Use of recombinant mesenchymal stem cell in preparation of immunosuppressant
An immunosuppressant and mesenchymal stem cell technology, which is applied in the field of stem cell therapy, can solve the problems of increased tumor recurrence, weak immune regulation ability, and large amount of cells, and achieve the effects of suppressing immune response, reducing the risk of tumor recurrence, and reducing embolism
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
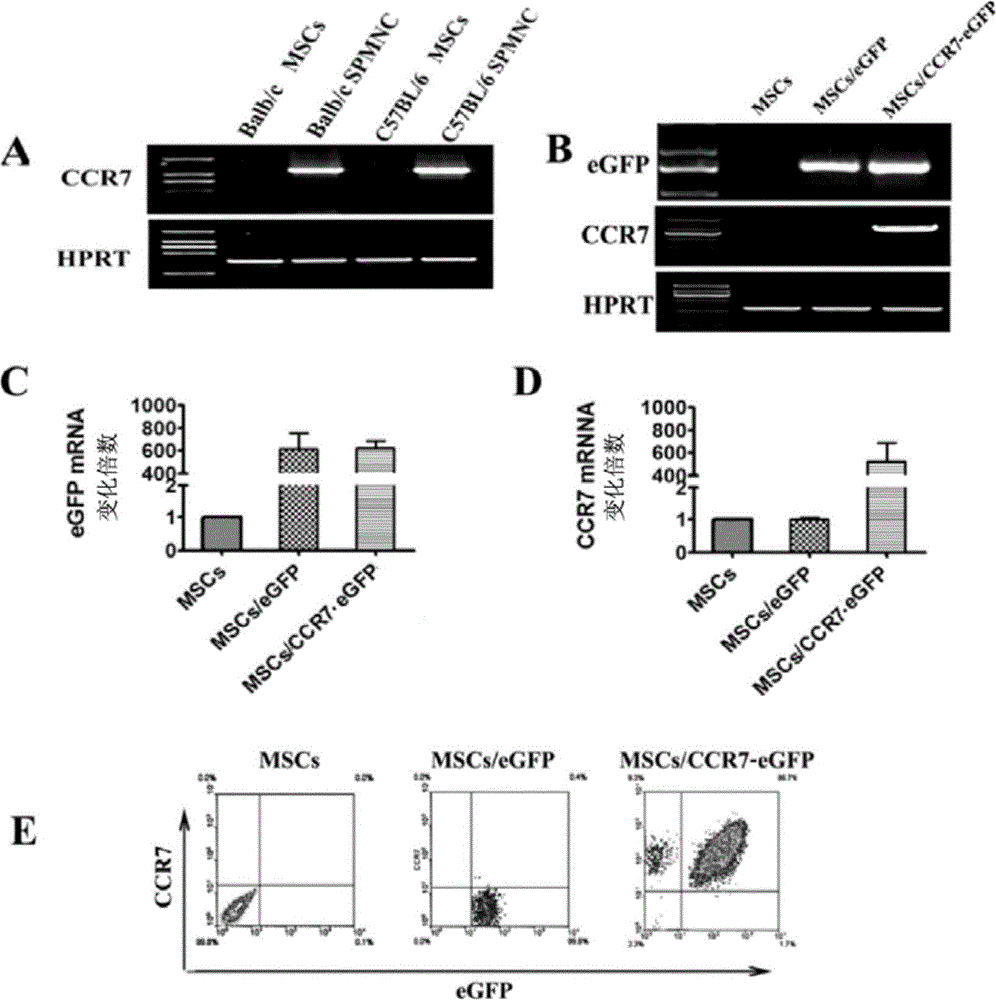
[0042] Example 1 Preparation of recombinant mesenchymal stem cells expressing chemokine receptor CCR7
[0043] 1. Isolation and culture of mouse bone marrow MSCs
[0044] The sample is 2-3 weeks old C57BL / 6 mice (Experimental Animal Center, Academy of Military Medical Sciences), the mice were killed by neck dislocation, the mice were soaked in 75% alcohol to sterilize the mice → the mouse tibia and femur were separated under aseptic conditions and placed in a plate →Use sterile gauze to rub the attached muscle and remove the metaphysis →Rinse the bone marrow cavity repeatedly with PBS+10% FCS (Hyclone) to flush out the bone marrow cells →Inoculate in a culture bottle, so that the bone fragments are evenly distributed on the bottom of the bottle, and the culture system Containing αt-MEM (Gibco) + 10% FCS (Hyclone) + 100U / ml penicillin + 100U / ml streptomycin, 37°C, 5% CO 2 Cultivate under saturated humidity conditions → change the medium once every 3 days → when the cells are c...
Embodiment 2
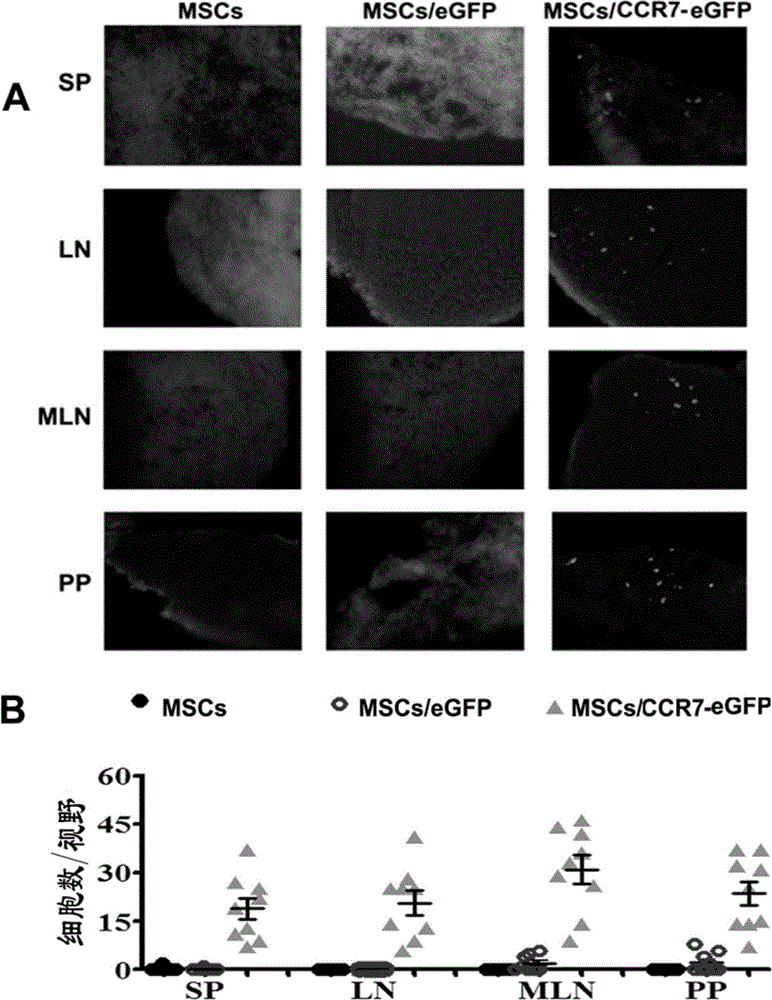
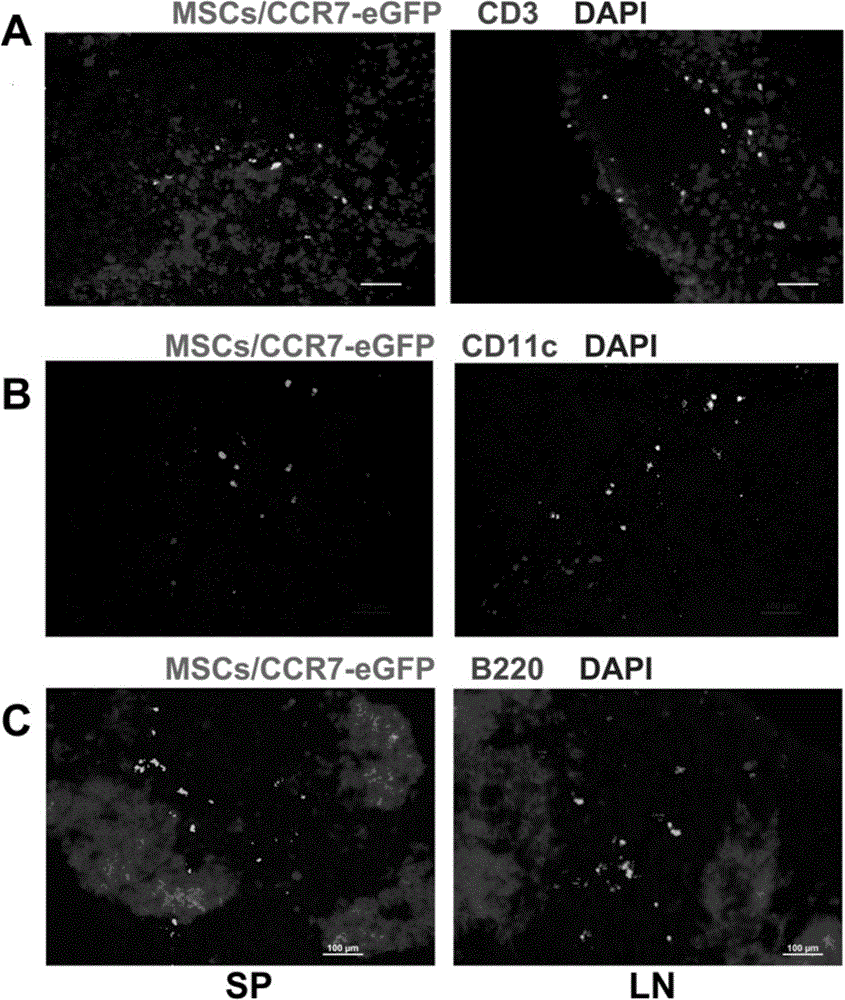
[0059] Example 2 Detection of migration ability and enrichment area of MSCs to secondary lymphoid organs in vivo
[0060] 1. Detection of migration ability of MSCs to secondary lymphoid organs in vivo
[0061] MSCs, MSCs / eGFP or MSCs / CCR7-eGFP (1×10 6 ) three groups of mice (4 mice in each group); 5 days after the infusion, the spleen (SP), lymph node (LN), mesenteric lymph node (MLN) and PP node were taken for frozen sections, and DAPI was used for nuclear labeling. Green fluorescent cells were observed under a fluorescence microscope. figure 2 A The results showed that MSCs / eGFP did not have the ability to migrate to secondary lymphoid organs, while MSCs / eGFP-CCR7 had a large distribution in SP, LN, MLN, and PP. figure 2 Calculate the eGFP of each organ as shown in B +The frequency of distribution of MSCs in various tissues and organs in vivo (the number of green fluorescence / high power field) quantitatively shows that the infused MSCs / eGFP does not have the ability t...
Embodiment 3
[0064] Example 3 Detection of MSCs / CCR7-eGFP immune regulation effect in vivo and its impact on tumor growth
[0065] 1. Inhibition of GvHD by MSCs / CCR7-eGFP
[0066] GvHD is a typical T cell-mediated immune disease. Establishment of mouse GvHD model: Take 20-24g BALB / c male mice, collect splenocytes and bone marrow cells, and count; 2×10 7 Splenocytes+1×10 7 Bone marrow cells / 0.25mlPBS were injected into the tail vein of 22-24g C57BL / 6 male mice irradiated with 9Gy to establish aGvHD model (GvHD group). On this basis, a total of 1×10 5 or 1×10 6 MSCs / eGFP, 1 x 10 5 or 1×10 6 MSCs / CCR7-eGFP. 1×10 6 Mice co-infused with MSCs / eGFP tail vein are called GvHD+MSCs / eGFP mice; 1×10 6 Mice with tail vein co-infusion of MSCs / CCR7-eGFP were called GvHD+MSCs / CCR7-eGFP mice.
[0067] After infusion, the survival and status of the mice in the three groups were observed daily, and the survival curves were recorded to observe the immune regulation effect of MSCs in vivo, and the le...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com