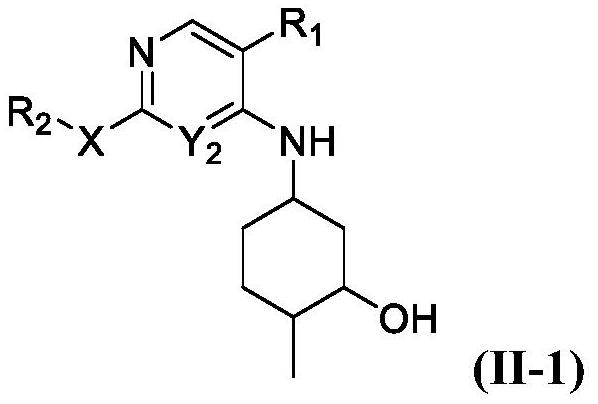
JNK inhibitor as well as pharmaceutical composition and application thereof
A compound and solvate technology, applied in the field of compounds that can inhibit the activity of JNK, can solve problems such as the complexity of liver fibrosis reversal process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0381] Embodiment 1: the preparation of compound (T001A) and (T001)
[0382]
[0383] 1.1 Preparation of compound (T001-2)
[0384] To a solution of 5-bromo-2,4-dichloropyrimidine (4g, 17.4mmol) in acetonitrile (40ml) was added sodium methylthiolate (2.4g, 34.8mmol) at room temperature. After the addition was complete, stir overnight at room temperature. Water was added and extracted with ethyl acetate. The organic phase was collected, dried and concentrated under reduced pressure, and then purified by silica gel column chromatography (EA / PE=1 / 50) to obtain 3.48 g of white solid, with a yield of 82.8%.
[0385] 1 H NMR (400MHz, CDCl 3 ): δ=8.31(s, 1H), 2.59(s, 3H).
[0386] 1.2 Preparation of compound (T001-3)
[0387] Compound T001-2 (3.48 g, 14.54 mmol), dioxane (10 ml) and tert-butylamine (6.38 g, 87.2 mmol) were added to the sealed tube. After the addition was completed, the tube was sealed and reacted at 100°C for 72 hours. The completion of the reaction was mo...
Embodiment 2
[0399] Embodiment 2: the preparation of compound (T002)
[0400] Hydroxylamine hydrochloride (114 mg, 1.65 mmol) and triethylamine (166 mg, 1.65 mmol) were added to a solution of compound T001A (100 mg, 0.33 mmol) in ethanol (2 ml) at room temperature. After heating to 80°C for 2 hours, it was cooled to room temperature, concentrated under reduced pressure, purified by preparative high performance liquid chromatography (ammonium bicarbonate method), and freeze-dried to obtain 100 mg of white solid with a yield of 90%.
[0401] 1 H NMR (400MHz, DMSO-d 6 ):δ=9.48(s,1H),8.43-8.34(m,1H),8.09(s,1H),6.17(s,1H),5.72(s,1H),4.57-4.56(m,1H), 3.90-3.87(m,1H),2.98-2.96(m,1H),2.17-2.14(m,1H),1.95-1.92(m,1H),1.69-1.65(m,1H),1.36(s,9H ), 1.23-0.98 (m, 4H), 0.94 (d, J=6Hz, 3H); Rt=3.214min, [M+H]+=337.2.
Embodiment 3
[0402] Embodiment 3: the preparation of compound (T003)
[0403]
[0404] To a solution of compound T002 (50 mg, 0.148 mmol) in methanol (3 ml) was added 2 drops of acetic acid, 2 drops of acetic anhydride and wet palladium on carbon (about 10 mg) at room temperature. After the addition was completed, the mixture was replaced with nitrogen and then with hydrogen, and stirred overnight at room temperature. After concentration under reduced pressure, it was purified by preparative high-performance liquid chromatography (ammonium bicarbonate method), and after freeze-drying, 17.1 mg of a yellow solid was obtained, with a yield of 35.8%.
[0405] 1 H NMR (400MHz, DMSO-d 6 ):δ=10.62-10.60(br,1H),6.17(s,1H),6.57-6.28(brs,1H),6.24-6.07(brs,1H),6.04-5.96(brs,2H),4.55-4.54 (m,1H),3.87-3.84(m,1H),2.98-2.93(m,1H),2.15-2.12(m,1H),1.92-1.90(m,1H),1.67-1.63(m,1H) , 1.36(s, 9H), 1.20-0.97(m, 4H), 0.93(d, J=6.4Hz, 3H); Rt=2.846min, [M+H]+=321.2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com