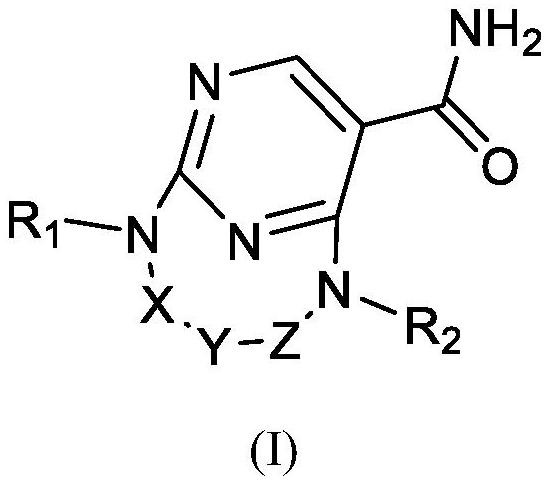
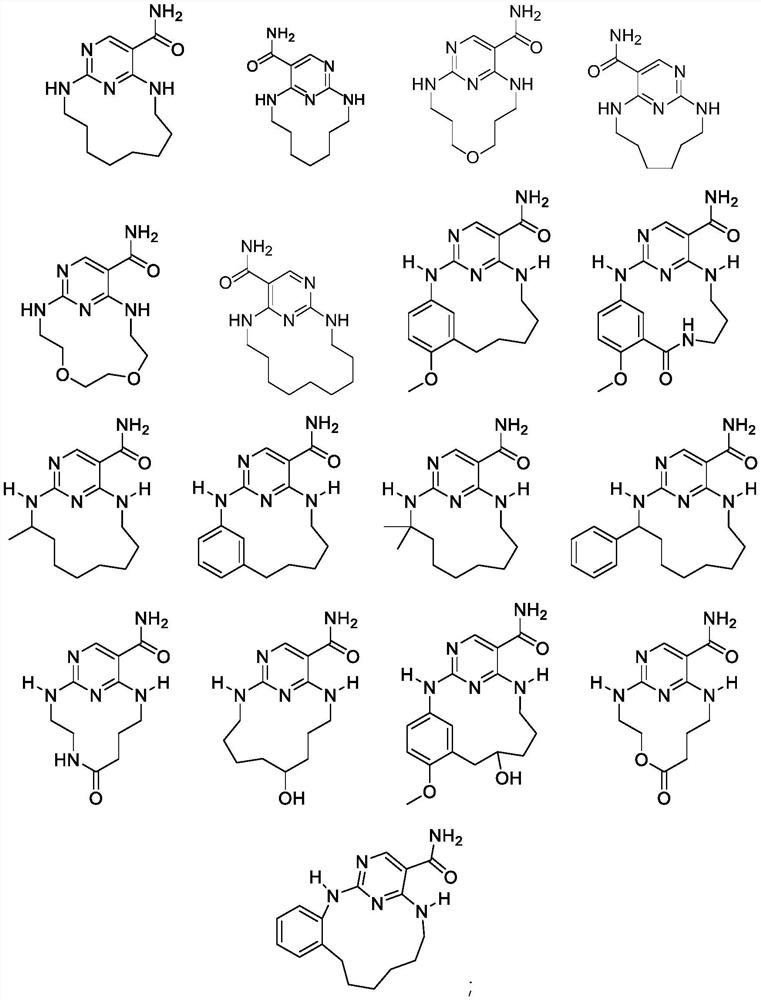
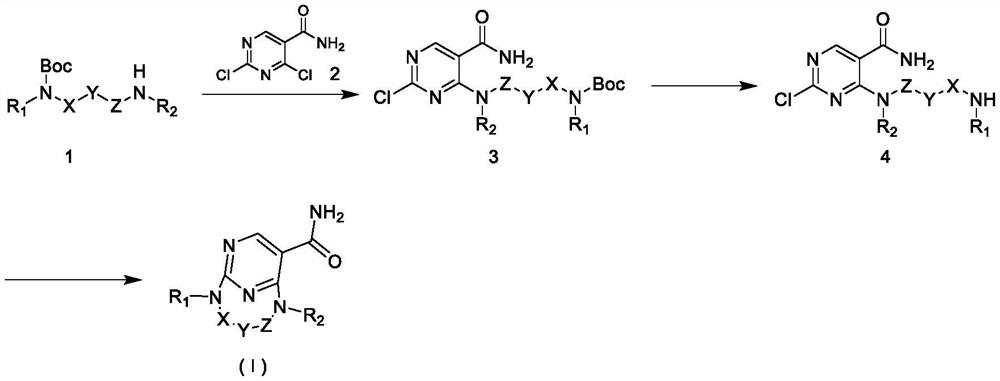
JNK inhibitor, pharmaceutical composition and application
A technology of solvates and polymorphs, applied in the field of compounds that can inhibit JNK activity, can solve the problems of complicated liver fibrosis reversal process and no drugs on the market
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0119] Example 1: 2,11-diaza-1(2,4)-pyrimidinecycloundecane-1 5 - Preparation of formamide (L001)
[0120]
[0121] 1.1 Preparation of compound (8-aminooctyl) tert-butyl carbamate (L001-2)
[0122] 1,8-Octanediamine (2 g, 13.86 mmol) and DIEA (537 mg, 4.16 mmol) were dissolved in DCM (50 mL), and to the solution was slowly added dropwise (Boc) 2 A solution of O (106 mg, 4.85 mmol) in DCM (20 mL) was added dropwise, and the reaction was stirred at room temperature for 12 hours. After the reaction was completed, the reaction solution was adjusted to pH=3~4, extracted with water (50mL*3), the aqueous phase was combined, then the aqueous phase was adjusted to pH=9~10, then extracted with DCM (50mL*3), the organic phase was collected, and no After drying with sodium sulfate, spin-dry to obtain 600 mg of white solid product, which is the title compound L001-2, yield: 12%, LC-MS [M+H] + :245.0.
[0123] 1.2 Preparation of compound (8-((5-carbamoyl-2-chloropyrimidin-4-yl)amino)...
Embodiment 2
[0130] Example 2: Compound 2,10-diaza-1(2,4)-pyrimidinylcyclodecane-1 5 - Preparation of formamide (L002)
[0131]
[0132] 2.1 Preparation of compound (7-aminoheptyl) tert-butyl carbamate (L002-2)
[0133] L002-1 (2.0g, 15.36mmol) and (Boc) 2 O (0.67g, 3.07mmol) was dissolved in DCM (100mL), and the reaction solution was reacted at room temperature for 4h. TLC (DCM:MeOH=10:1) showed that the reaction was complete. The reaction solution was added dropwise to DCM (100mL) and water (50mL), the aqueous phase was extracted with DCM (100mL*3), the organic phases were combined, washed with saturated brine (100mL), dried over anhydrous sodium sulfate, filtered, and the filtrate was concentrated Column (DCM:MeOH=20:1, adding 1% ammonia water) gave 0.6 g of colorless liquid, which was the title compound L002-2.
[0134] 2.2 Preparation of compound (7-((5-carbamoyl-2-chloropyrimidin-4-yl)amino)heptyl)carbamate tert-butyl ester (L002-3)
[0135] L002-2 (0.60g, 2.60mmol), 2,4-dich...
Embodiment 3
[0141] Example 3: Compound 6-oxa2,10-diaza-1(2,4)-pyrimidinylcyclodecane-1 5 - Preparation of formamide (L003)
[0142]
[0143] 3.1 Preparation of compound (3-(3-aminopropoxy)propyl) tert-butyl carbamate (L003-2)
[0144] 2,2'-Oxybis(ethylamine) (750 mg, 5.7 mmol) and DIEA (220 mg, 1.7 mmol) were dissolved in DCM (50 mL), and to the solution was slowly added dropwise (Boc) 2 A solution of O (433 mg, 2 mmol) in DCM (20 mL) was added dropwise, and the reaction was stirred at room temperature for 12 hours. After completion of the reaction, dilute hydrochloric acid to adjust pH=3~4, extract with water (50mL*3), combine the aqueous phase, then adjust the pH of the aqueous phase=9~10, then extract with DCM (50mL*3), collect the organic phase, After drying over sodium sulfate, spin-dry to obtain 250 mg of a white solid product, which is the title compound L003-2, yield: 19%.
[0145] 3.2 Preparation of compound (3-(3-((5-carbamoyl-2-chloropyrimidin-4-yl)amino)propoxy)propyl)ca...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com