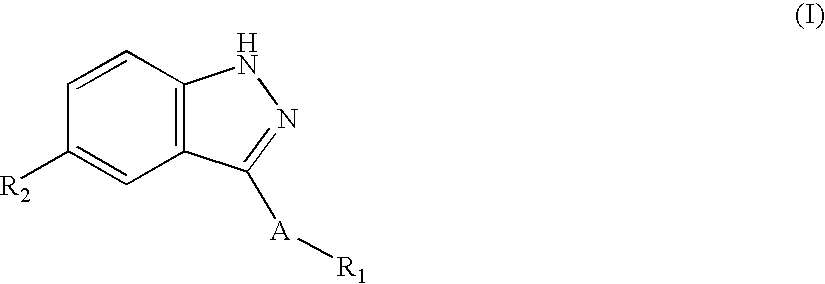
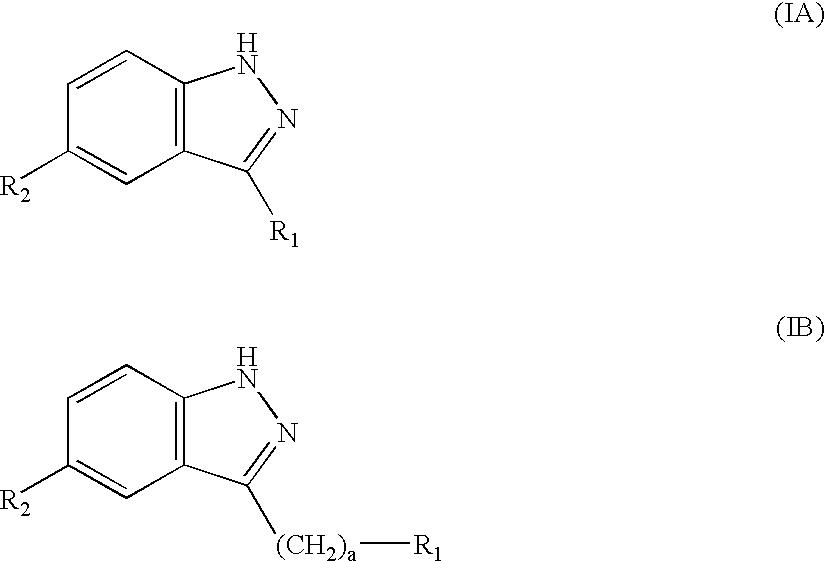
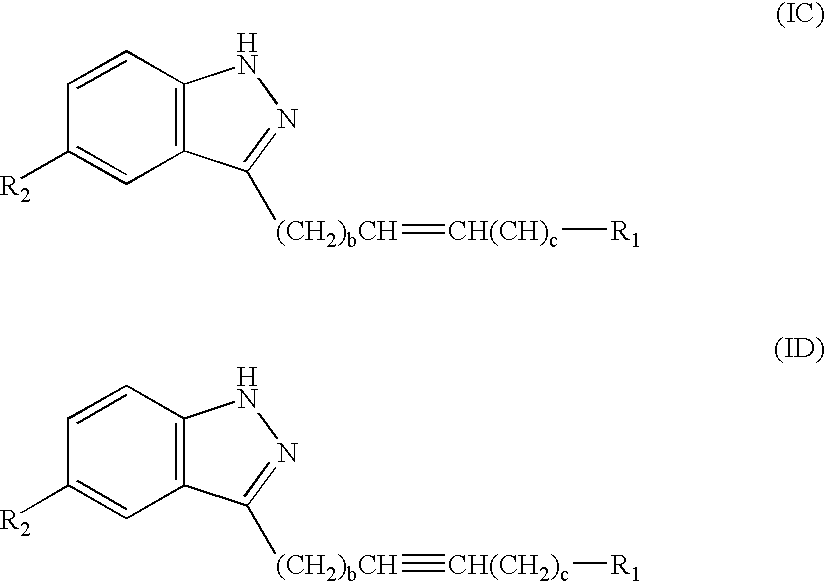
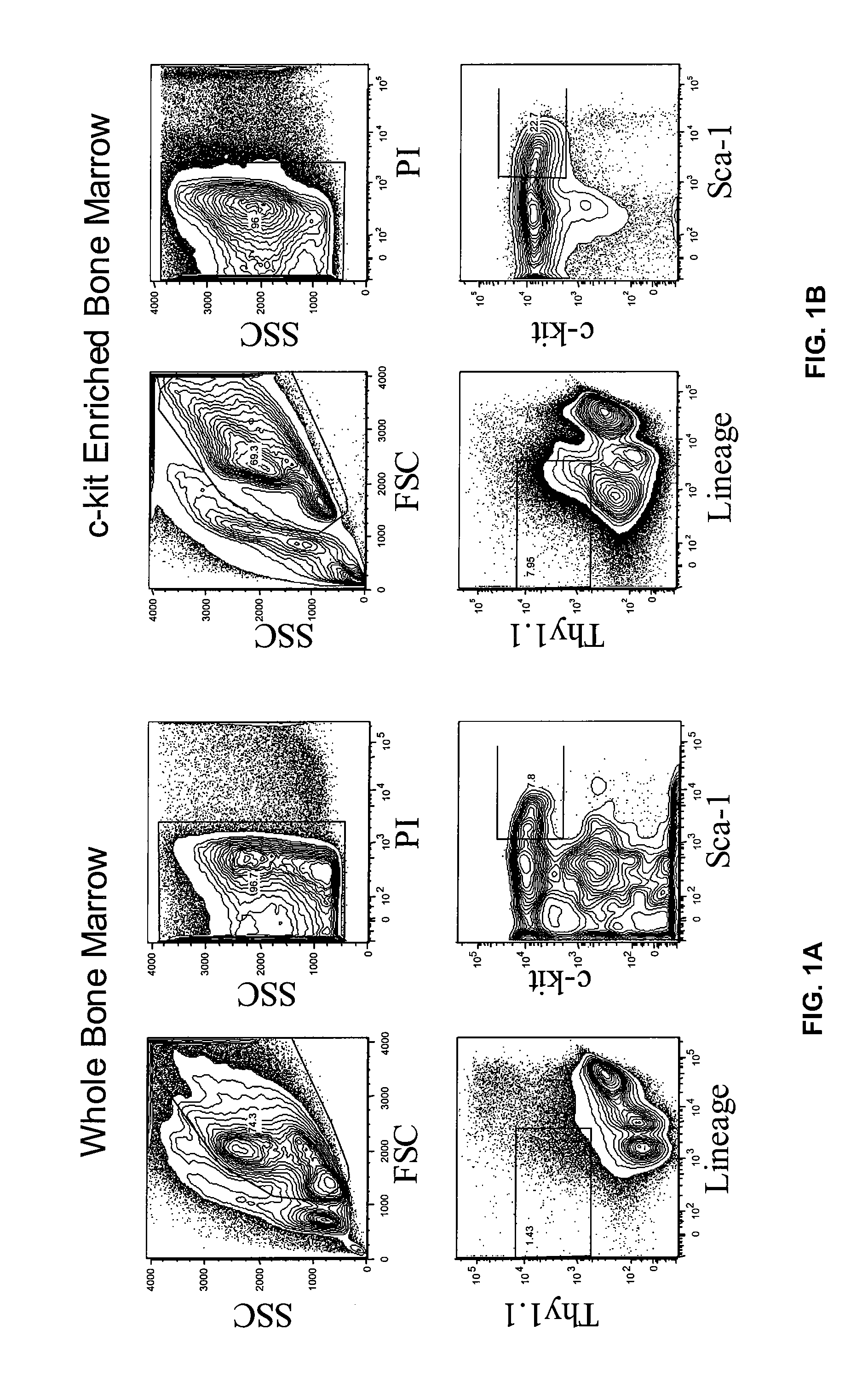
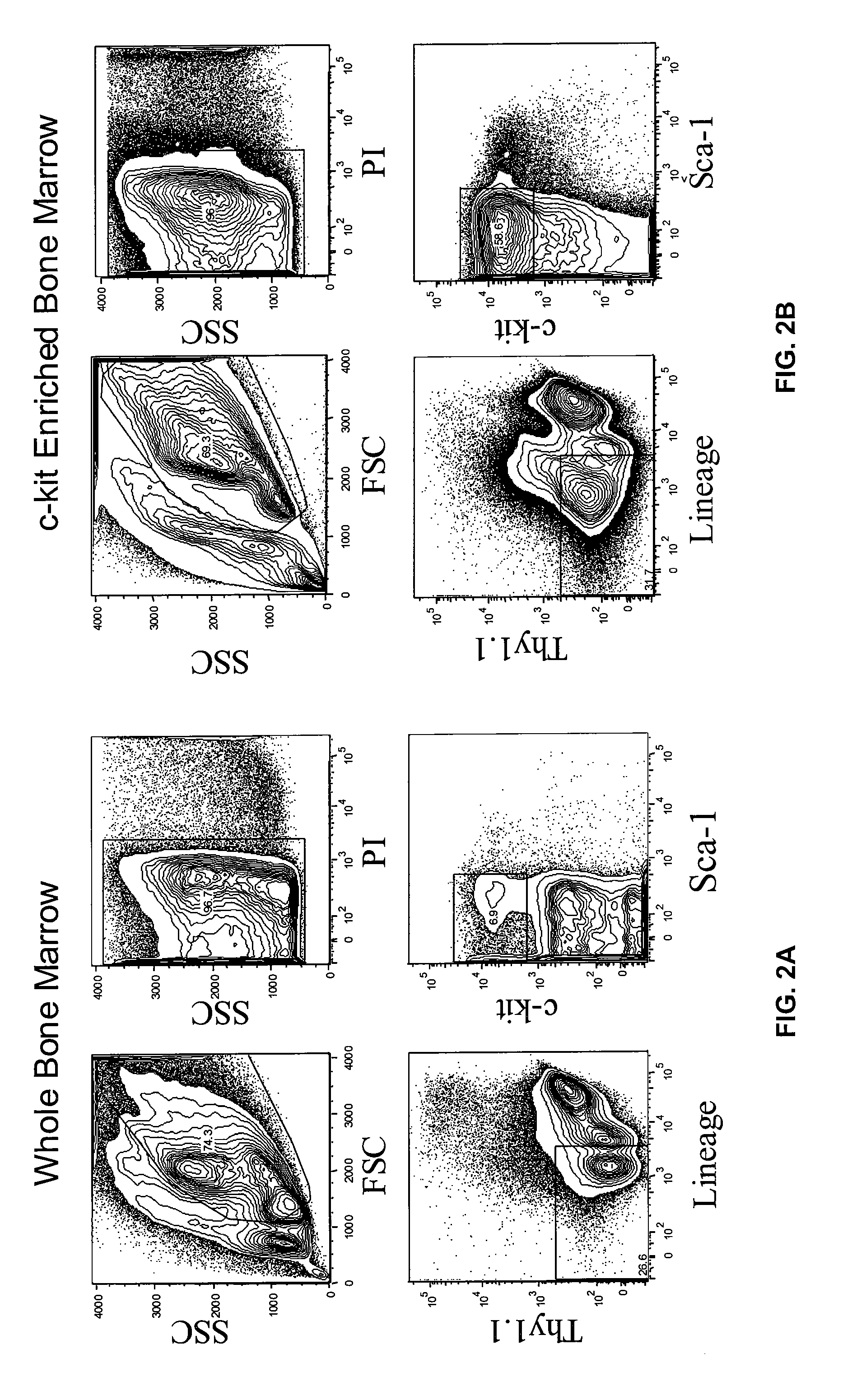
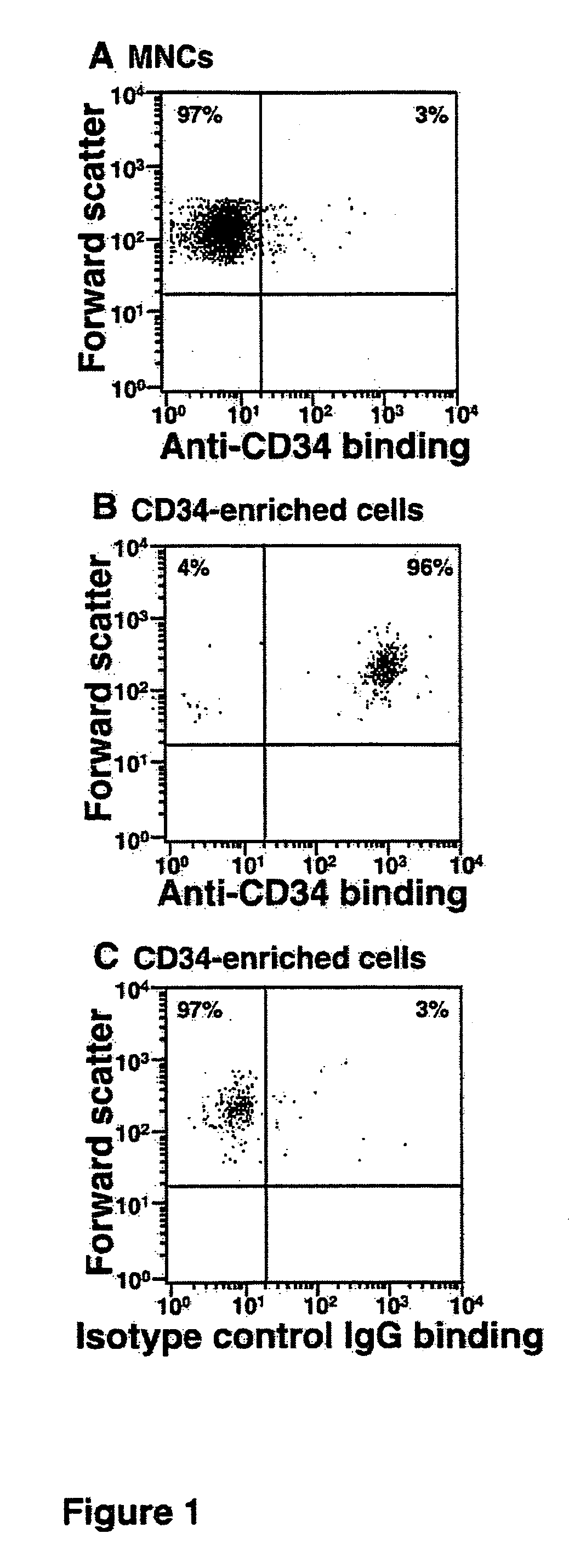
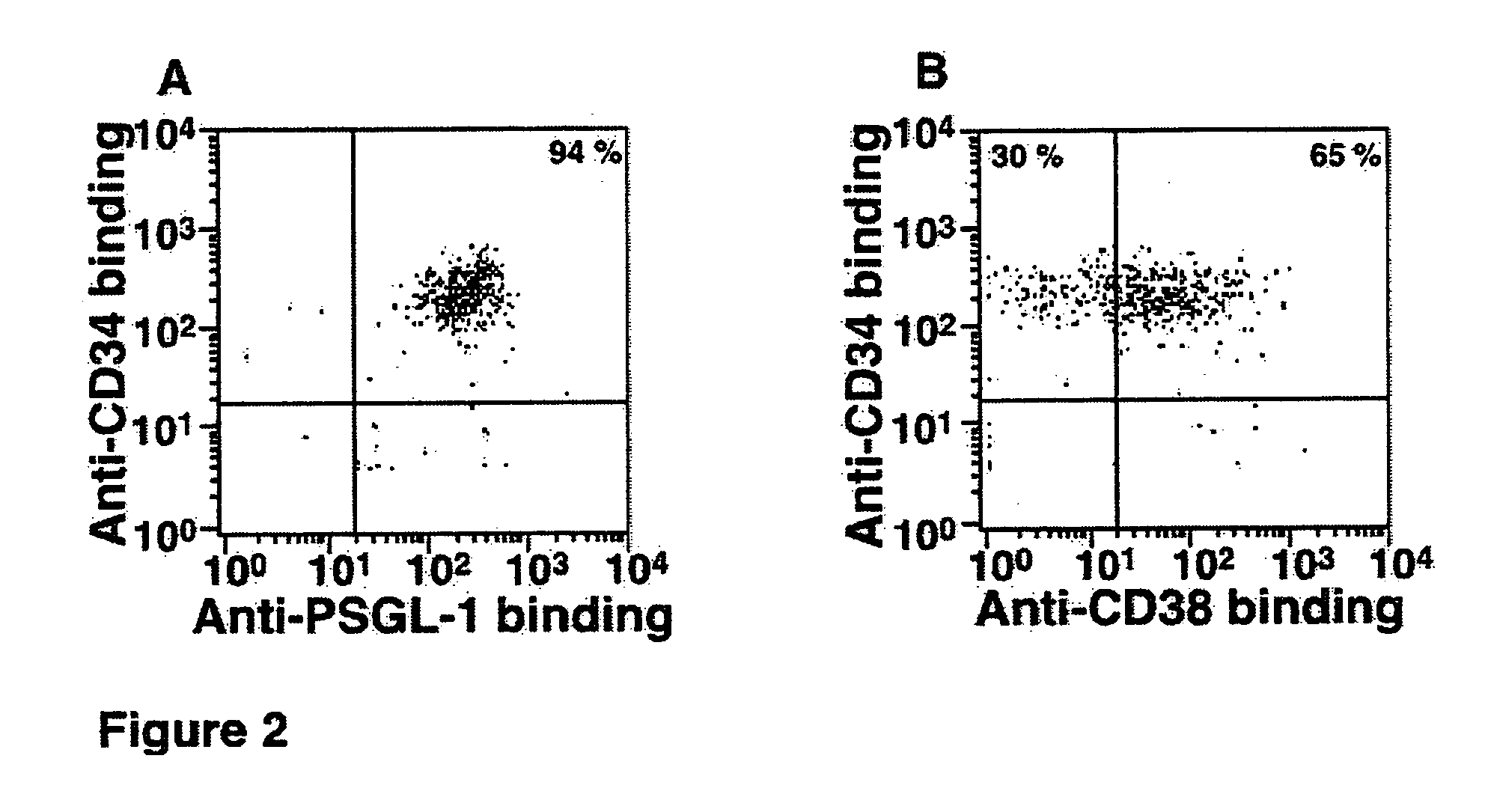
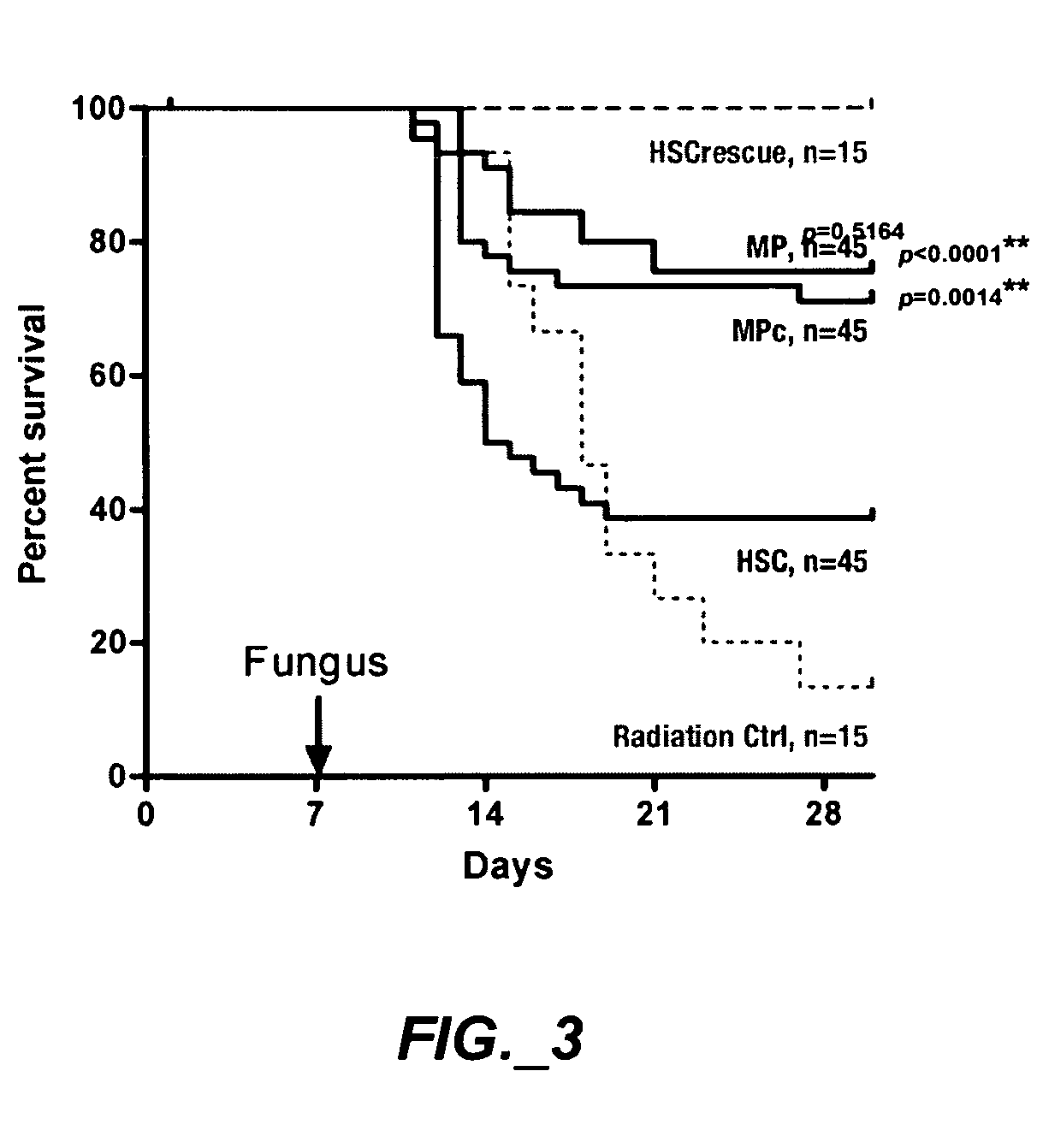
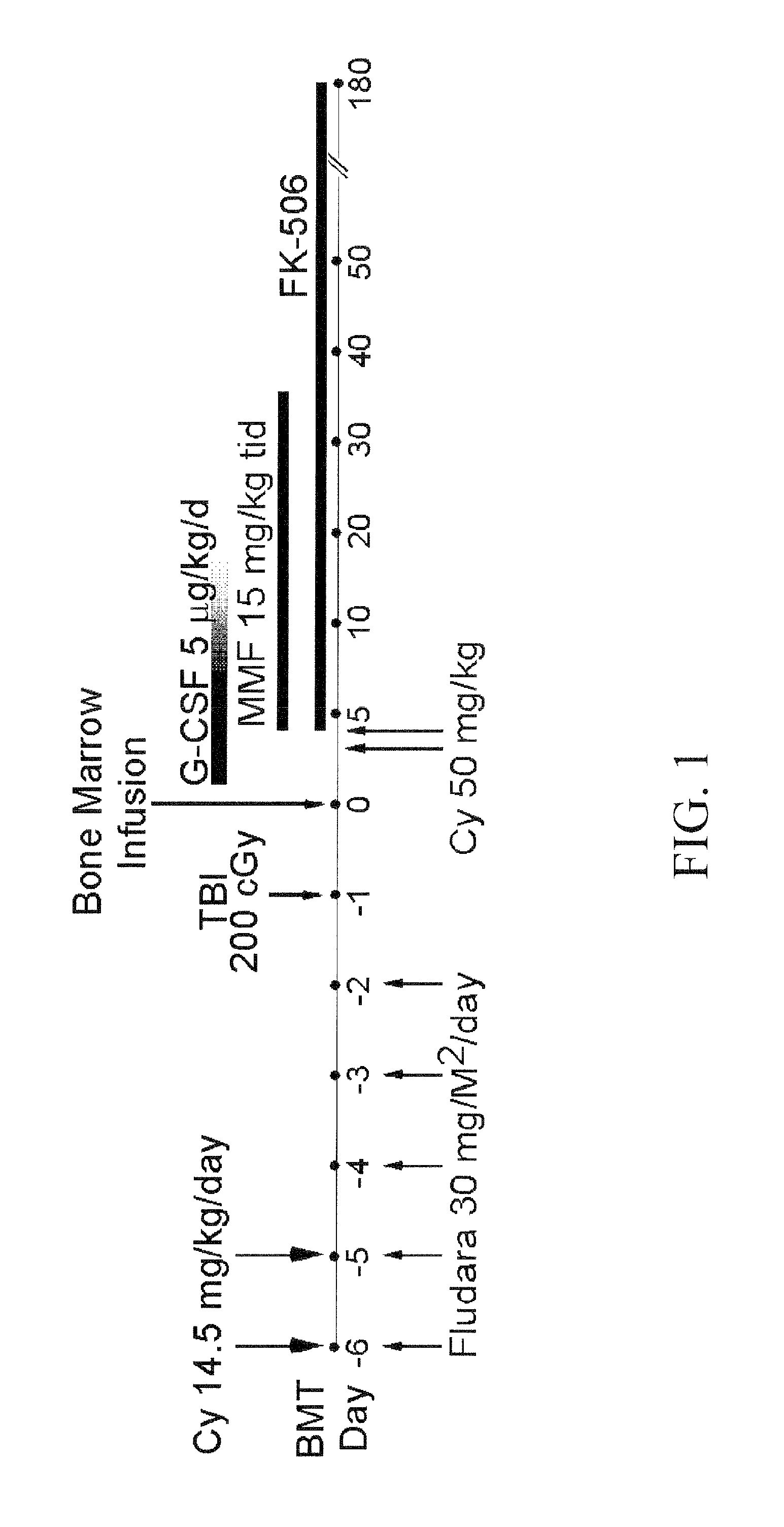
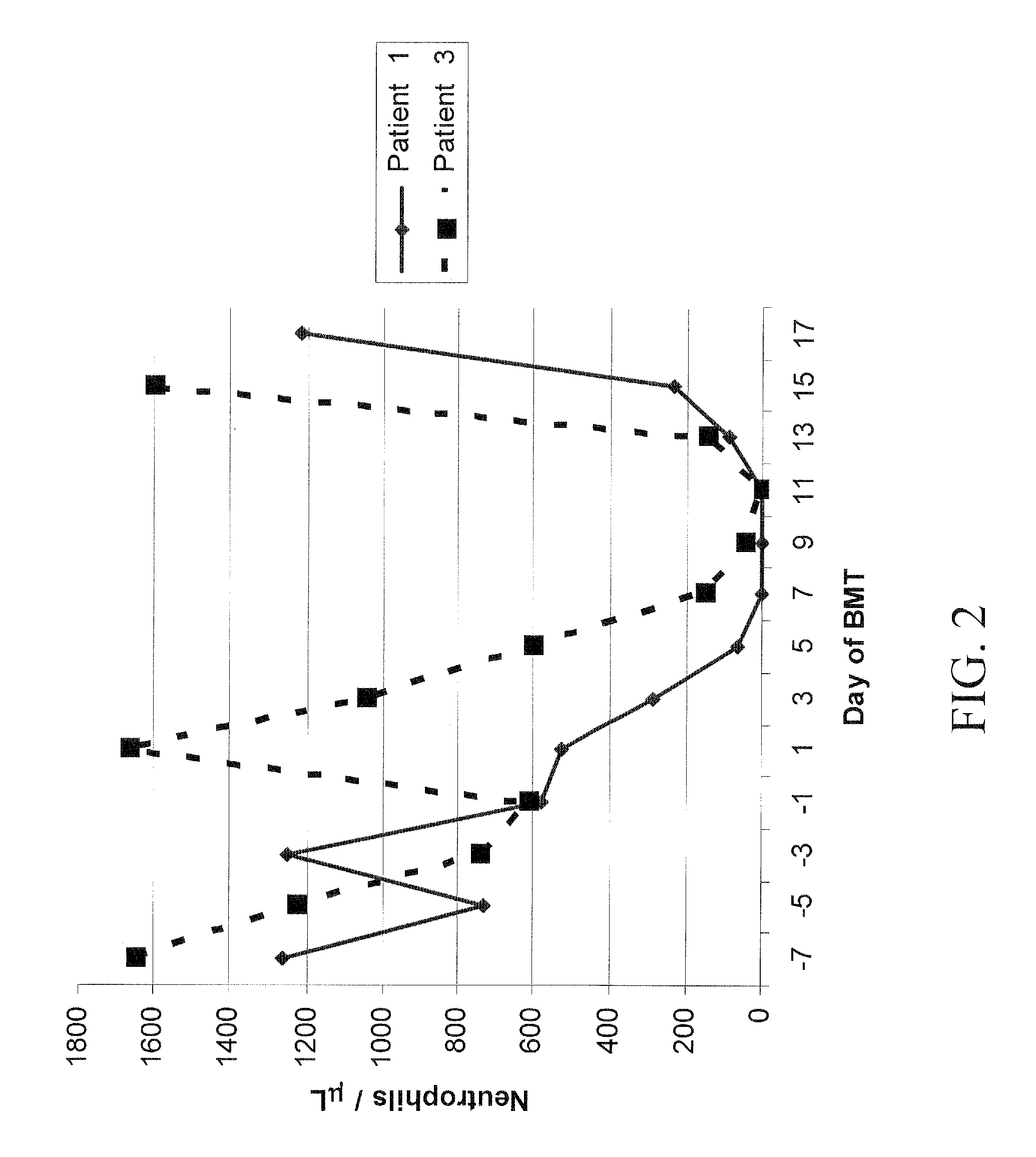
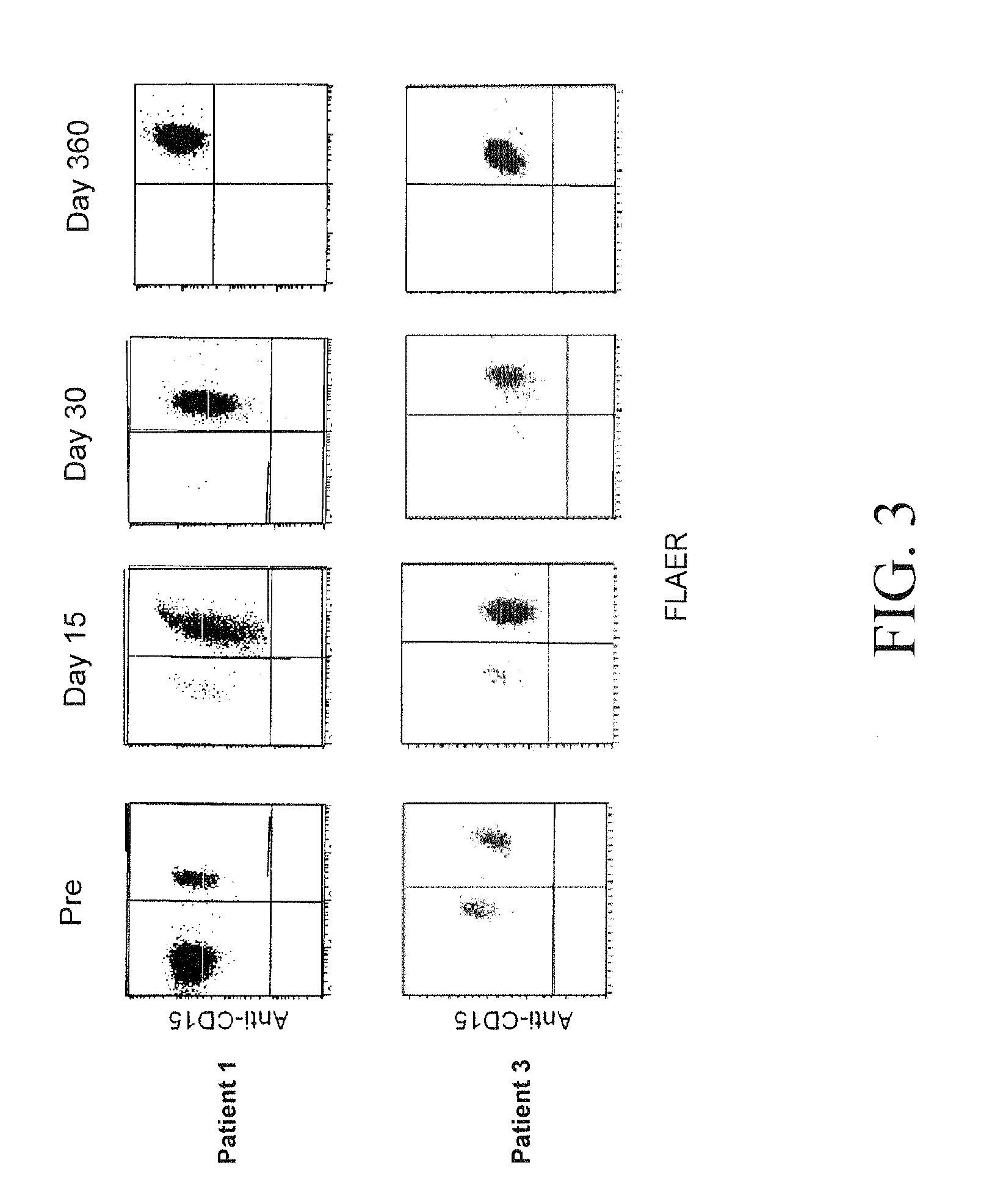
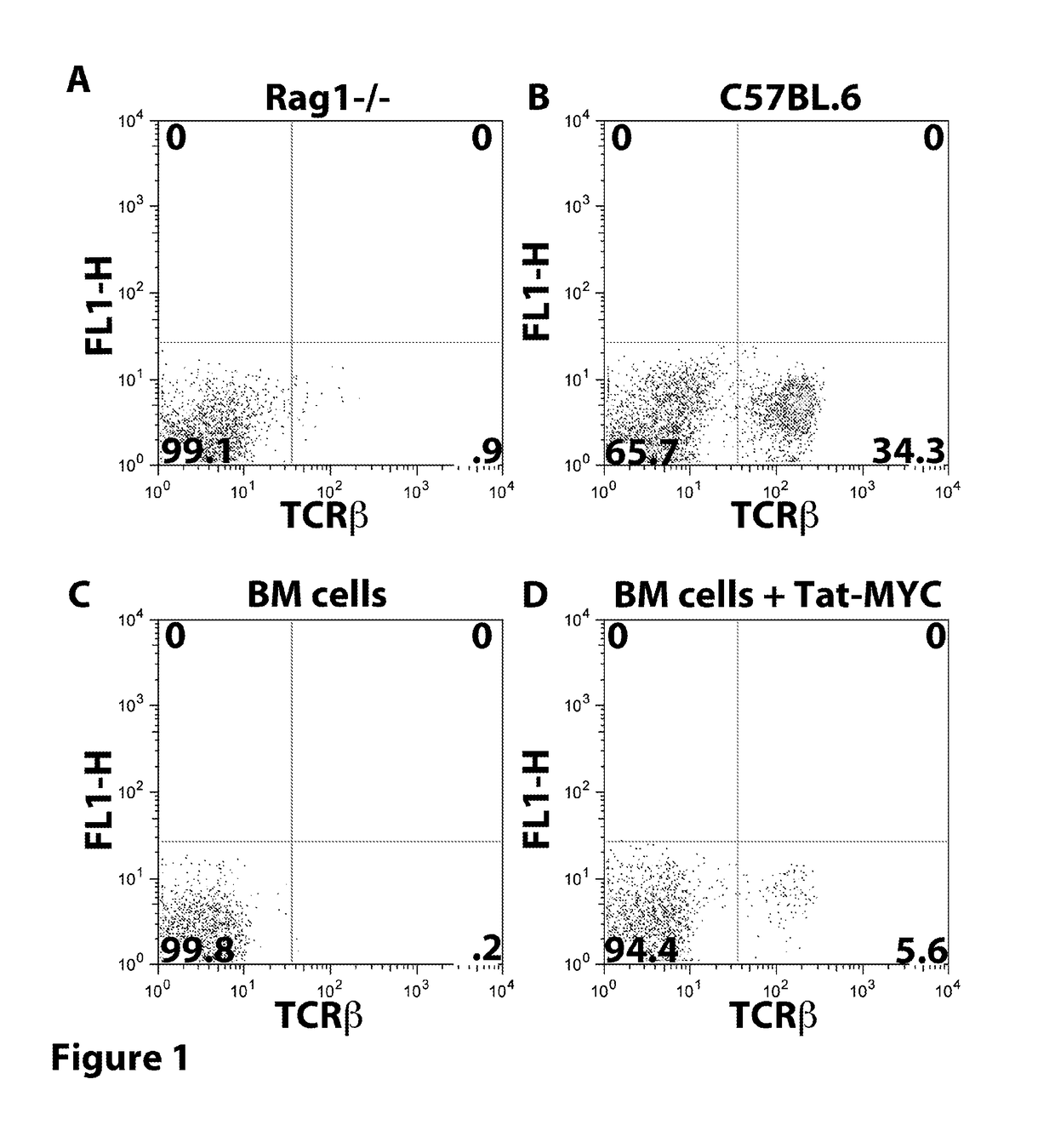
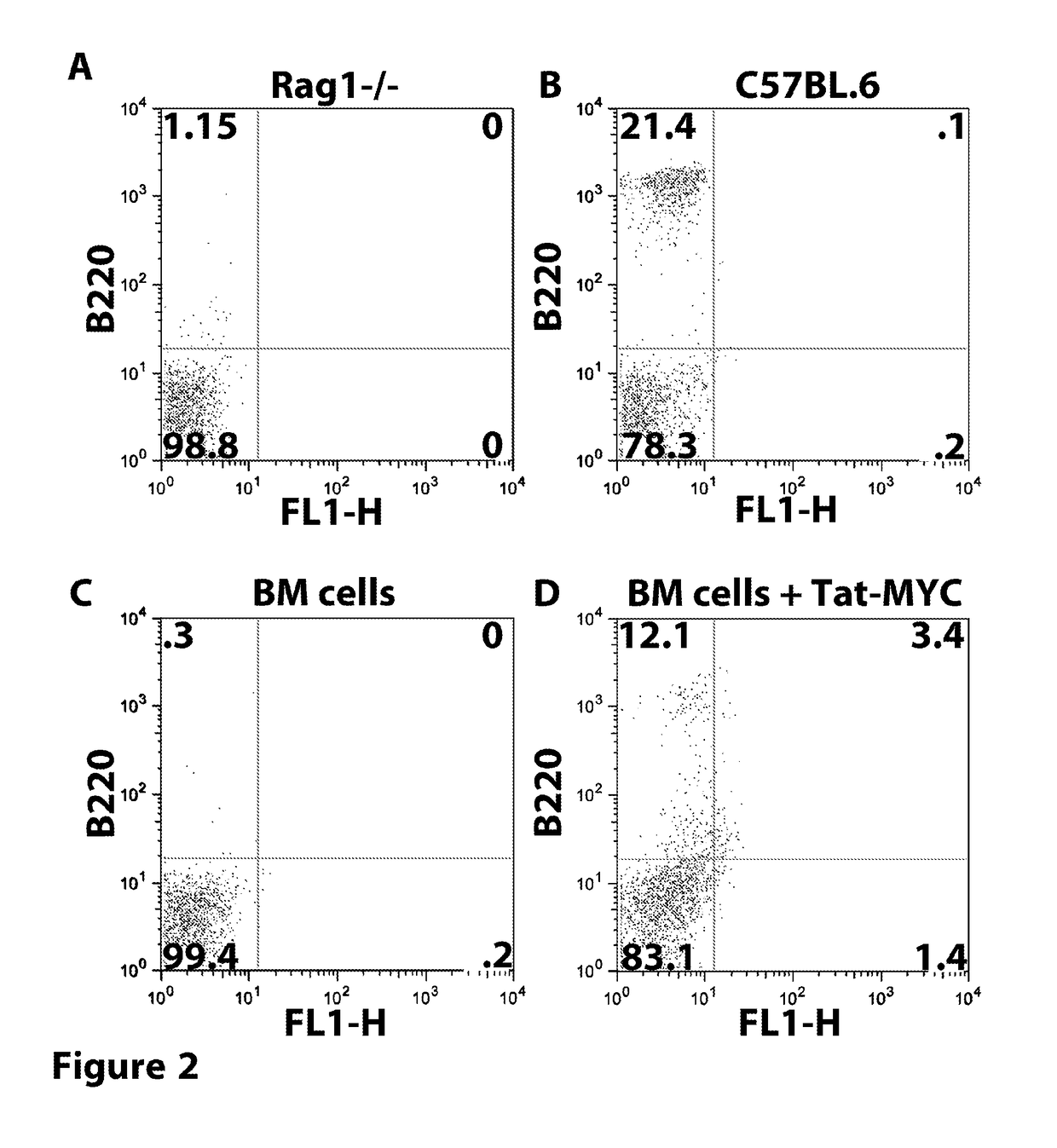
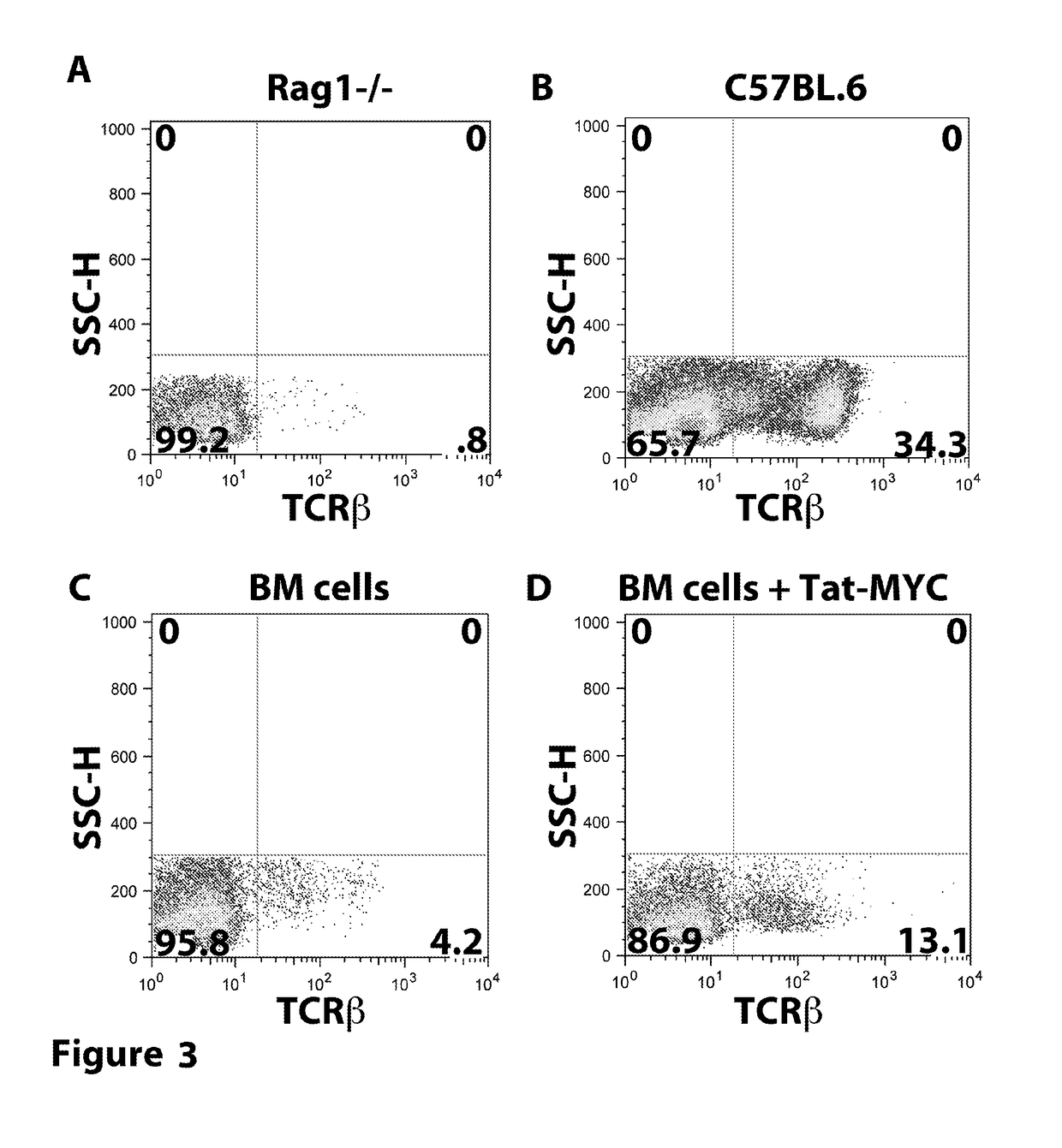
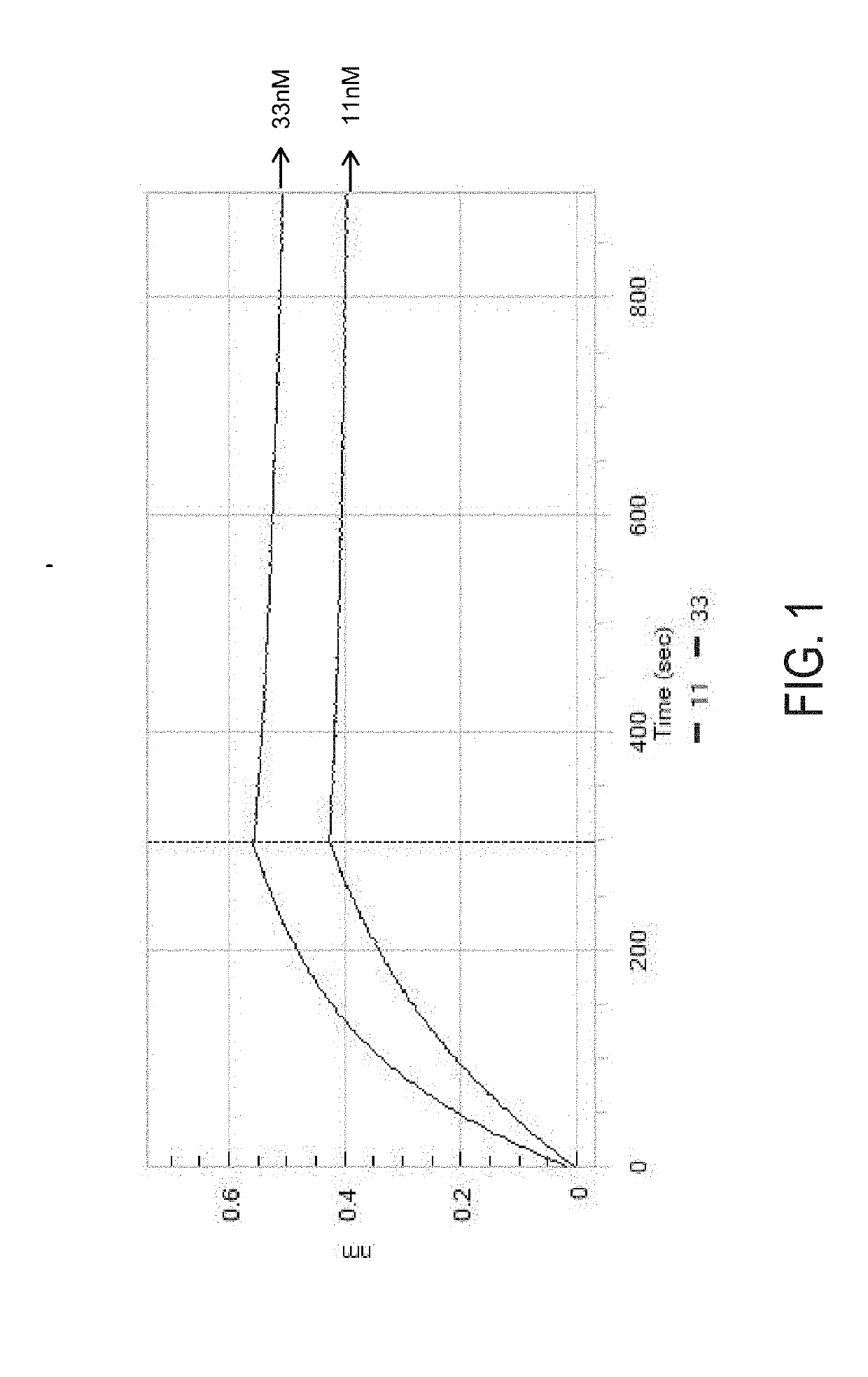
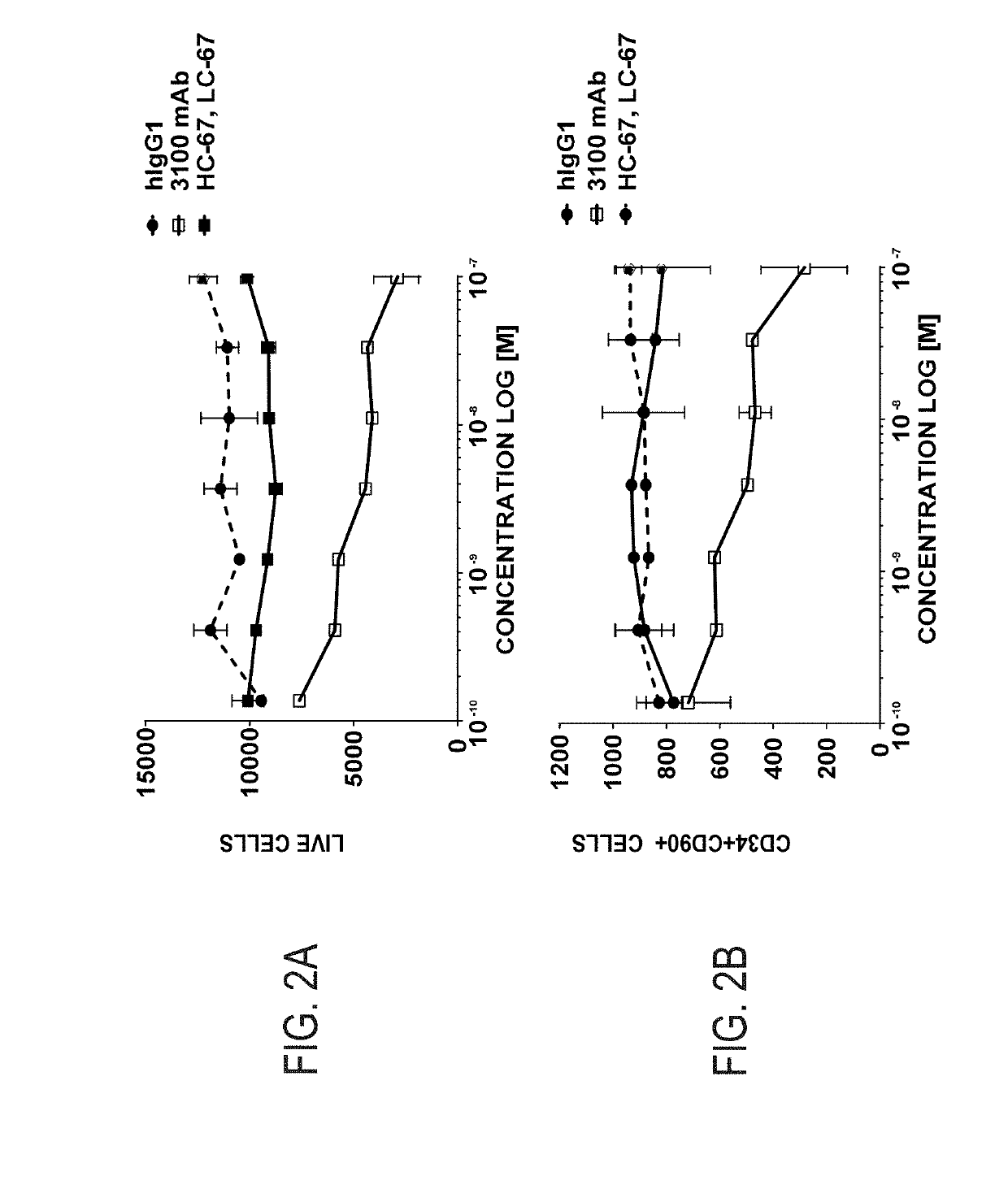
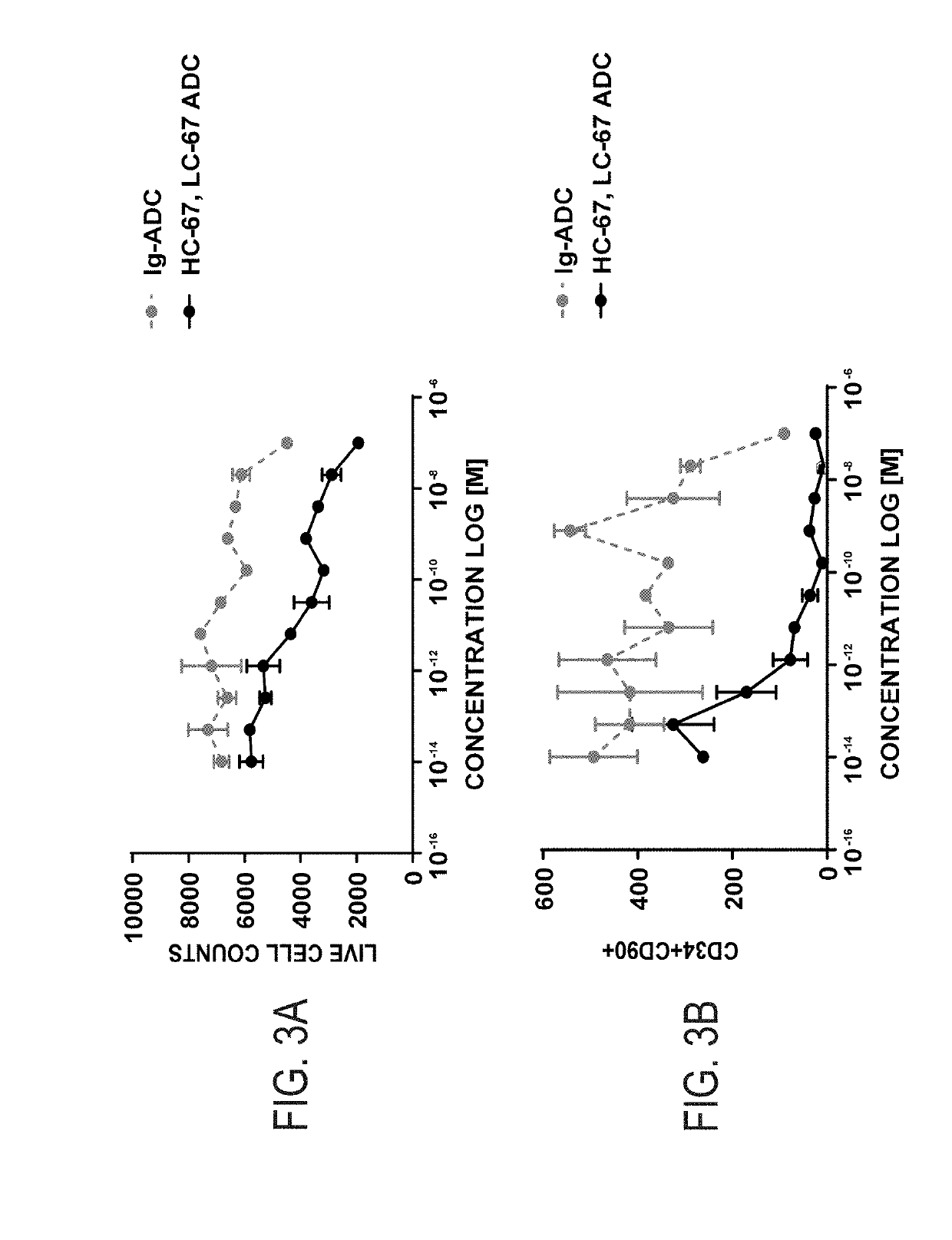
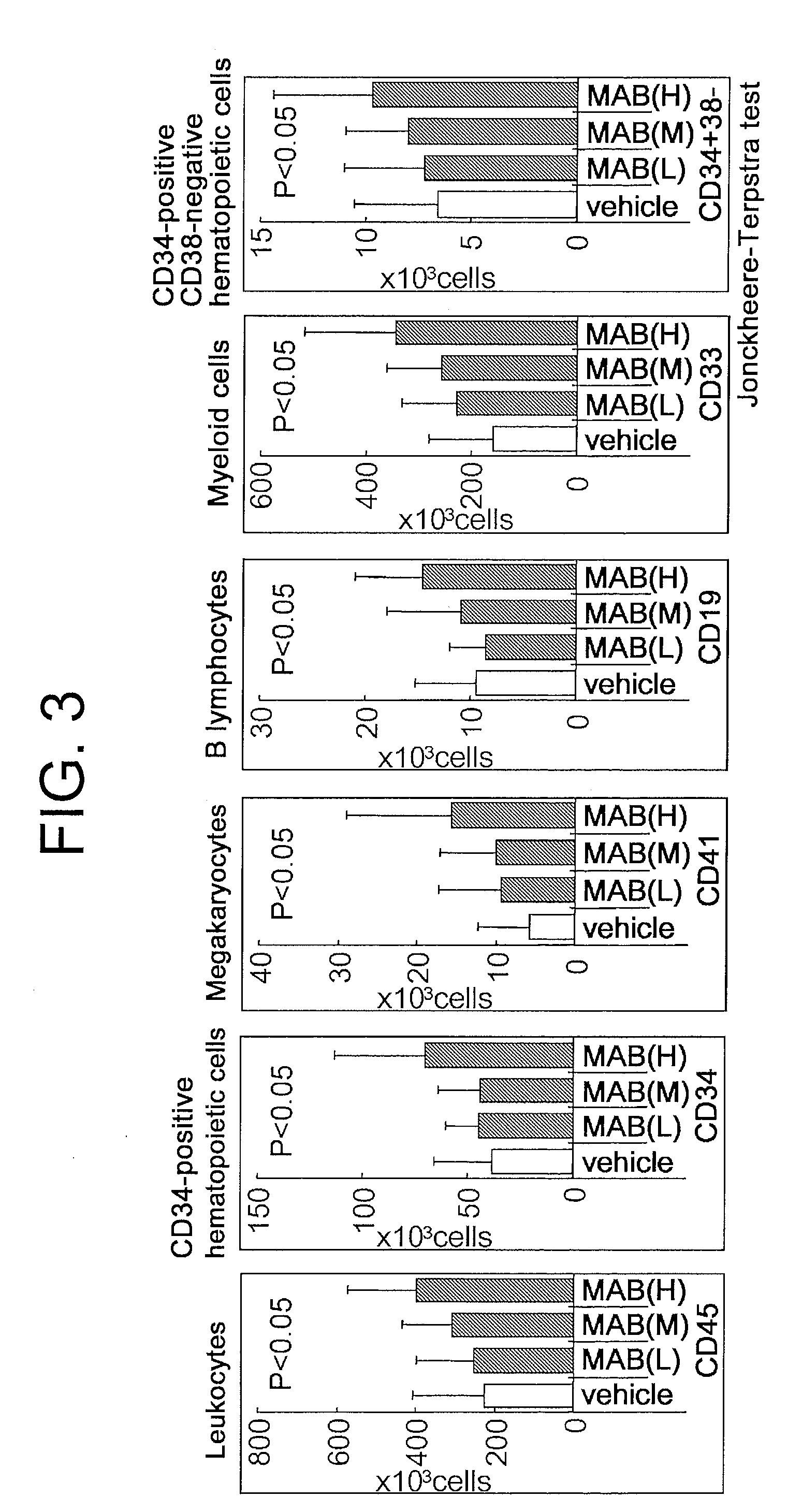
Patents
Literature
271 results about "Hematopoietic stem cell transplantation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Hematopoietic stem cell transplantation (HSCT) is the transplantation of multipotent hematopoietic stem cells, usually derived from bone marrow, peripheral blood, or umbilical cord blood. It may be autologous (the patient's own stem cells are used), allogeneic (the stem cells come from a donor) or syngeneic (from an identical twin).
Combination therapy for treating, preventing or managing proliferative disorders and cancers
InactiveUS20040067953A1Improve efficiencyImprove toleranceBiocideHeavy metal active ingredientsCancer preventionBiologically-Based Therapy
The present invention relates to methods and compositions designed for the treatment, management or prevention of cancer. The methods of the invention comprise the administration of an effective amount of one or more inhibitors of JNK in combination with the administration of an effective amount of one or more other agents useful for cancer therapy. The invention also provides pharmaceutical compositions comprising one or more inhibitors of JNK in combination with one or more other agents useful for cancer therapy. In particular, the invention is directed to methods of treatment and prevention of cancer by the administration of an effective amount of one or more inhibitors of JNK in combination with standard and experimental chemotherapies, hormonal therapies, bone marrow transplants, stem cell replacement therapies, biological therapies / immunotherapies and / or radiation therapies for treatment or prevention of cancer. Also included are methods of treatment of cancer by the administration of one or more inhibitors of JNK in combination with surgery, alone or in further combination with standard and experimental chemotherapies, hormonal therapies, bone marrow transplants, stem cell replacement therapies, biological therapies / immunotherapies and / or radiation therapies.
Owner:SIGNAL PHARMA LLC
Gene expression analysis of pluri-differentiated mesenchymal progenitor cells and methods for diagnosing a leukemic disease state
Pluri-differentiated human mesenchymal progenitor cells (MPCs) are isolated. A method isolates and purifies human mesenchymal progenitor cells from Dexter-type cultures for characterization of and uses, particularly therapeutic uses for such cells. Specifically, isolated MPCs can be used for diagnostic purposes, to enhance the engraftment of hematopoietic progenitor cells, enhance bone marrow transplantation, or aid in the treatment or prevention of graft versus host disease.
Owner:SOUTH FLORIDA UNIVESITY OF
Cellular compositions which facilitate engraftment of hematopoietic stem cells while minimizing the risk of gvhd
The present invention provides for cellular compositions which facilitate engraftment of hematopoietic stem cells from a syngeneic, allogeneic or xenogeneic donor. The cellular compositions of the invention facilitate engraftment while minimizing the risk of graft versus host disease in the graft recipient. According to a preferred embodiment of the invention, a cell composition is provided, which cell composition comprises hematopoietic stem cells, such as CD34+ cells and / or facilitating cells, in combination with αβ TCR+ T cells. The invention also relates to methods of using the cellular compositions of the invention to induce donor specific tolerance in a recipient, thus allowing the transplantation of donor organs, cells and tissues. Also disclosed are methods of treating leukemia and cancer as well as infectious diseases caused by viruses.
Owner:JEWISH HOSPITAL HEALTHCARE SERVICES
Predicting relapse of chronic lymphocytic leukemia patients treated by allogeneic stem cell transplantation
InactiveUS20150247201A1Increase the number ofMicrobiological testing/measurementLibrary screeningFold changeMedicine
The invention is directed to a prognostic indicator for CLL patients who have undergone an allogeneic stem cell transplant (SCT). The indicator is based on a method of monitoring levels and changes in levels of correlating clonotypes of the CLLs at successive time points. The prognostic indicator applies to patients who have survived for at least one year from an allogeneic SCT and includes criteria based on the following two measurements: (a) frequency of CLL correlating clonotypes (e.g. in terms of number per 106 clonotypes) in an initial clonotype profile (from peripheral blood), and (b) fold change in such CLL correlating clonotype number between such initial measurement and a successively measured clonotype profile.
Owner:ADAPTIVE BIOTECH
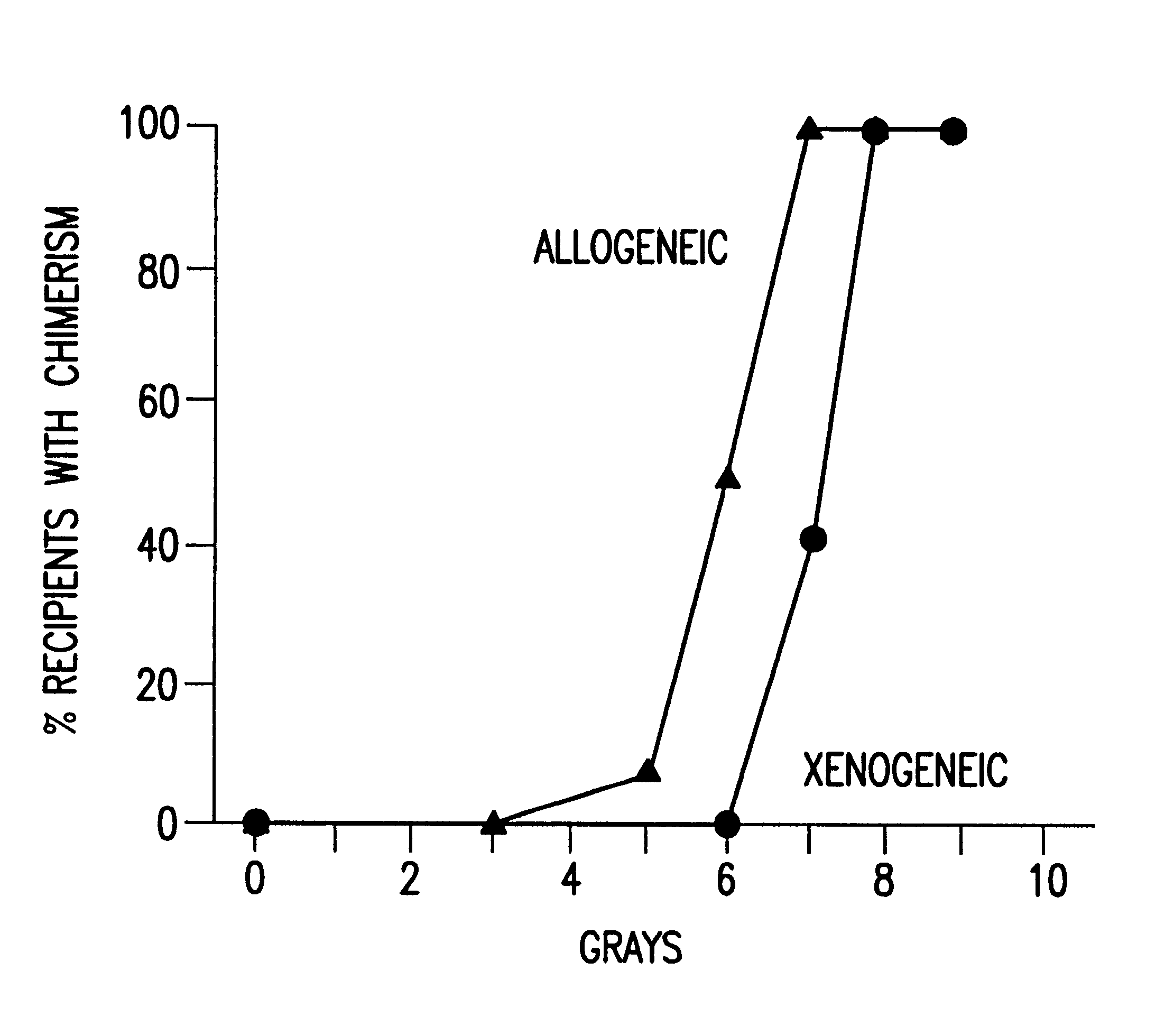
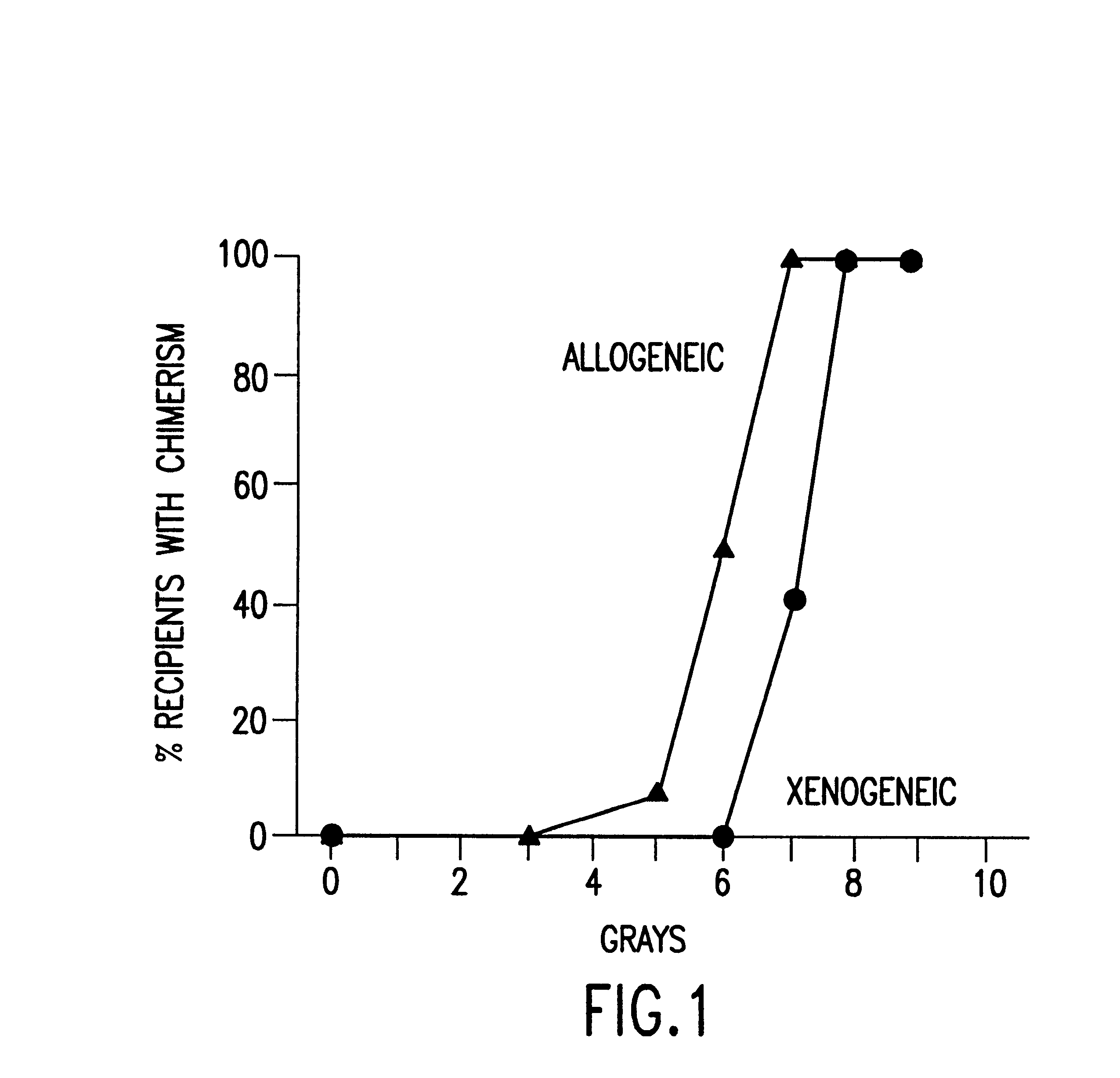
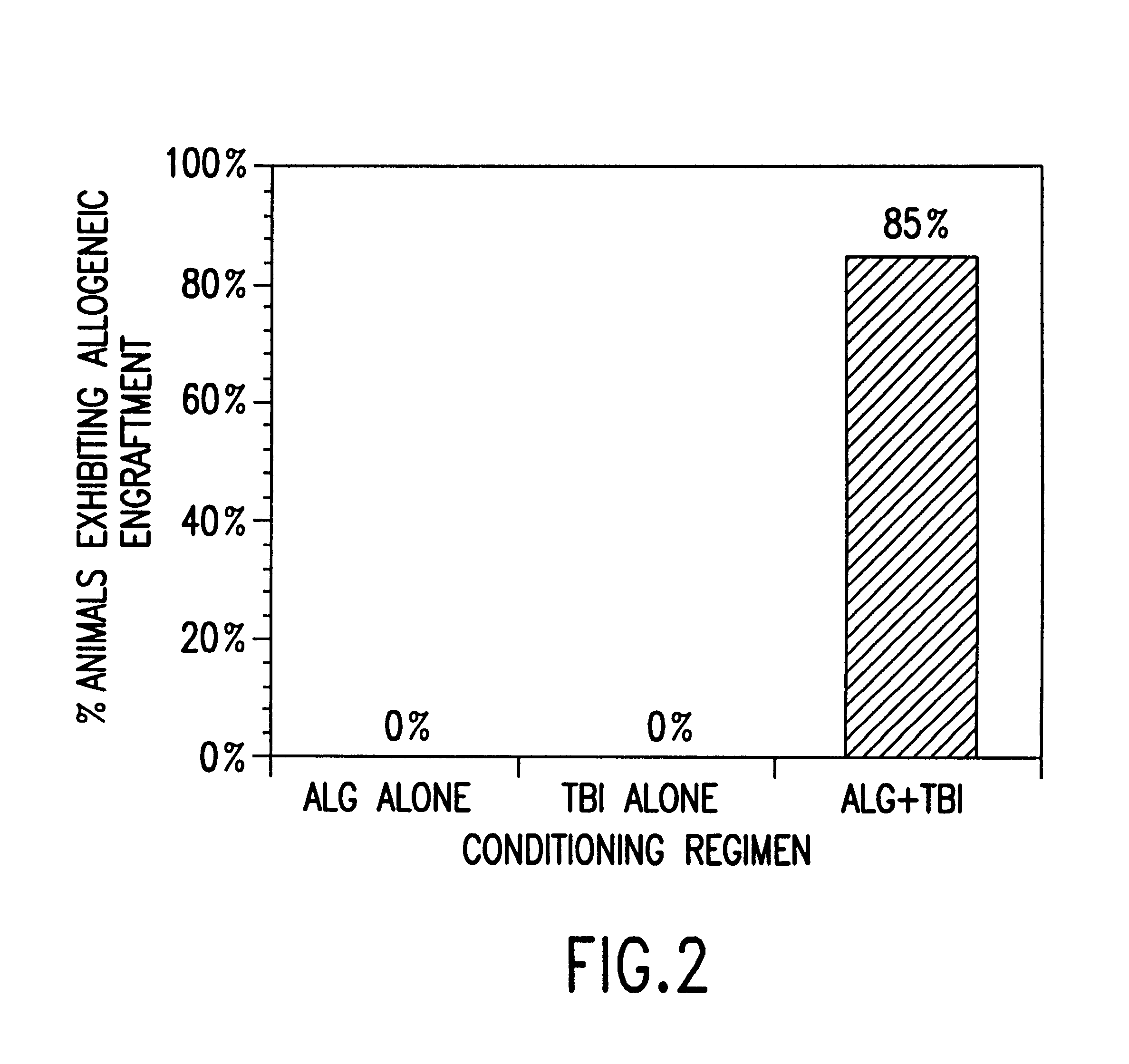
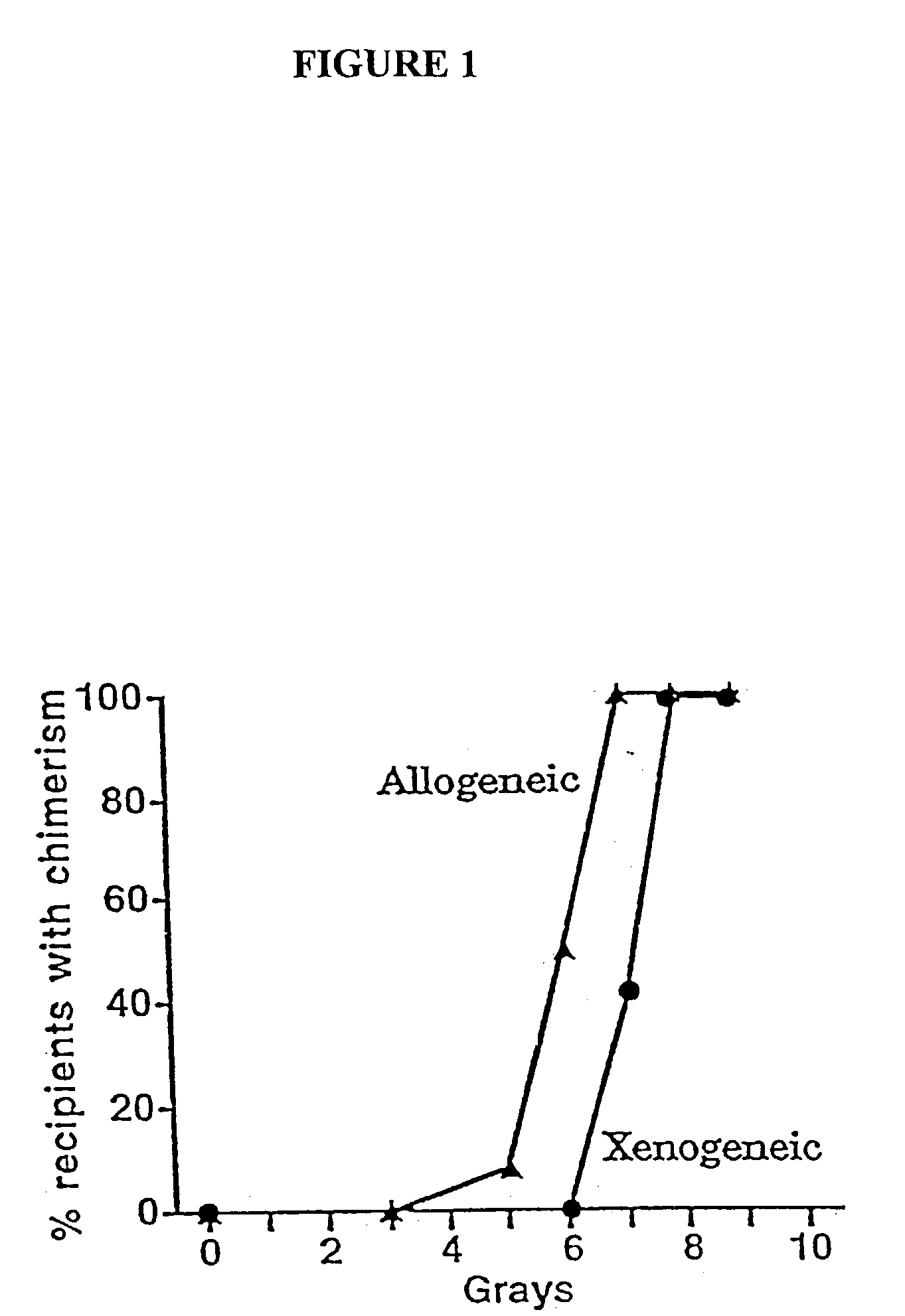
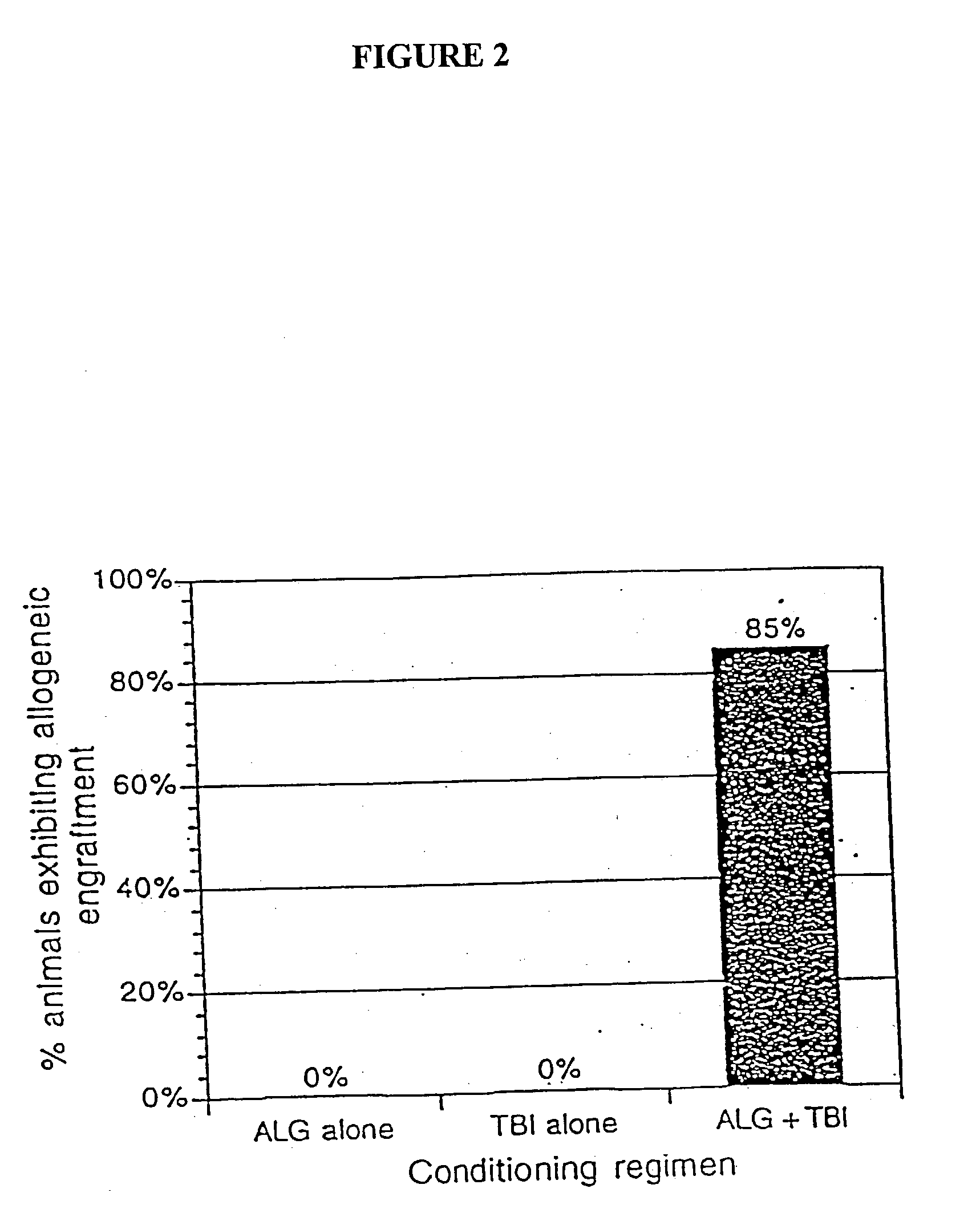
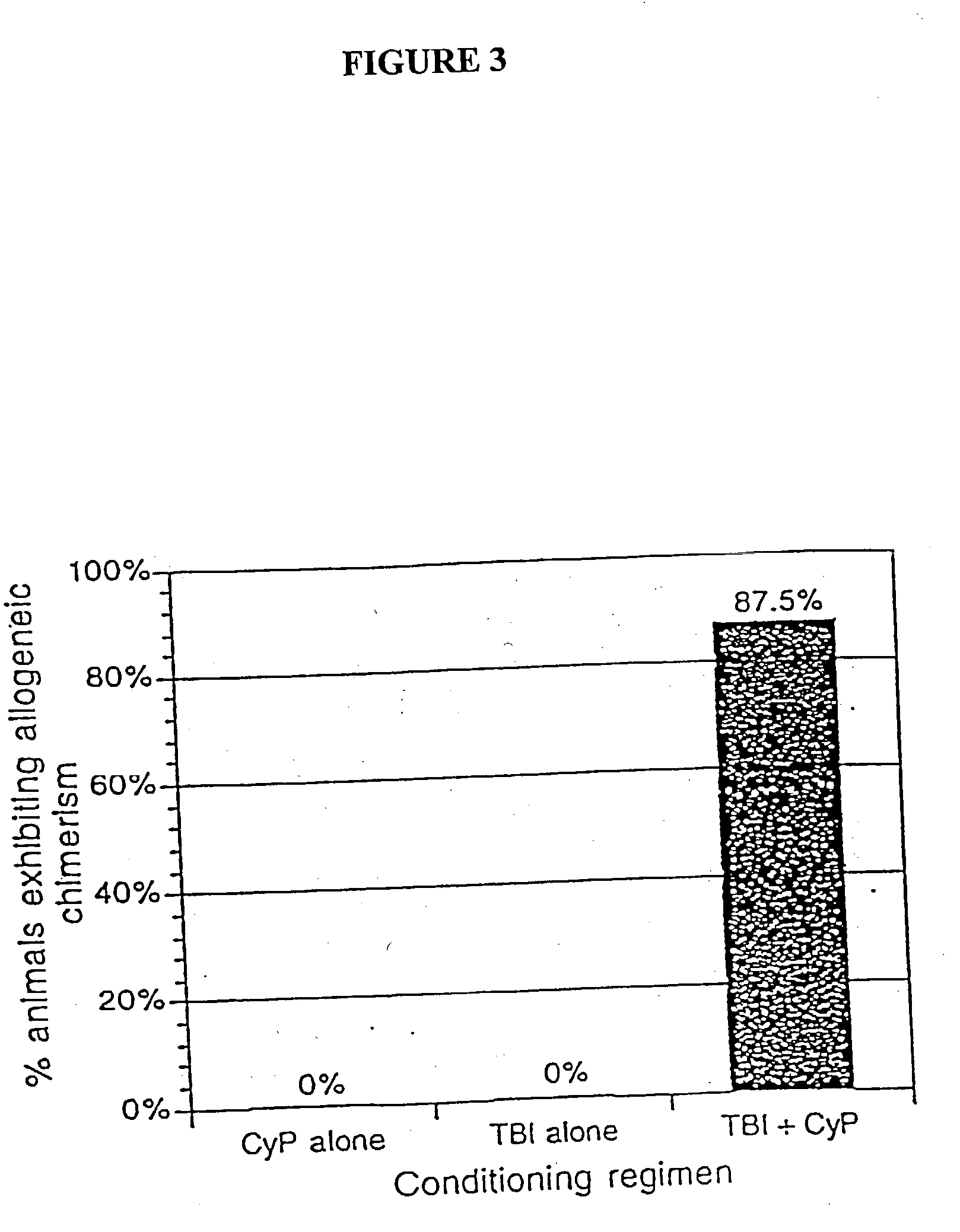
Non-lethal methods for conditioning a recipient for bone marrow transplantation
InactiveUS6217867B1Reduce proliferationDiminished cytotoxic activityBiocideOrganic active ingredientsGackstroemiaAcquired immunodeficiency
The present invention relates to non-lethal methods of conditioning a recipient for bone marrow transplantation. In particular, it relates to the use of nonlethal doses of total body irradiation, total lymphoid irradiation, cell type-specific or cell marker-specific antibodies, especially antibodies directed to bone marrow stromal cell markers, NK cells, or the CD8 cell marker, cytotoxic drugs, or a combination thereof. The methods of the invention have a wide range of applications, including, but not limited to, the conditioning of an individual for hematopoietic reconstitution by bone marrow transplantation for the treatment of hematologic malignancies, hematologic disorders, autoimmunity, infectious diseases such as acquired immunodeficiency syndrome, and the engraftment of bone marrow cells to induce tolerance for solid organ, tissue and cellular transplantation.
Owner:PITTSBURGH UNIV OF
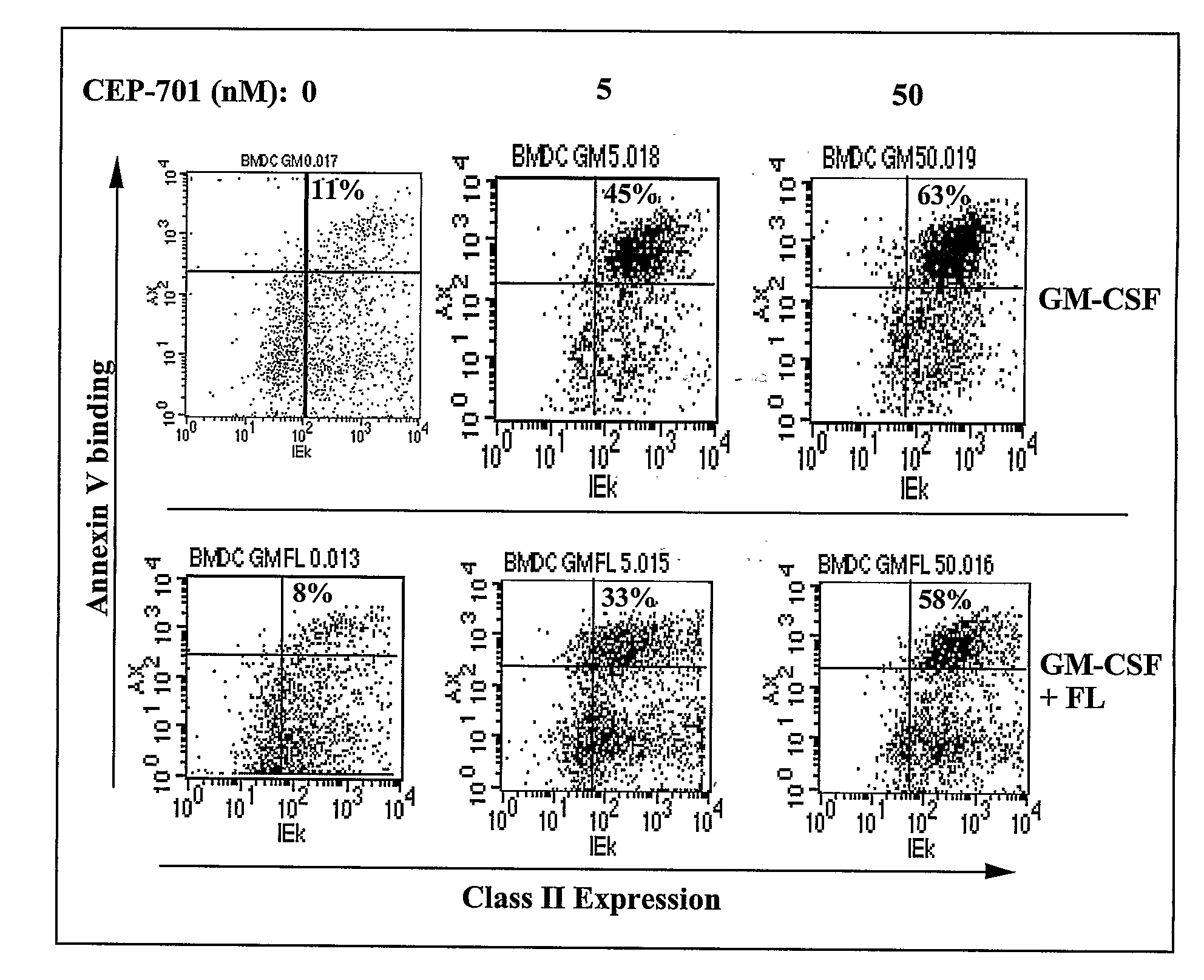
Flt3 inhibitors for immune suppression
InactiveUS20090054358A1Suppressing response of cellReduce in quantityBiocideNervous disorderAcquired immunodeficiencySystemic lupus erythematosus
New methods are provided for suppressing the immune system and for treating immune related disorders. Therapies of the invention include administration of an FLT3 inhibitor compound to a subject in need thereof, such as a subject suffering from organ rejection, bone marrow transplant rejection, acquired immune deficiency syndrome, arthritis, aplastic anemia, graft-versus-host disease, Graves' disease, established experimental allergic encephalitomyelitis, multiple sclerosis, lupus, or a neurological disorder. Methods are also provided for screening therapeutic agents for treating immune disorders, including the use of a mouse having an elevated level of FLT3 receptor activity.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Compositions and methods for eliminating undesired subpopulations of T cells in patients with immunological defects related to autoimmunity and organ or hematopoietic stem cell transplantation
InactiveUS20050084967A1Reduce riskReduce severityMicrobiological testing/measurementArtificial cell constructsAutoimmune responsesHematopoietic stem cell transplantation
The present invention relates generally to methods for stimulating T cells, and more particularly, to methods to eliminate undesired (e.g. autoreactive, alloreactive, pathogenic) subpopulations of T cells from a mixed population of T cells, thereby restoring the normal immune repertoire of said T cells. The present invention also relates to compositions of cells, including stimulated T cells having restored immune repertoire and uses thereof.
Owner:LIFE TECH CORP
Human mesenchymal progenitor cell
InactiveUS6936281B2BiocideGenetic material ingredientsProgenitorHematopoietic stem cell transplantation
There is provided an isolated pluri-differentiated human mesenchymal progenitor cells (MPCs), a method for isolating and purifying human mesenchymal progenitor cells from Dexter-type cultures, and characterization of and uses, particularly therapeutic uses for such cells. Specifically, there is provided isolated MPCs which can be used for diagnostic purposes, to enhance the engraftment of hematopoietic progenitor cells, enhance bone marrow transplantation, or aid in the treatment or prevention of graft versus host disease.
Owner:UNIV OF SOUTH FLORIDA
Cell population with enhanced transplantation activity
InactiveUS20100303766A1Excellent migration ability and engraftment abilityRestore damageBiocideNervous system cellsDamages tissueHematopoietic stem cell transplantation
A cell having excellent migration ability and engraftment ability to tissues is required for the restoration and the like of damaged tissues making use of a hematopoietic stem cell transplantation or a stem cell transplantation. By the present invention, a cell population having excellent migration ability and engraftment ability to tissues, a pharmaceutical preparation comprising said cell and a transplantation method of said cell are provided. The cell of the present invention is useful for hematopoietic stem cell transplantation and restoration of damaged tissues using stem cell transplantation and the like.
Owner:KYOWA HAKKO KIRIN CO LTD
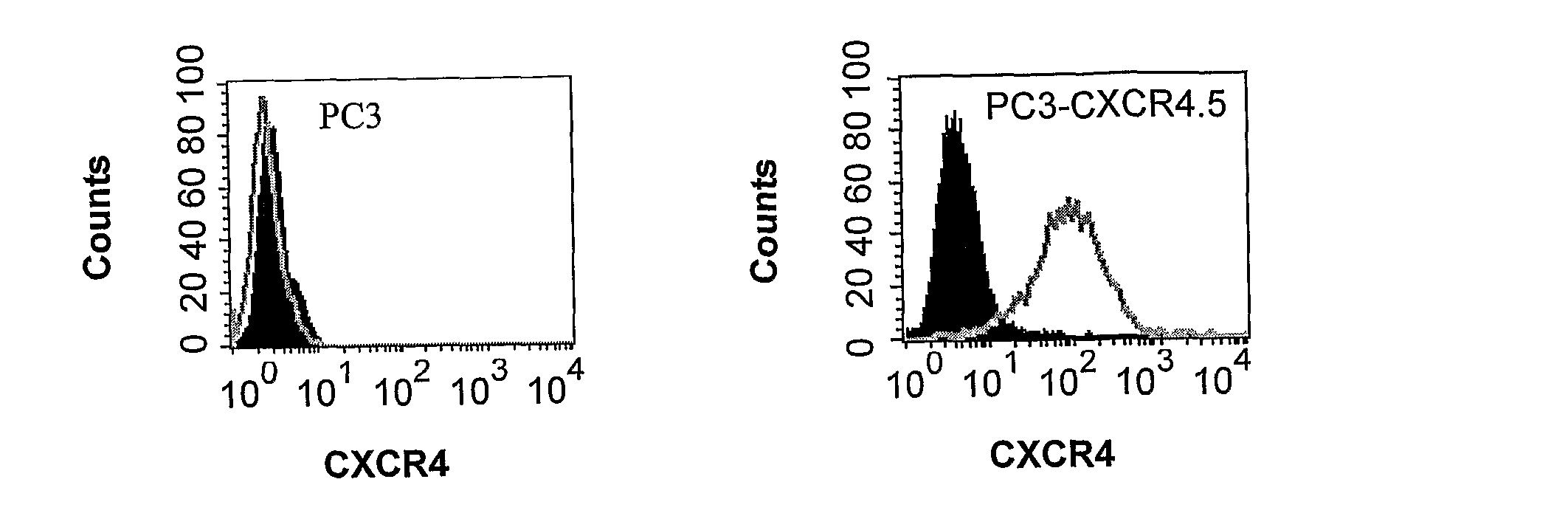
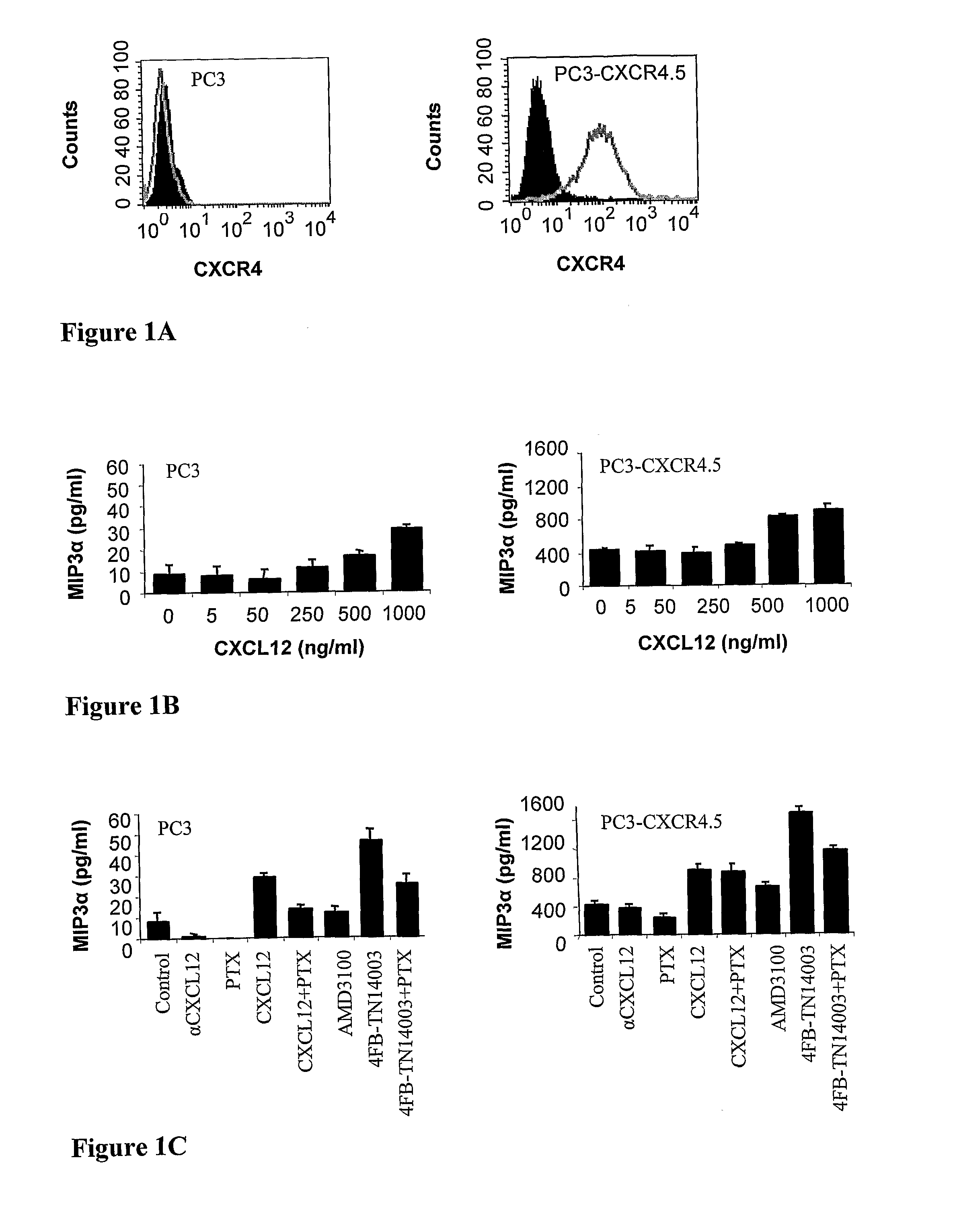
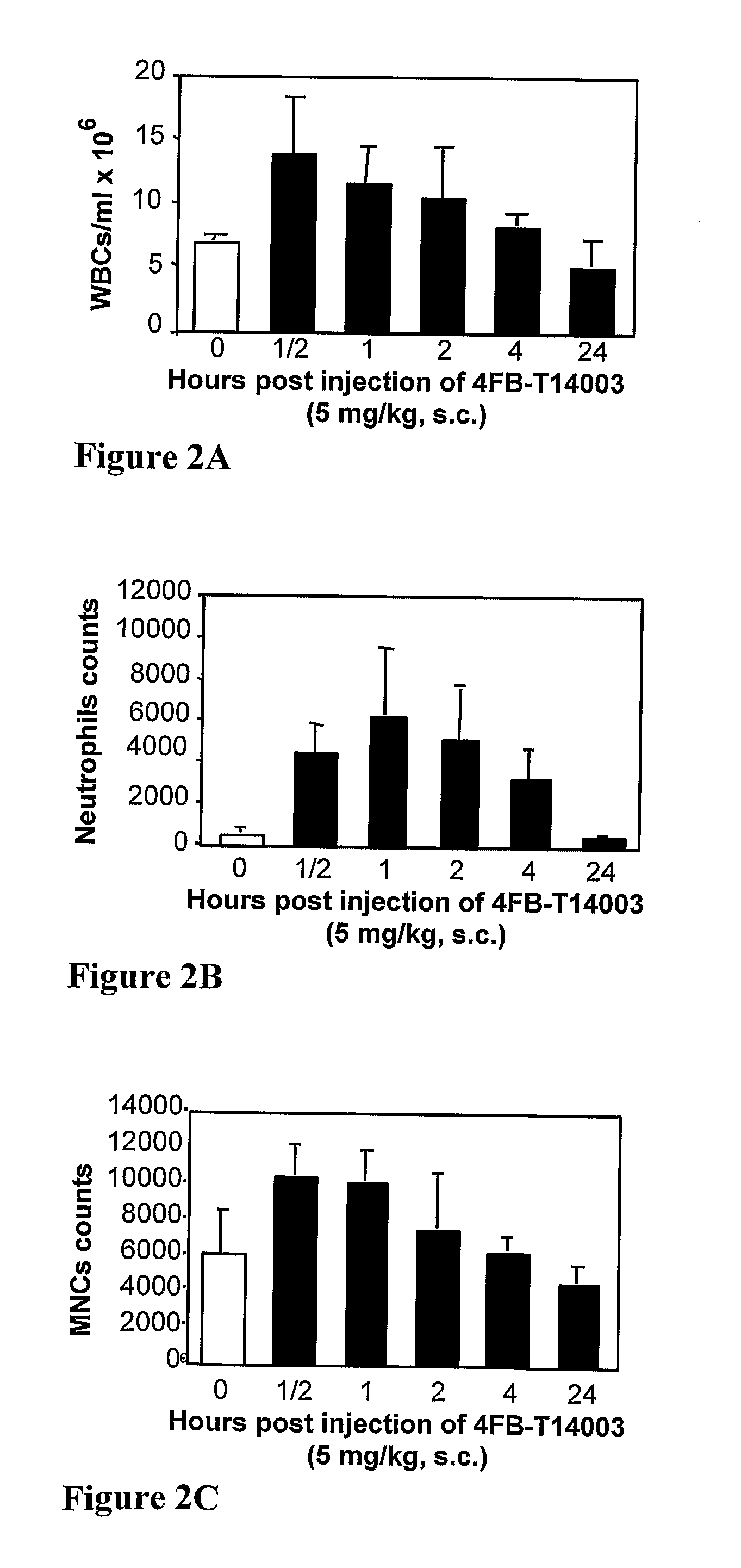
T-140 peptide analogs having cxcr4 super-agonist activity for bone marrow recovery
ActiveUS20100166715A1Improve safety and efficiencyPromote recoveryBiocidePeptide/protein ingredientsCXCR4Hematopoietic stem cell transplantation
The present invention is directed to novel therapeutic uses of T-140 analog peptides and compositions comprising same. Specifically, the invention provides compositions and methods useful for providing improved bone marrow transplantation and in the treatment of other conditions wherein bone marrow depletion or suppression is involved.
Owner:BIOKINE THERAPEUTICS LTD
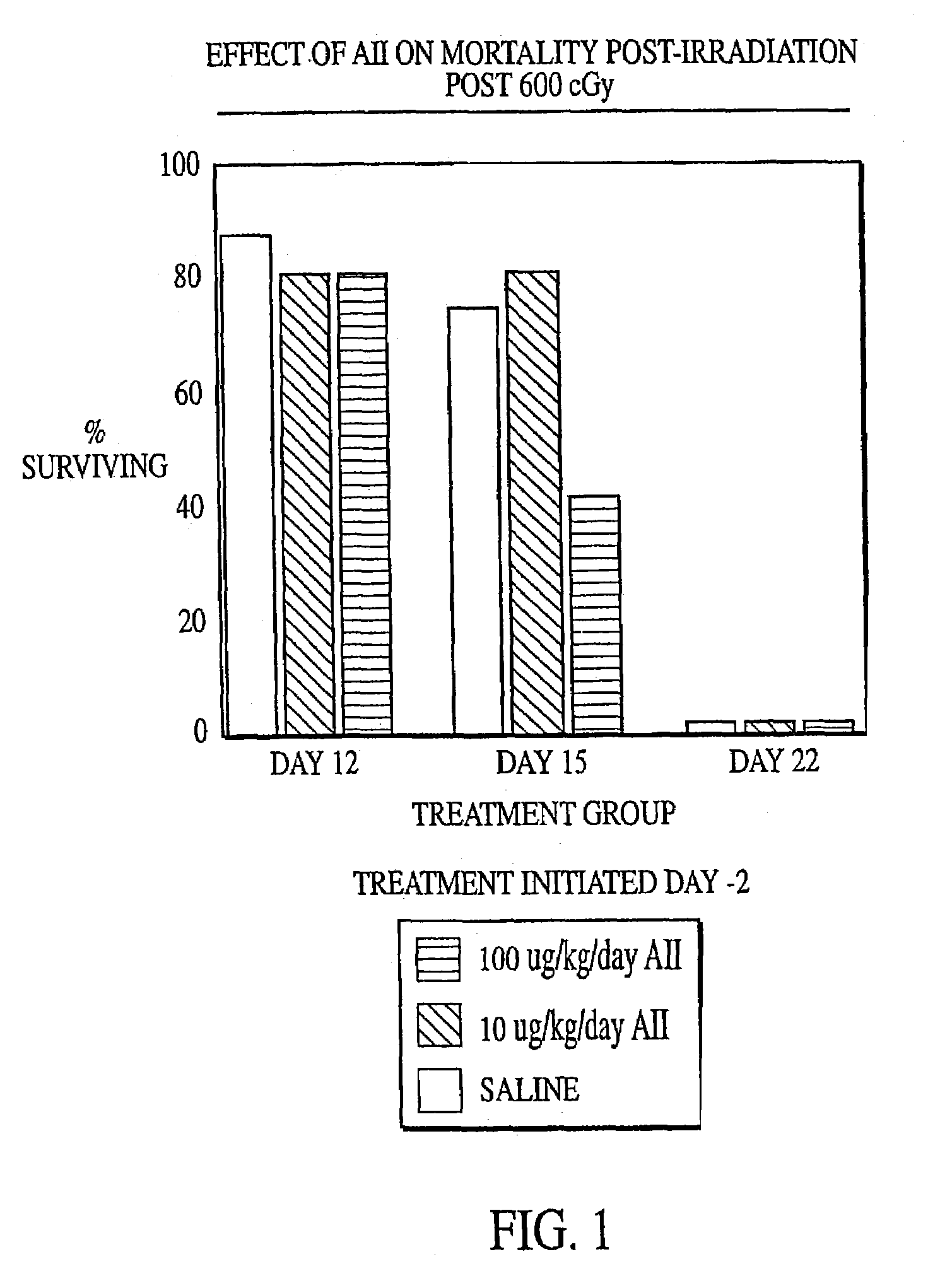
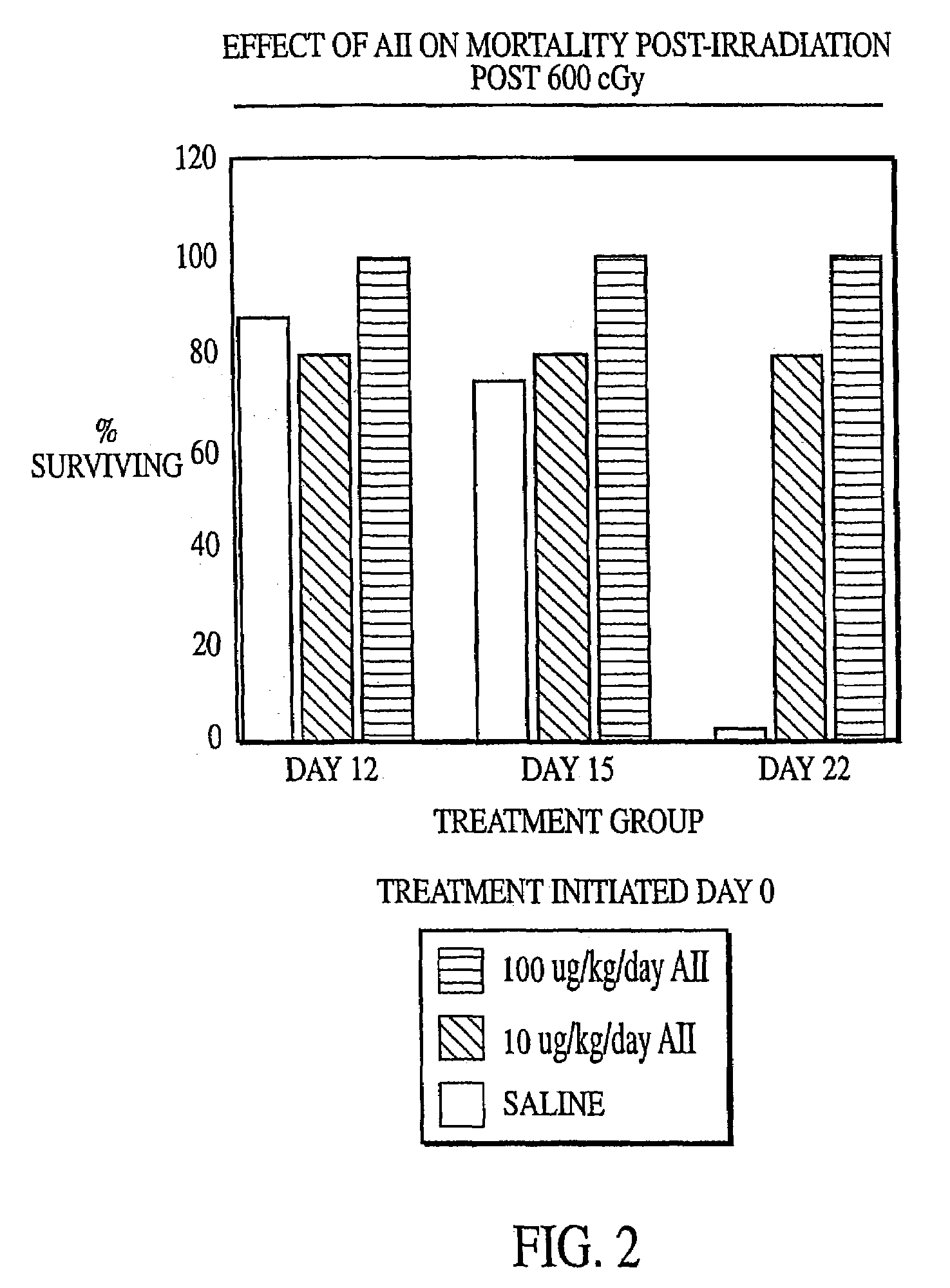
Radiation therapy methods
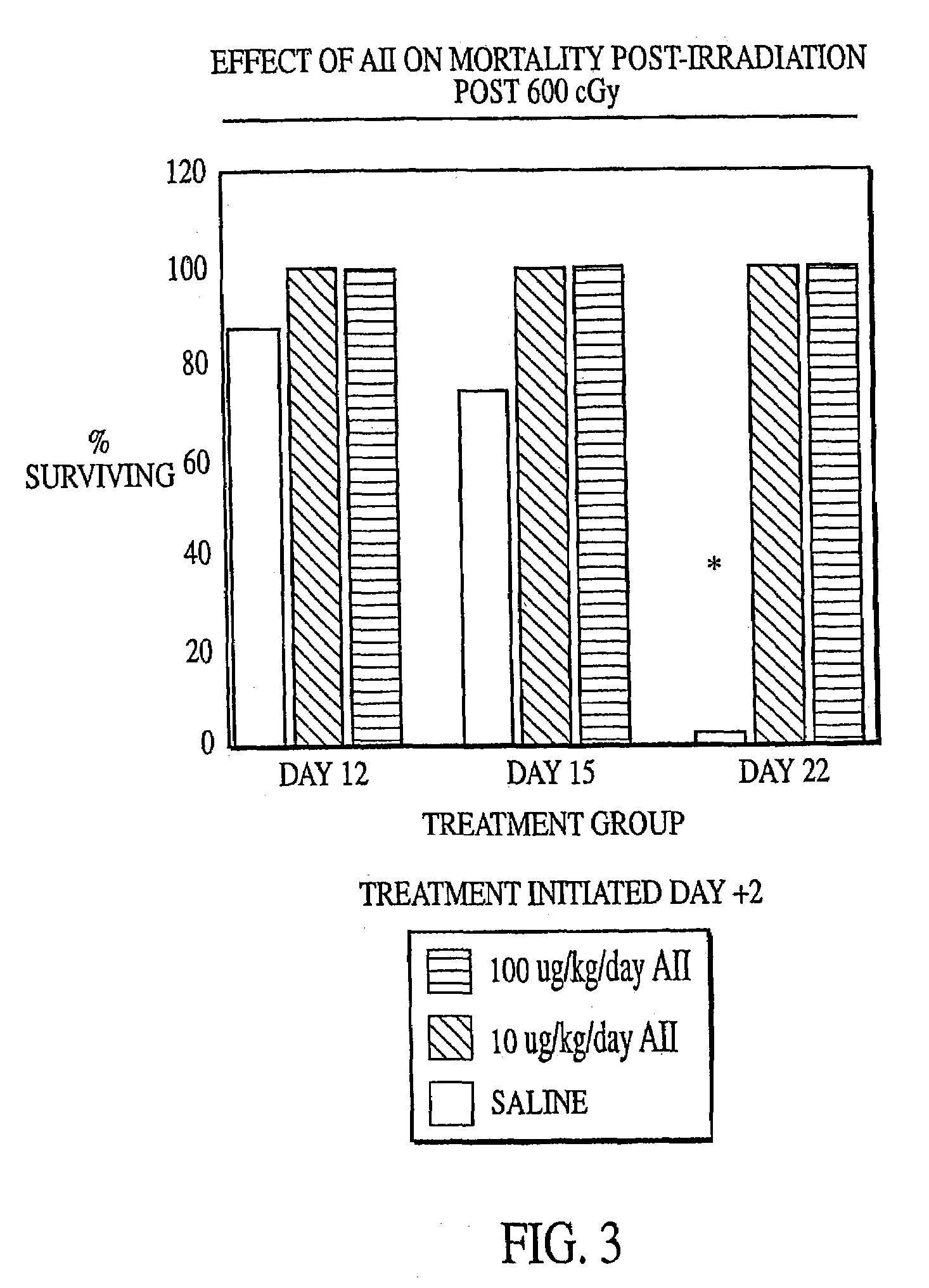
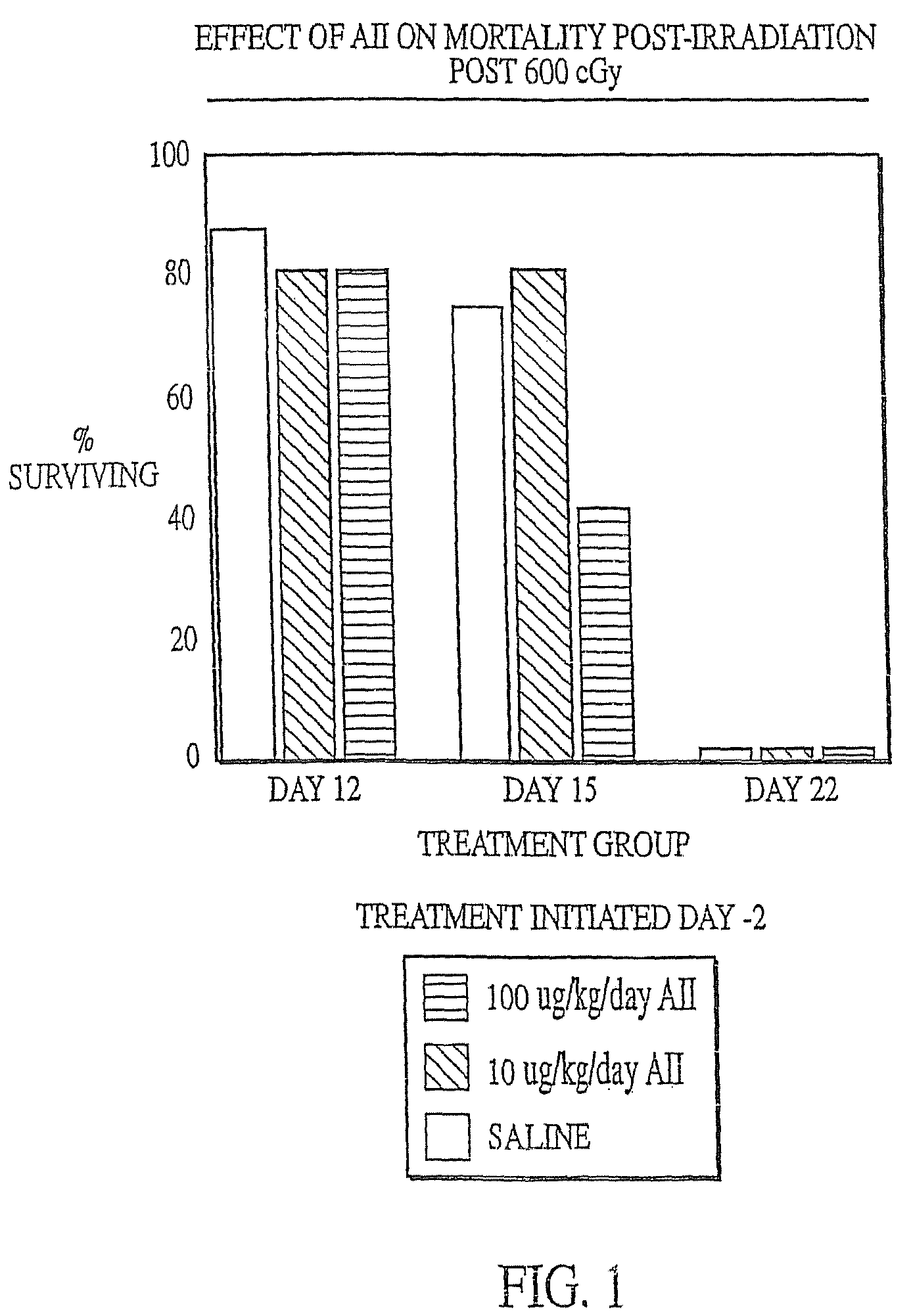
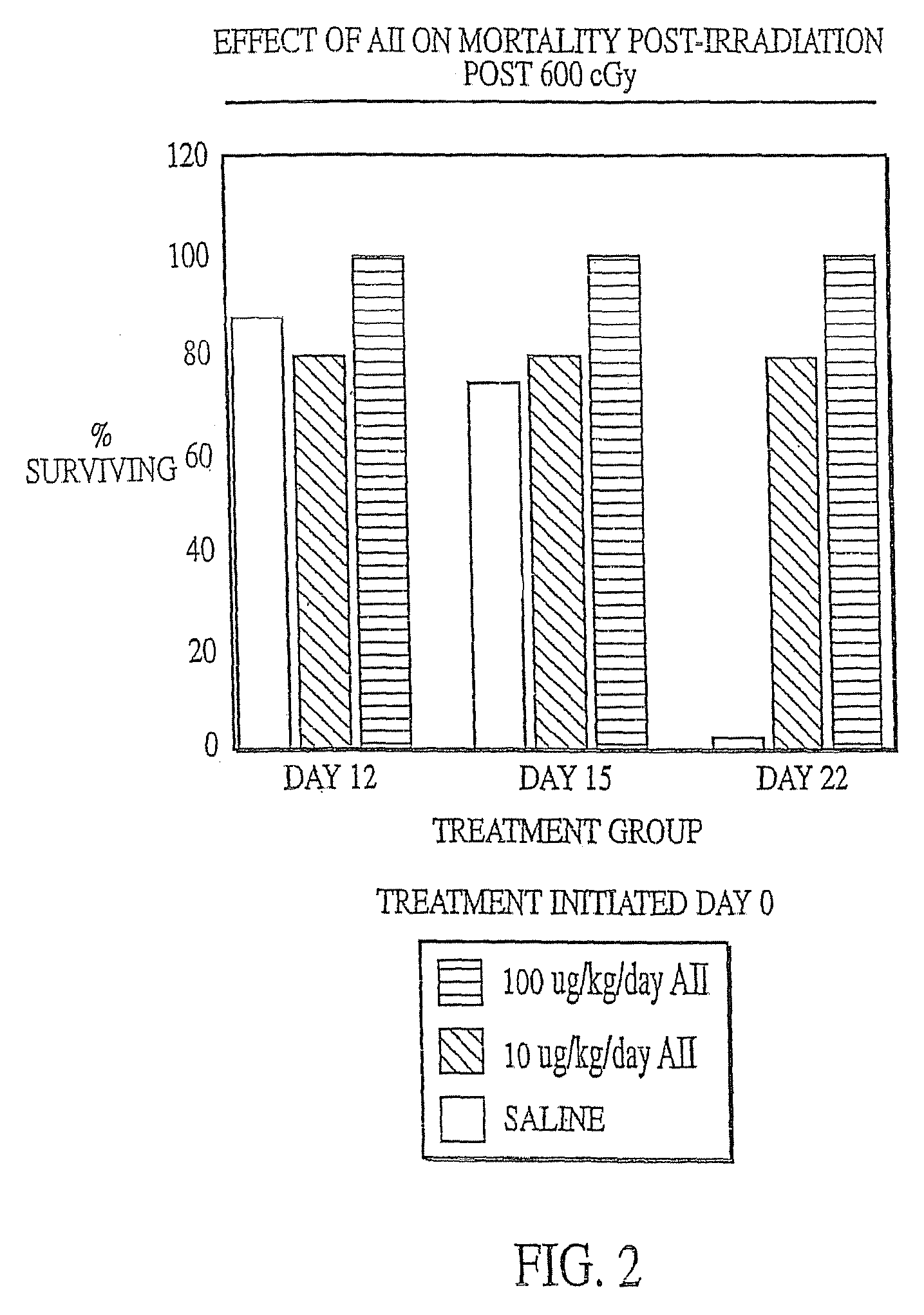
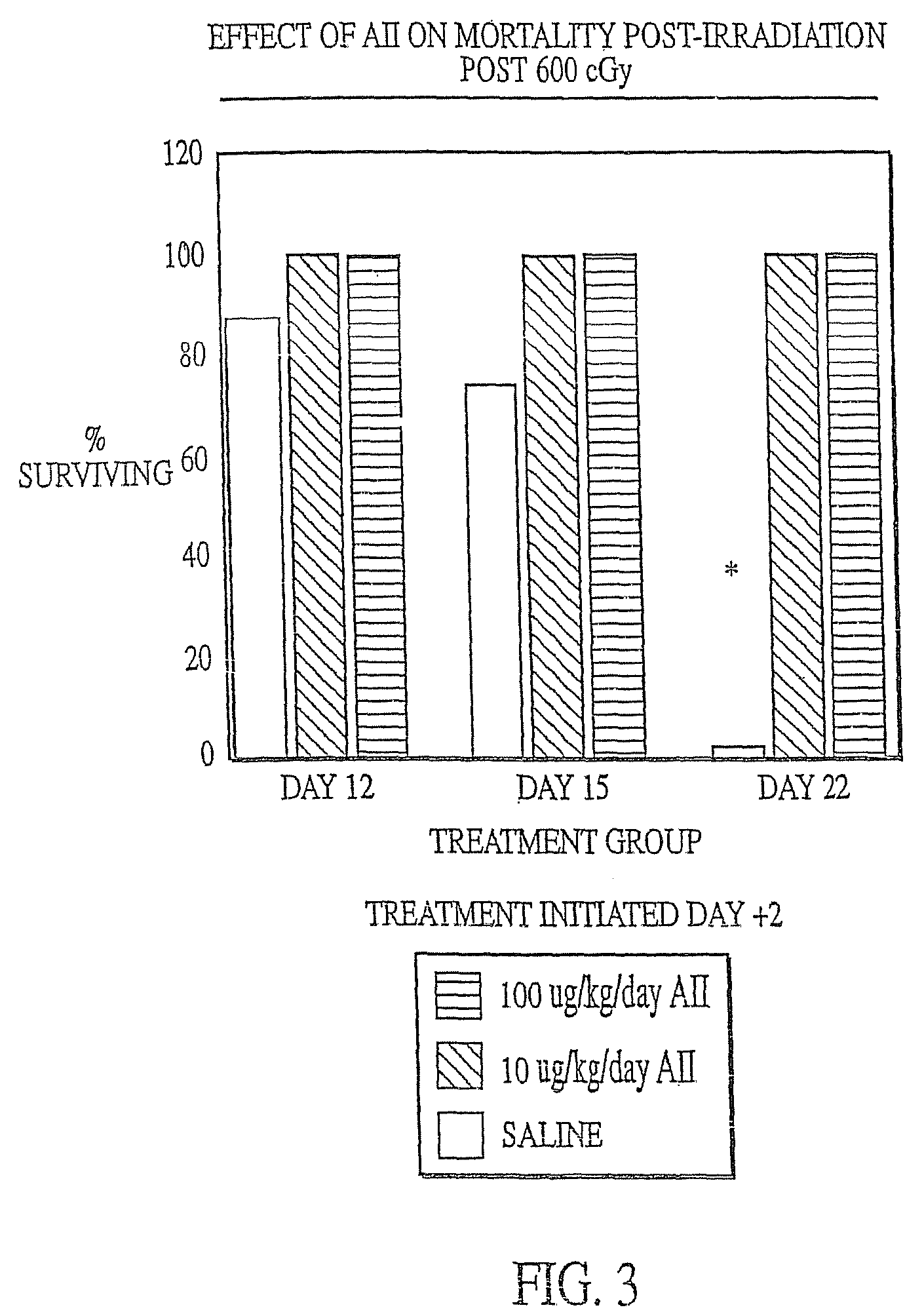
InactiveUS7173011B2Reduce tissue damageImprove efficiencyAngiotensinsPeptide/protein ingredientsMegakaryocyte productionAngiotensinogen mrna
The present invention provides methods and kits for mitigating radiation induced tissue damage, improving the effectiveness of radiation therapy, to support bone marrow transplantation, and promoting megakaryocyte production and mobilization and platelet production, each method comprising the administration of an effective amount of angiotensinogen, angiotensin I (AI), AI analogues, AI fragments and analogues thereof, angiotensin II (AII), AII analogues, AII fragments or analogues thereof or AII AT2 type 2 receptor agonists.
Owner:UNIV OF SOUTHERN CALIFORNIA
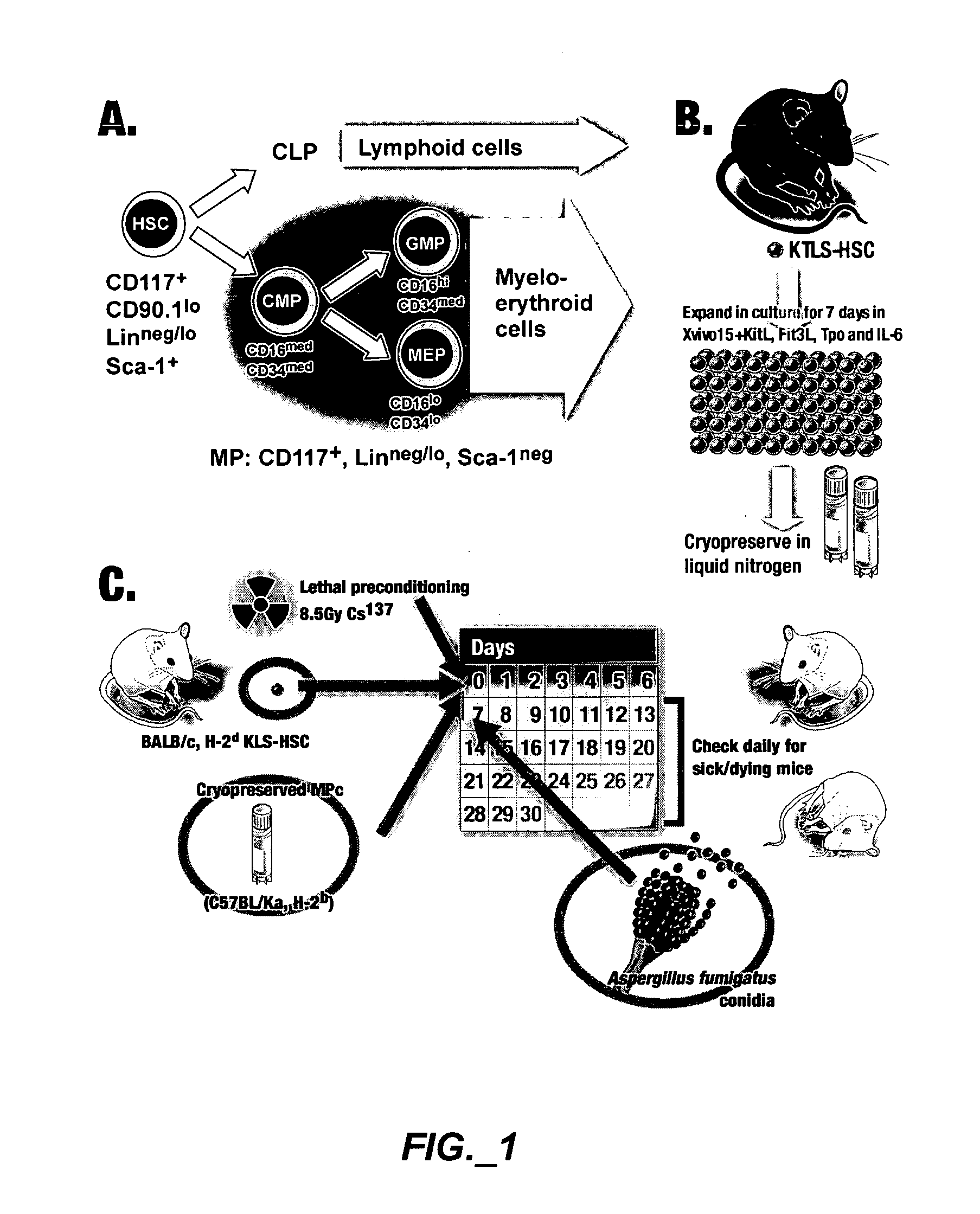
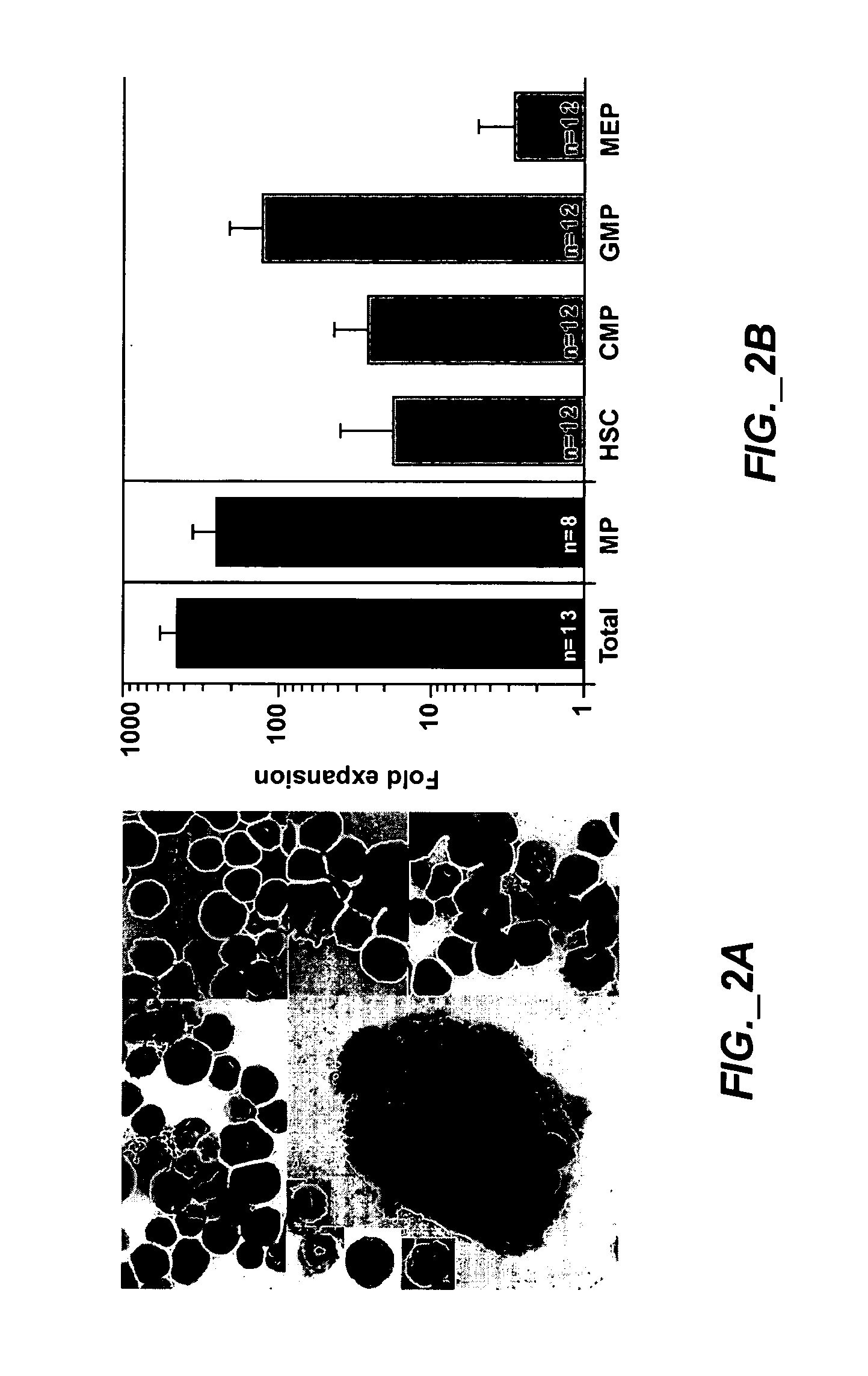
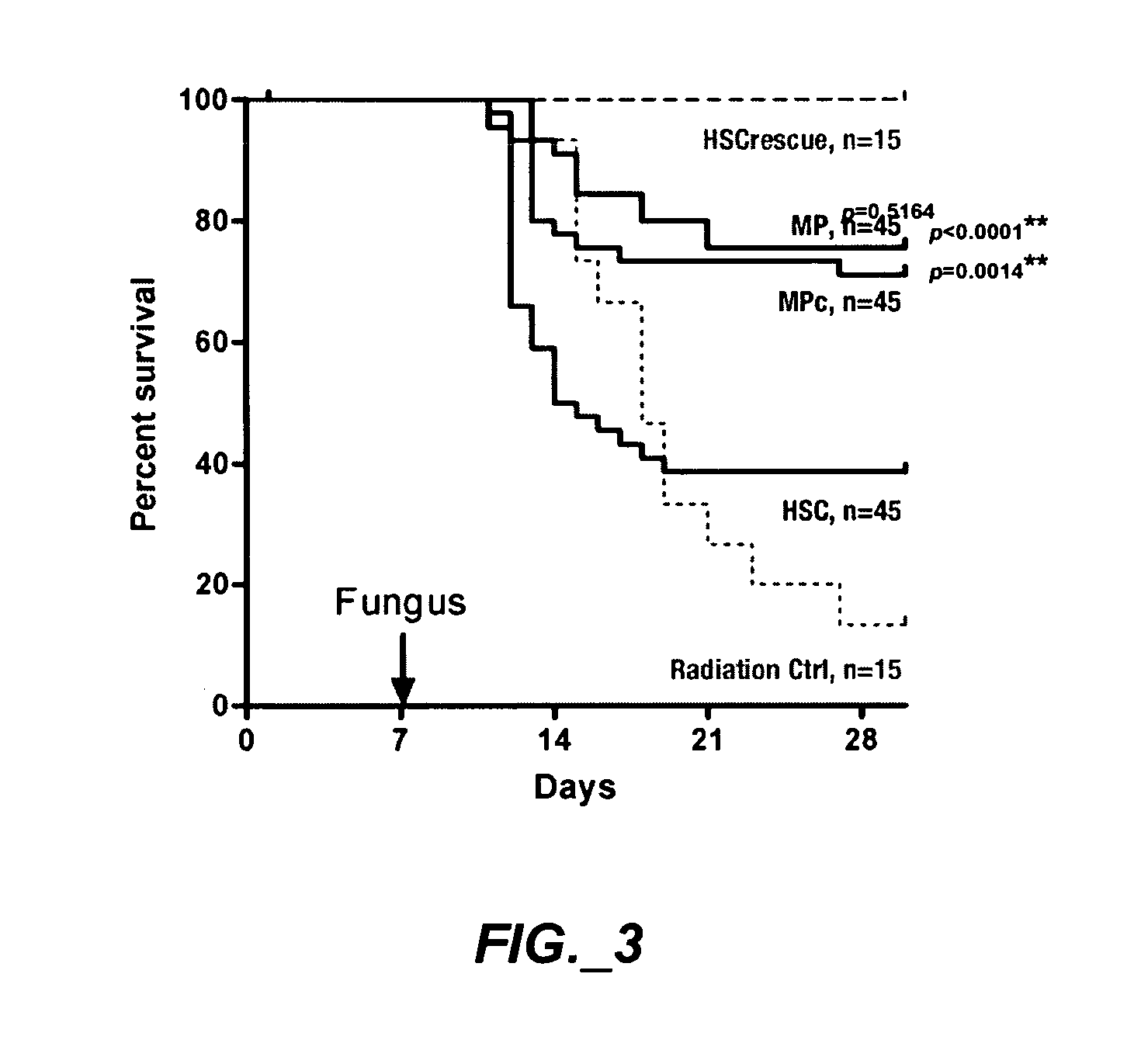
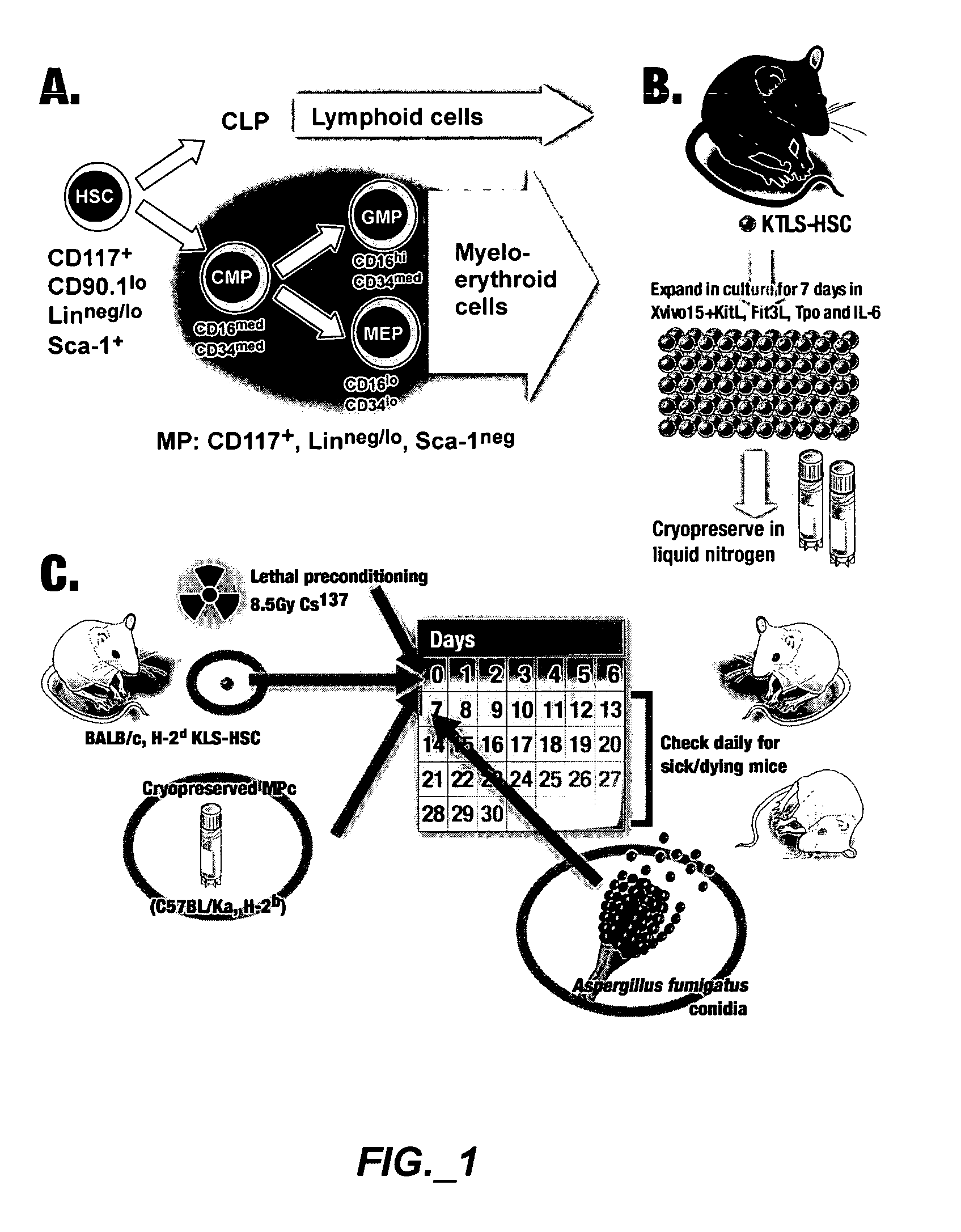
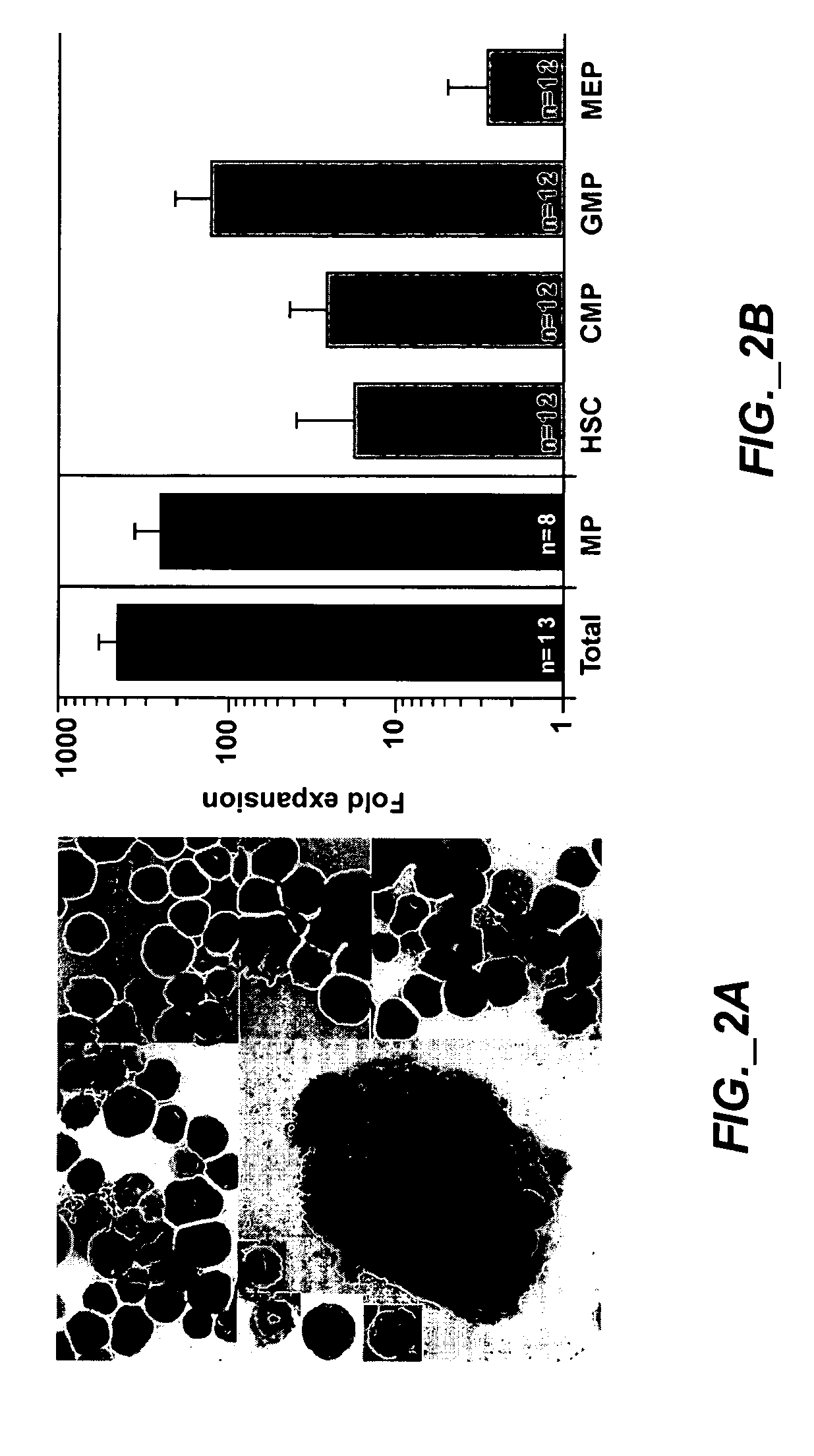
Methods of expanding myeloid cell populations and uses thereof
ActiveUS20060134783A1Increase differentiationAntibacterial agentsAntimycoticsMyeloid Progenitor CellsHematopoietic stem cell transplantation
The present disclosure relates to a method of expanding myeloid progenitor cells by culturing an initial population of cells in a medium comprising a mixture of cytokines and growth factors that promote growth and expansion of the myeloid progenitor cells. The expanded cell population provides a source of cells as therapeutic treatments for neutropenia and / or thrombocytopenia arising in patients subjected to myeloablative therapy and hematopoietic stem cell transplantation.
Owner:CELLERANT THERAPEUTICS INC
Non-lethal methods for conditioning a recipient for bone marrow transplantation
InactiveUS20030165475A1Diminished proliferative and cytotoxic activityDosage of TBI can be further reducedBiocideEnergy modified materialsAcquired immunodeficiencyGackstroemia
The present invention relates to non-lethal methods of conditioning a recipient for bone marrow transplantation. In particular, it relates to the use of nonlethal doses of total body irradiation, total lymphoid irradiation, cell type-specific or cell marker-specific antibodies, especially antibodies directed to bone marrow stromal cell markers or the CD8 cell marker, cytotoxic drugs, or a combination thereof. The methods of the invention have a wide range of applications, including, but not limited to, the conditioning of an individual for hematopoietic reconstitution by bone marrow transplantation for the treatment of hematologic malignancies, hematologic disorders, autoimmunity, infectious diseases such as acquired immunodeficiency syndrome, and the engraftment of bone marrow cells to induce tolerance for solid organ, tissue and cellular transplantation.
Owner:ILDSTAD SUZANNE T
Methods and Compositions for Enhancing Engraftment of Hematopoietic Stem Cells
ActiveUS20070237752A1Easy to implantIncrease heightBiocideMammal material medical ingredientsMyeloid Progenitor CellsHematopoietic stem cell transplantation
The present disclosure relates to the field of hematopoietic stem cell transplantation. More specifically, methods, compositions and kits for improving engraftment of stem cell transplants by administering myeloid progenitor cells are provided.
Owner:CELLERANT THERAPEUTICS INC
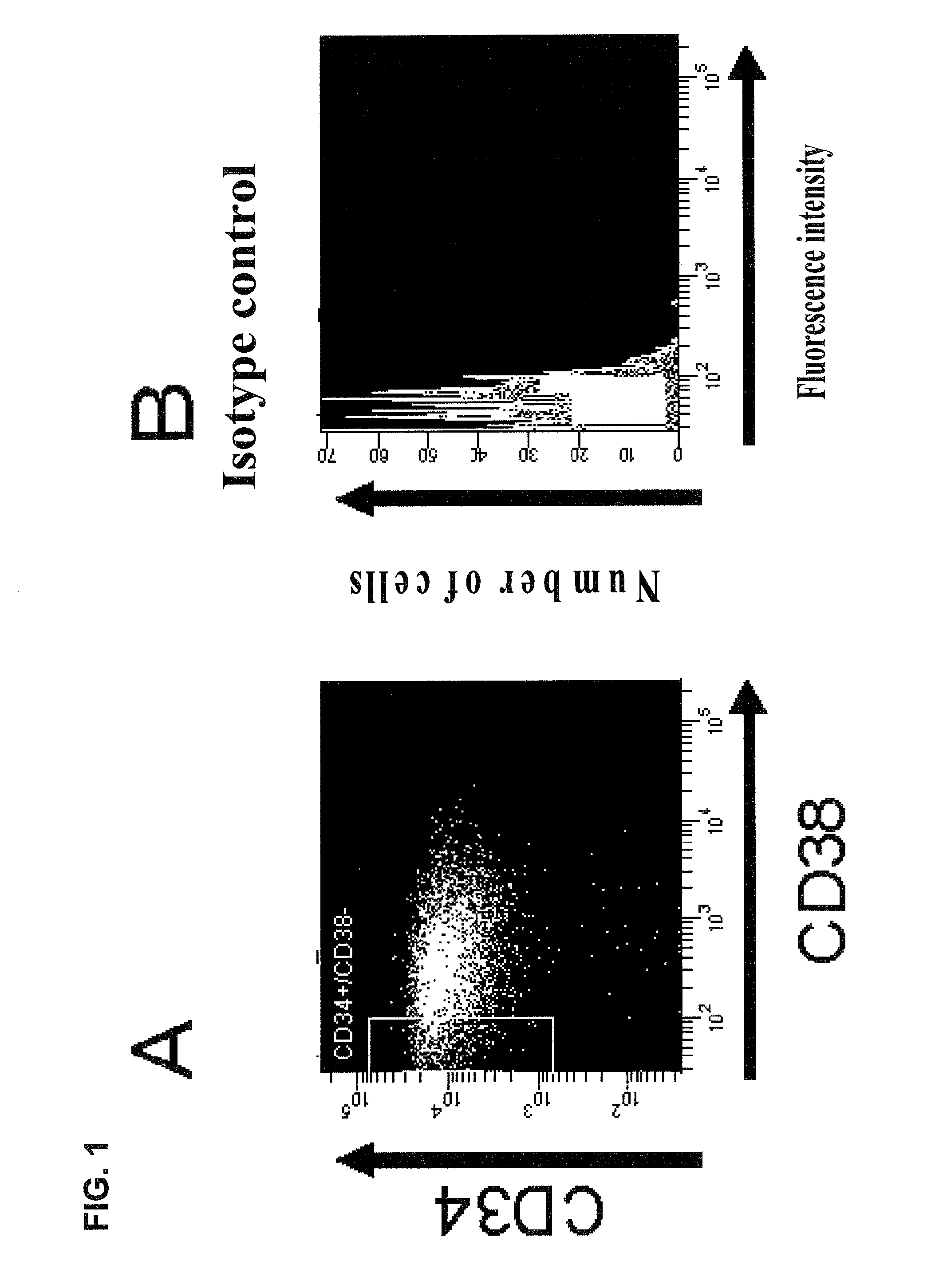
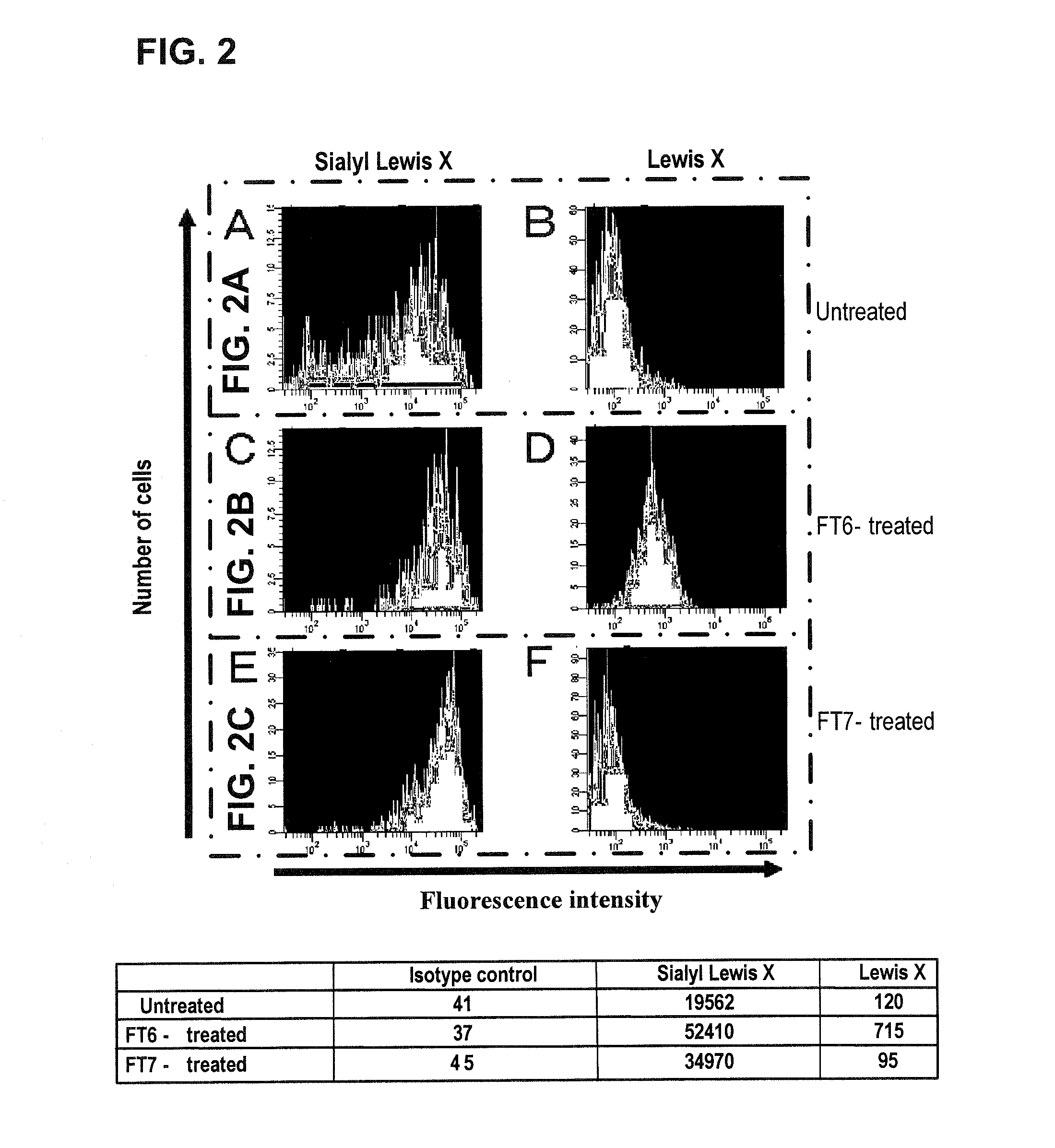
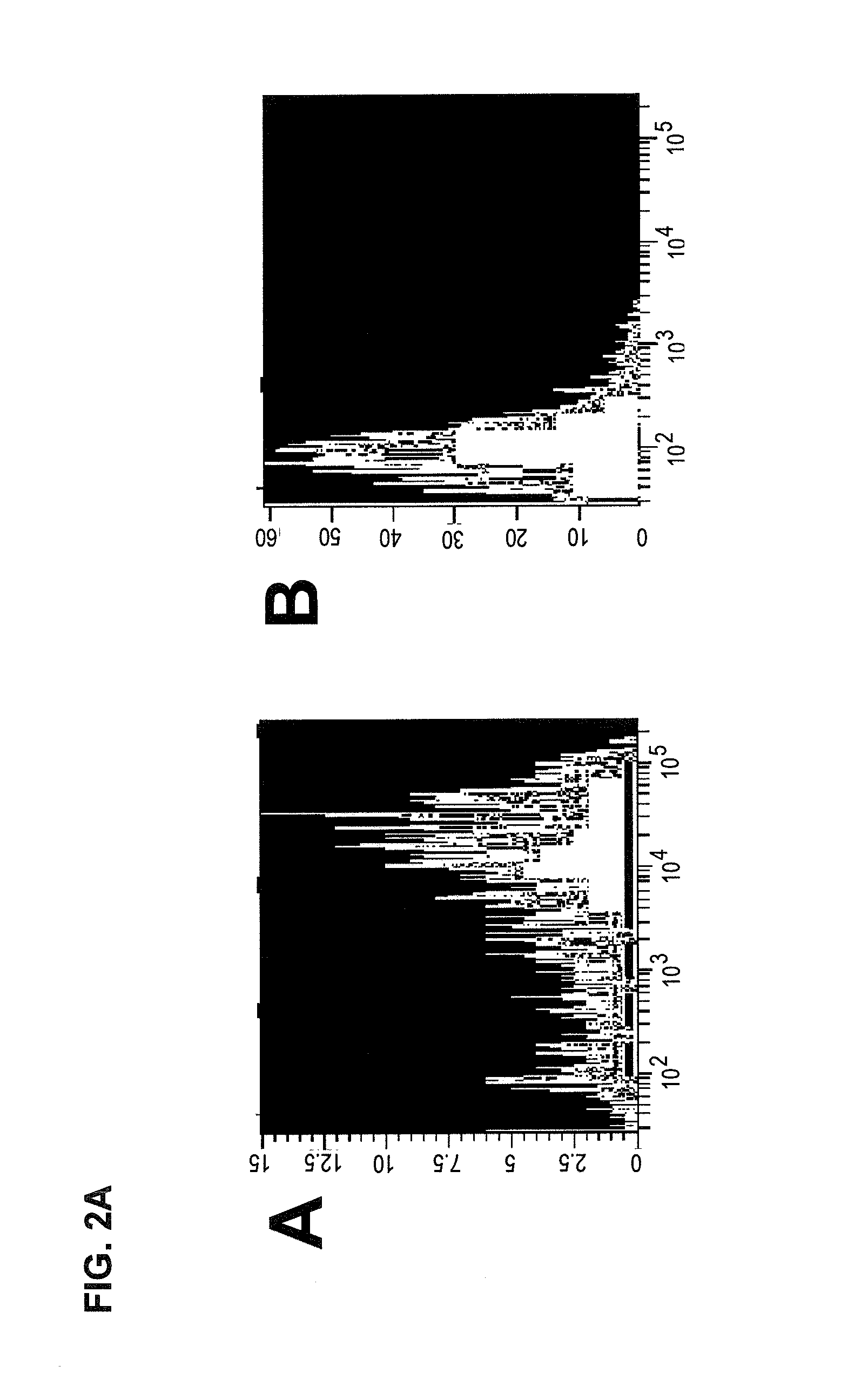
Hematopoietic stem cells treated by in vitro fucosylation and methods of use
A method of in vitro fucosylation of selectin ligands on cord blood-derived hematopoietic stem cells for bone marrow transplantation is disclosed. In this method, an effective amount of an α1,3-fucosyltransferase, e.g., α1,3-fucosyltransferase VI, is used in vitro to treat cord blood-derived hematopoietic stem cells to convert non-functional PSGL-1 or other ligands on the cell surface into functional forms that bind selectins, especially P-selectin or E-selectin. The treated cells have enhanced effectiveness in reconstituting bone marrow in patients in need of such therapy.
Owner:OKLAHOMA MEDICAL RES FOUND
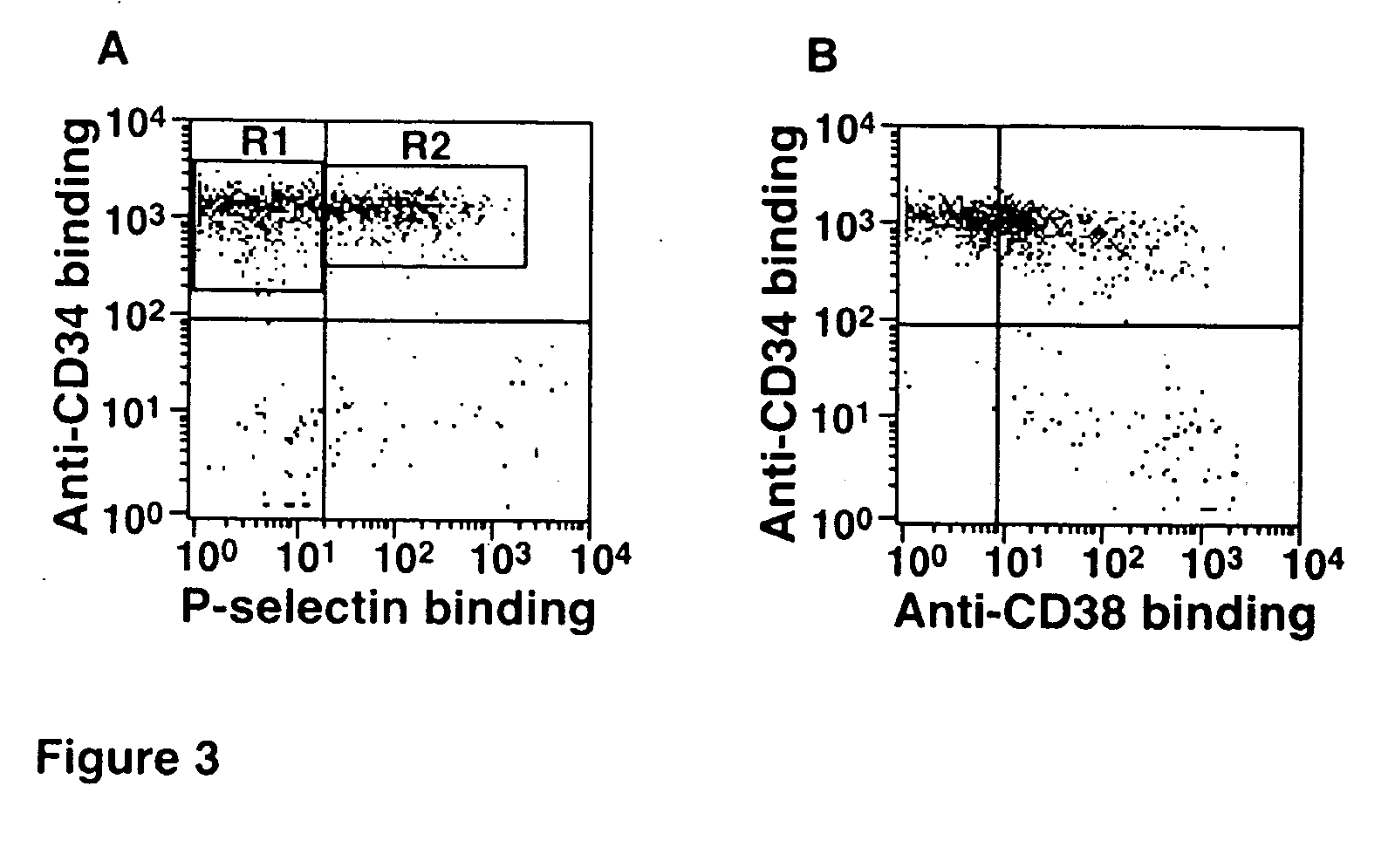
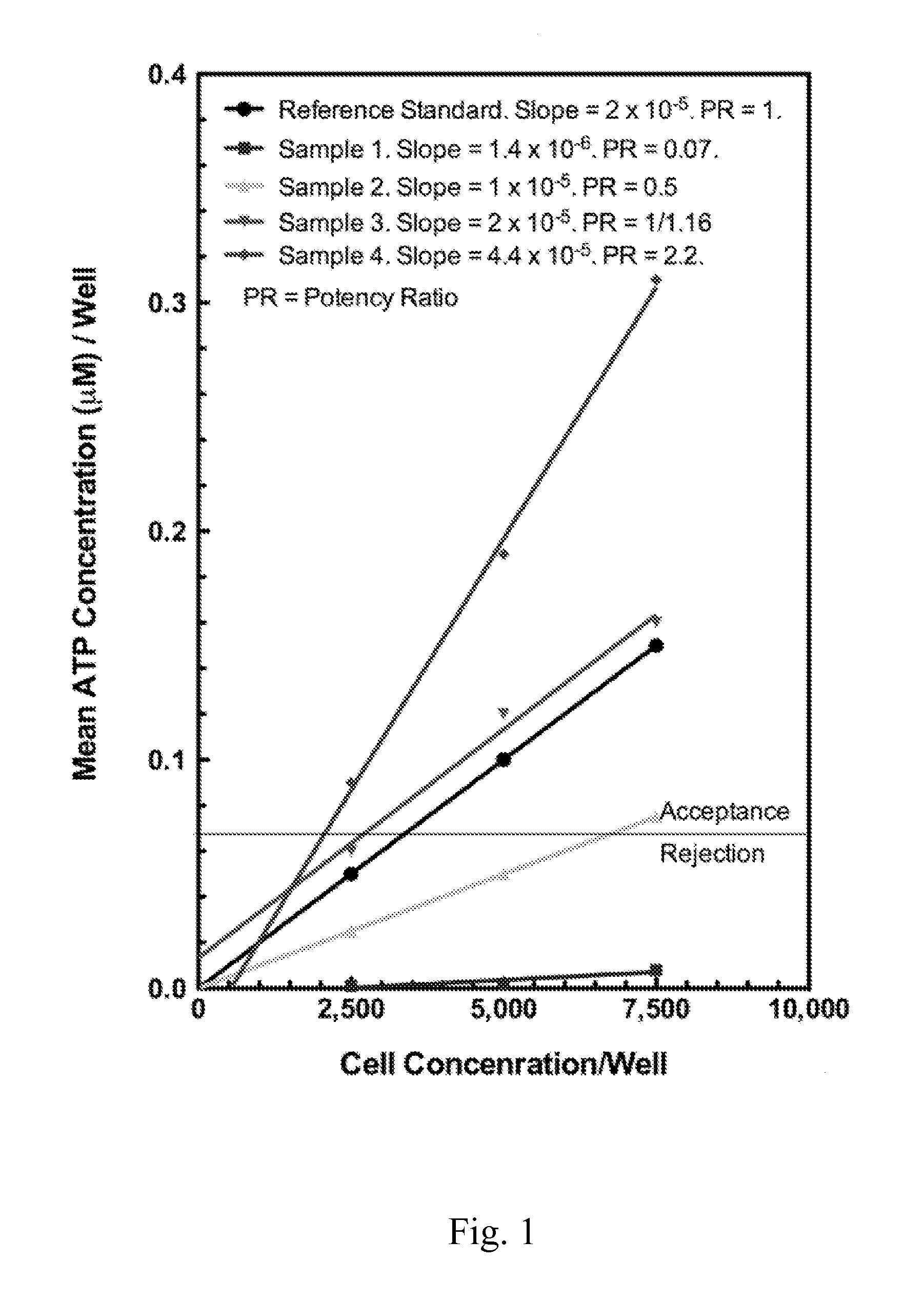
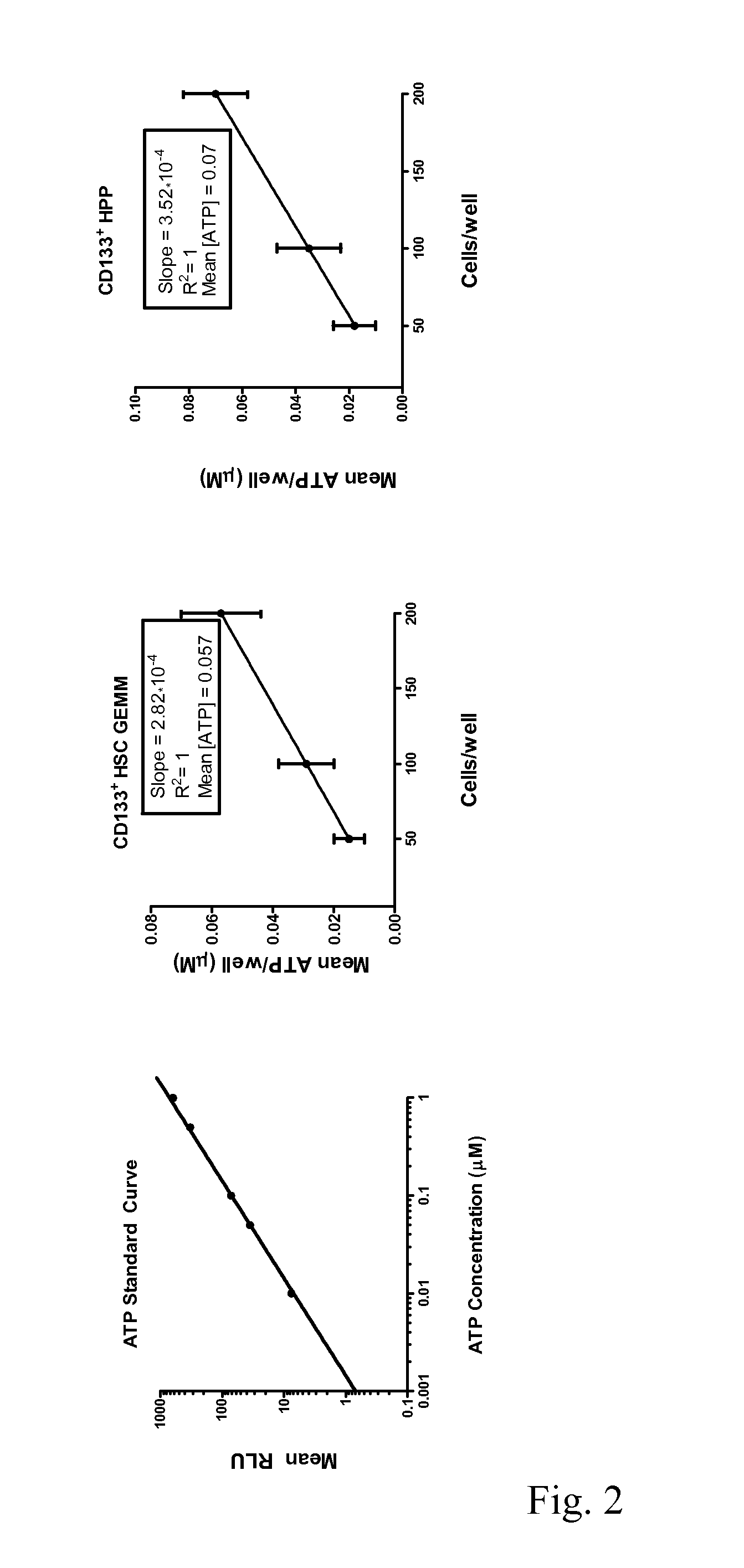
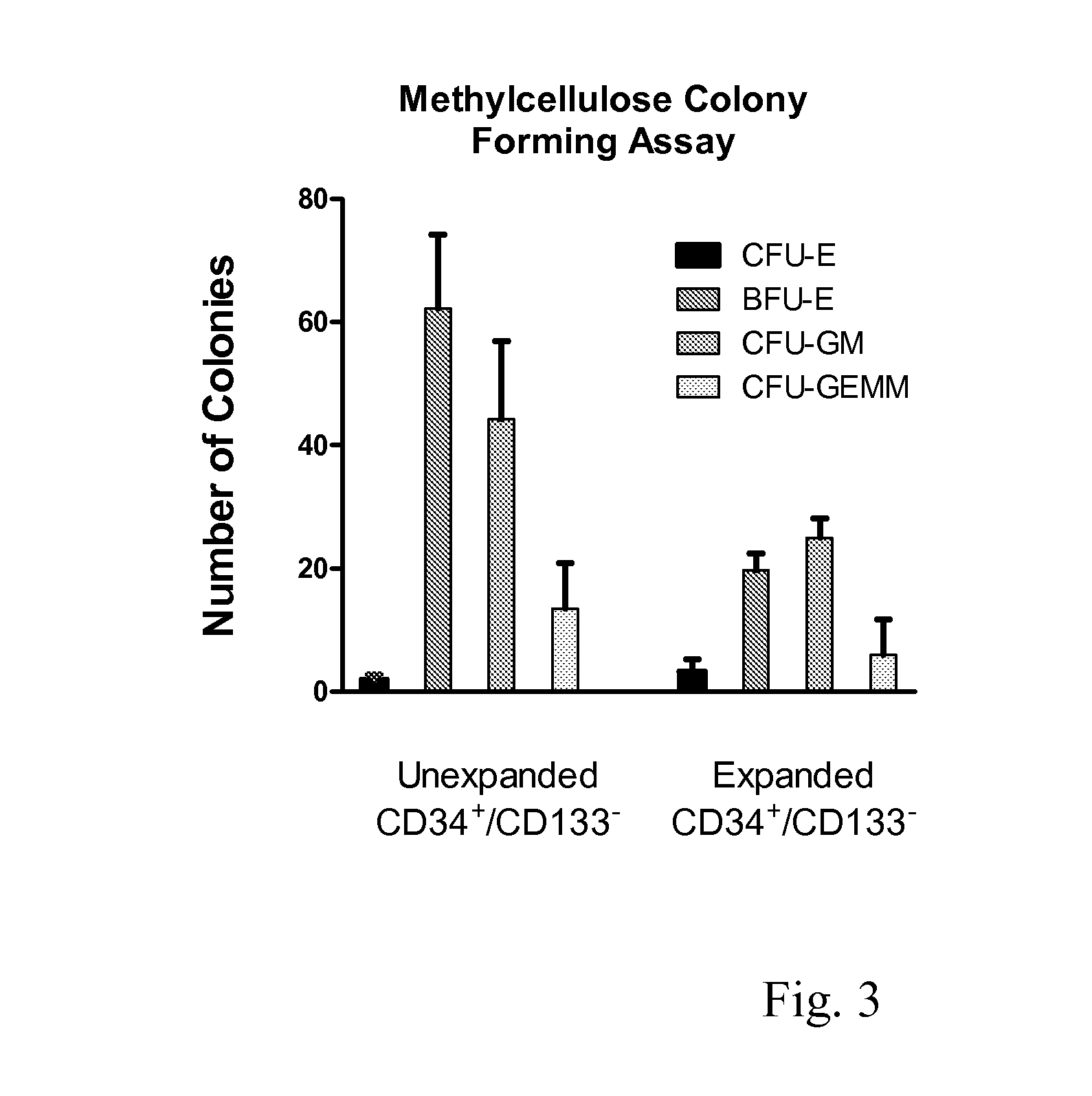
Enhanced Hematopoietic Stem Cell Engraftment
InactiveUS20120093782A1Enhance transplant potentialTherapeutic applicationBiocideMammal material medical ingredientsCord blood stem cellHematopoietic cell
The invention relates to improved products, processes, and therapeutic methods relating to hematopoietic stem cells and hematopoietic stem cell transplantation. Included are methods for improving transplant efficiency of cord blood units comprising use of mixtures of expanded CD34+ / CD 133− HSCs and unexpanded CD133+ HSCs for IBM administration.
Owner:GROVE ROBERT I +1
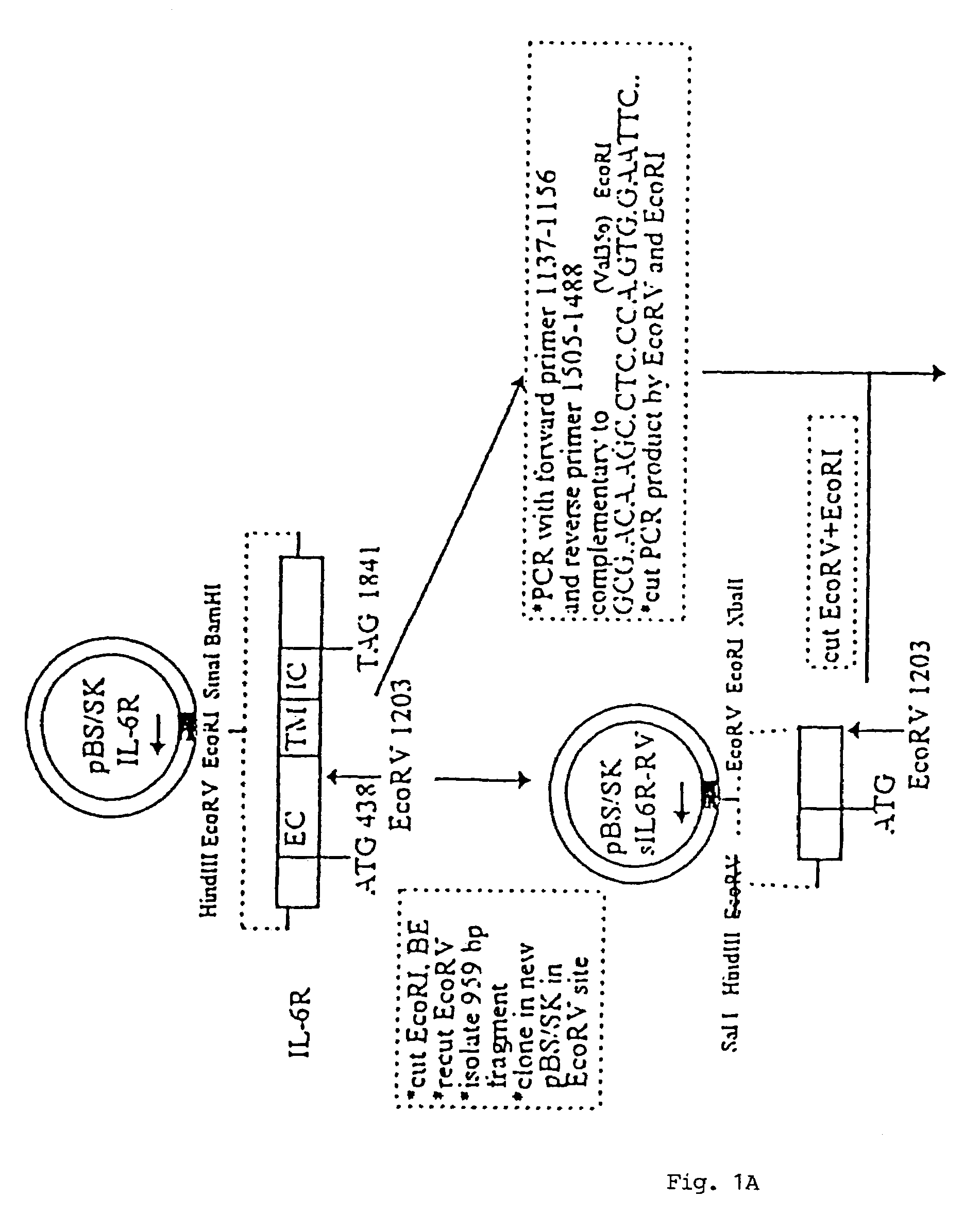
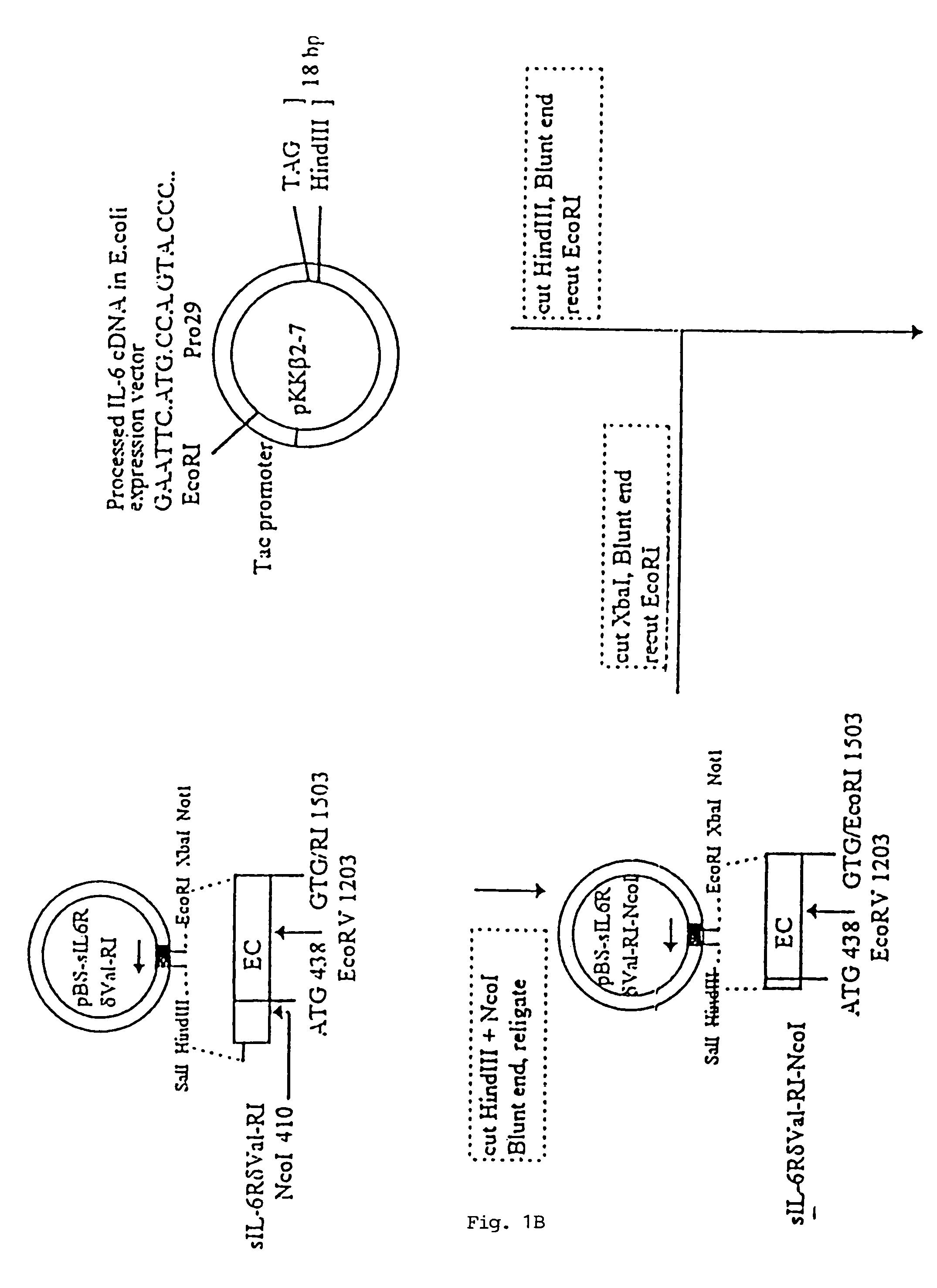
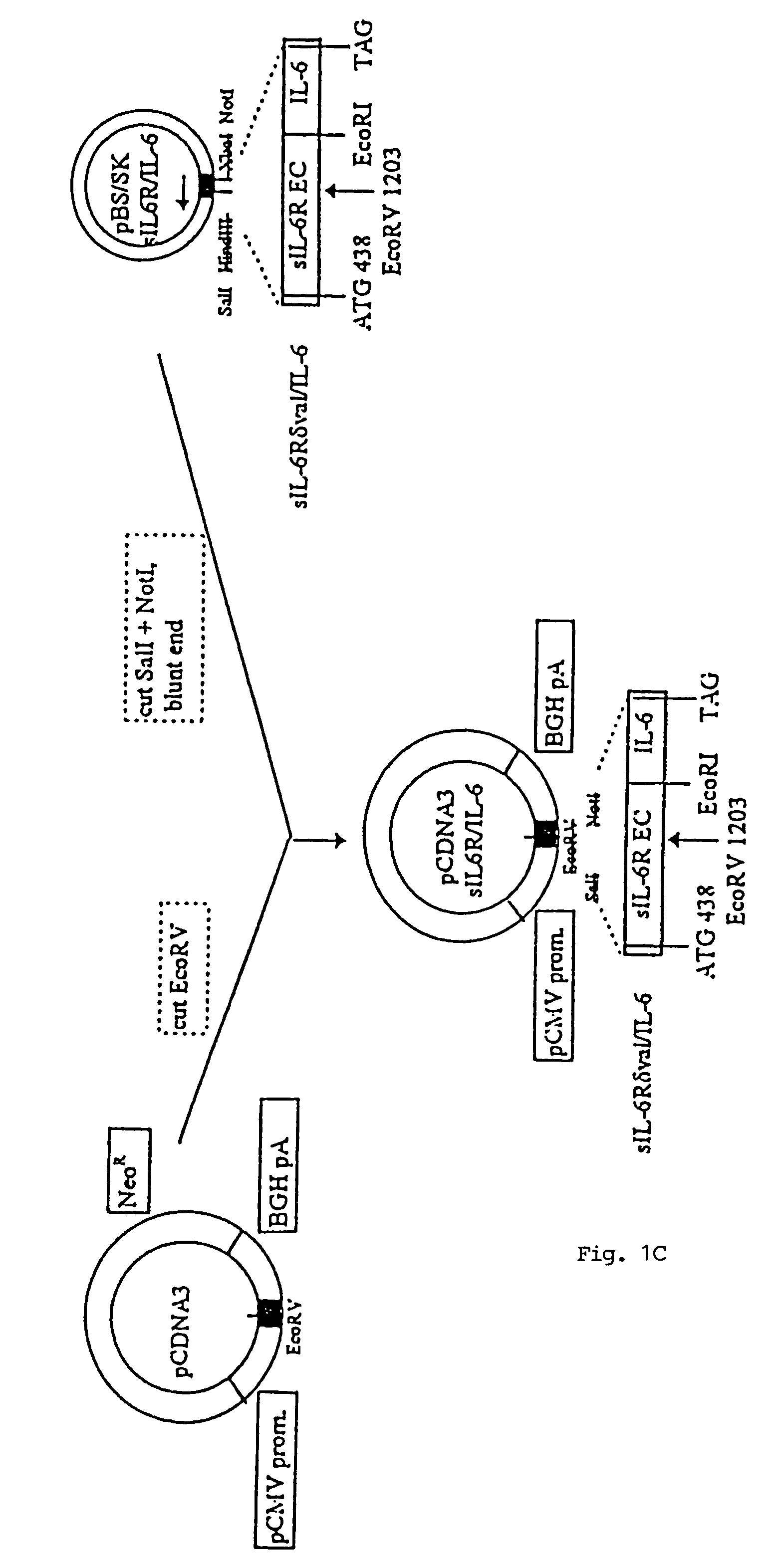
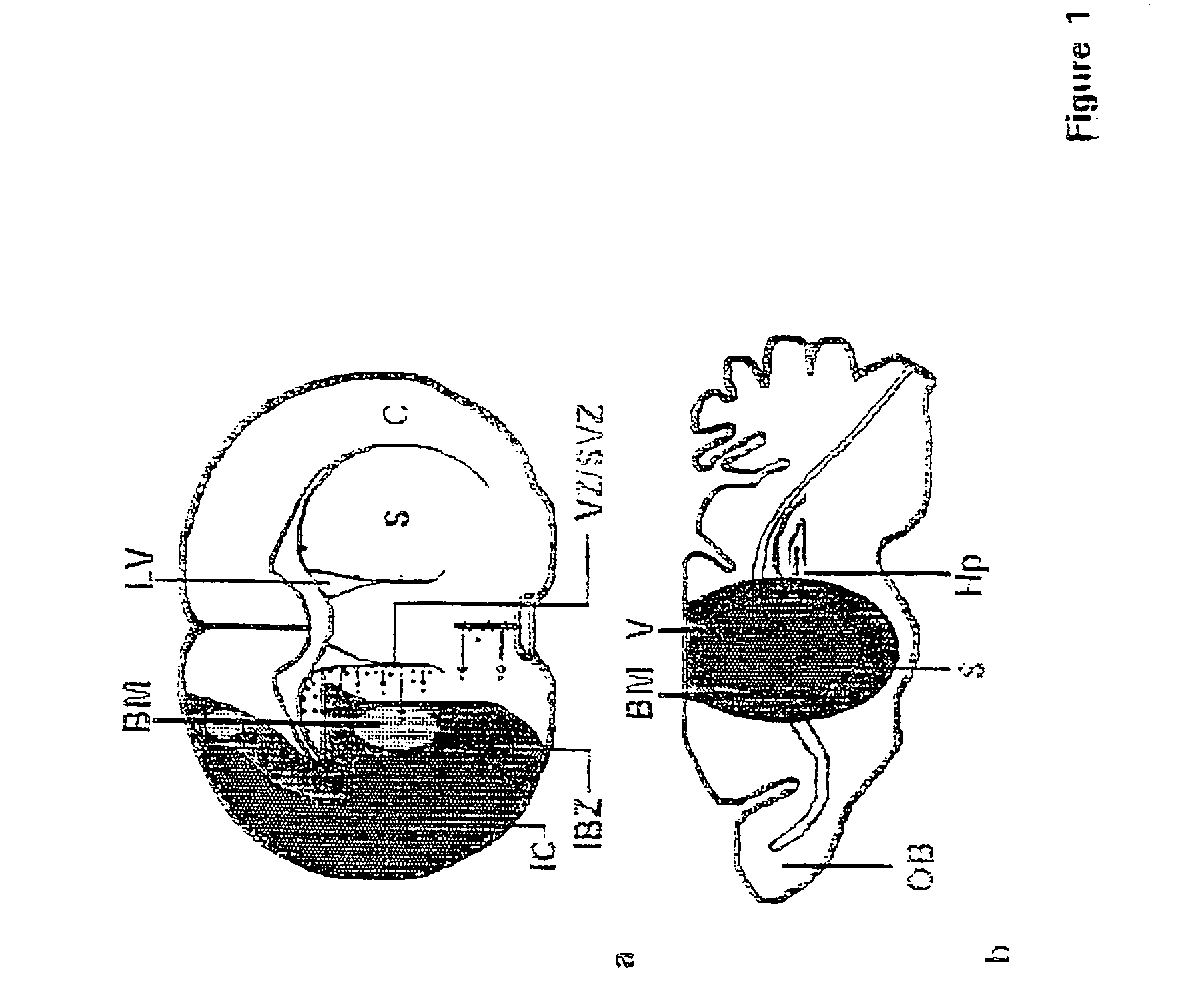
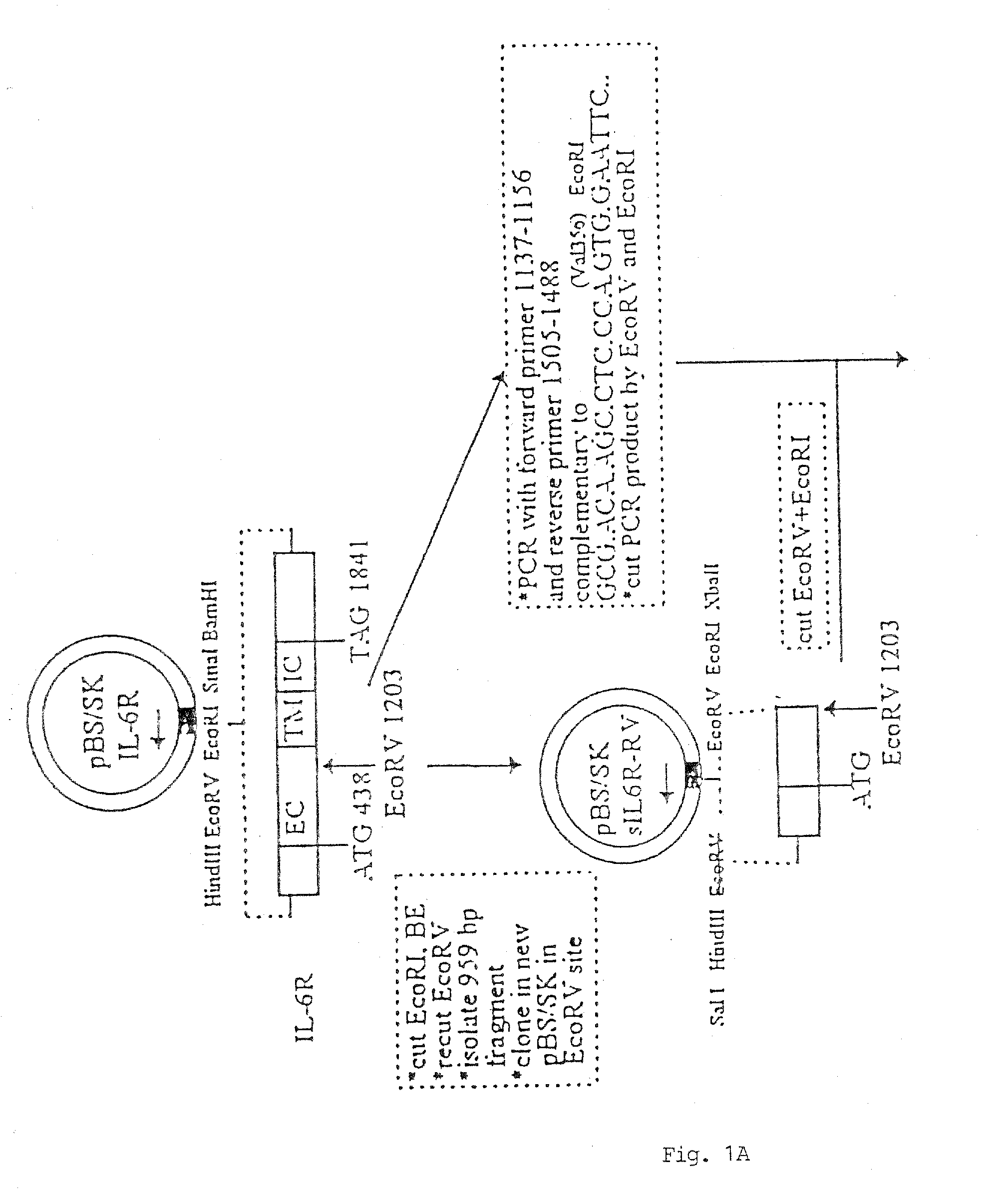
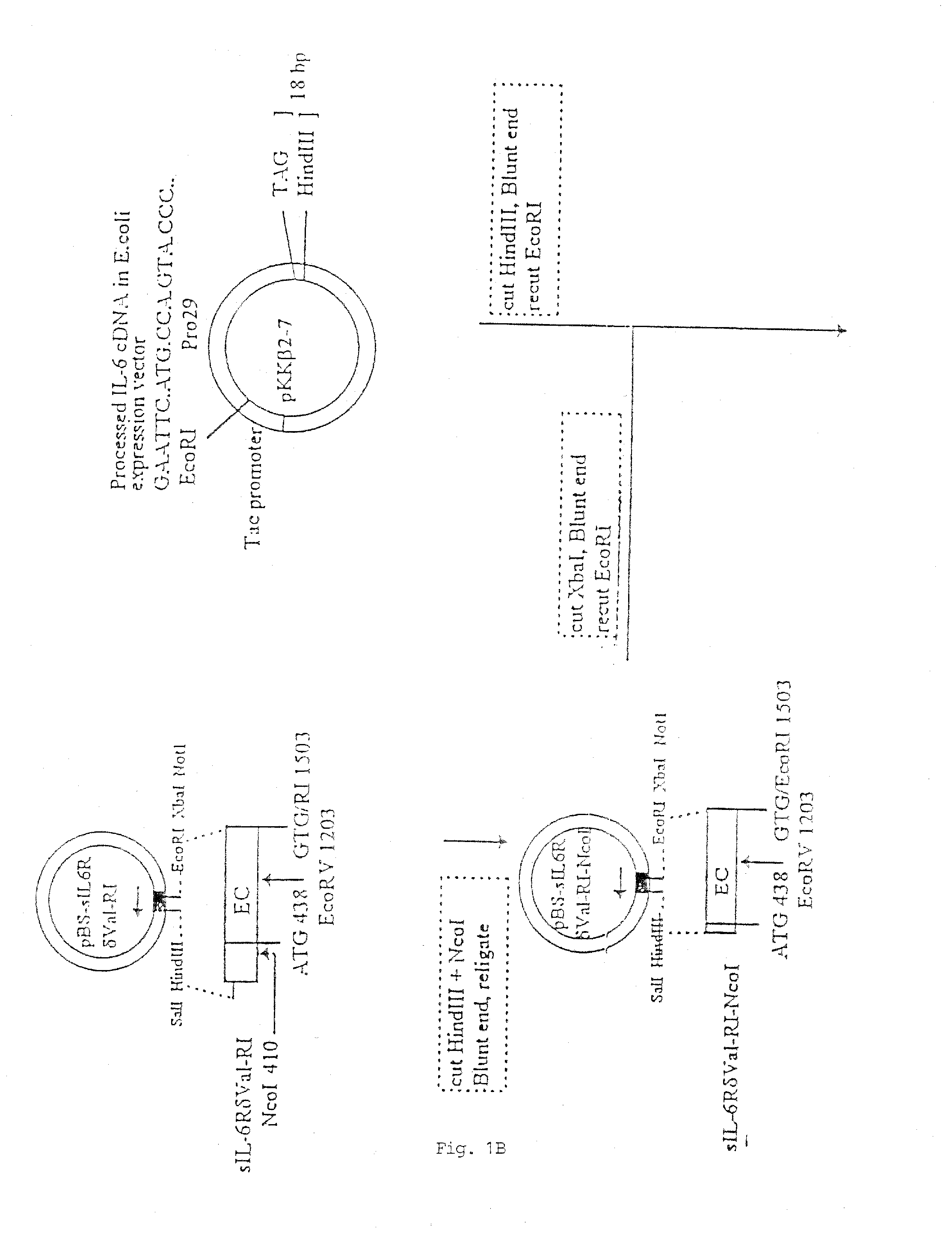
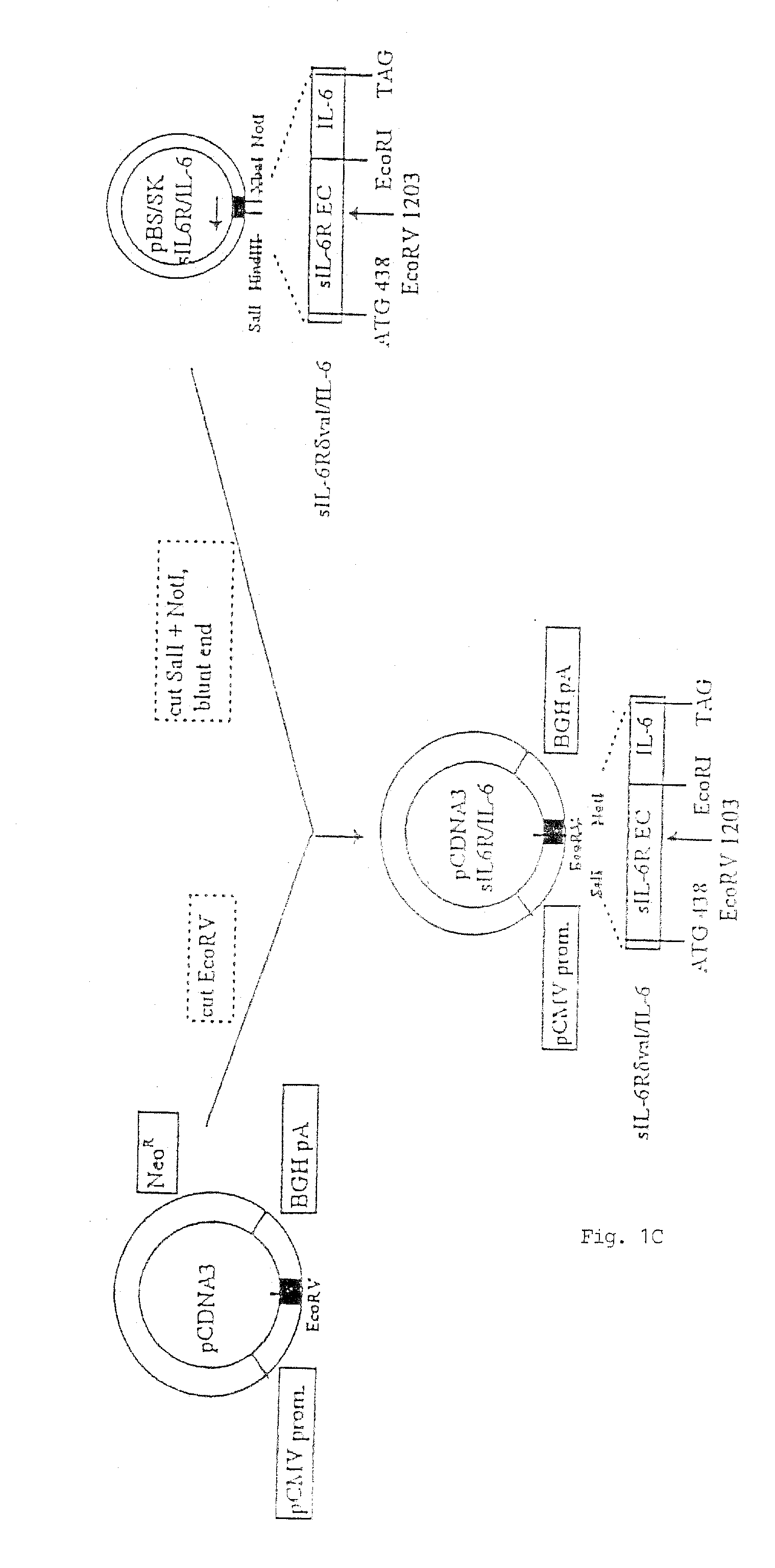
Chimeric interleukin-6 soluble receptor/ligand proteins
InactiveUS7198781B1Risk minimizationReduce potential immunogenicityNervous disorderPeptide/protein ingredientsDiseaseInterleukin 6
Chimeric proteins constructed from the fusion of the naturally occurring form of the soluble IL-6 receptor and IL-6 which are useful for treatment of cancer and liver disorders, enhancement of bone marrow transplantation, and treatment of other IL-6 related conditions are provided.
Owner:YEDA RES & DEV CO LTD
Bone marrow transplantation for treatment of stroke
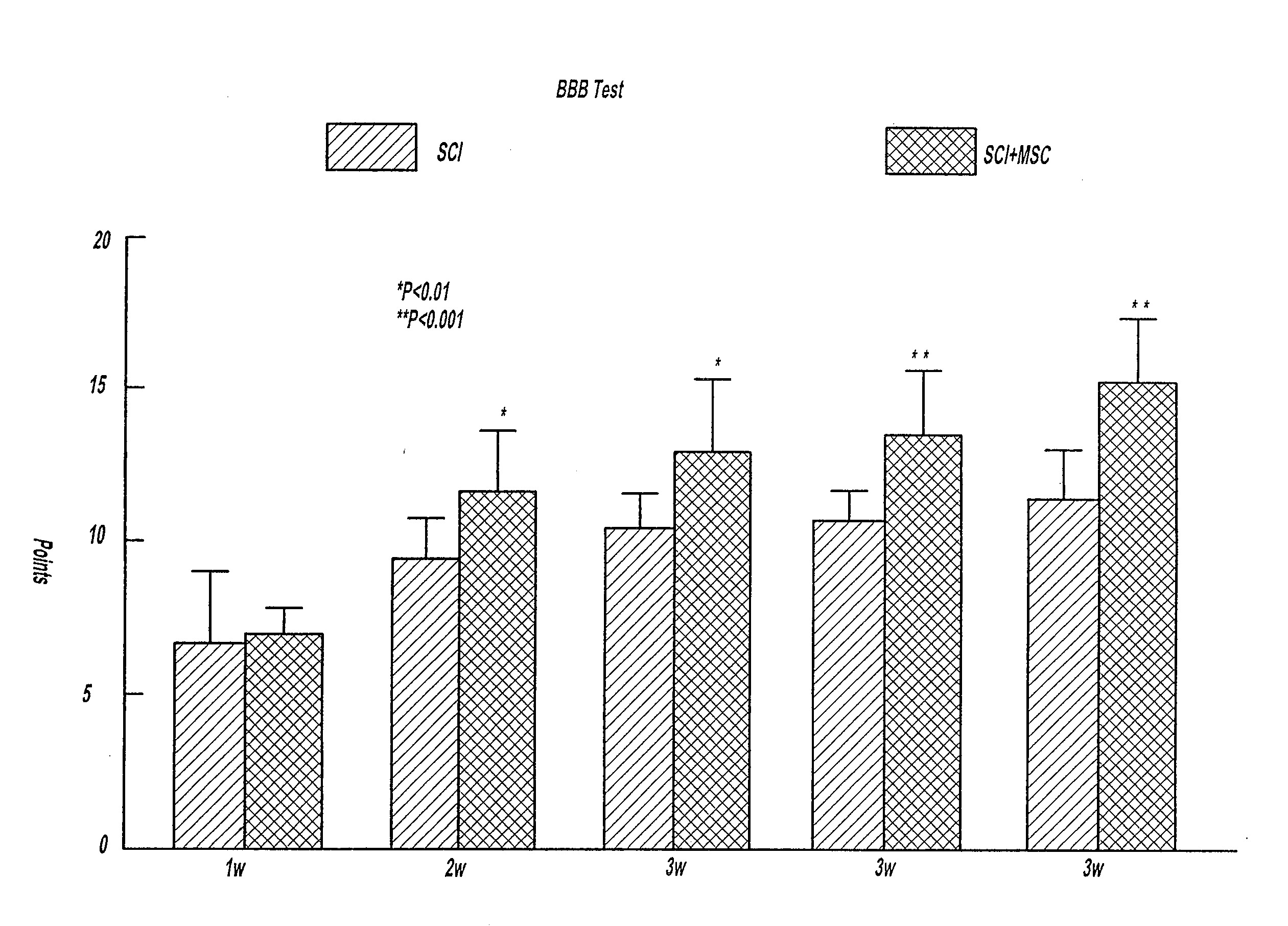
InactiveUS20090162327A1Reduces functional deficitRelieve symptomsBiocideNervous disorderDiseaseNeural cell
There is provided a treatment for patients suffering from neurodegenerative disease or neural injury including the steps of transplanting cultured bone marrow cells into the spinal cord or brain or injecting intravascularly bone marrow cells of a patient in need. Also provided is a method of activating the differentiation of neural cells in an injured brain including the steps of transplanting bone marrow cells adjacent to the injured brain cells and activating the endogenous central nervous system stem cells to differentiate into neurons. A method of treating injured brain or spinal cord cells is also provided including the steps of transplanting bone marrow cells near the injured brain cells and generating new neurons at the location of transplantation. A method of treating injured brain or spinal cord cells with a composite of MSCs and neurospheres.
Owner:HENRY FORD HEALTH SYST
Chimeric Interleukin-6 Soluble Receptor/Ligand Protein, Analogs Thereof and Uses Thereof
InactiveUS20070172455A1Risk minimizationReduce potential immunogenicityNervous disorderPeptide/protein ingredientsDiseaseInterleukin 6
Owner:YEDA RES & DEV CO LTD
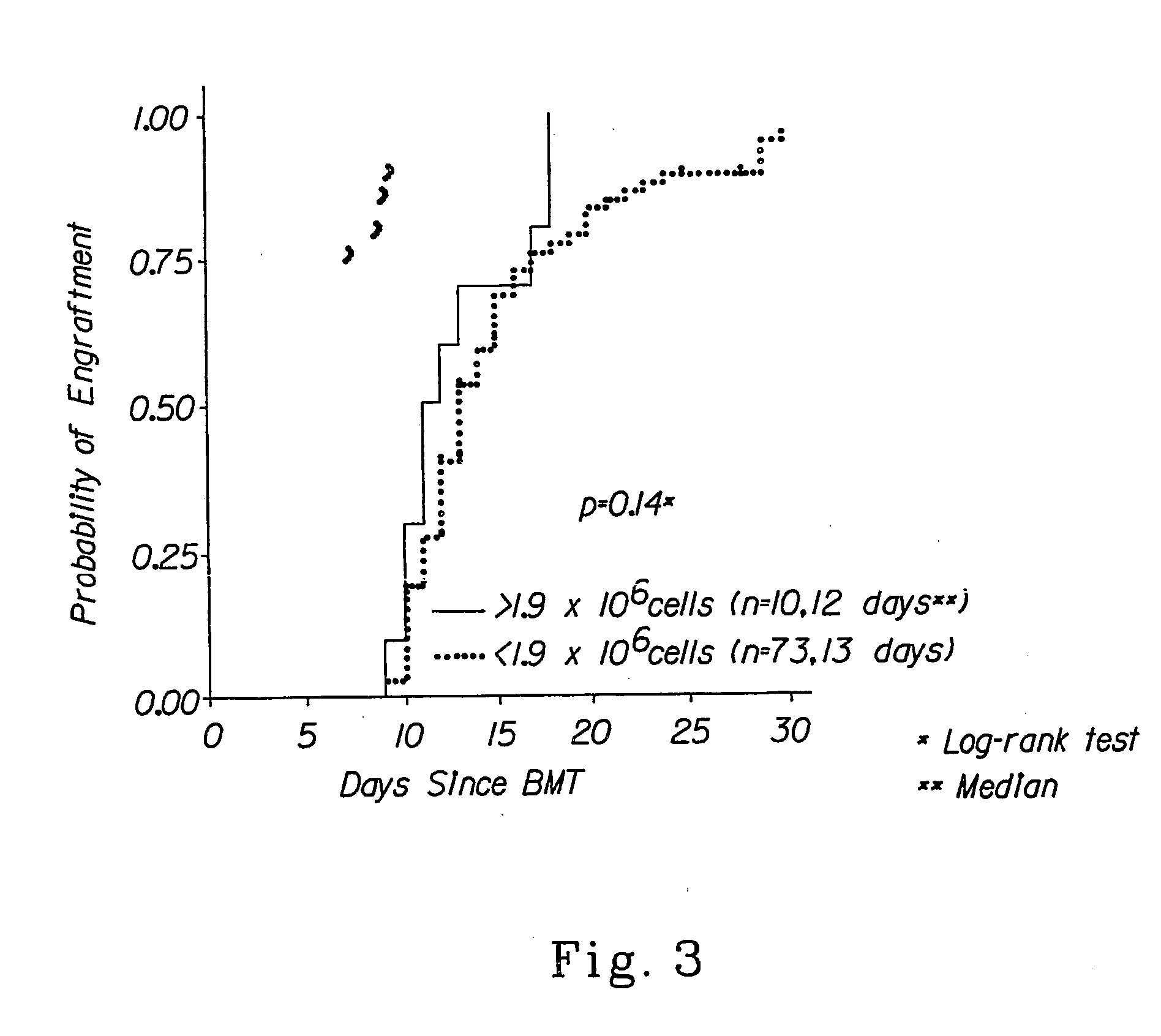
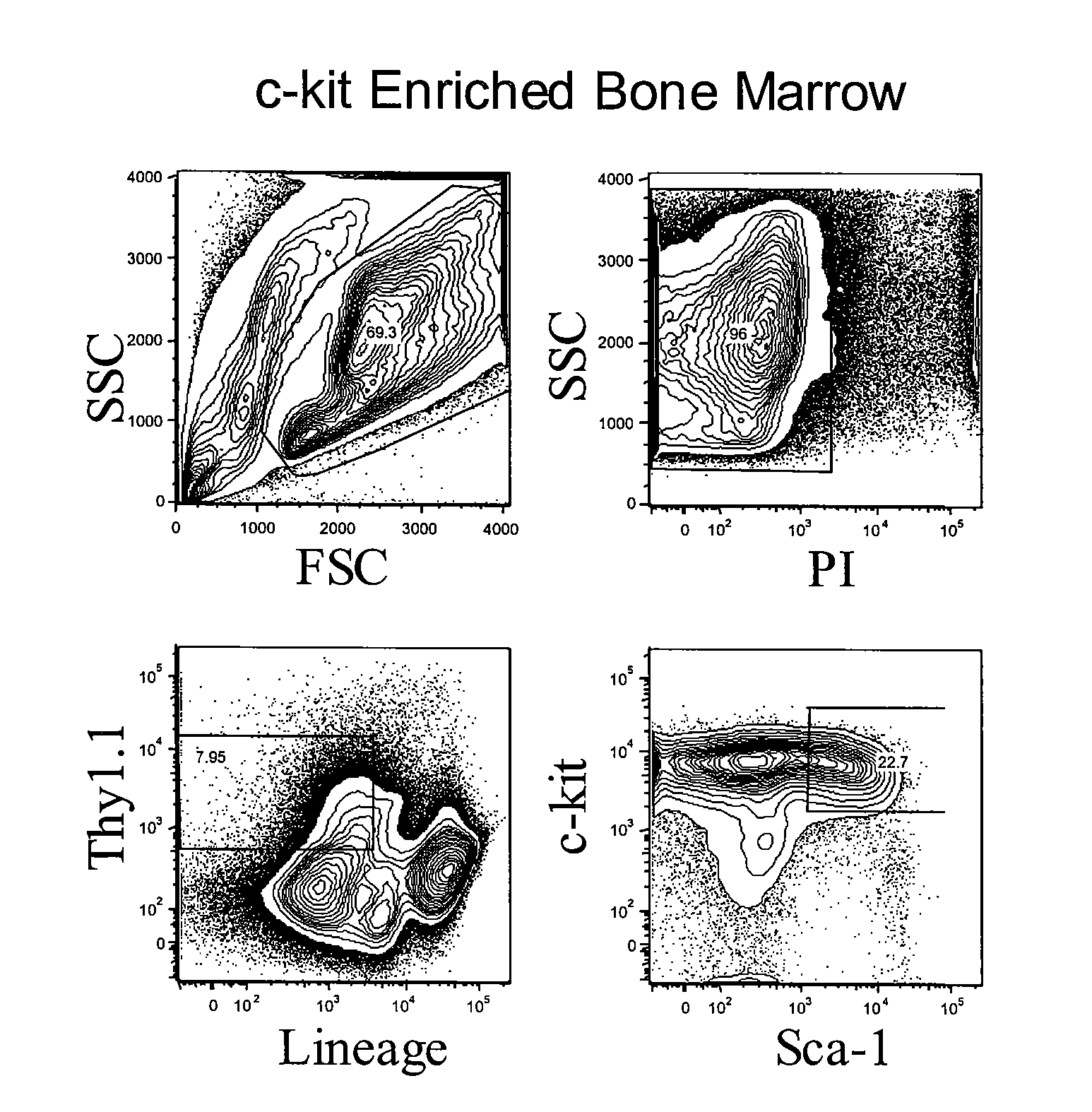
Method to determine an engrafting cell dose of hematopoietic stem cell transplant units
InactiveUS6852534B2Accurate assessmentAutomatically performBiocideDead animal preservationCord blood stem cellCell dose
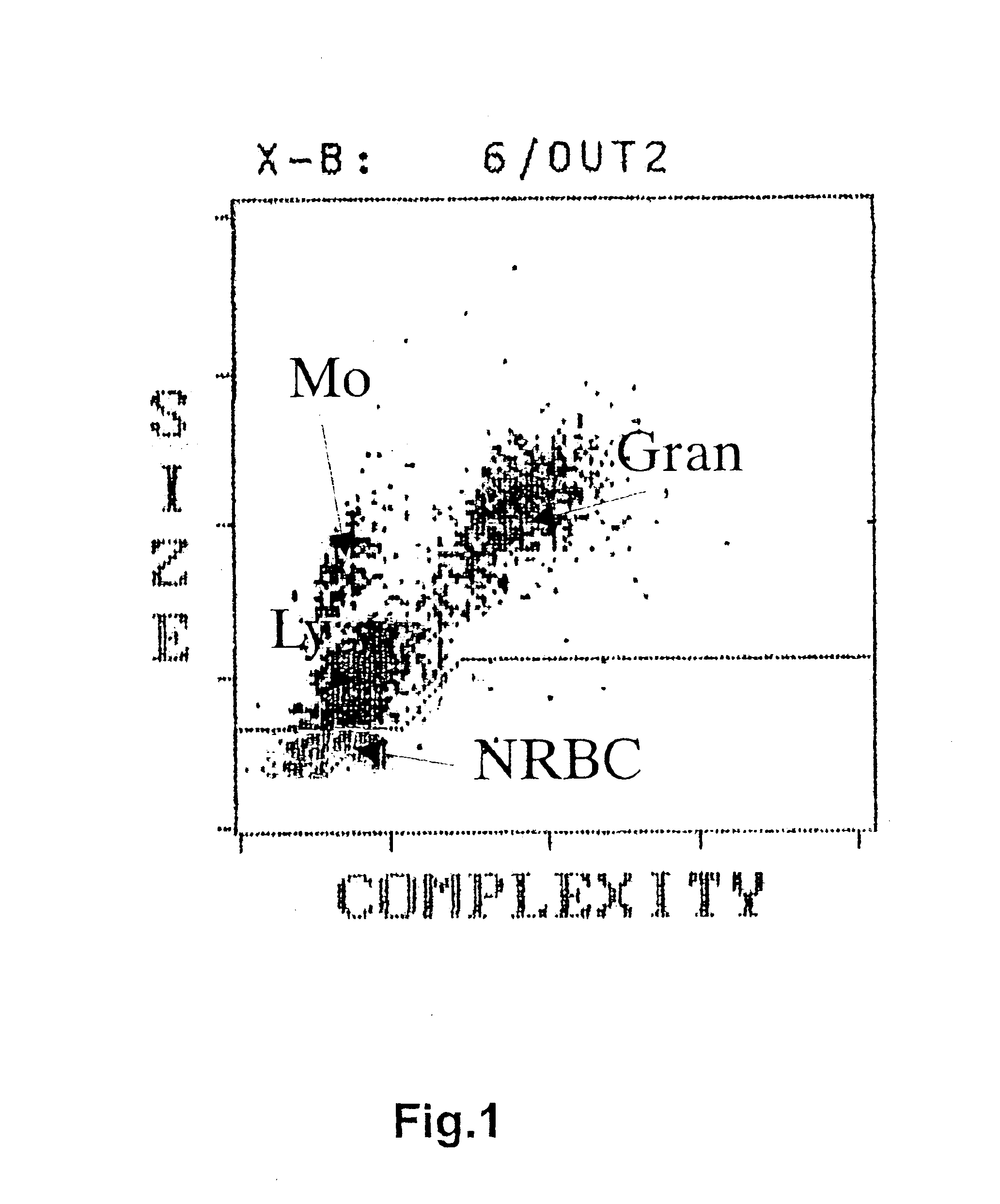
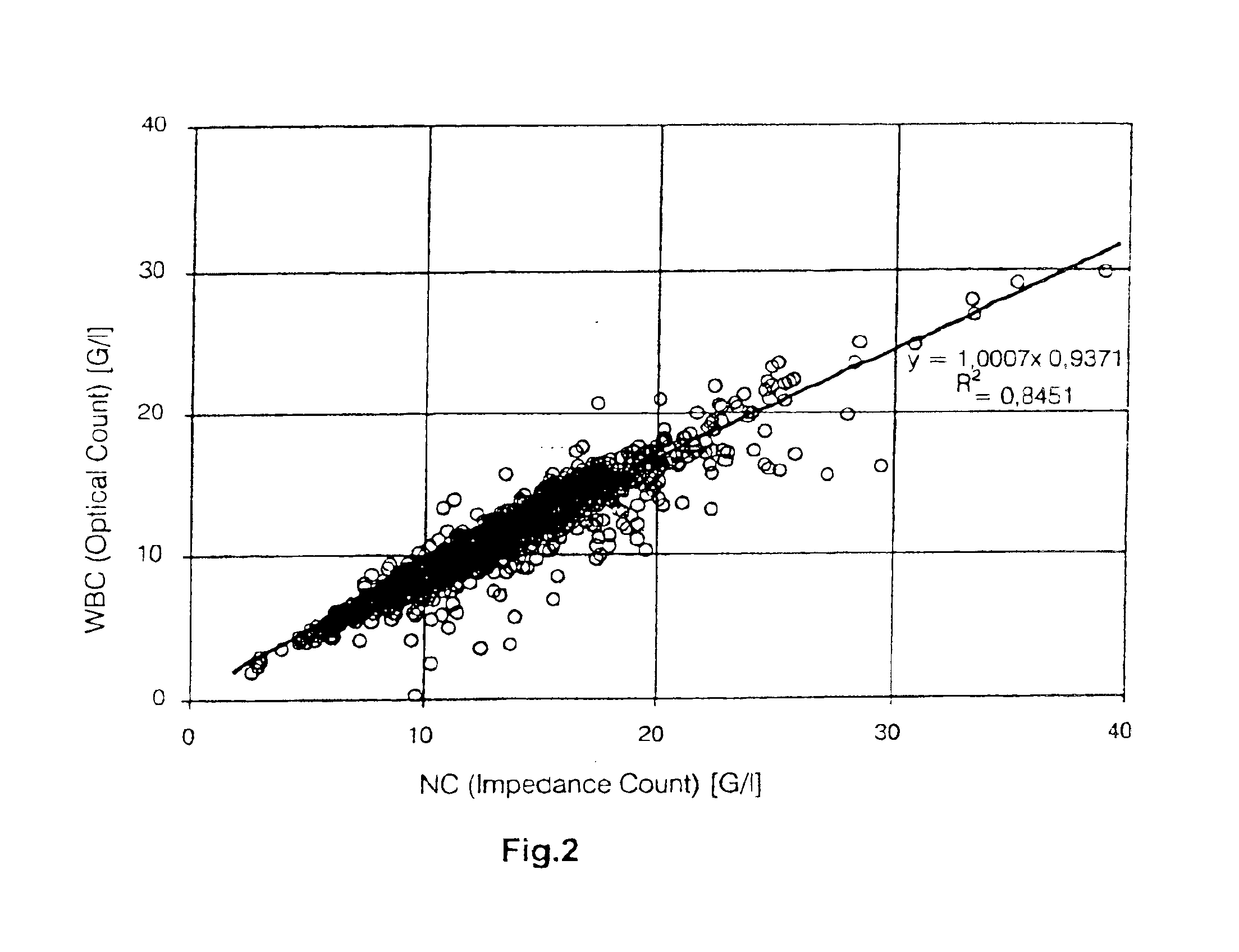
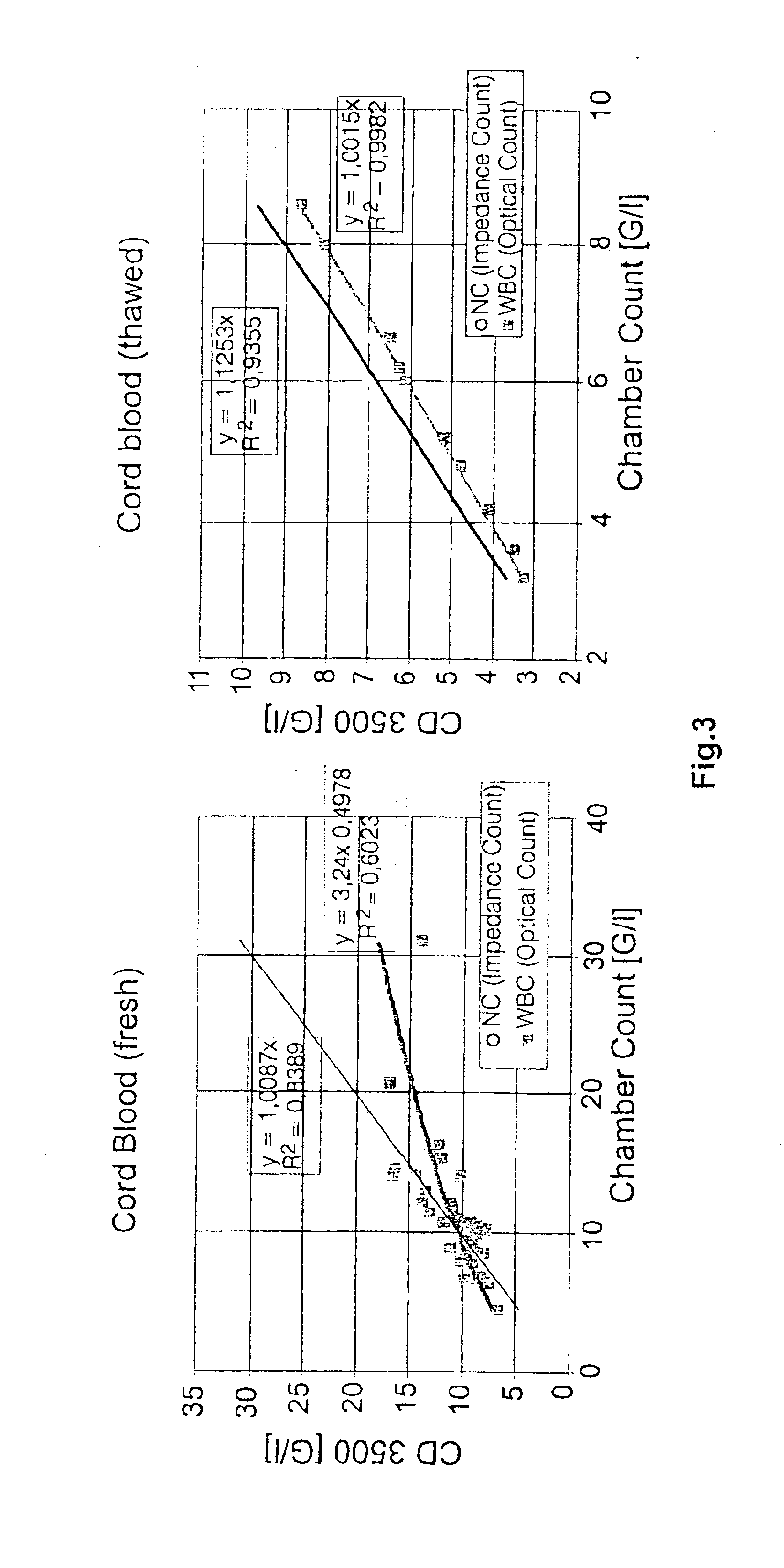
A method to determine an engrafting cell dose of hematopoietic stem cell transplant units from transplant sources having nucleated cells selected from the group consisting of cord blood, bone marrow, peripheral blood comprising the steps of[0002]subjecting the source to a substantially complete erythrocyte lysis,[0003]measuring in a cell counter a signal corresponding selectively to white blood cells,[0004]assessing essentially quantitatively nucleated red blood cell (NRBC) count as part of the total nucleated cell (NC) count,[0005]and determining the number of white blood cells (WBCs) as transplant relevant cells.
Owner:KOURION THERAPEUTICS
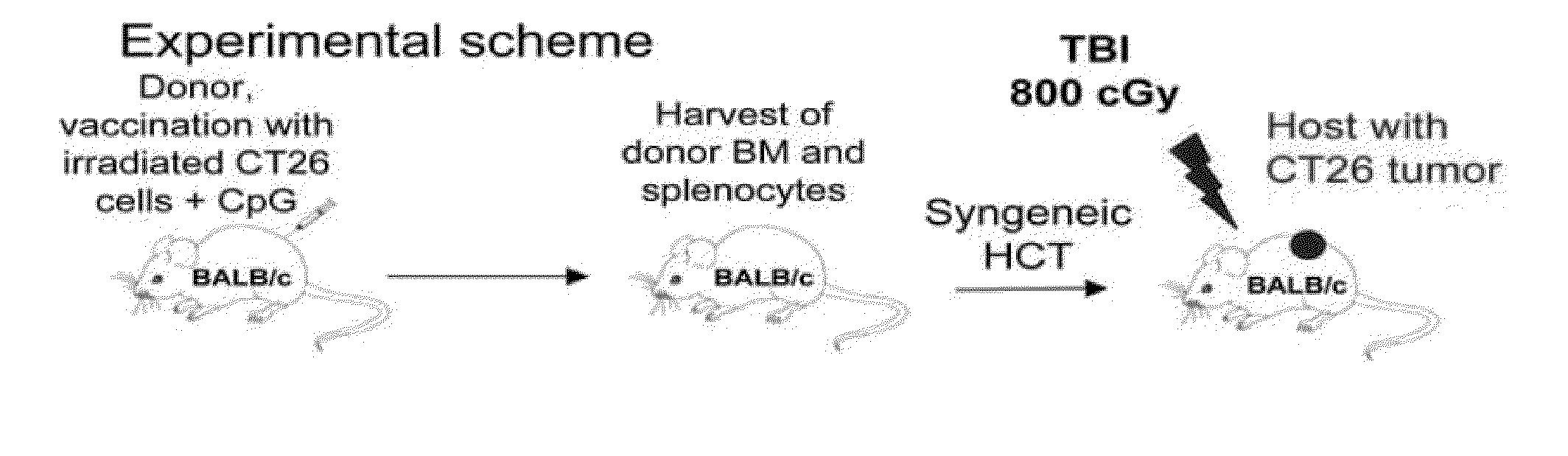
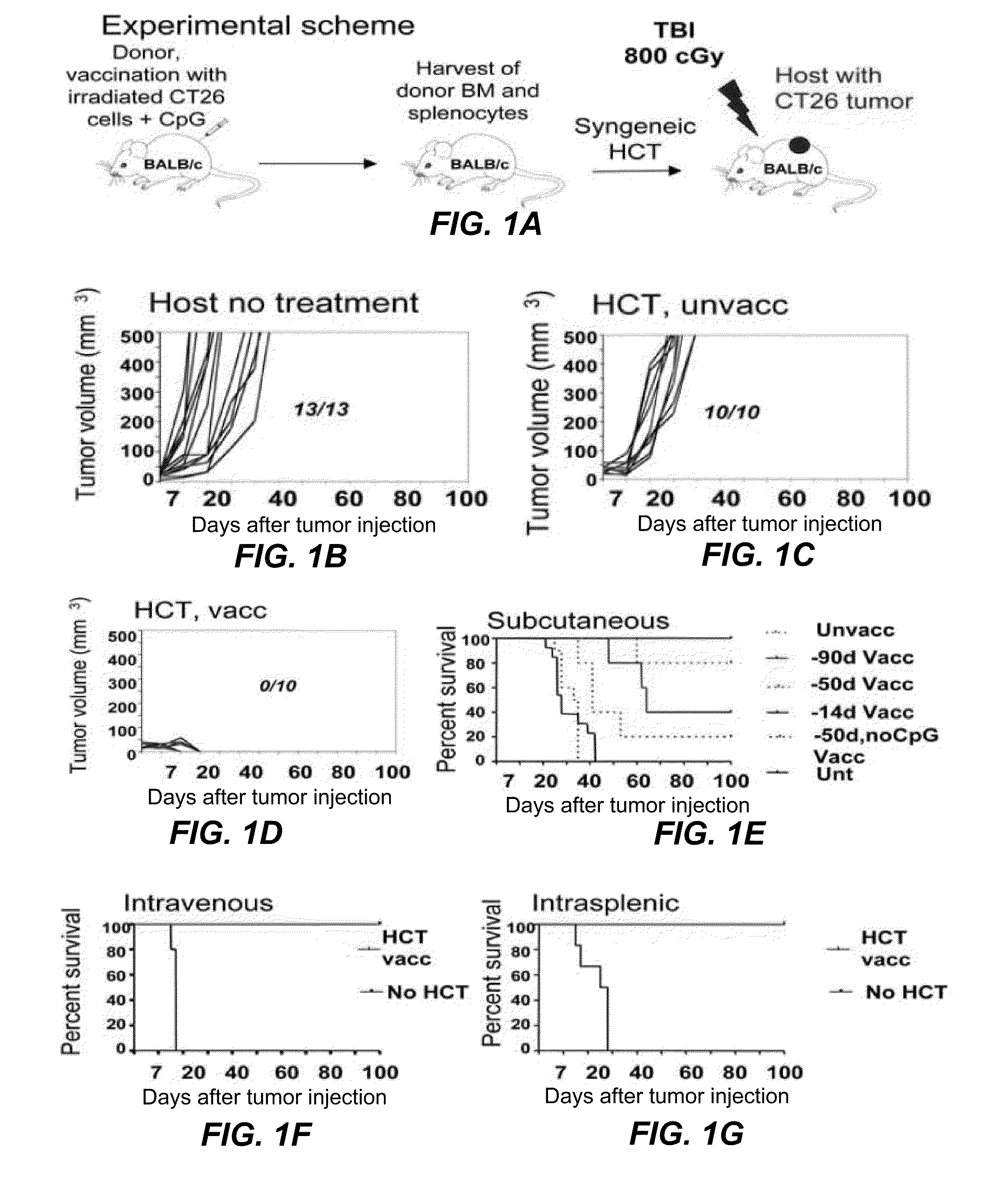
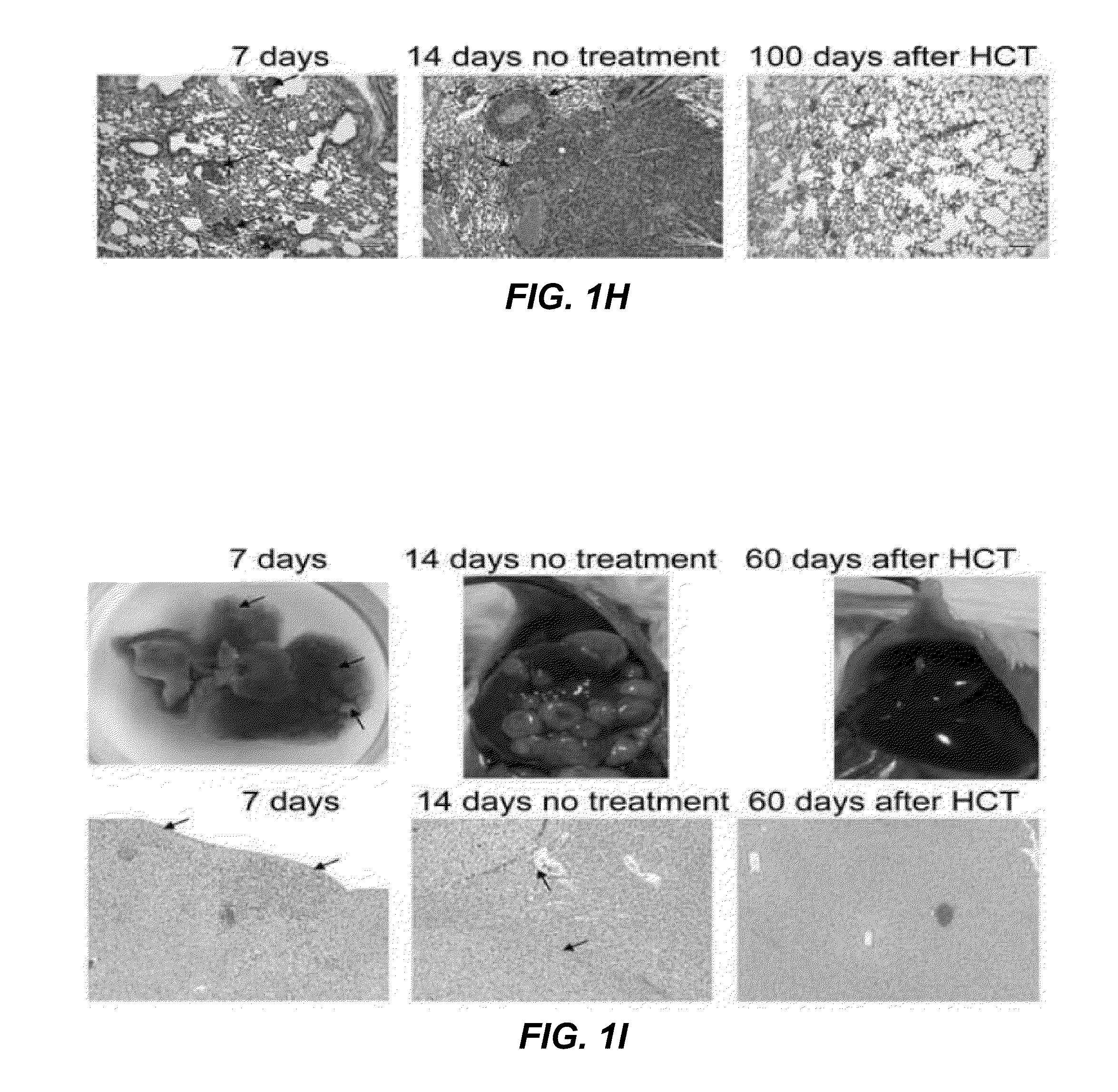
Tumor vaccination in combination with hematopoietic cell transplantation for cancer therapy
ActiveUS20110129503A1Eliminate side effectsBiocideEnergy modified materialsAbnormal tissue growthVaccination
In one aspect, the present invention provides a method for treating cancer comprising tumor cell vaccination in combination with hematopoietic and immune cell transplantation. In some embodiments, the method involves autologous tumor cell vaccination prior to autologous hematopoietic and immune cell transplantation. In another aspect, the present invention provides a method of purifying tumor cells from a subject in preparation for vaccination.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Methods of expanding myeloid cell populations and uses thereof
ActiveUS8252587B2Antibacterial agentsAntimycoticsMyeloid Progenitor CellsHematopoietic stem cell transplantation
The present disclosure relates to a method of expanding myeloid progenitor cells by culturing an initial population of cells in a medium comprising a mixture of cytokines and growth factors that promote growth and expansion of the myeloid progenitor cells. The expanded cell population provides a source of cells as therapeutic treatments for neutropenia and / or thrombocytopenia arising in patients subjected to myeloablative therapy and hematopoietic stem cell transplantation.
Owner:CELLERANT THERAPEUTICS INC
Use of high-dose, post-transplantation oxazaphosphorine drugs for reduction of transplant rejection
InactiveUS20120148577A1Reduce transplant rejectionReduce rejectionBiocidePeptide/protein ingredientsGackstroemiaSickle cell anemia
A lymphocytotoxic, but hematopoietic stem cell-sparing, high-dose amount of an oxazaphosphorine drug such as, for example, cyclophosphamide, administered post-transplantation can be used to reduce transplant rejection, including graft-versus-host-disease (GVHD). In some embodiments, the transplants are bone marrow transplants or hematopoietic stem cell transplants carried out for the treatment of hematologic disorders, including hematologic malignancies and non-malignant hematologic disorders. In some embodiments, the transplants are carried out for the treatment of hereditary hemoglobinopathies, such as sickle cell anemia and thalassemia.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE +1
Enhanced reconstitution and autoreconstitution of the hematopoietic compartment
ActiveUS9789135B2Increase the number ofReduce riskPeptide/protein ingredientsAntibody mimetics/scaffoldsProtein transduction domainHematopoietic stem cell transplantation
Owner:TAIGA BIOTECH
Compositions and methods for the depletion of cd117+ cells
ActiveUS20190144558A1Peptide/protein ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsDiseaseCancer cell
The invention provides compositions and methods useful for the depletion of CD117+ cells and for the treatment of various hematopoietic diseases, metabolic disorders, cancers, and autoimmune diseases, among others. Described herein are antibodies, antigen-binding fragments, and conjugates thereof that can be applied to effect the treatment of these conditions, for instance, by depleting a population of CD117+ cells in a patient, such as a human. The compositions and methods described herein can be used to treat a disorder directly, for instance, by depleting a population of CD117+ cancer cells or autoimmune cells. The compositions and methods described herein can also be used to prepare a patient for hematopoietic stem cell transplant therapy and to improve the engraftment of hematopoietic stem cell transplants by selectively depleting endogenous hematopoietic stem cells prior to the transplant procedure.
Owner:CRISPR THERAPEUTICS AG
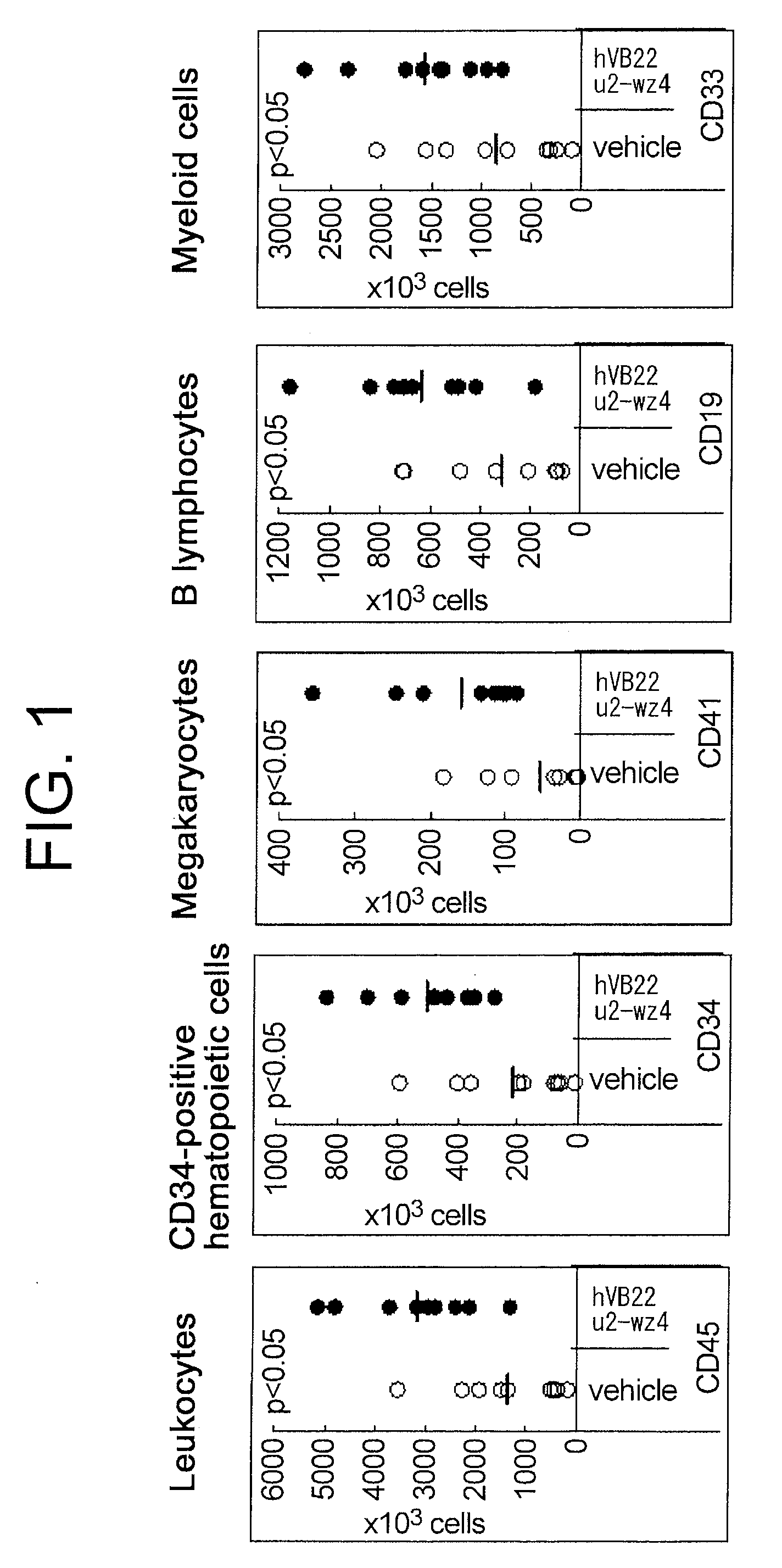
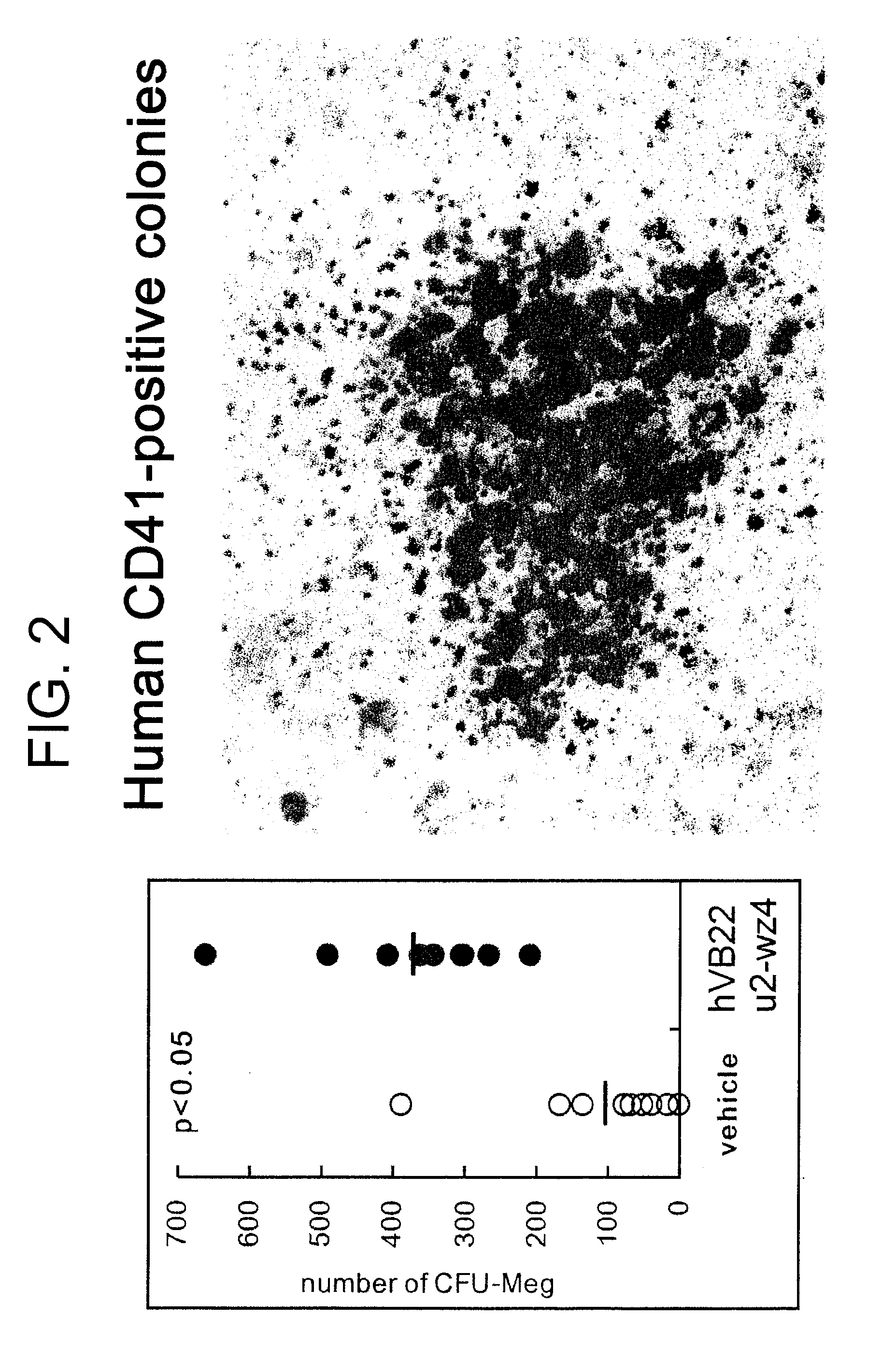
Agents for Promoting the Growth of Hematopoietic Stem Cells
The present inventors discovered that the administration of an agonistic minibody (VB22B sc(Fv)2) against the TPO receptor resulted in not only the induction of human megakaryocyte-specific differentiation (increase in platelet precursor cells), but also the engraftment of transplanted hematopoietic stem cells derived from human cord blood (CD34-positive cells) and significant increase in multi-lineage hematopoietic precursor cells. TPO and TPO receptor agonists can be used as agents for promoting the growth of CD34-positive hematopoietic cells or agents for promoting the engraftment of transplanted cells in the bone marrow, which can be effective when administered alone (without using G-CSF and erythropoietin in combination) after hematopoietic stem cell transplantation (in particular, cord blood transplantation). Furthermore, TPO and TPO receptor agonists can be used as agents for promoting the growth and / or differentiation of multilineage hematopoietic precursor cells and agents for promoting the recovery of multilineage hematopoiesis.
Owner:CHUGAI PHARMA CO LTD
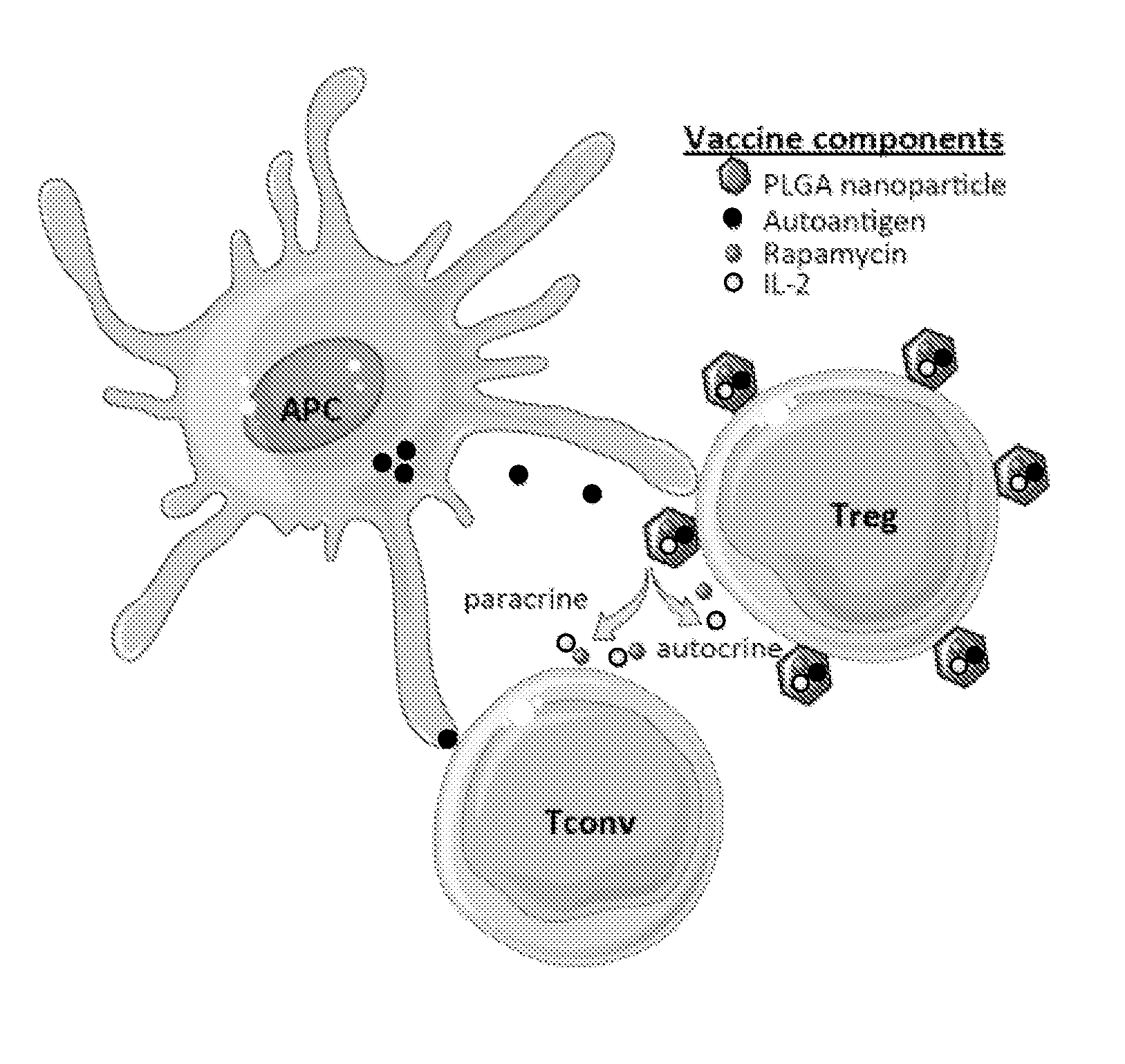
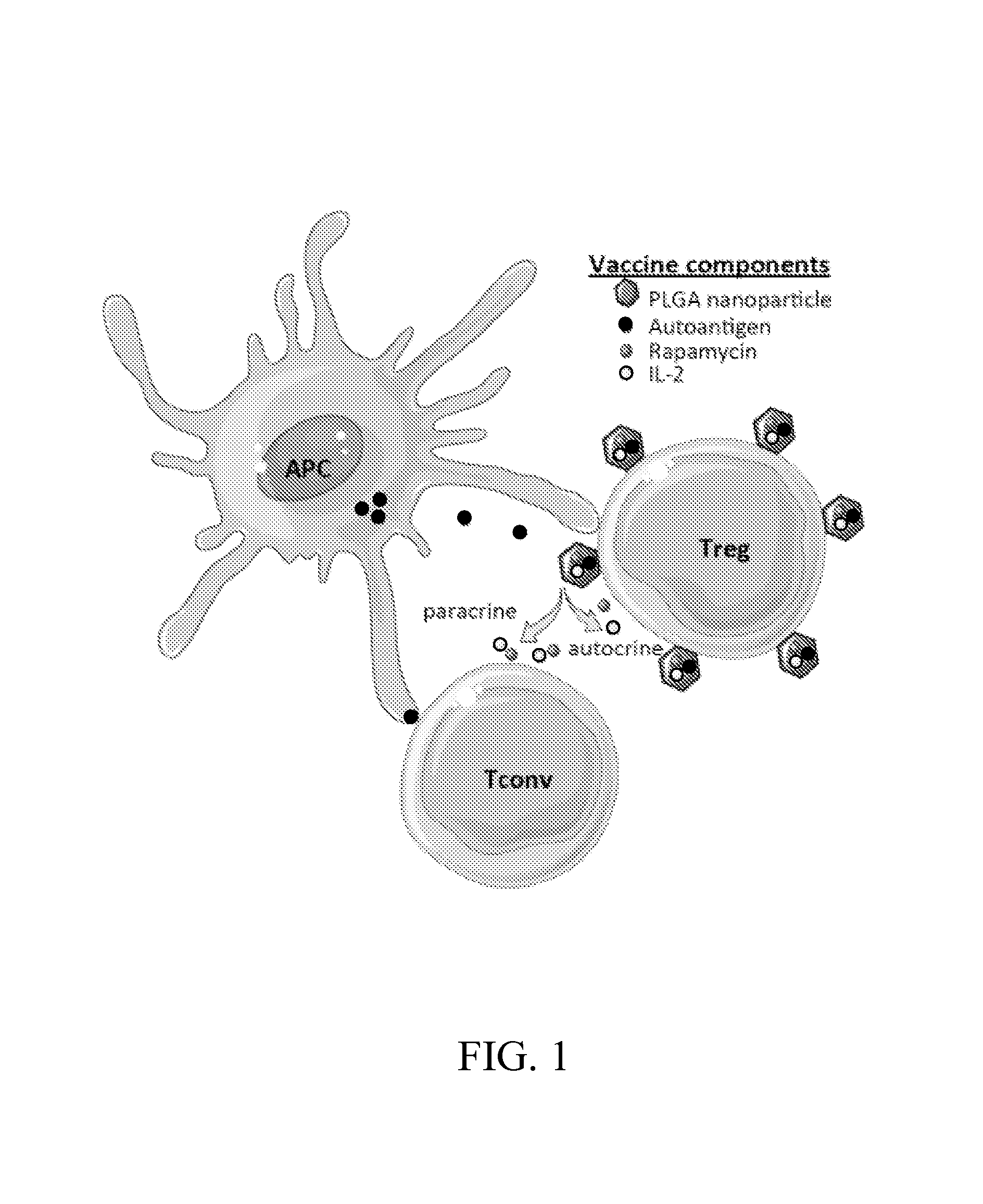
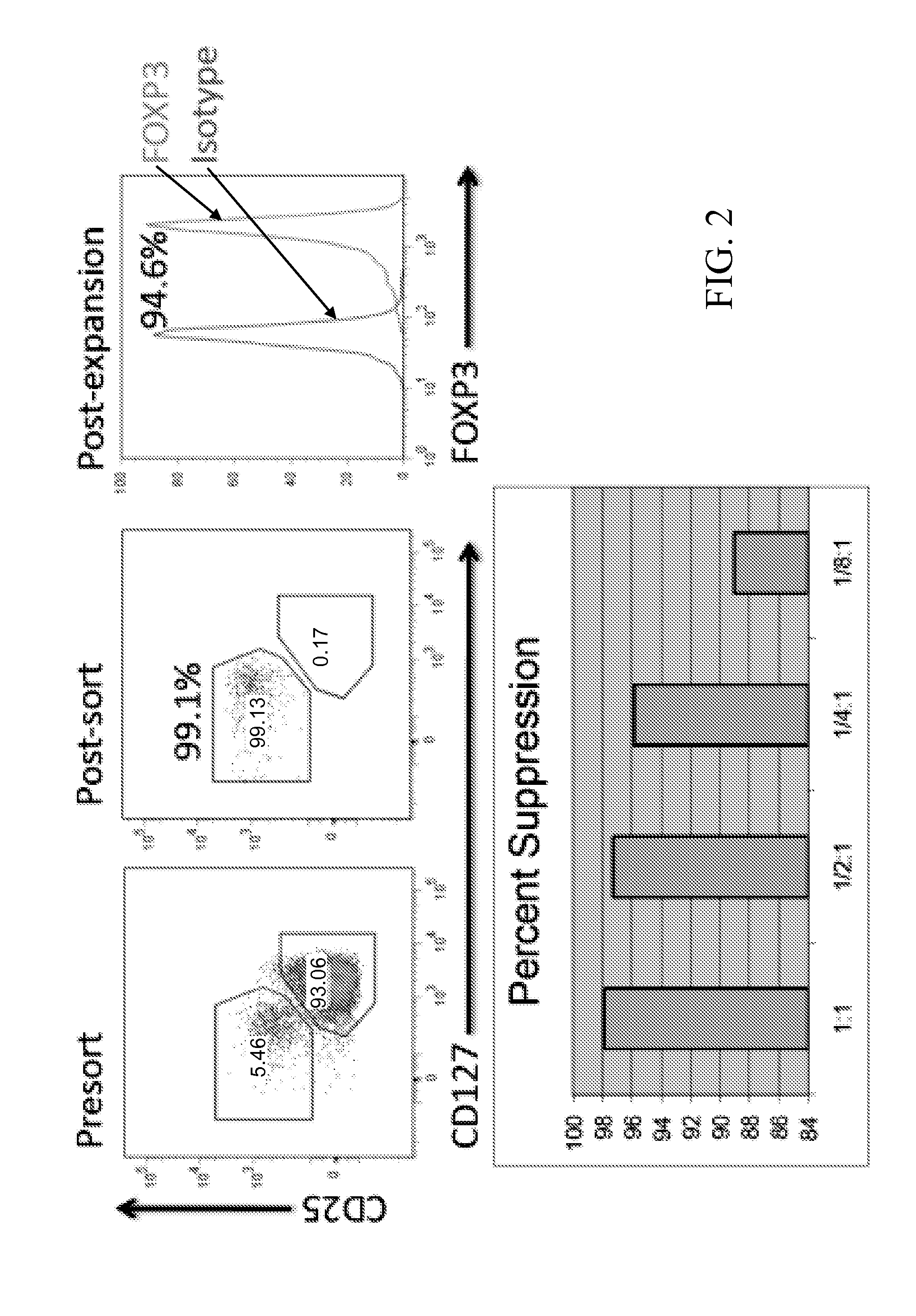
Materials and methods for modulating immune responses
The present invention provides nanoparticle-coupled tolerogenic Treg cell therapy for treatment of immune and / or autoimmune disorders. In certain specific embodiments, the present invention can be used in the prevention and / or treatment of autoimmune diseases including, but not limited to, type 1 diabetes, lupus erythematosus (SLE), multiple sclerosis (MS), inflammatory bowel disease (IBD), rheumatoid arthritis, oophoritis, and autoimmune pathology associated with Graft versus Host Disease (GvHD) following hematopoietic stem cell transplantation.
Owner:UNIV OF FLORIDA RES FOUNDATION INC
Use of recombinant mesenchymal stem cell in preparation of immunosuppressant
ActiveCN104800243ASuppress immune responseRetain anti-tumor abilityUnknown materialsOther foreign material introduction processesAbnormal tissue growthImmunologic disorders
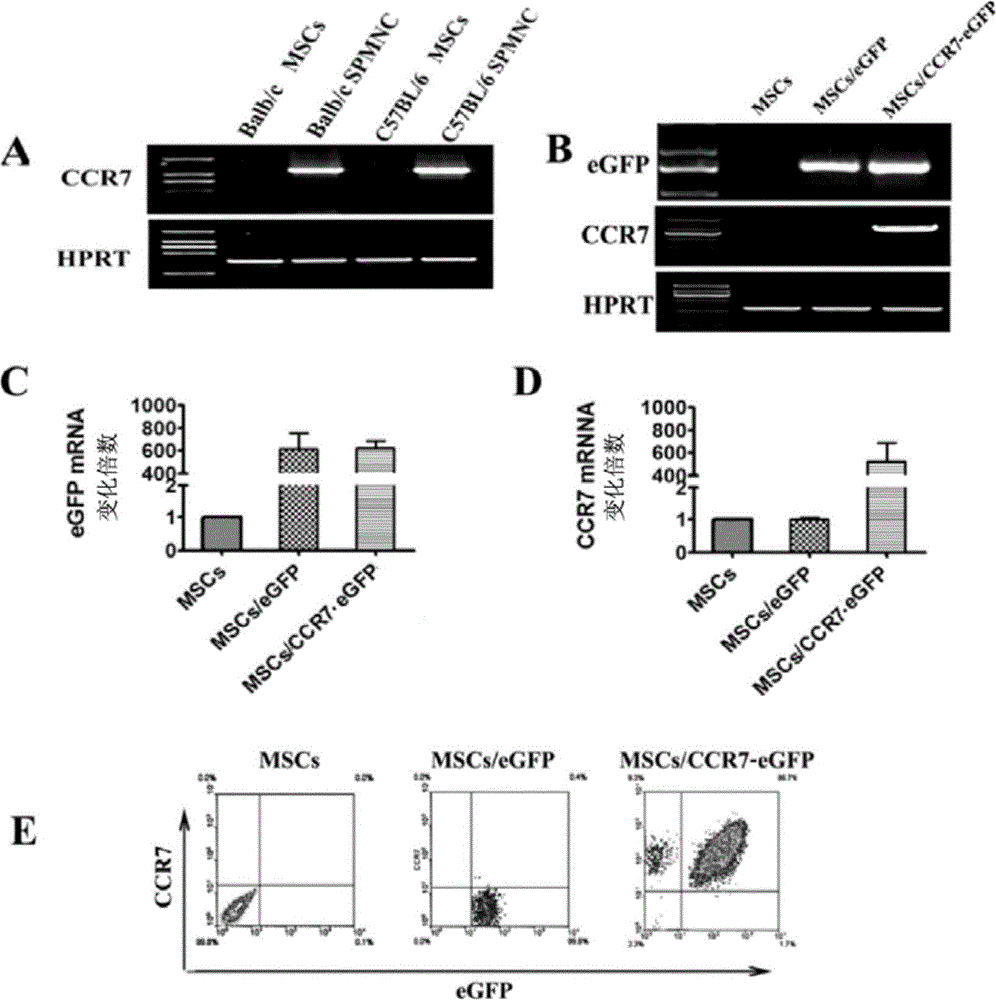
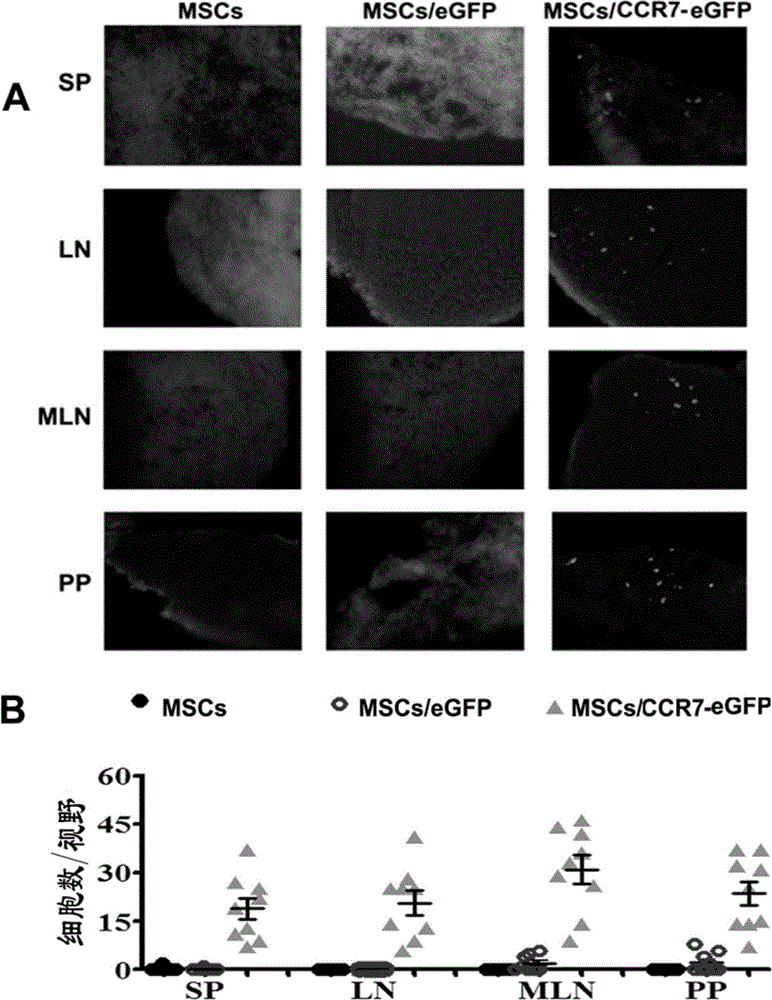
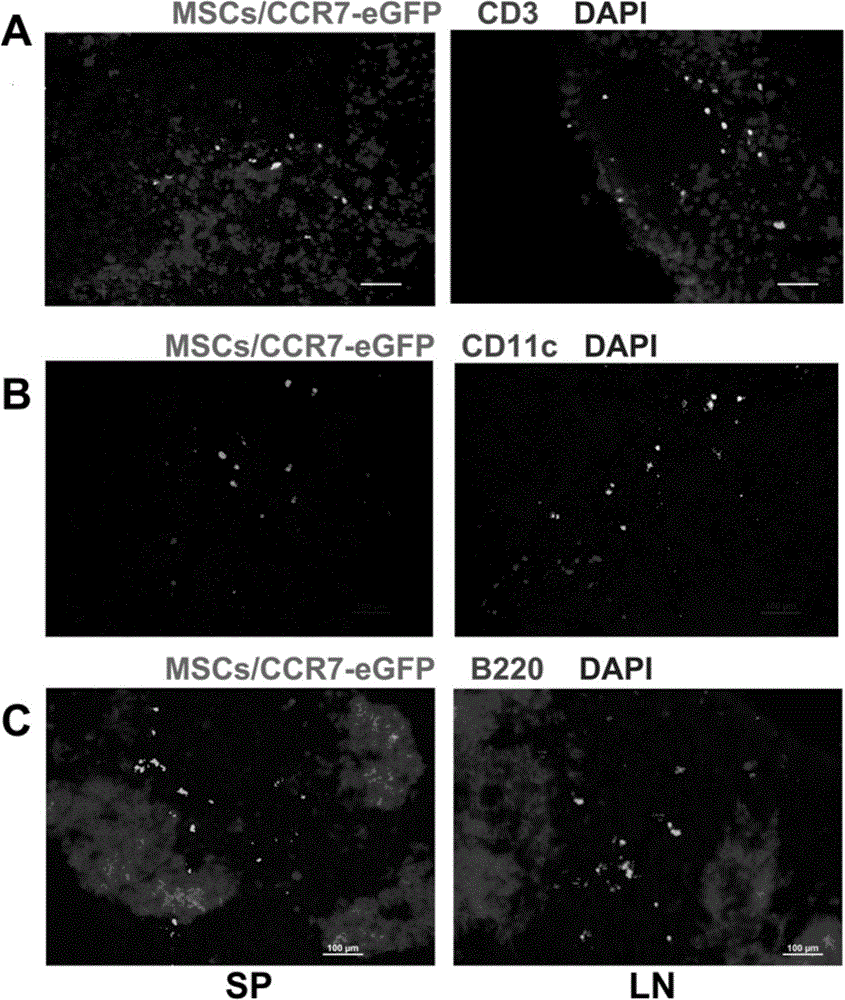
The invention discloses a use of a recombinant mesenchymal stem cell in preparation of an immunosuppressant. The invention relates to the recombinant mesenchymal stem cell capable of expressing a chemokine receptor CCR7 on a cytomembrane. After infusion, the recombinant mesenchymal stem cells can be specifically transferred to a secondary lymphatic organ, and a large amount of the recombinant mesenchymal stem cells are gathered in a T cell enrichment region in the secondary lymphatic organ and can efficiently inhibit T cell immunoreaction. Through use of the recombinant mesenchymal stem cell in treatment on graft-versus-host disease after bone marrow transplantation, immunological rejection after organ transplantation and autoimmune diseases, a cost is reduced, a cell use amount is reduced and side-effects are relieved. In treatment, the recombinant mesenchymal stem cell retains tumor cell killing effects and provides a high efficiency and beneficial novel approach for clinical treatment on graft-versus-host disease after bone marrow transplantation, immunological rejection after organ transplantation and autoimmune diseases of tumor high-risk groups.
Owner:INST OF BASIC MEDICAL SCI ACAD OF MILITARY MEDICAL SCI OF PLA
KIR and ligand genetic typing experimental method
InactiveCN108624665ASmall sample sizePromote amplificationMicrobiological testing/measurementDiseaseNatural Killer Cell Inhibitory Receptors
The invention discloses a KIR and ligand genetic typing experimental method. By the technology, the time and labor are saved, and meanwhile, DNA sample capacity is further saved. A multi-PCR technology is adopted, meanwhile, 36 pairs of primers are further combined according to the sizes of PCR products, and finally, amplified reaction is finished in 12 reaction holes. The KIR and ligand genetic typing experimental method is conveniently applied to amplification of 96 pore plates, conventional Taq enzyme is used, typing of all KIR genes and ligands thereof can be finished at a time under the same reaction conditions, and the practicality of the KIR and ligand genetic typing experimental method is greatly improved. Two pairs of primers are used for a KIR gene, the accuracy is improved, anda false negative result is avoided. The KIR gene can be combined to MHC-I type ligand molecules on the surfaces of targeting cells, inhibiting or activating signals are transmitted to regulate the activity of NK cells and T cells, and the KIR and ligand genetic typing experimental method plays an important regulation role in hematopoietic stem cell transplantation, feto-matemal tolerance, anti-infectious immunity, tumor immunity and autoimmune diseases. Therefore, KIR genetic typing facilitates understanding of influences of KIR to tumor immunity, hematopoietic stem cell transplantation and autoimmune diseases.
Owner:韩瑜
Radiation therapy methods
InactiveUS7776828B2Reduce tissue damageImprove efficiencyPeptide/protein ingredientsEnergy modified materialsMegakaryocyte productionAngiotensinogen mrna
Owner:UNIV OF SOUTHERN CALIFORNIA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com