Agents for Promoting the Growth of Hematopoietic Stem Cells
a technology of stem cells and agents, applied in the direction of antibody medical ingredients, drug compositions, extracellular fluid disorders, etc., can solve the problems of not reporting whether cd34-positive cells survive, or mention human cord blood, etc., to promote the growth of cd34-positive hematopoietic cells, enhance the engraftment of transplanted cells, and induce significant growth
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
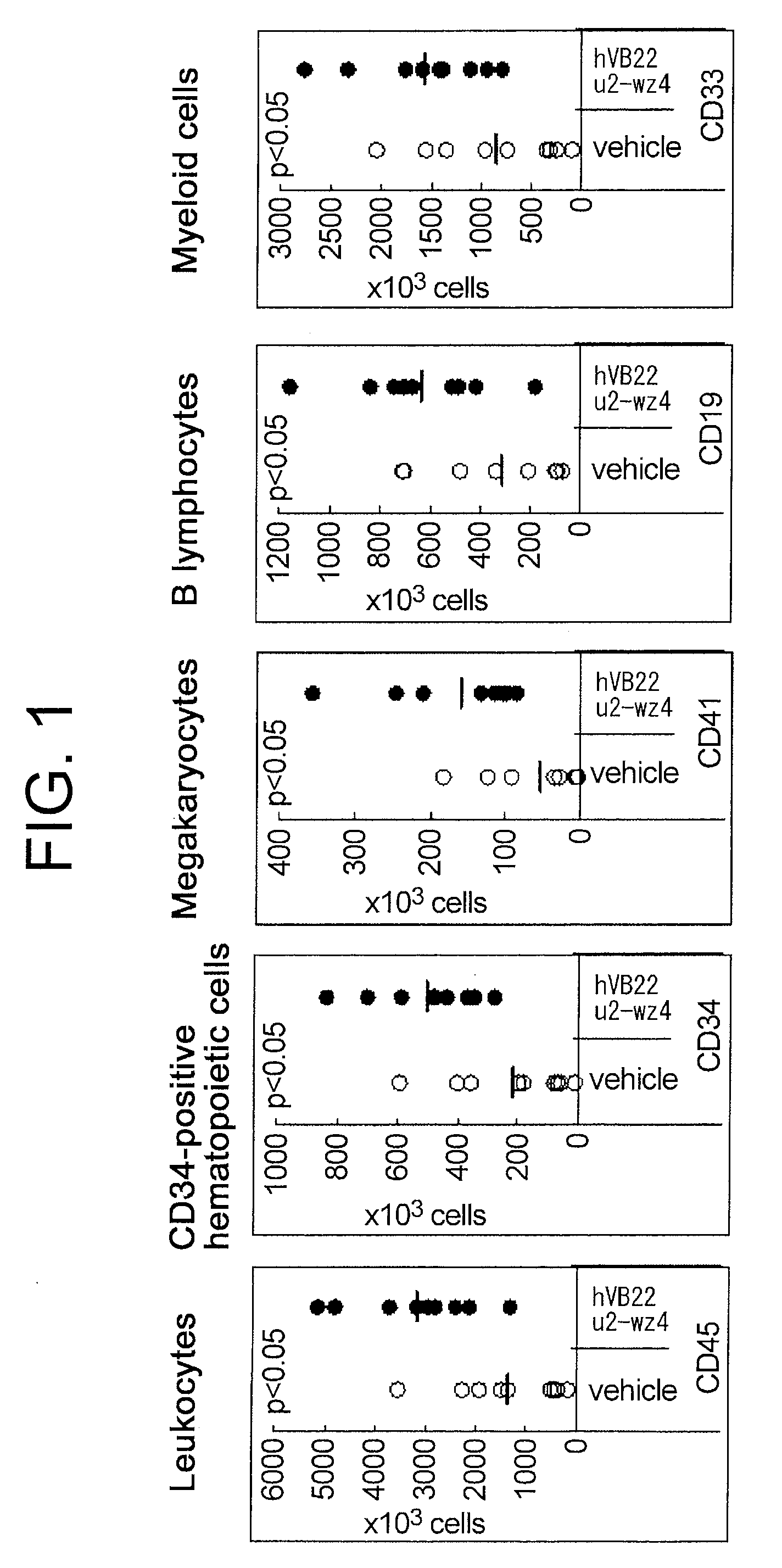
Effect of the TPO Receptor Agonist on the Engraftment Number of Different Human Blood Cell Lineages in the Bone Marrow of Mice Transplanted with Human Cord Blood-Derived Hematopoietic Stem Cells
[0149]The experiments were carried out by the method described below to assess the effect of the sc(Fv)2 antibody (hVB22 u2-wz4: sc(Fv)2) comprising the amino acid sequence of SEQ ID NO: 73 on the engraftment number of different human blood cell lineages in the bone marrow of mice transplanted with human cord blood-derived hematopoietic stem cells at early stages. The sc(Fv)2 antibody comprising the amino acid sequence of SEQ ID NO: 73 can be prepared by the method described in WO 2005 / 056604.
Methods
[0150]Mice used were acclimatized six-week-old male NOD.CB17-Prkdc / J. After systemic irradiation with 3.0 Gy of X-ray, an anti-asialo-GM1 antibody was intraperitoneally administered to the mice once every ten days from the day of irradiation. 5×104 human cord blood-derived CD34-positive cells were...
example 2
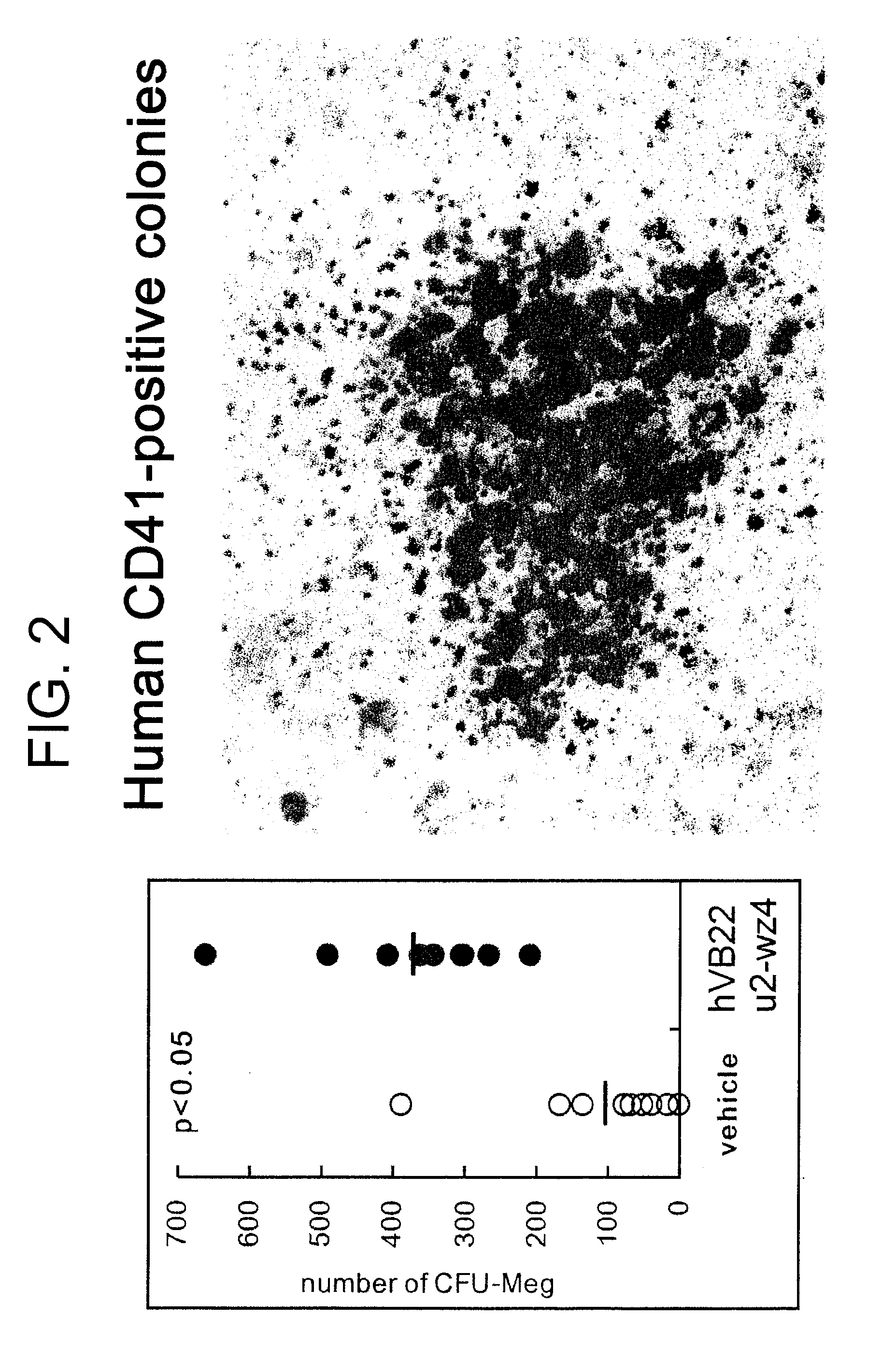
Effect of the TPO Receptor Agonist on the Number of Human CFU-Meg Colonies in the Bone Marrow of Mice Transplanted with Human Cord Blood-Derived Hematopoietic Stem Cells
[0152]The following experiments were carried out to assess the effect of the sc(Fv)2 antibody of SEQ ID NO: 73 on the number of human CFU-Meg colonies after transplantation of human cord blood-derived hematopoietic stem cells.
Methods
[0153]Mice used were acclimatized six-week-old male NOD.CB17-Prkdc / J. After systemic irradiation with 3.0 Gy of X-ray, an anti-asialo-GM1 antibody was intraperitoneally administered to the mice once every ten days from the day of irradiation. 5×104 human cord blood-derived CD34-positive cells were transplanted to each mouse at the caudal vein one day after irradiation. From the day after transplantation, the sc(Fv)2 antibody of SEQ ID NO: 73 was administered every day for ten consecutive days, and then after that, administration was conducted for five days followed by two days of break. T...
example 3
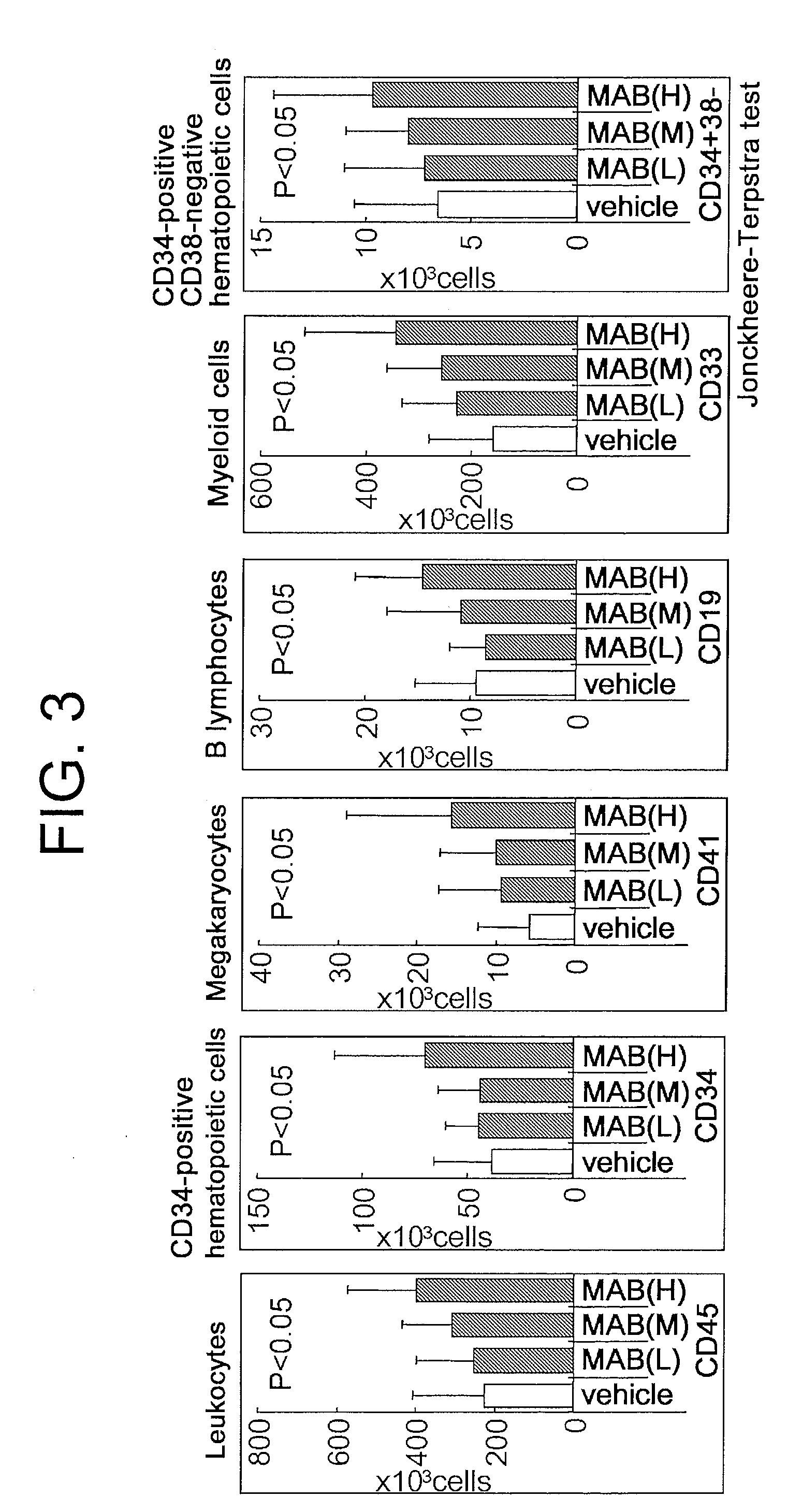
Dose-Dependent Effect of the TPO Receptor Agonist on the Engraftment Number of Different Human Blood Cell Lineages in the Bone Marrow of Mice Transplanted with Human Cord Blood-Derived Hematopoietic Stem Cells
[0155]The following experiments were carried out to assess the dose-dependent effect of the sc(Fv)2 antibody of SEQ ID NO: 73 on the engraftment number of different human blood cell lineages in the bone marrow of mice transplanted with human cord blood-derived hematopoietic stem cells.
Methods
[0156]Mice used were acclimatized six-week-old male NOD.CB17-Prkdc / J. After systemic irradiation with 3.0 Gy of X-ray, an anti-asialo-GM1 antibody was intraperitoneally administered to the mice on the day of irradiation and eight days after irradiation. 5×104 human cord blood-derived CD34-positive cells were transplanted to each mouse at the caudal vein the day after irradiation. The sc(Fv)2 antibody of SEQ ID NO: 73 was administered every day for ten consecutive days from the day after the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| frequencies | aaaaa | aaaaa |
| molecular-weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com