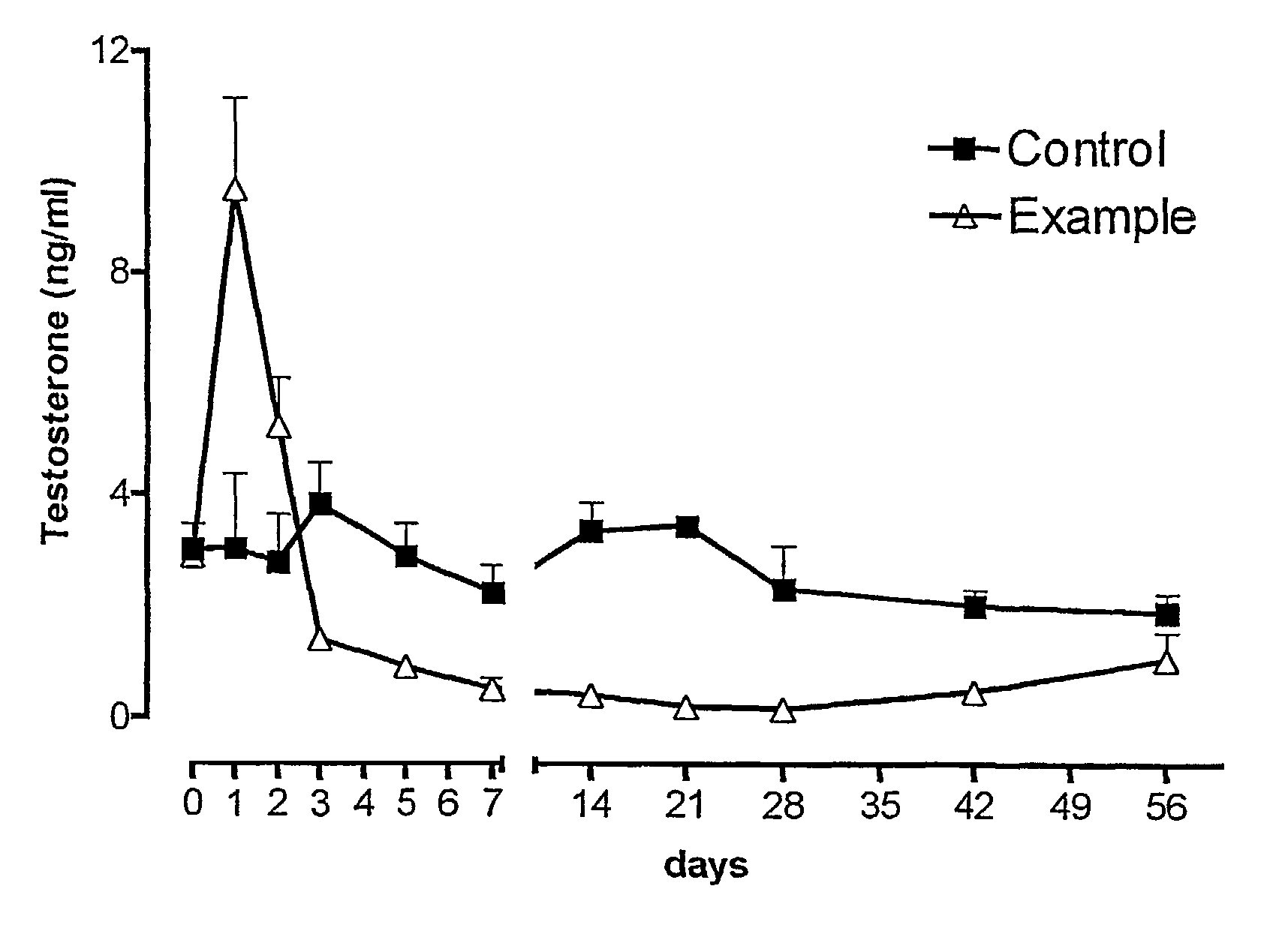
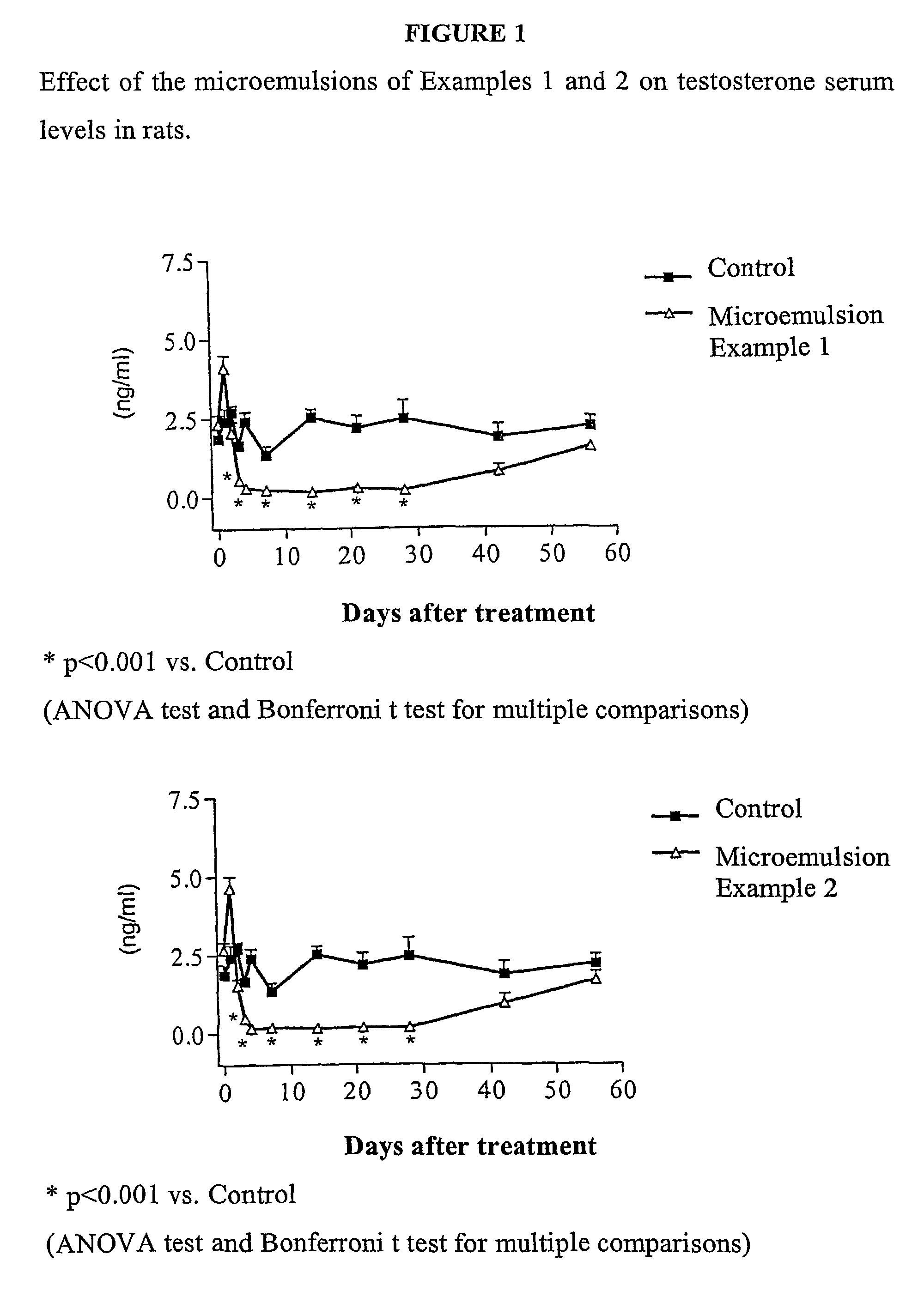
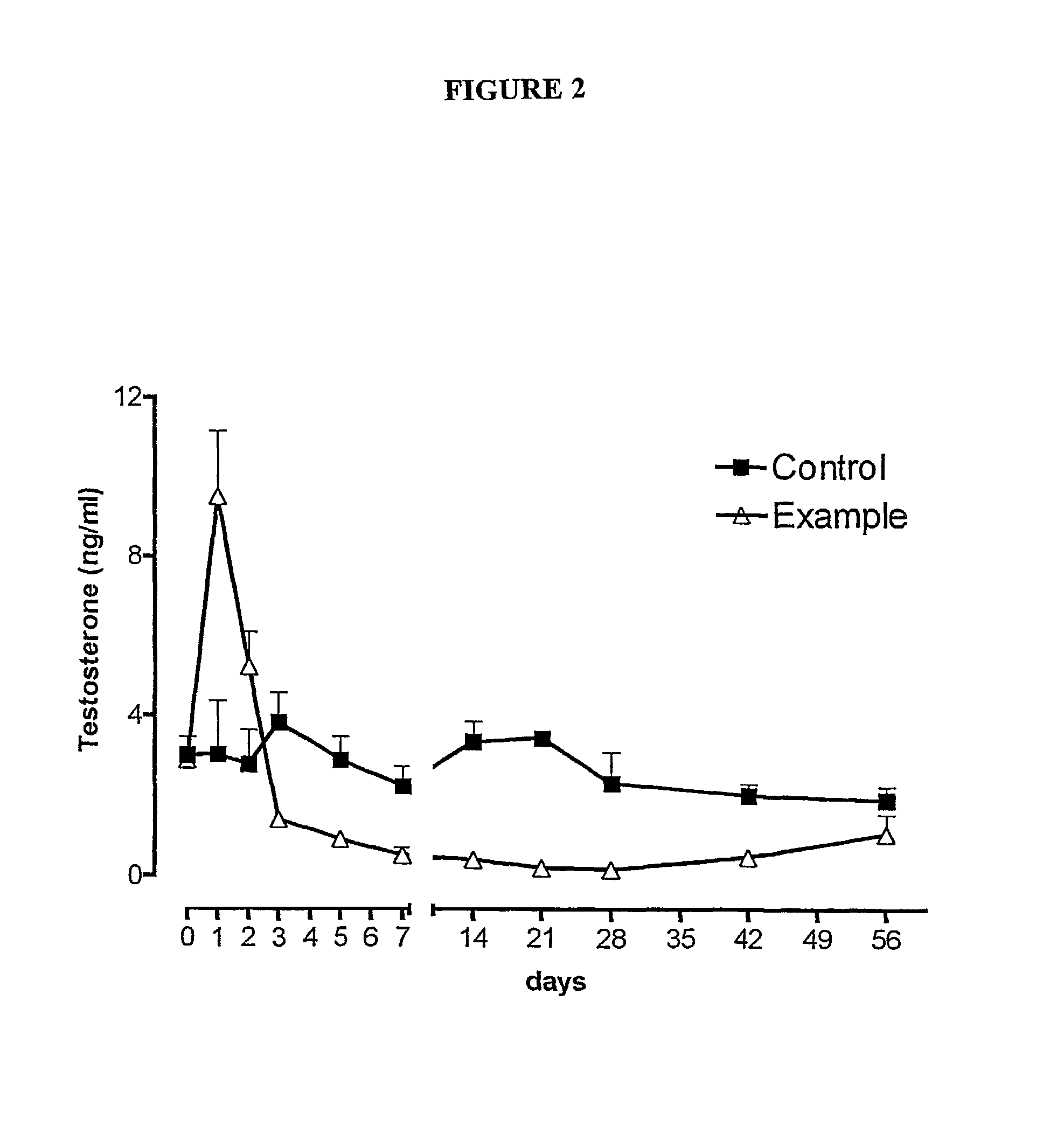
Sustained release pharmaceutical compositions for the parenteral administration of hydrophilic compounds
a technology of hydrophilic compounds and pharmaceutical compositions, which is applied in the direction of drug compositions, somatostatins, peptides, etc., can solve the problems of conversely unacceptable intolerantness in the form of persistent ulcerations, and achieve the effect of sustained release of hydrophilic active ingredients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 2
Preparation of a w / o Microemulsion Containing Leuprolide Acetate
[0045]The procedure of Example 1 is followed, but solubilizing 600 mg of Leuprolide acetate in 10 ml of water.
example 3
Preparation of a w / o Microemulsion Containing Leuprolide Acetate
[0046]The procedure of Example 1 is followed, but without adding 200 mg of lysine. Calculated pH is about 3.
example 4
Preparation of a w / o Microemulsion Containing Leuprolide Acetate
[0047]The procedure of Example 1 is followed, but solubilizing 900 mg of Leuprolide acetate in 10 ml of water.
PUM
| Property | Measurement | Unit |
|---|---|---|
| droplet diameter | aaaaa | aaaaa |
| diameters | aaaaa | aaaaa |
| diameters | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com