Viral immunotherapy drug compound and purpose thereof
A technology of immunotherapy drugs and antiviral drugs, applied in the field of biomedicine, can solve the problems of ineffective improvement of primary immune response, GM-CSF cannot provide adjuvant activity, etc., to prevent re-infection, easy to promote, and enhance immune response Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
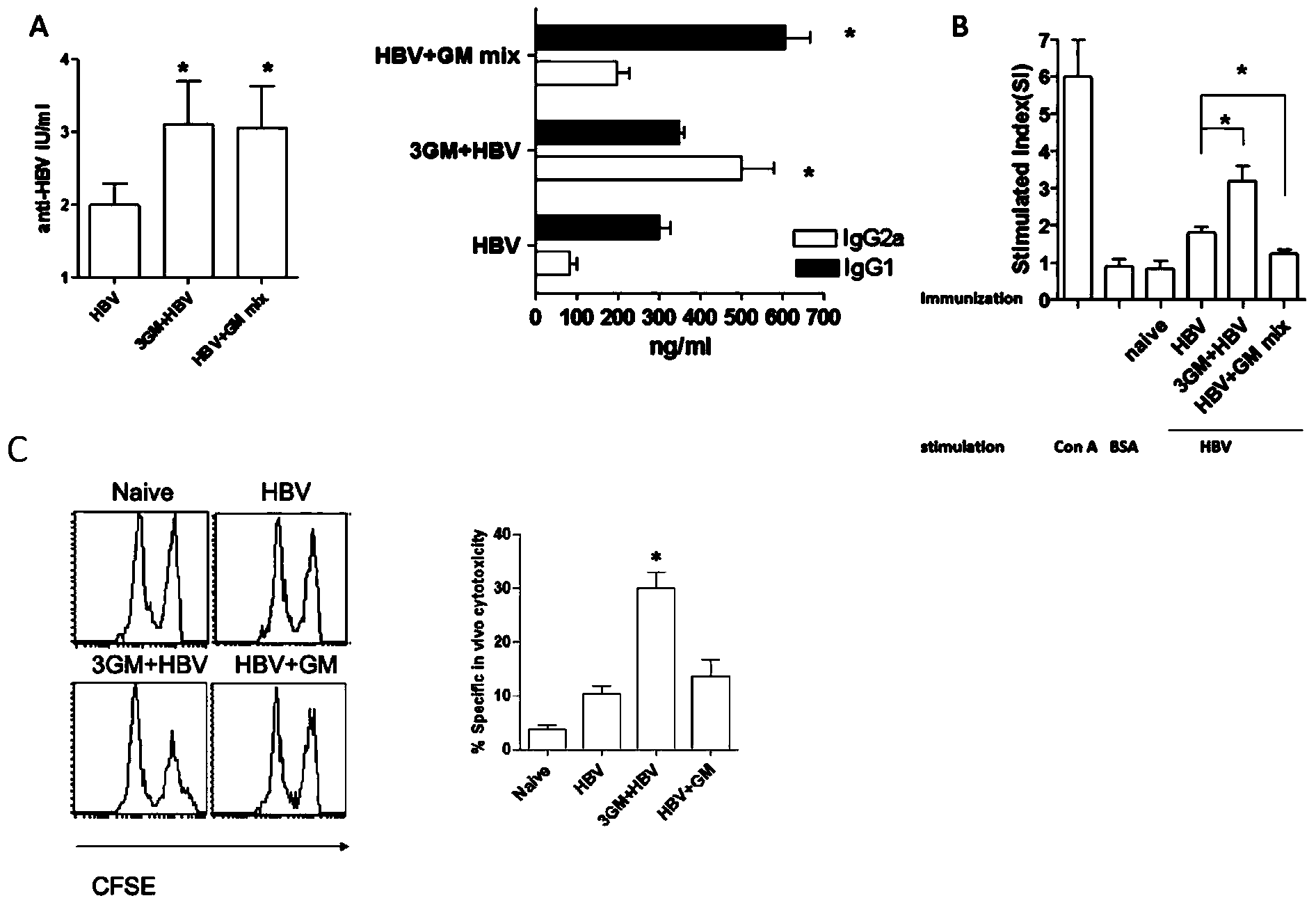
[0062] Unless otherwise specified, the experimental data in the examples of the present invention are the average values obtained from each mouse in each group. Example 1: The immunization strategy of GM-CSF combined with recombinant hepatitis B vaccine enhances the immune response of C57BL / 6 to recombinant hepatitis B subunit vaccine
[0063] Materials and Instruments:
[0064] Experimental materials: Genetic engineering (CHO) hepatitis B vaccine [Recombinant Hepatitis B Vaccine (CHO)], 10ug / 1.0mL, recombinant human granulocyte macrophage colony-stimulating factor for injection [Recombinant Human Granulocyte / Macrophage Clolony-Stimulating Factor for Injection], 300ug / support, CHO-expressed genetically engineered hepatitis B vaccine stock solution (HBsAg stock solution) were provided by North China Pharmaceutical Group Jintan Biotechnology Co., Ltd.
[0065] Main kits and instruments: RPMI1640 culture medium (WISENT company); fetal bovine serum (Tianjin Haoyang Biological P...
Embodiment 2
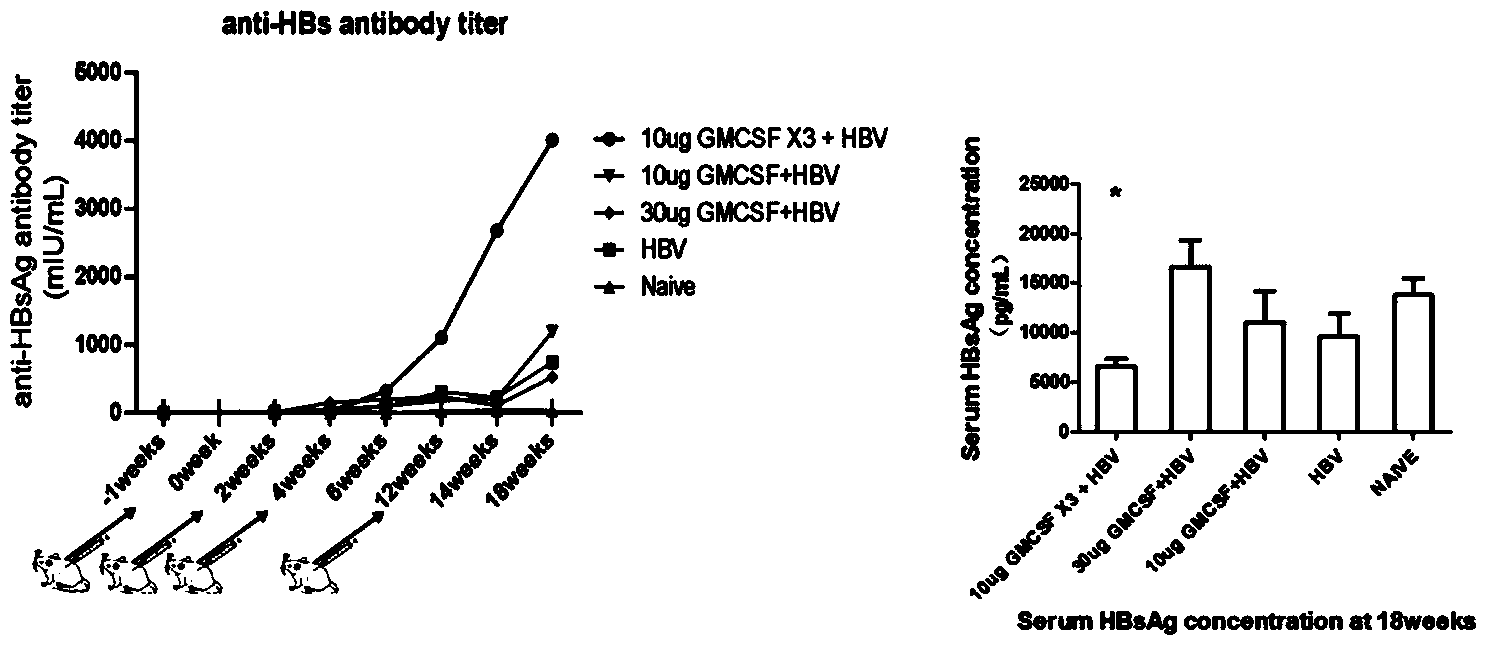
[0131] Example 2: The immunization strategy of GM-CSF combined with recombinant hepatitis B vaccine breaks the immune tolerance of HBsAg transgenic mice and induces anti-HBsAg humoral immune response in transgenic mice
[0132] Materials and Instruments:
[0133] HBsAg transgenic mice (C57BL / 6J-Tg(AlblHBV)44Bri / Jf4J) were purchased from Shanghai Public Health Clinical Center affiliated to Fudan University. "Hepatitis B surface antigen enzyme-linked immunoassay diagnostic kit" and hepatitis B surface antigen standard were purchased from Beijing Jinhao Pharmaceutical Co., Ltd. Other experimental materials, main reagents and instruments are the same as in Example 1.
[0134] experiment method:
[0135] Animal grouping and immunization methods:
[0136] 35 HBsAg transgenic mice (C57BL / 6J-Tg(AlblHBV)44Bri / Jf4J) (initial serum HBsAg concentration is 5000-10000pg / ml), were randomly divided into 5 groups, 7 mice in each group, and the experimental grouping was performed according ...
Embodiment 3
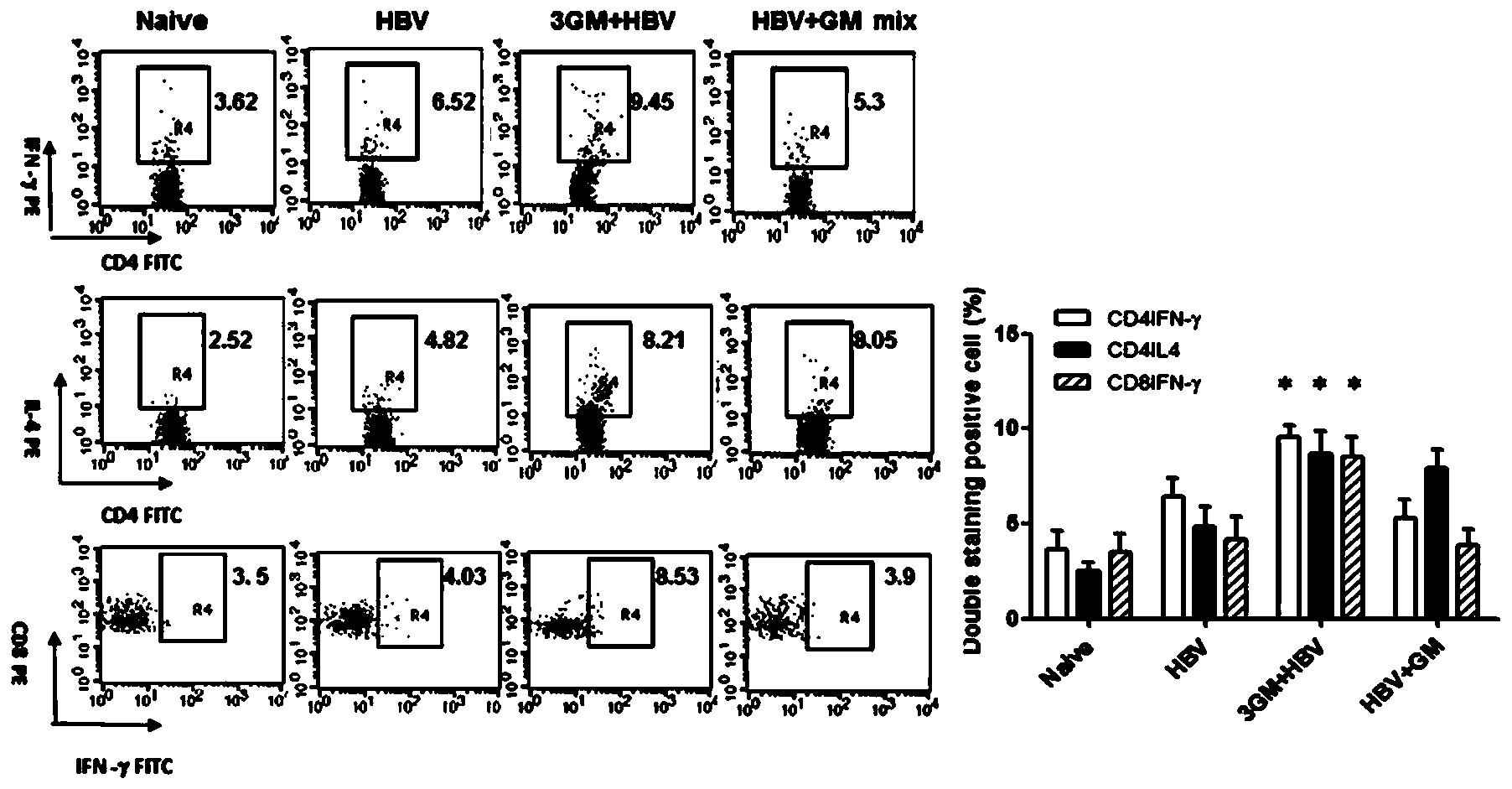
[0157] Example 3: GM-CSF Combined with Recombinant Hepatitis B Vaccine Immune Strategy Breaks Immune Tolerance of HBsAg Transgenic Mice Induces Anti-HBsAg Cellular Immune Response in Transgenic Mice and Eliminates HBsAg in Liver
[0158] Materials and Instruments:
[0159] HBsAg transgenic mice (C57BL / 6J-Tg(AlblHBV)44Bri / Jf4J) were purchased from Shanghai Public Health Clinical Center affiliated to Fudan University. The primary antibody and secondary antibody of hepatitis B surface S antigen immunohistochemistry were purchased from Shanghai Changdao Biotechnology Co., Ltd. Other experimental materials, main reagents and instruments are the same as in Example 1.
[0160] Animal grouping and immunization methods:
[0161] Thirty-five HBsAg transgenic mice (C57BL / 6J-Tg(AlblHBV)44Bri / Jf4J) (initial serum HBsAg concentration was 5000-10000pg / ml) were randomly divided into 5 groups with 5 mice in each group. The experimental groups are shown in the table below. Each group of vacc...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com