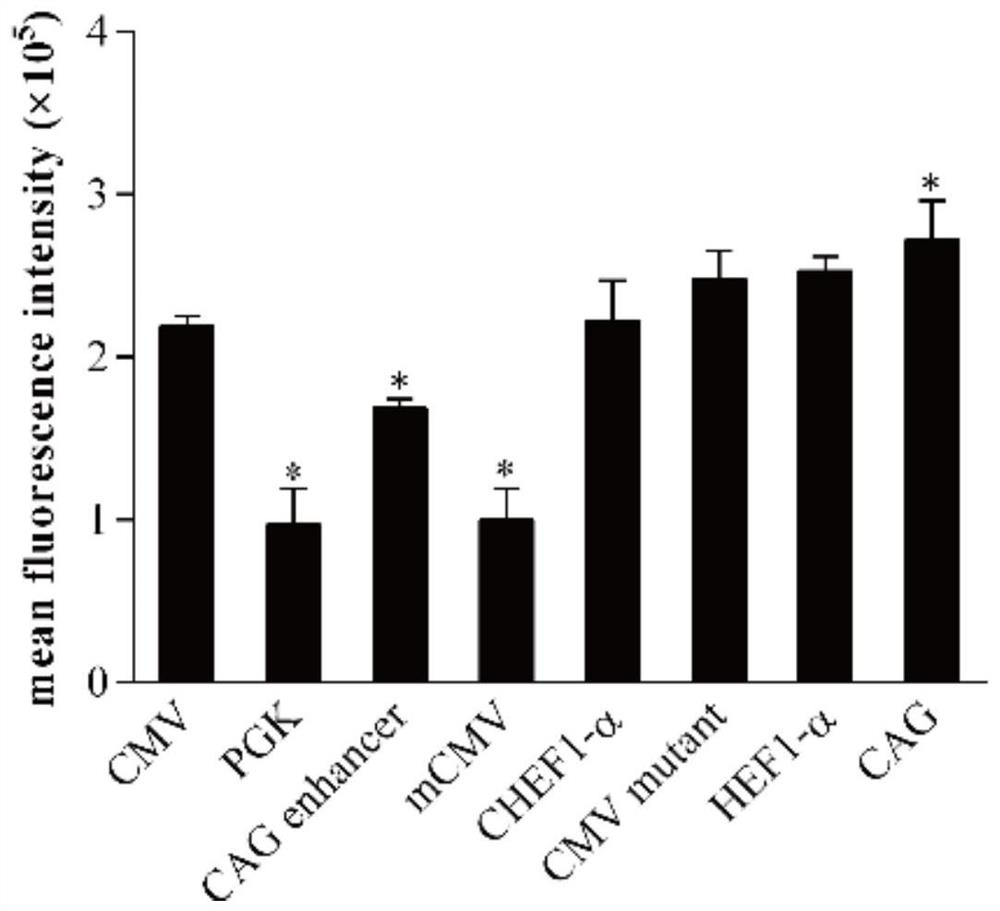
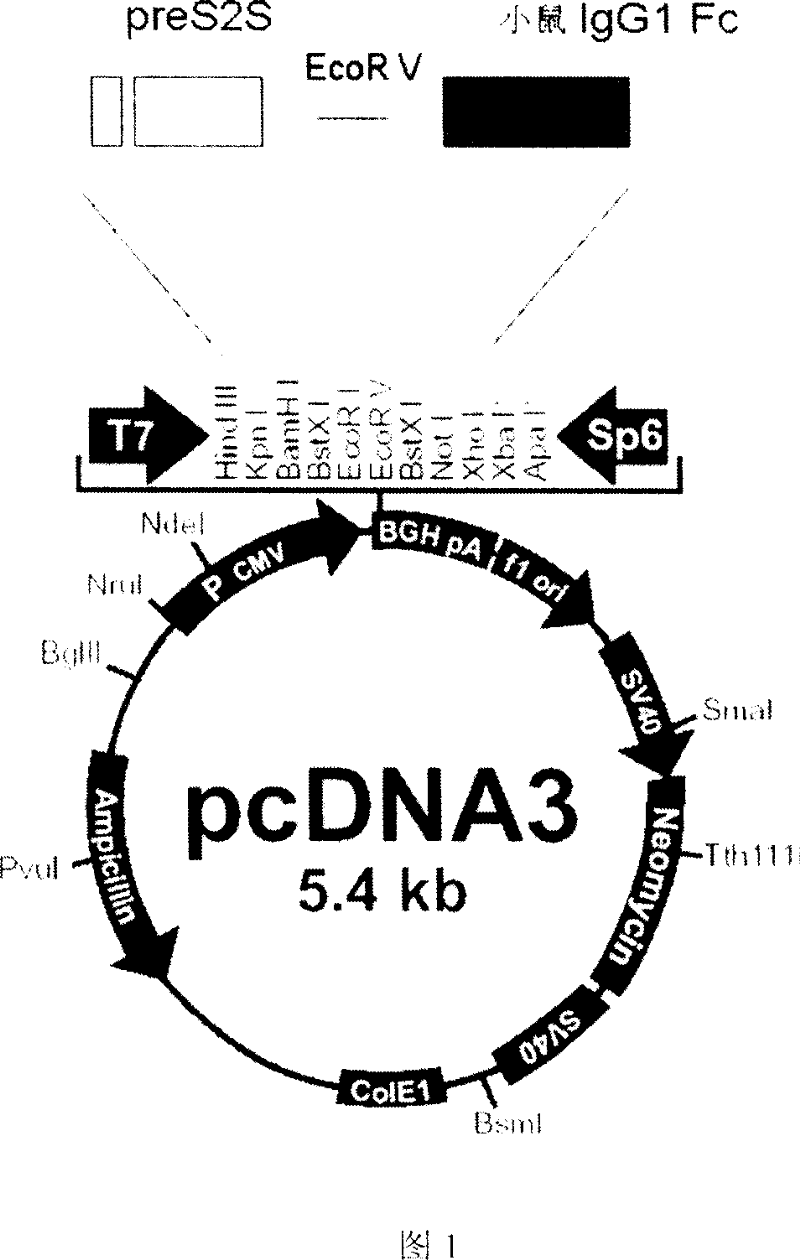
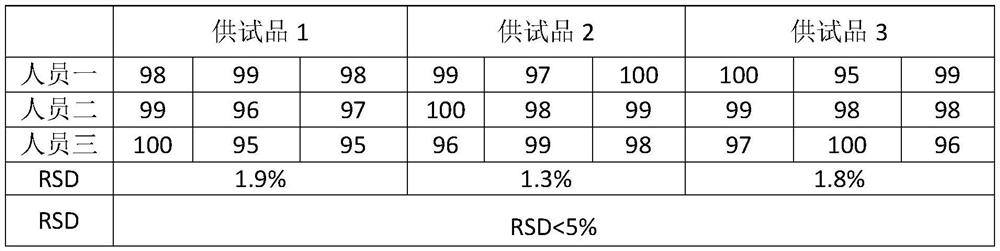
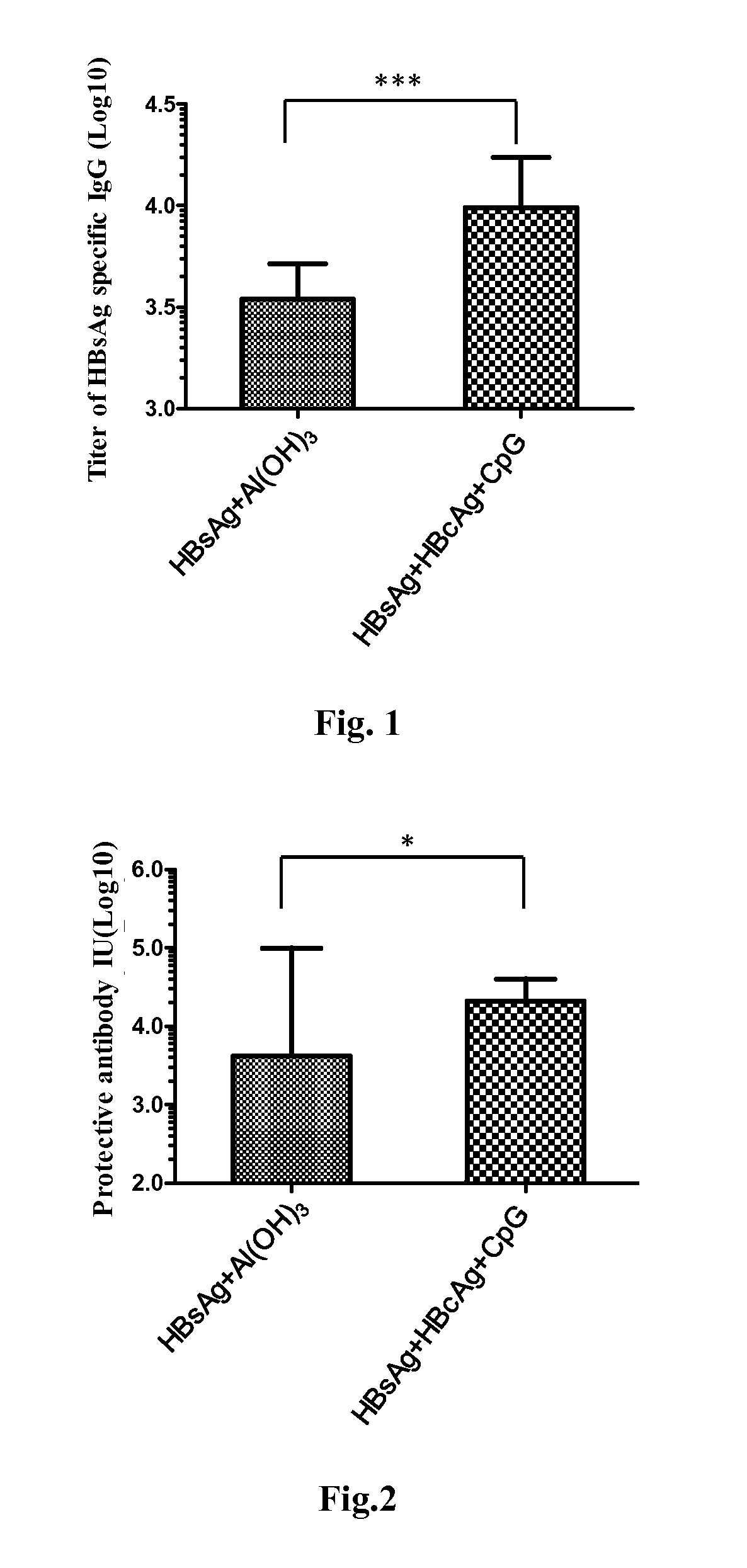
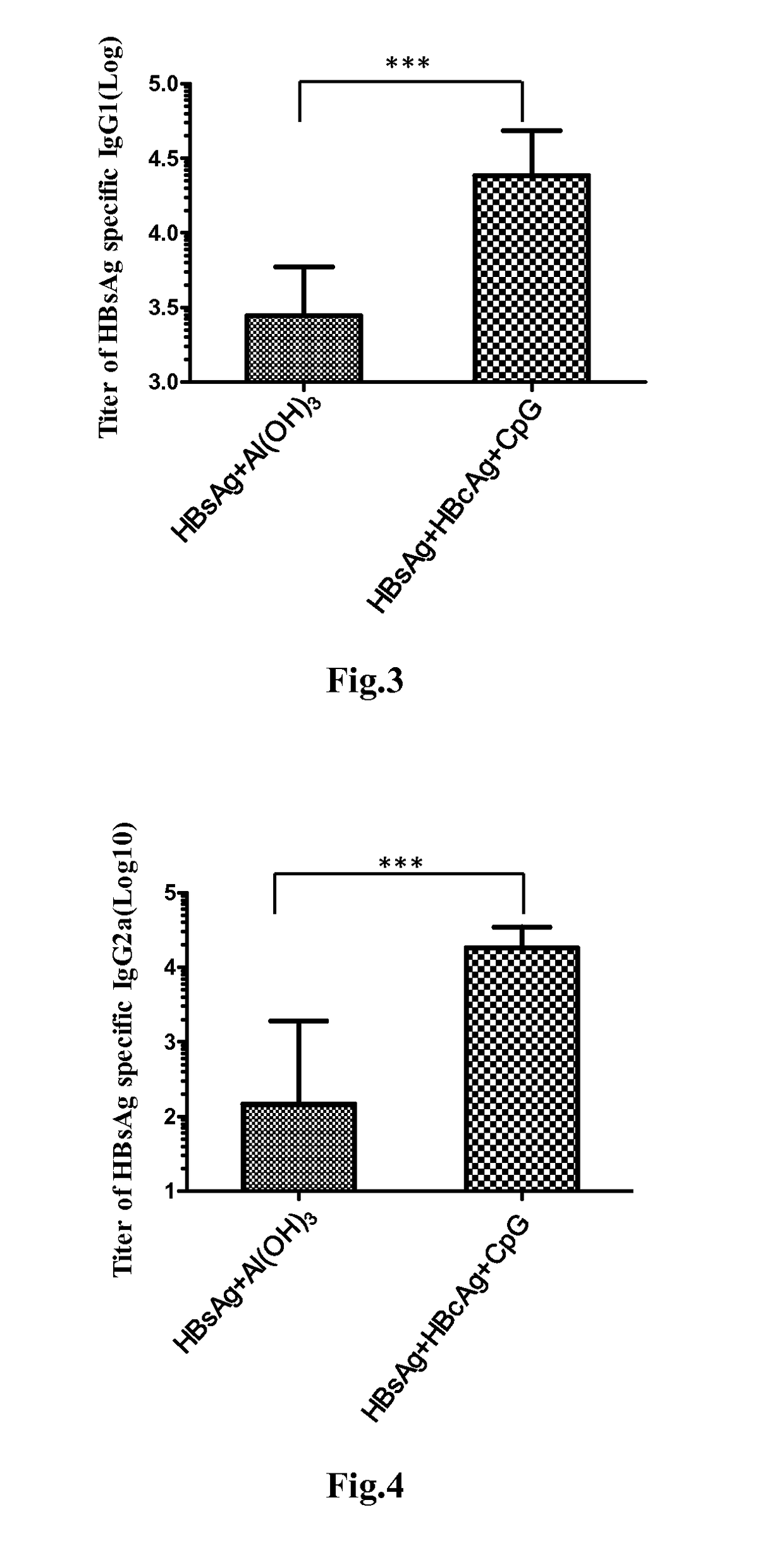
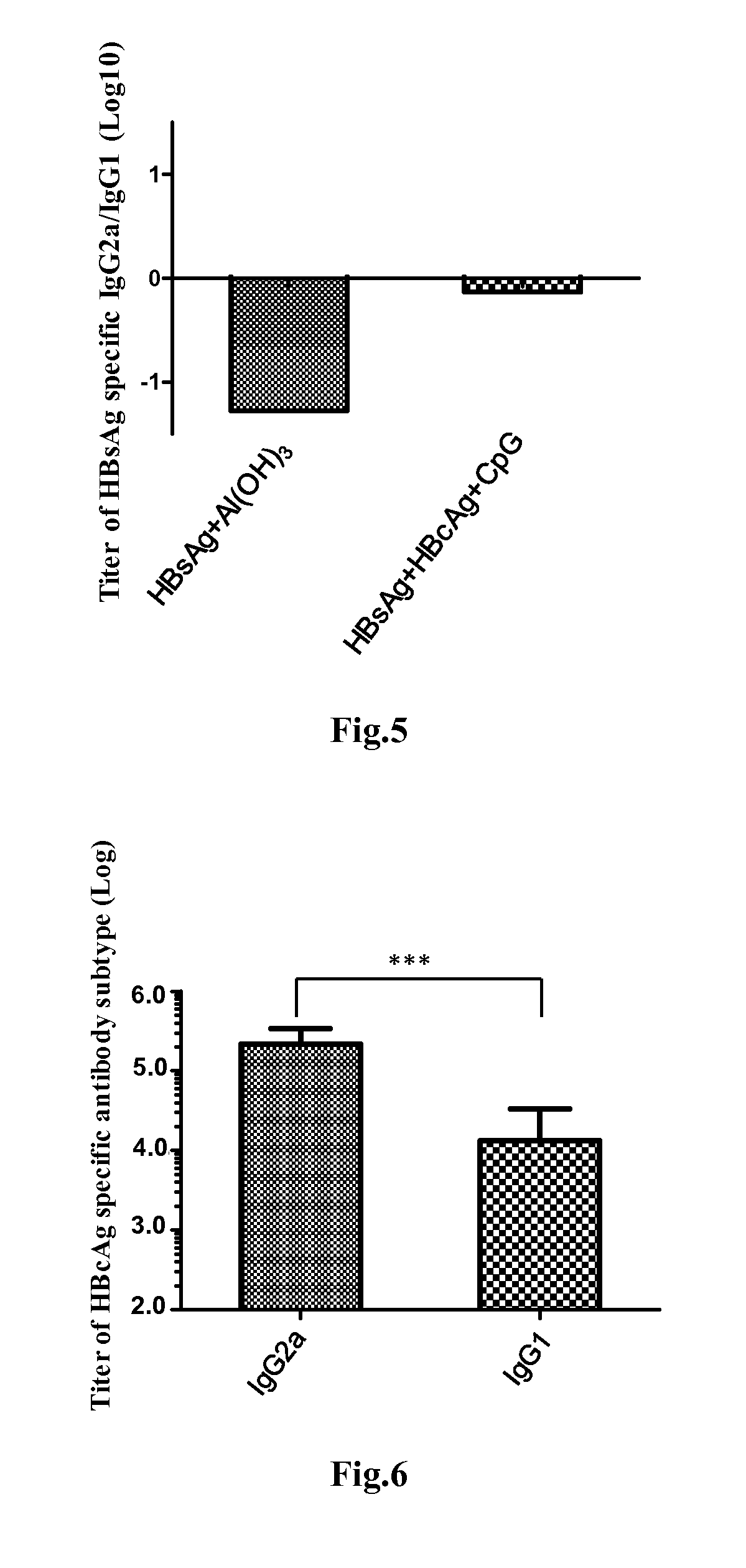
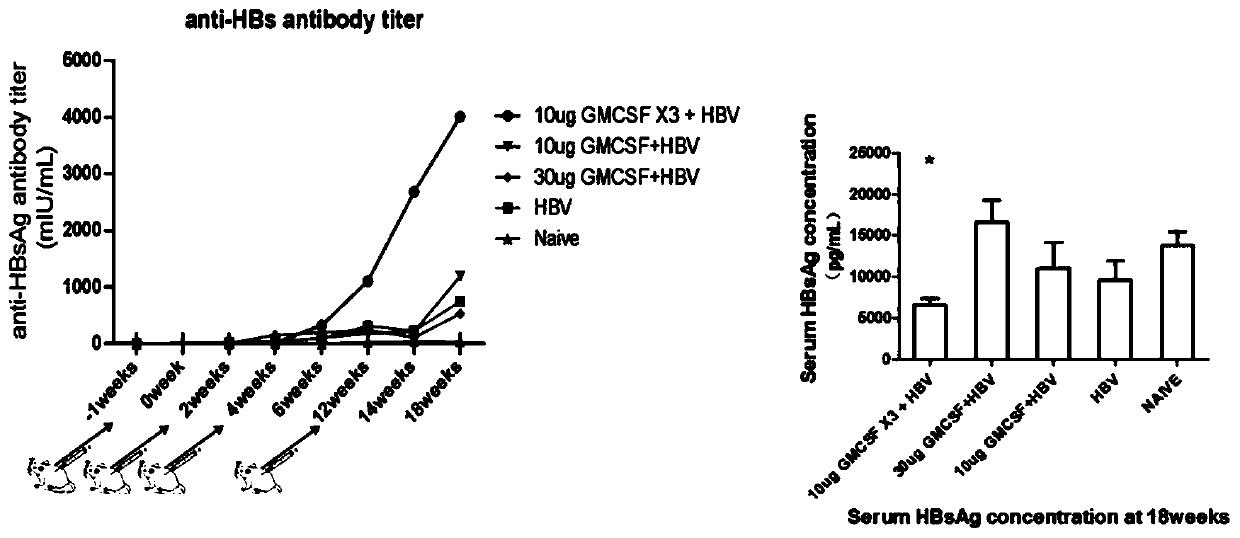
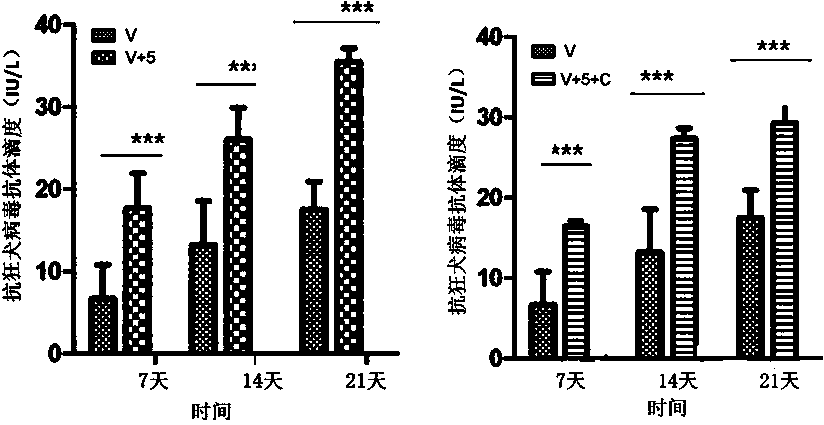
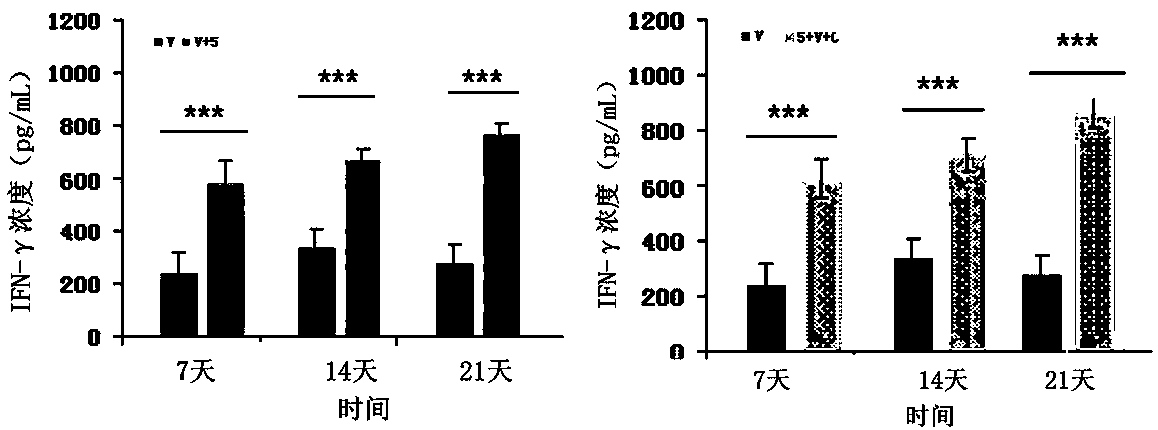
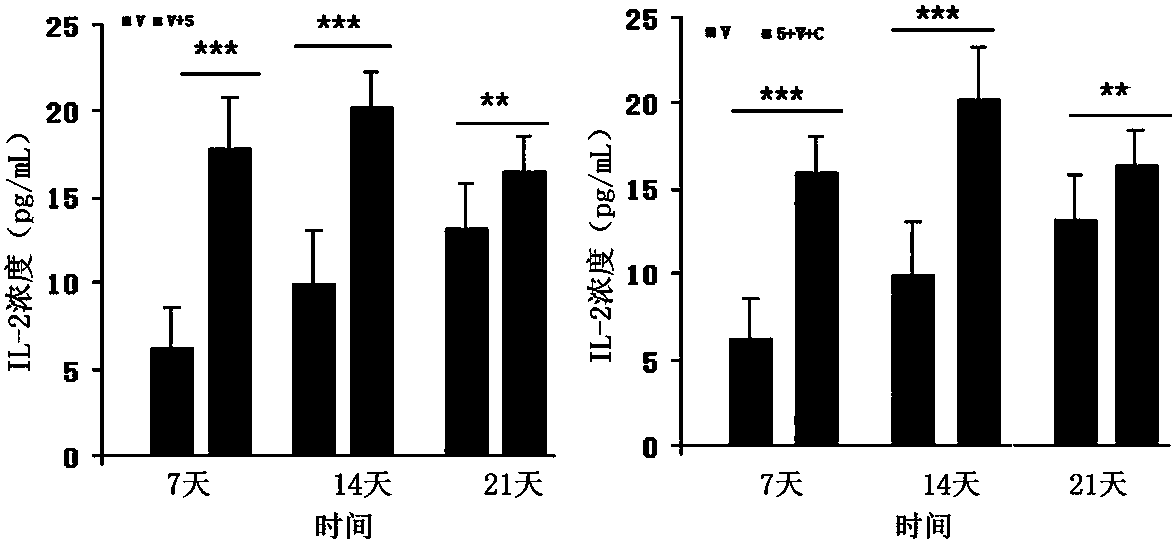
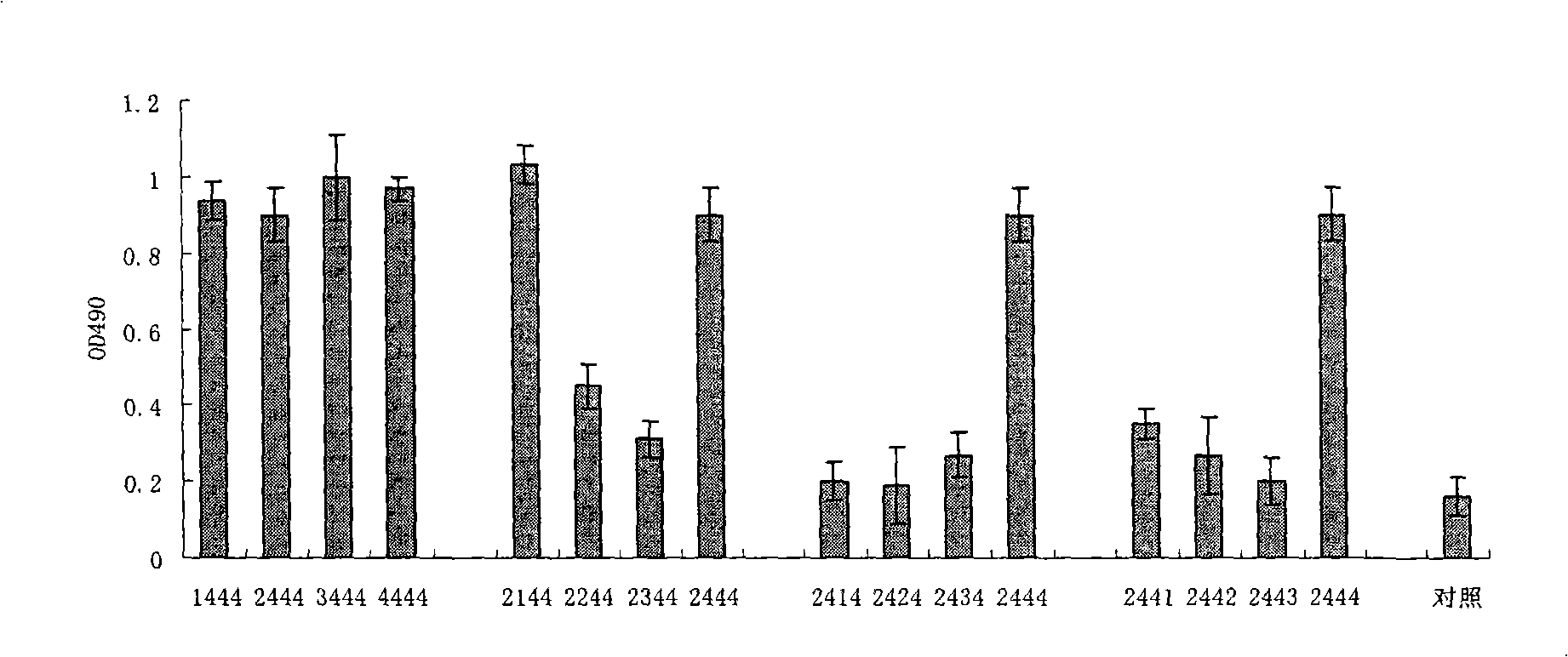Patents
Literature
49 results about "Hb vaccine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The hepatitis B vaccine — sometimes known by the trade name Recombivax HB — is used to prevent this infection. The vaccine is provided in three doses. The first dose can be taken on a date you choose. The second dose must be taken one month later.
Sulpho-oligodeoxynucleotide with immune stimulation activity and uses thereof
ActiveCN101492672AHigh activityEnhance immune responseOrganic active ingredientsSugar derivativesNucleotideGene engineering
The invention relates to a phosphothioate oligodeoxynucleotide with immunostimulation activity. A 5'-NTCGTT-3' primitive with two or more than two copies is arranged in the sequence thereof and the length thereof is of 15 to 35 nucleotide; wherein, CpG is unmethylated; and N does not represent A or G. The phosphothioate oligodeoxynucleotide has excellent immunostimulation activity both to a human body and a mouse immunocyte in vitro; and the immunostimulation activity in the human body thereof can be estimated according to the results of immunostimulation activity to the mouse. As a vaccine adjuvant, the phosphothioate oligodeoxynucleotide can remarkably enhance the immune response of the mouse to the hepatitis B vaccine of gene engineering, and can lead the immune response to lean to the direction of TH1, thus can be used for controlling and curing hypersusceptibility and infection. Meanwhile, the phosphothioate oligodeoxynucleotide of the invention also has excellent activity to inhibit the growth of tumors in the body of the mouse.
Owner:许洪林
HBV vaccine and a process of preparing the same
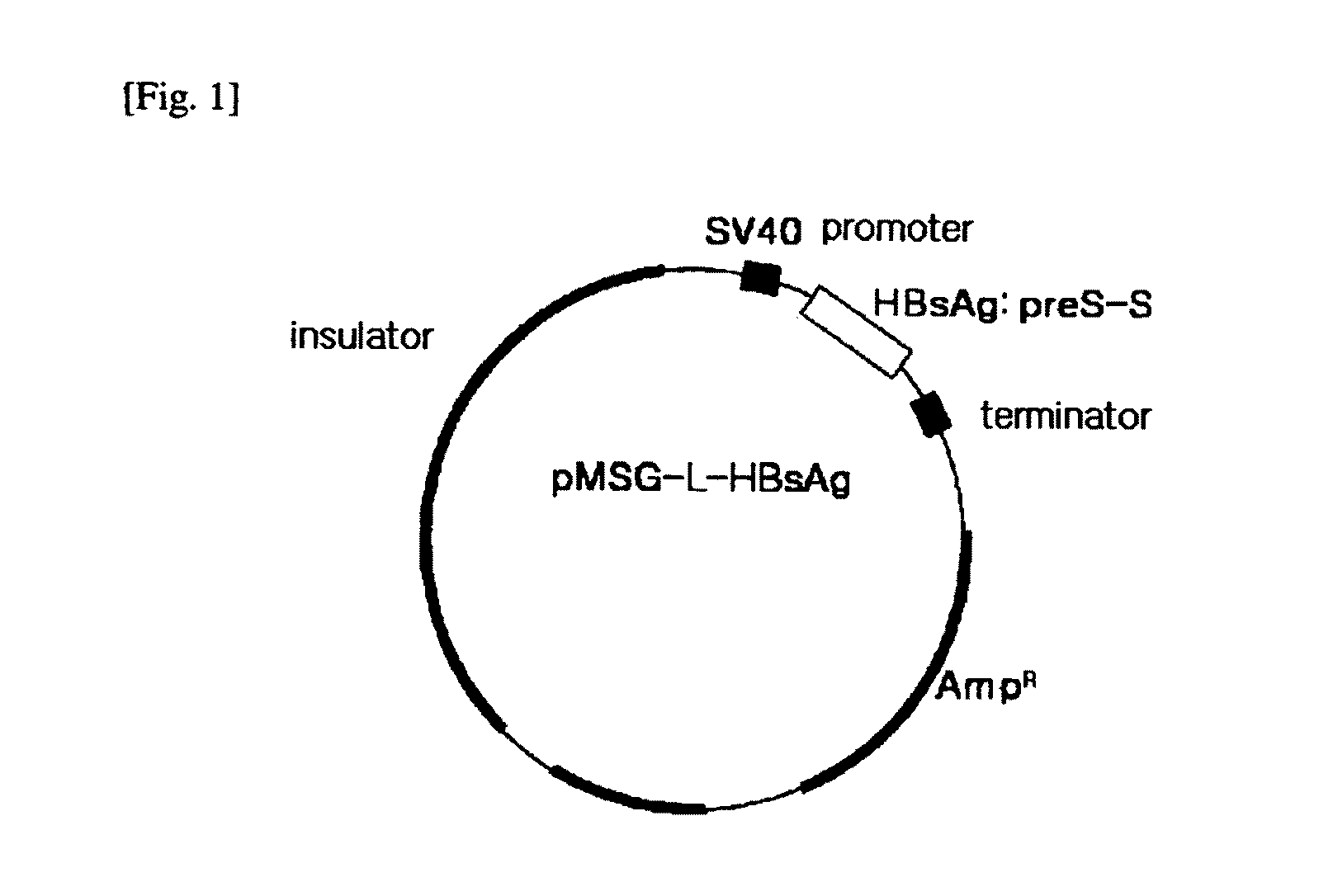
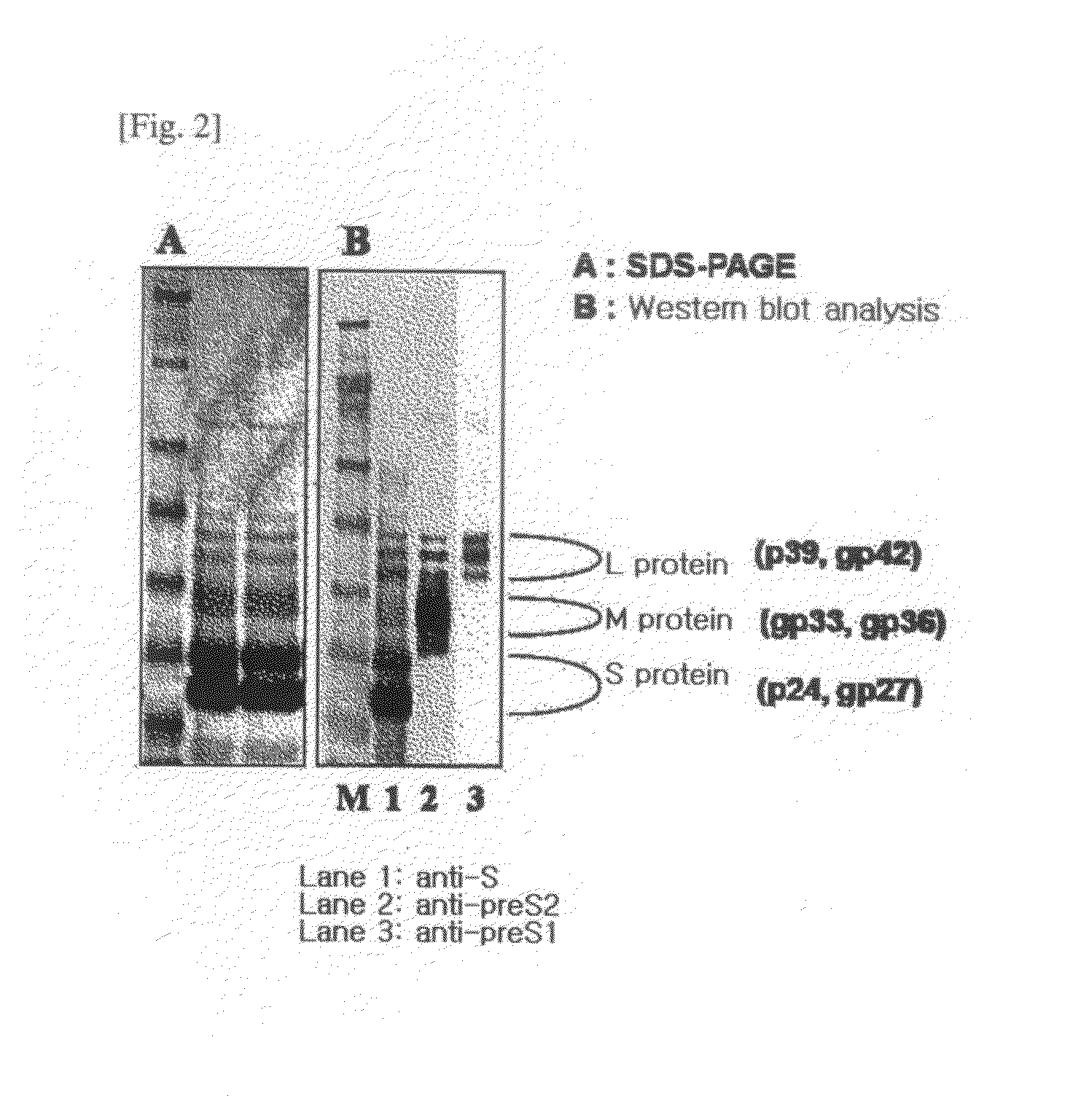
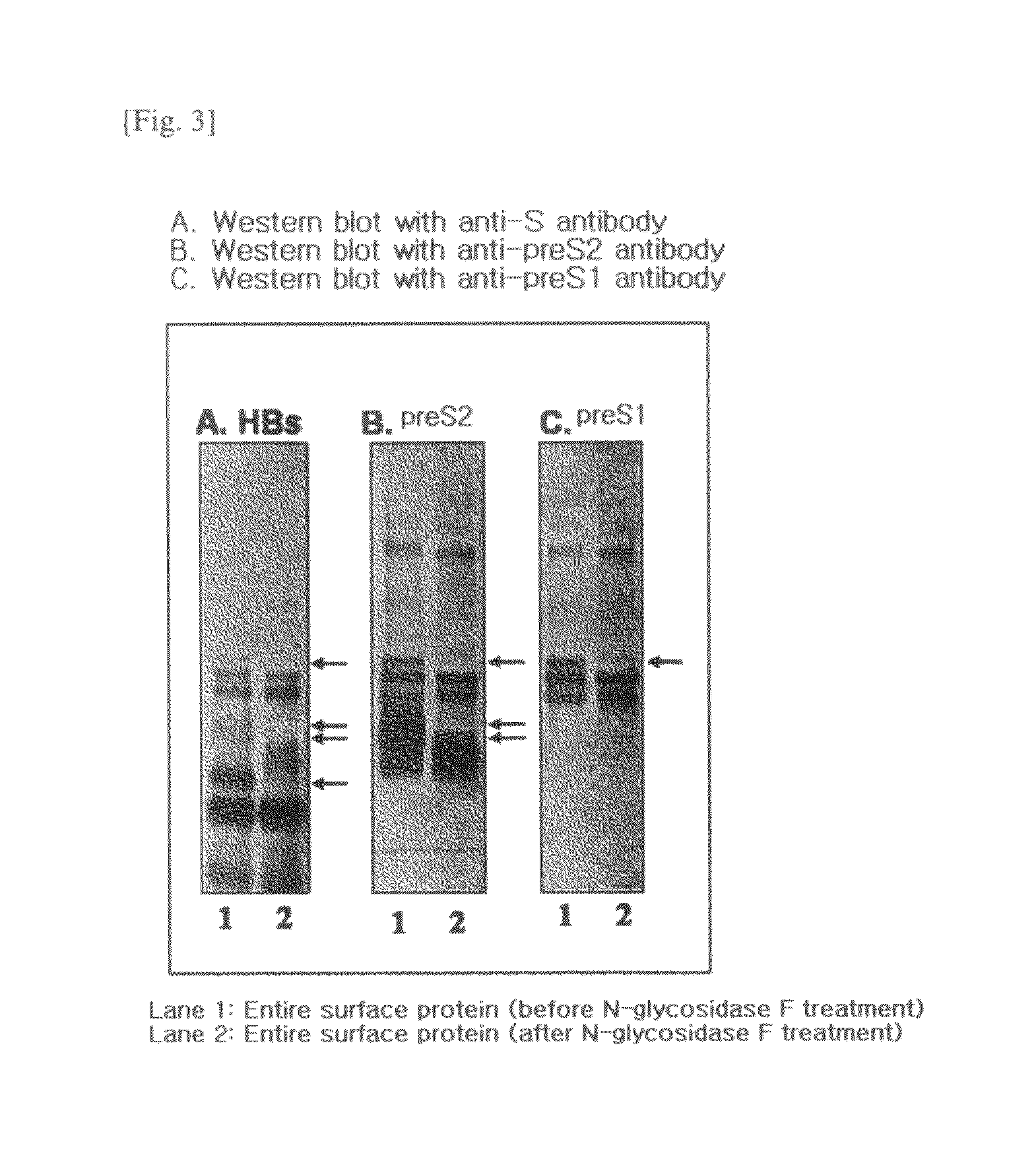
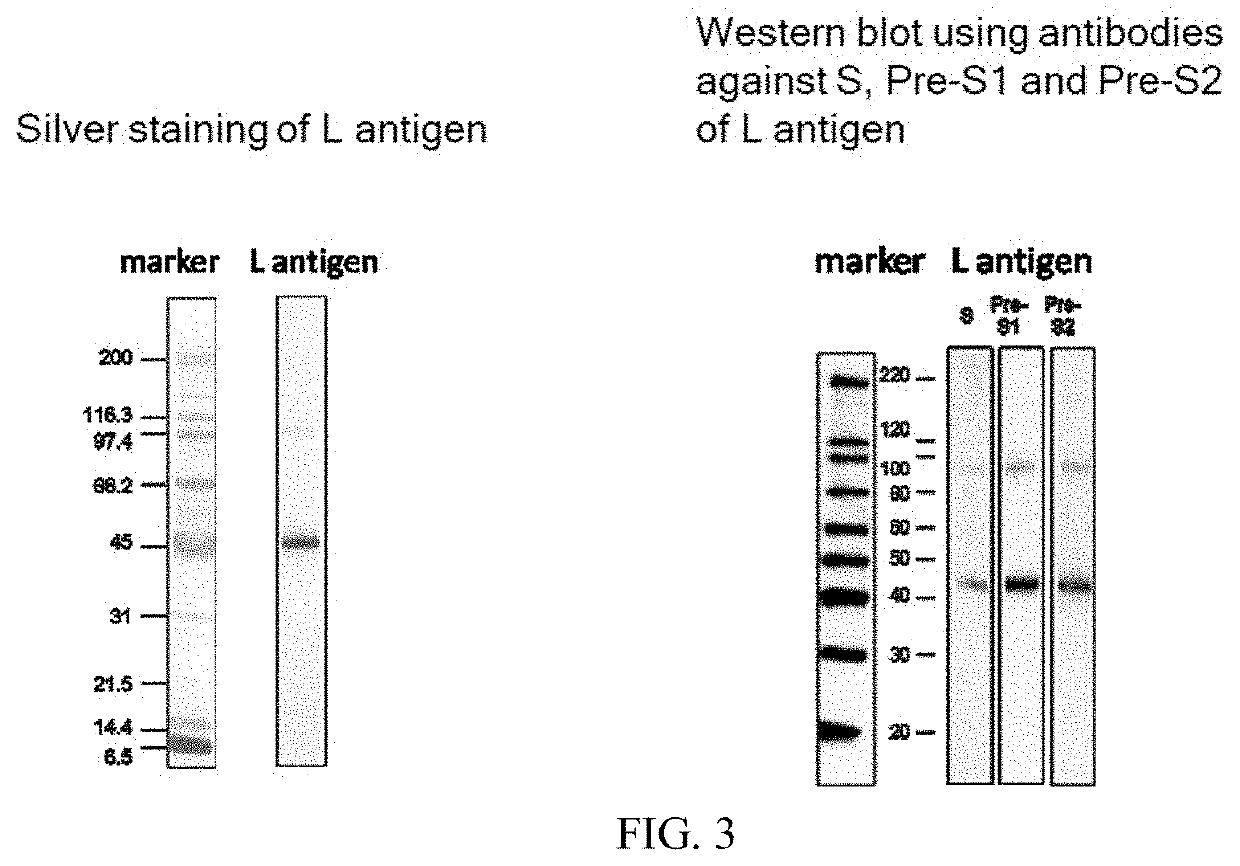
The present invention relates to an HBV vaccine comprising an entire hepatitis B surface antigen of L protein, M protein and S protein, in which the produced antigens form virus-like particles, and a multi-antigen vaccine further comprising an HBV core antigen in addition to the entire surface antigen, and a method for preparing the same. The vaccines provide various epitopes and have excellent immunogenicity to induce a strong humoral immune response as well as a cell-mediated immune response.
Owner:CHA VACCINE RES INST CO LTD
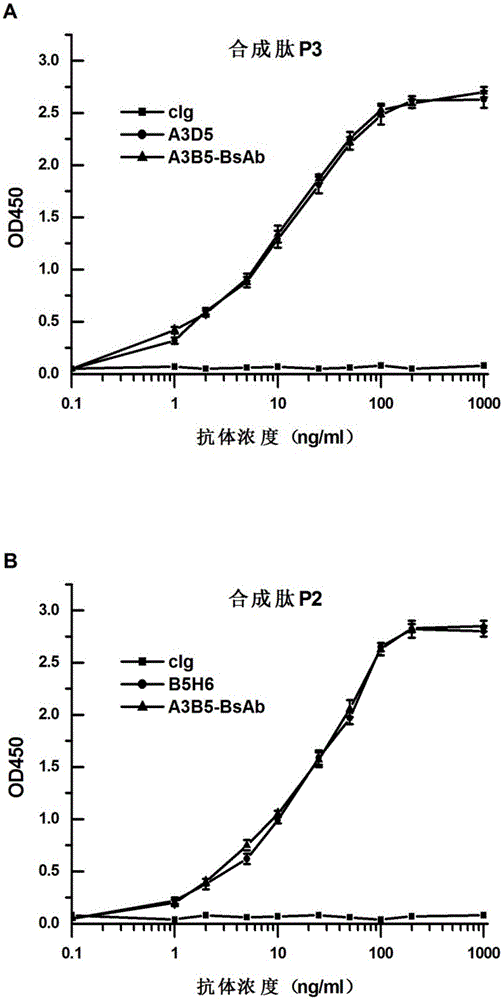
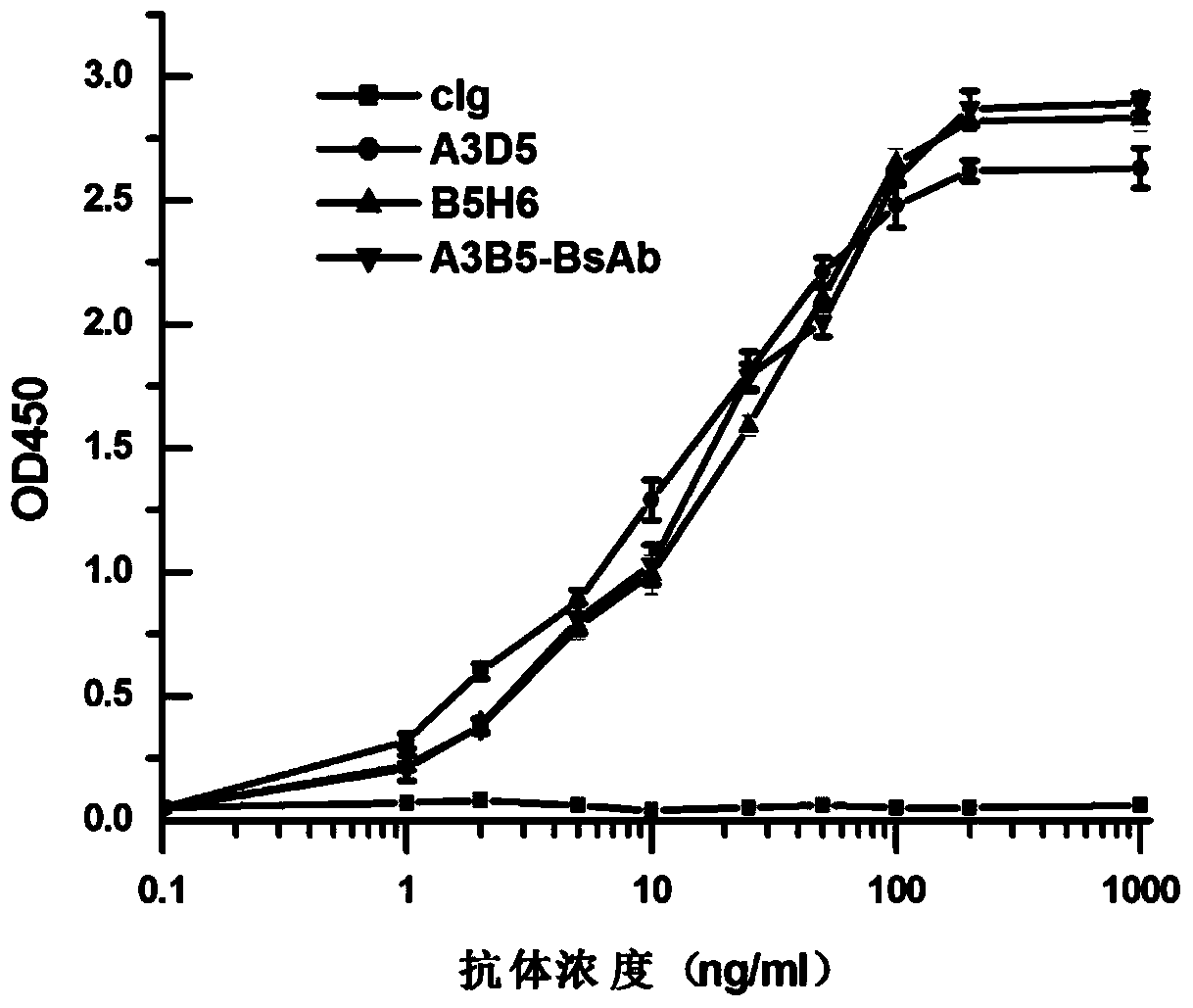
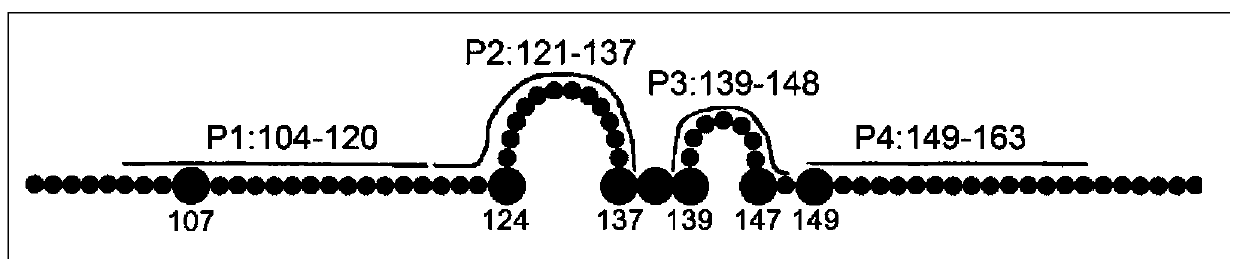
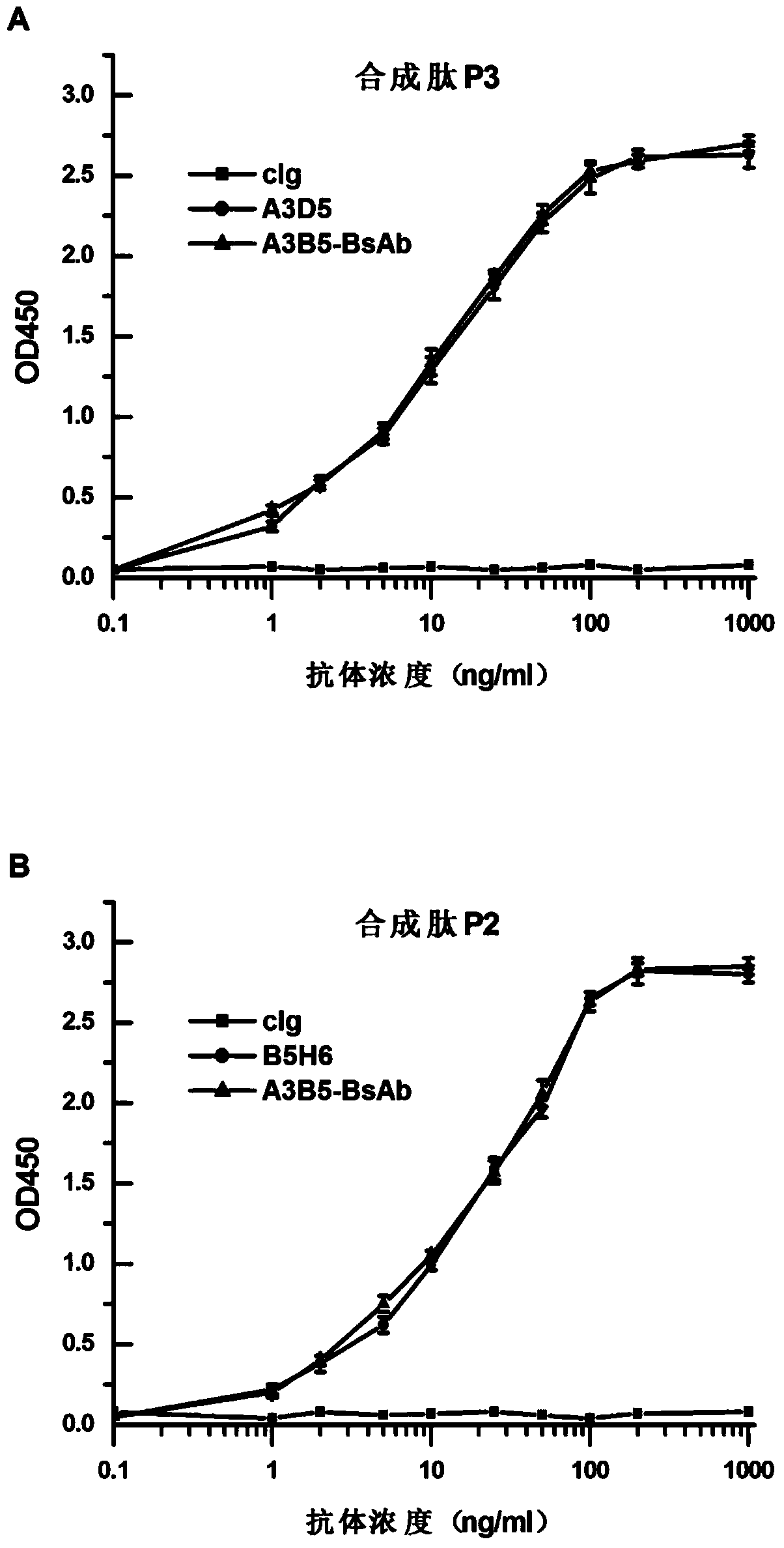
Bispecific antibody for hepatitis B surface protein, and use thereof
ActiveCN105061590AAvoid infectionCombined Application ExcellenceImmunoglobulins against virusesAntiviralsHepatitis a+b vaccineHumanized antibody
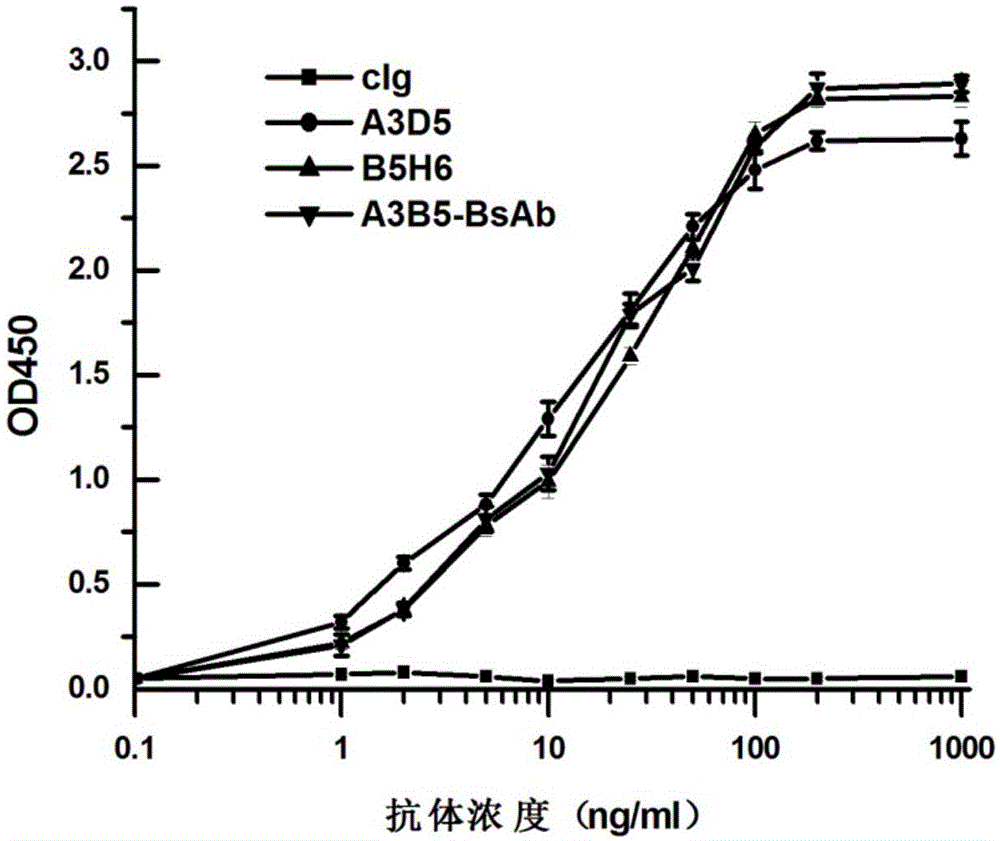
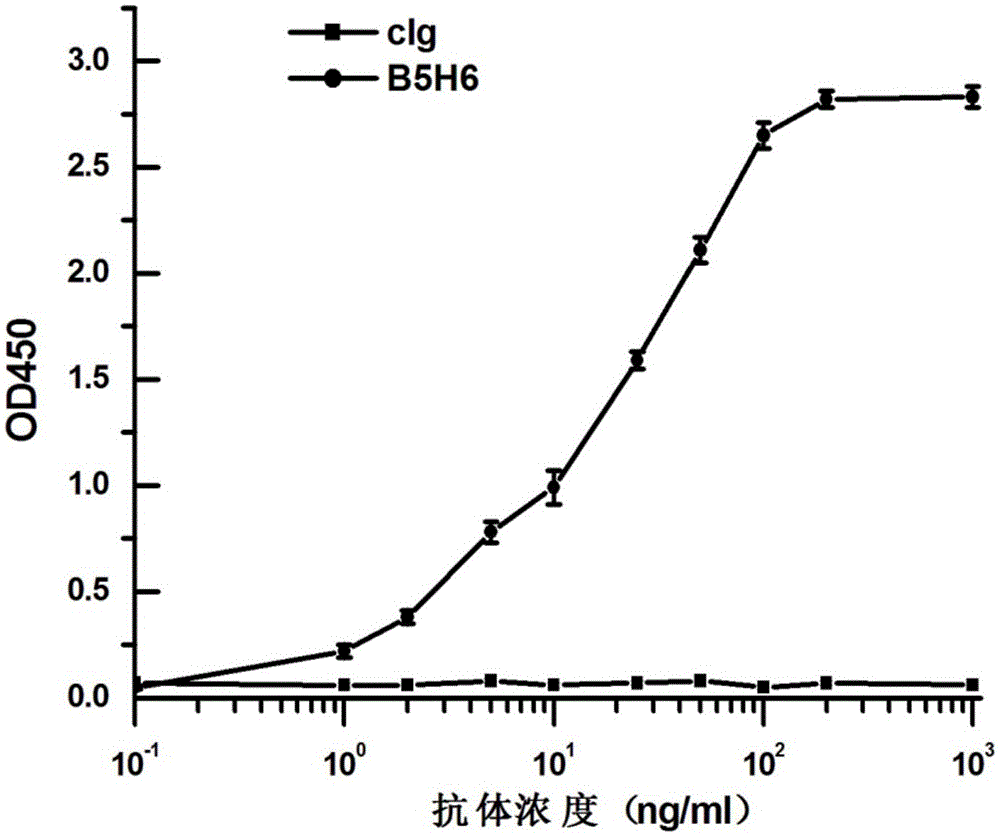
The invention provides a bispecific antibody A3B5-BsAb for a hepatitis B surface protein. The bispecific antibody is formed by combining an antibody A3D5 with an antibody B5H6. The bispecific antibody has better HBV virus neutralization ability and HBsAg release inhibition ability than single use of the antibody A3D5 and the antibody B5H6, and has strong synergistic effects, so the bispecific antibody is likely to prevent HBV infection related hepatitis, hepatic cirrhosis and liver cancer. The bispecific antibody is a completely humanized antibody obtained through cloning HBsAg specific memory B cells in the peripheral blood of hepatitis B vaccine inoculated volunteer, has lower immunogenicity than murine, chimeric and humanized antibodies, and can be used to prepare hepatitis B virus related hepatopathy prevention or treatment drugs or diagnose reagents.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Viral immunotherapy drug compound and purpose thereof
InactiveCN104338132AEnhance immune responseAvoid unresponsiveness andOrganic active ingredientsPeptide/protein ingredientsDiseaseAntiendomysial antibodies
The present invention involves a new viral immunotherapy drug complex, and in particular, a viral immunotherapy drug complex for persistent hepatitis B infection. The present drug consists of antiviral drugs, immunoregulating drugs and a recombinant hepatitis B vaccine, for use in treating hepatitis B and in particular chronic hepatitis B infections. Antiviral drugs of the described drug complex are selected among α-IFN and nucleosides; the immunoregulating drugs are selected from among GM-CSF and similar.
Owner:FUDAN UNIV
Recombinant protein and expressing method thereof in insect baculovirus expression system
InactiveCN104829732AEffective generationGenerating effective stimuliAntiviralsPharmaceutical non-active ingredientsBALB/cAntigen
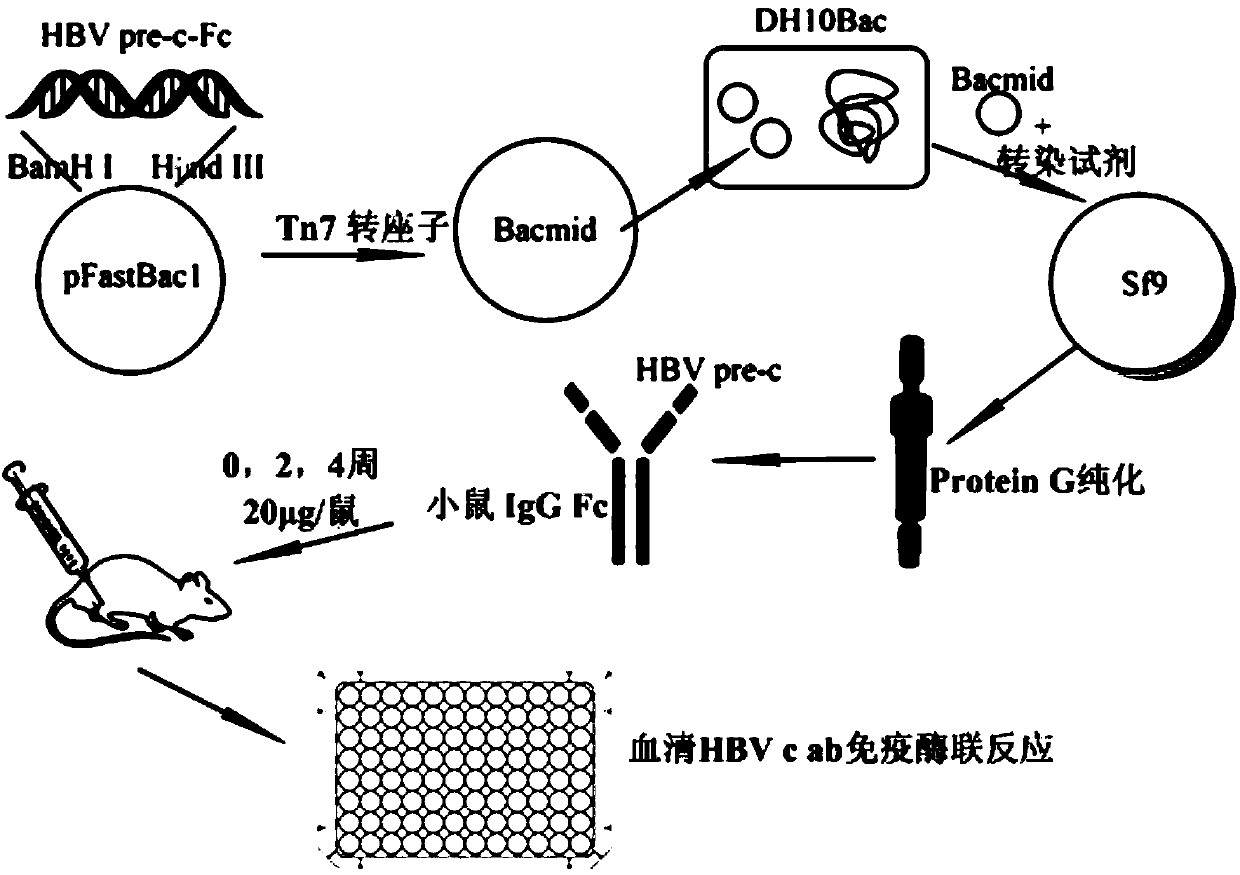
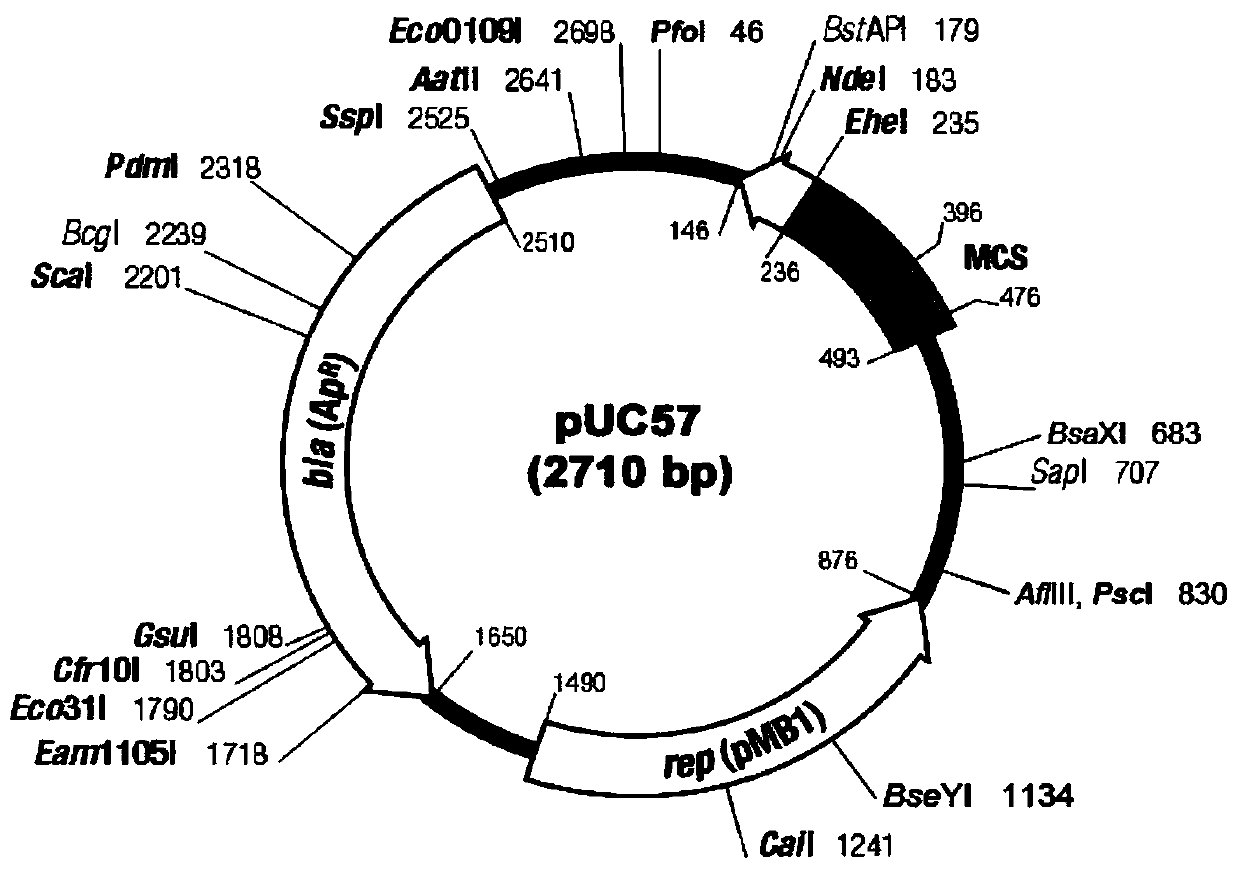
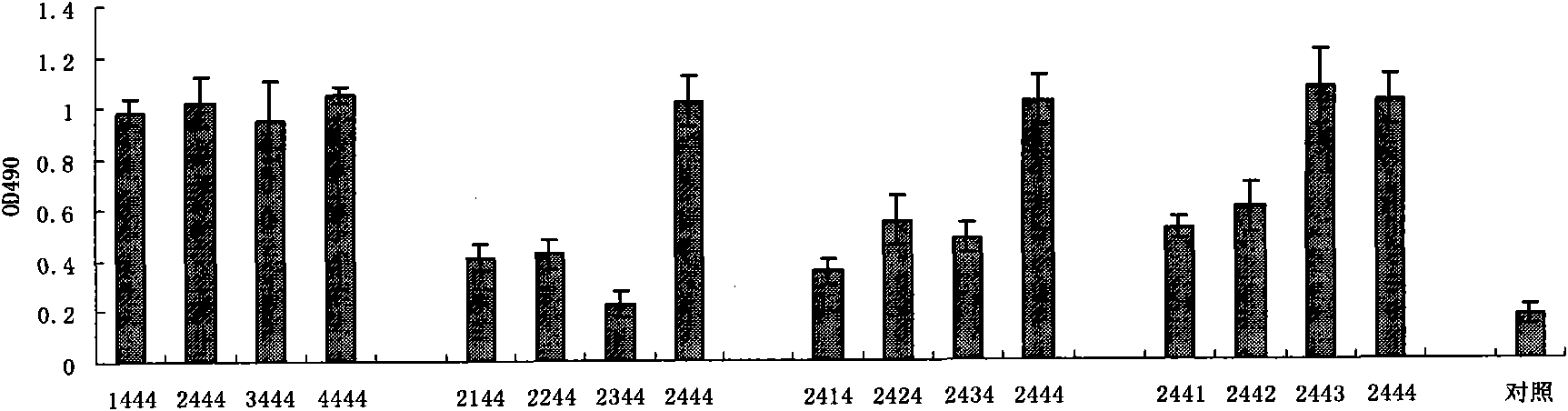
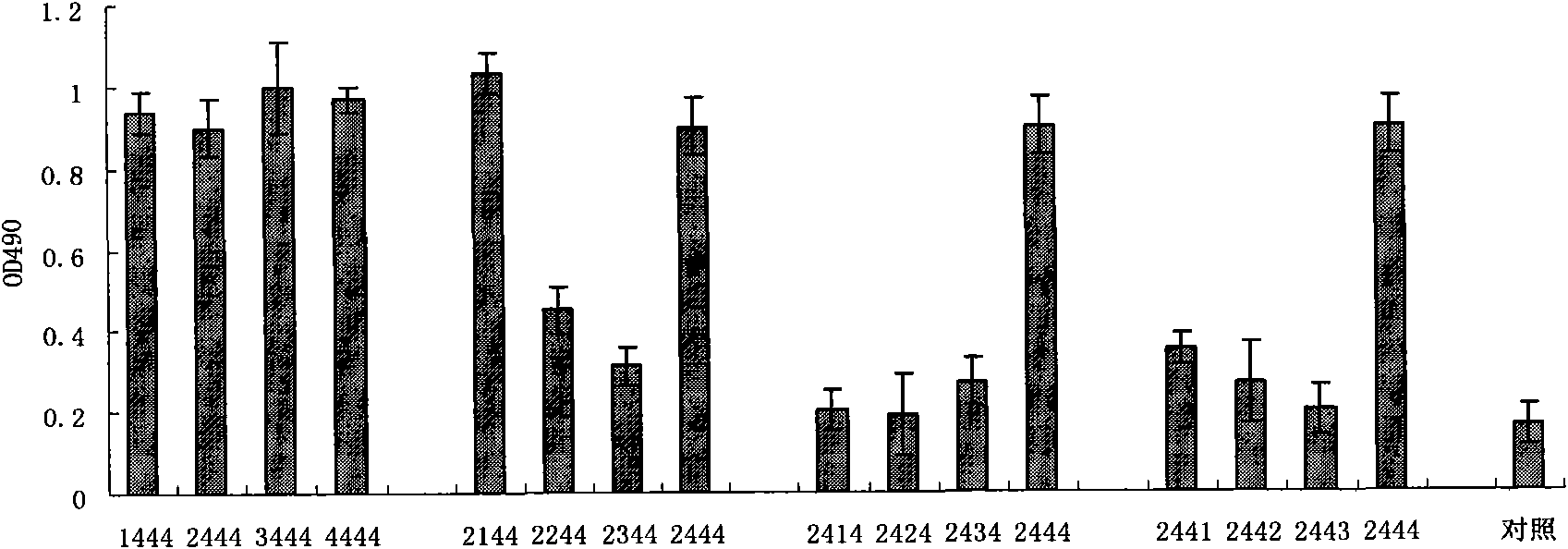
The invention discloses HBV pre-c-Fc recombinant protein and a coding nucleotide sequence thereof and discloses a recombinant vector containing a coding sequence of the protein and a host cell. The invention further discloses a recombinant baculovirus, a method of obtaining the recombinant baculovirus, and a method of expressing the HBV pre-c-Fc recombinant protein in an insect baculovirus expression system, and also discloses a recombinant baculovirus containing a HBV pre-c-Fc coding gene and applications of the HBV pre-c-Fc recombinant protein in preparation of vaccines or medicines preventing or treating hepatitis B. After a BALB / c mouse is subjected to injection immunization with the HBV pre-c-Fc recombinant protein, detection shows that the generating amount of a hepatitis B virus core protein antibody in serum of the mouse is far higher than that in a situation that an antigen used for preparing hepatitis B vaccines in traditional methods is used.
Owner:WENZHOU MEDICAL UNIV
Sulpho-oligodeoxynucleotide with immunostimulation activities and application thereof
ActiveCN101979566AHigh activityEnhance immune responseOrganic active ingredientsAntiinfectivesNucleotideAllergy
The invention relates to sulpho-oligodeoxynucleotide with immunostimulation activities. The sequence of the sulpho-oligodeoxynucleotide has two or more than two copied 5'-NTCGTT-3' elements having 15 to 35 nucleotides respectively, wherein CpG is non-methylated and N does not represent A or G. In vitro, the sulpho-oligodeoxynucleotide has high immunostimulation activities for the immunocytes of human and a mouse, and the immunostimulation activities of the sulpho-oligodeoxynucleotide in a human body can be evaluated according to the result of the immunostimulation activities in mouse. Used asa vaccine adjuvant, the sulpho-oligodeoxynucleotide can obviously strengthen the immune response of the mouse to a genetic engineering hepatitis B vaccine and make the immune response deflected to a TH1 direction, and thus, it is proved that the sulpho-oligodeoxynucleotide can be used for preventing and treating allergy and infection. Meanwhile, the sulpho-oligodeoxynucleotide has high tumor growth restraining activities in the mouse body.
Owner:苏州博特龙免疫技术有限公司
Hepatitis B vaccine for inducing organism to generate specific immunity in state of chronic hepatitis B virus infection
The invention discloses a hepatitis B vaccine, and in particular discloses a hepatitis B vaccine which contains an immunopotentiator and can induce an organism to generate specific immunity in a state of chronic hepatitis B virus infection. The hepatitis B vaccine disclosed by the invention contains a Toll-like receptor agonist serving as the immunopotentiator.
Owner:李金秋
Preparation method and application of completely humanized monoclonal antibody aiming at hepatitis B virus (HBV) surface protein
InactiveCN105017415AAvoid infectionBlock processImmunoglobulins against virusesAntiviralsHumanized antibodyA hepatitis b vaccine
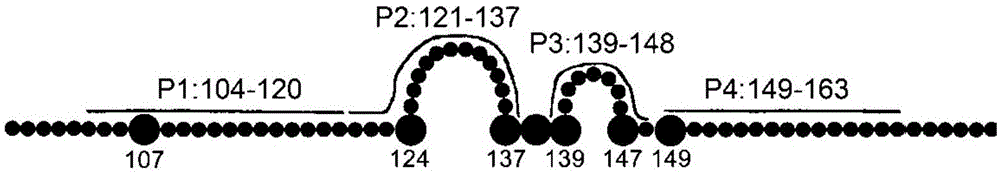
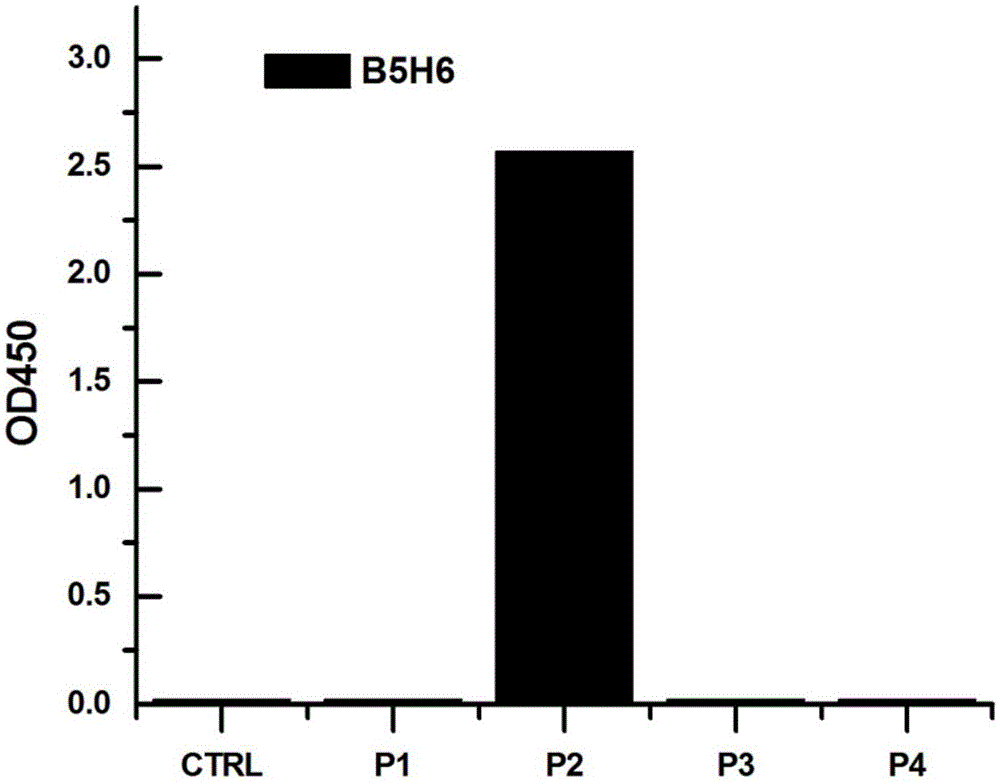
The invention provides a completely humanized monoclonal antibody B5H6 aiming at hepatitis B virus (HBV) surface protein and a gene for encoding the antibody. An experiment shows that the antibody B5H6 can be specifically combined with HBsAg protein, has relatively good HBV neutralization activity and then can resist the progresses of HBV infection related hepatitis, liver cirrhosis and liver cancers. Meanwhile, because the antibody is a completely humanized monoclonal antibody cloned from memory B cells with specific HBsAg in peripheral blood of a volunteer vaccinated with a hepatitis B vaccine, the antibody has immunogenicity lower than that of murine, chimeric and humanized antibodies, and can be used for preparing medicaments or diagnostic reagents for preventing or treating hepatitis B virus related hepatic diseases.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
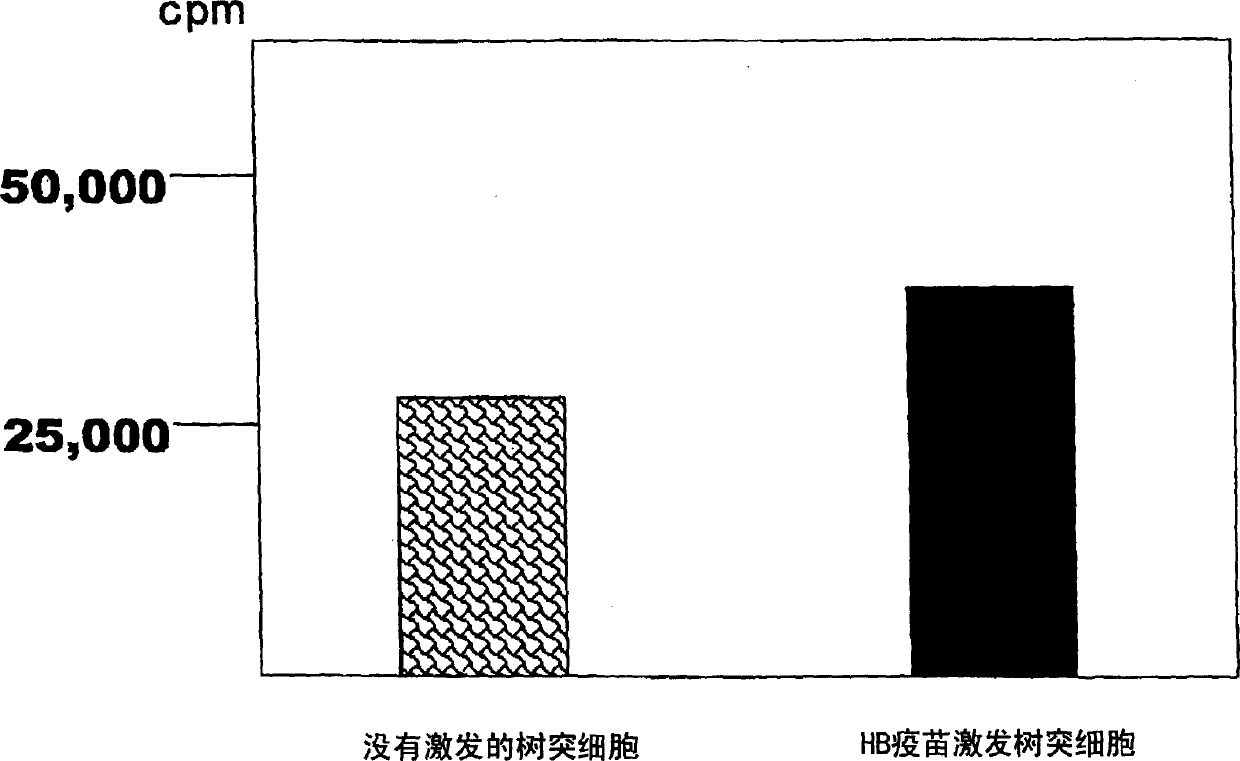
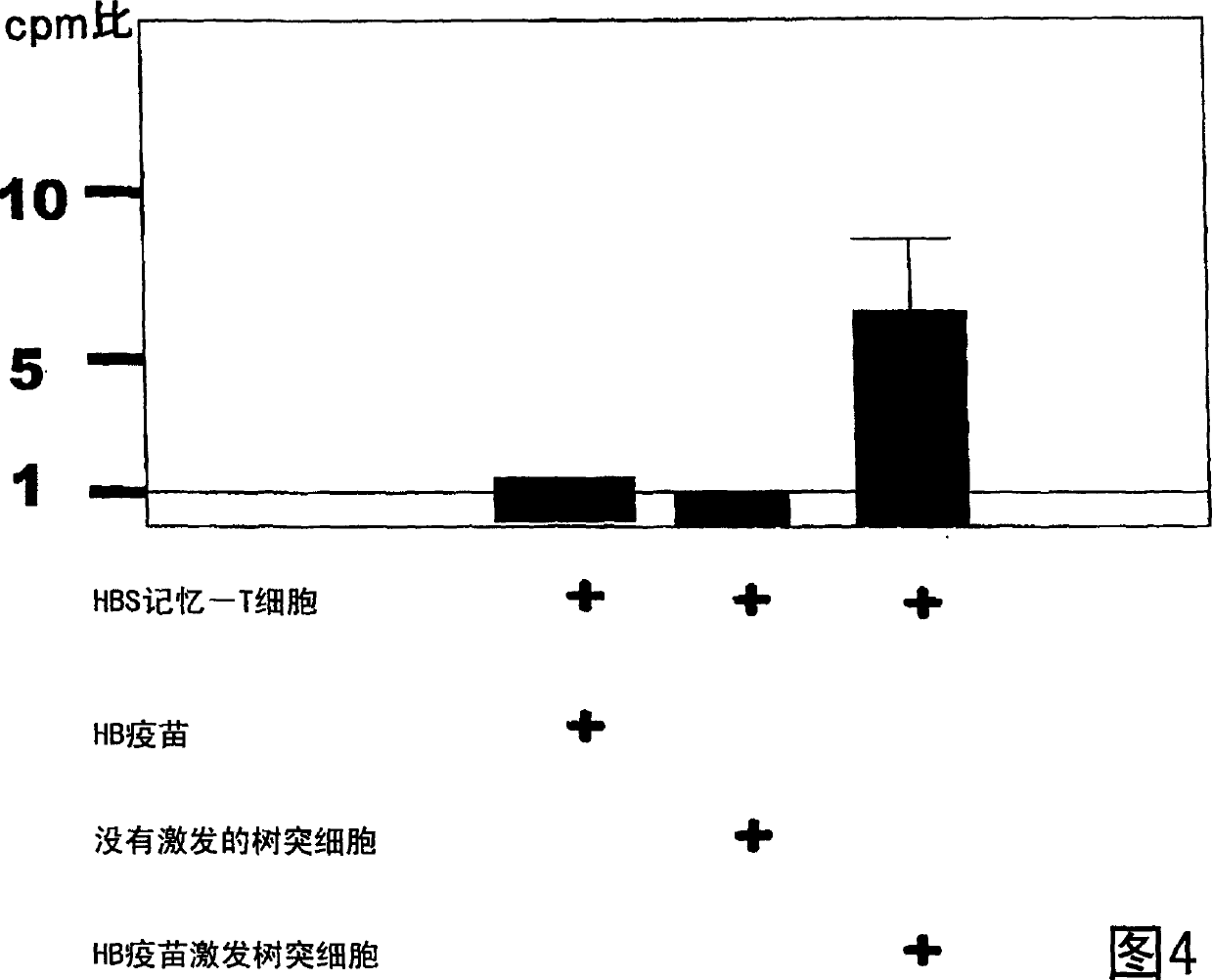
Dendritic cell obtained by antigen pulsing
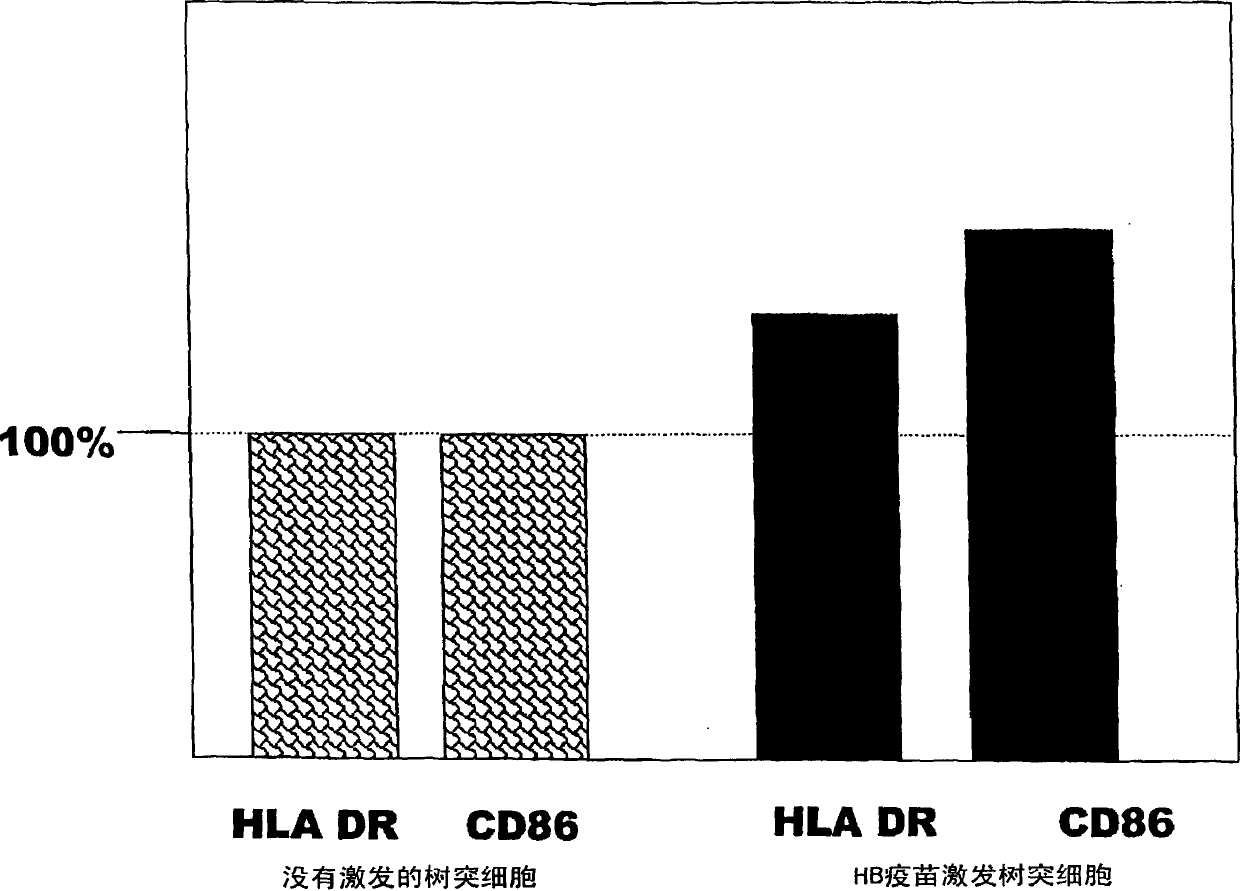
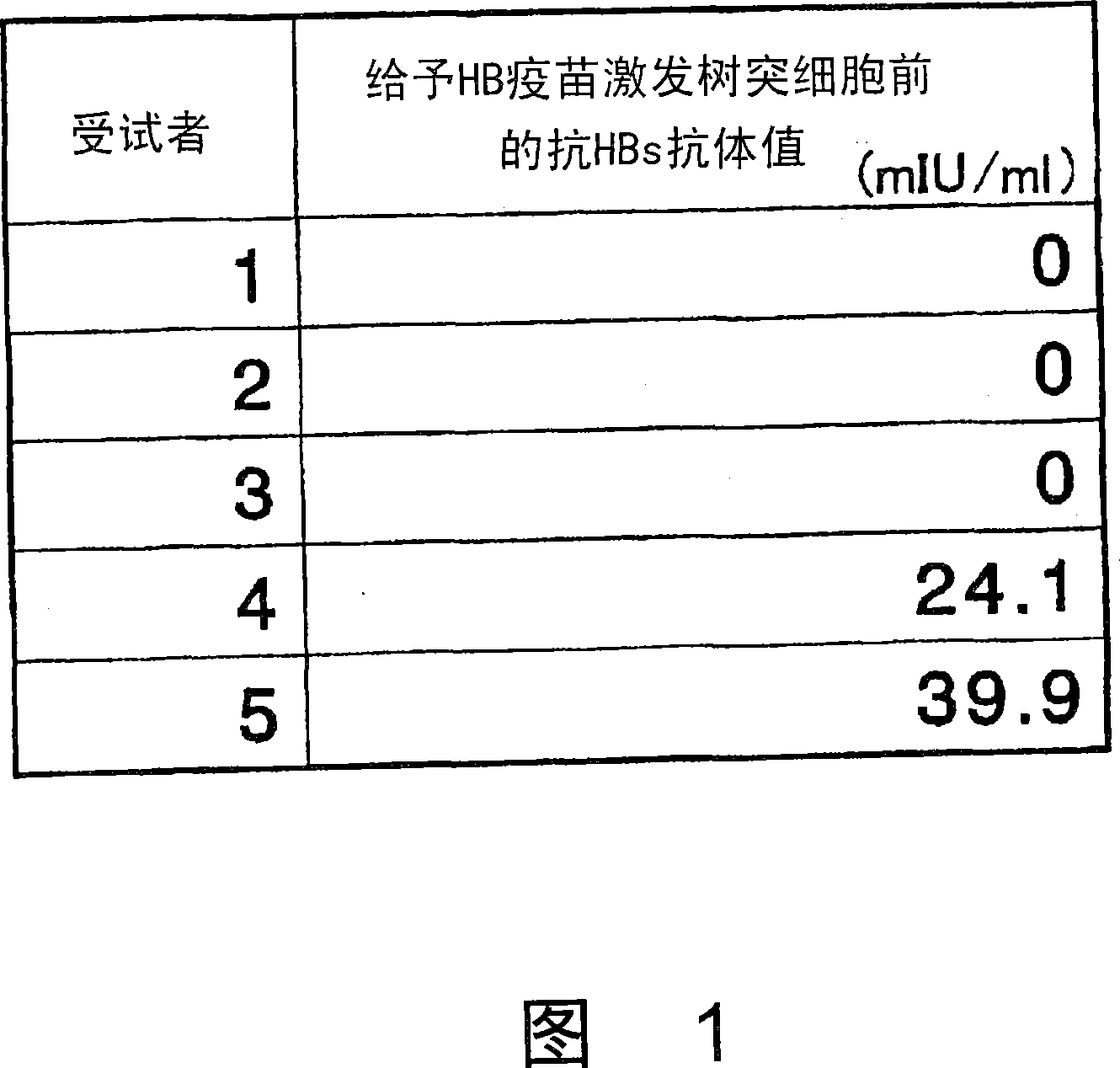
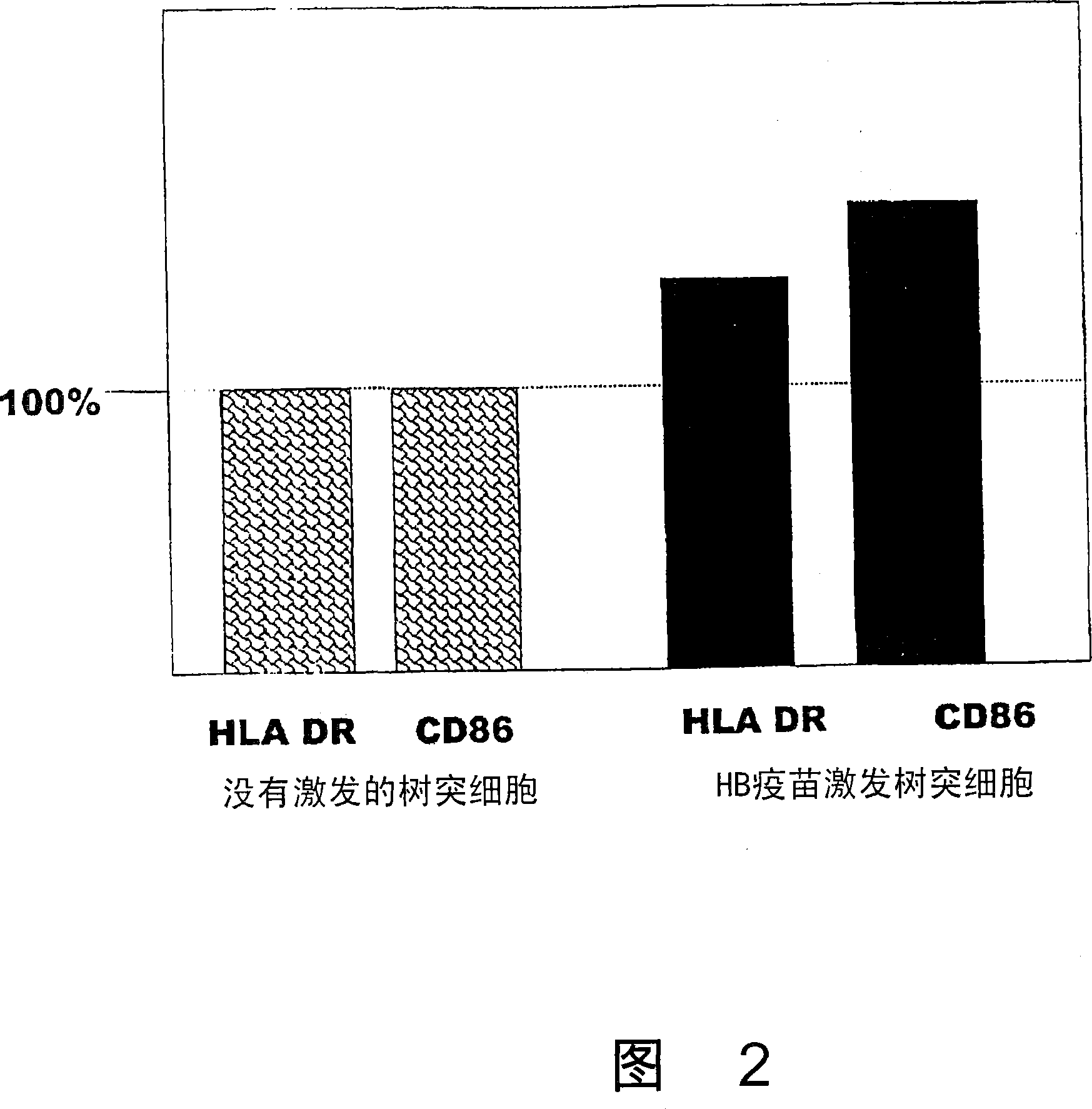
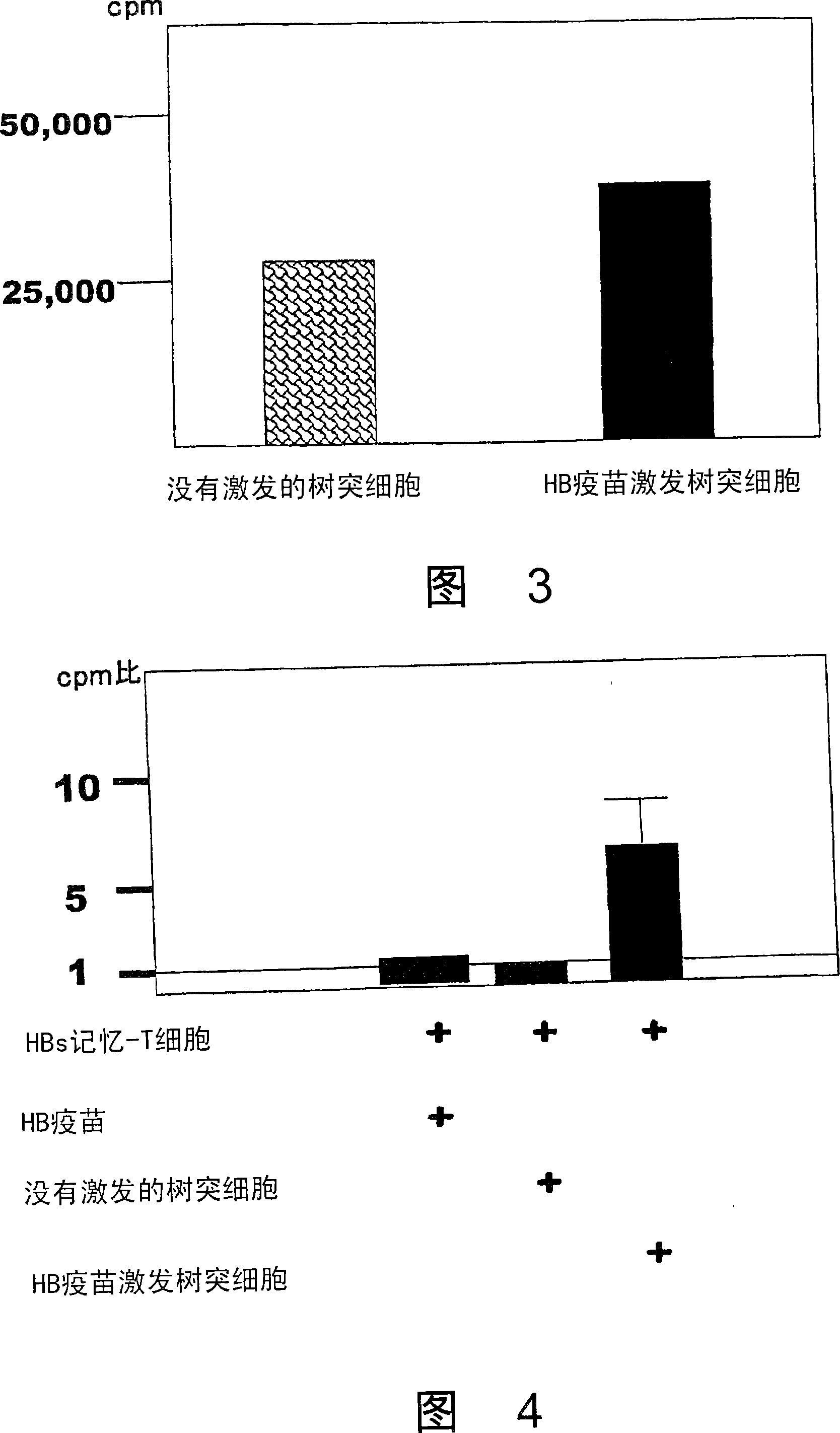
Dendritic cells effective in the prevention and curing of diseases, especially hepatitis B. Dendritic cells having HBs antigen presenting capability obtained by a process including the step of pulsing dendritic cells with HBs antigen are effective as a dendritic cell vaccine in the prevention and curing of hepatitis B. As the HBs antigen, use can be made of an HBs antigen contained in HB vaccine. Vaccine pulsed dendritic cells obtained by a process including the step of pulsing dendritic cells with a vaccine can be used as a dendritic cell vaccine in not only patients but also healthy subjects. As the vaccine for pulsing, use can be made of a wide variety of vaccines.
Owner:TECHNO NETWORK SHIKOKU
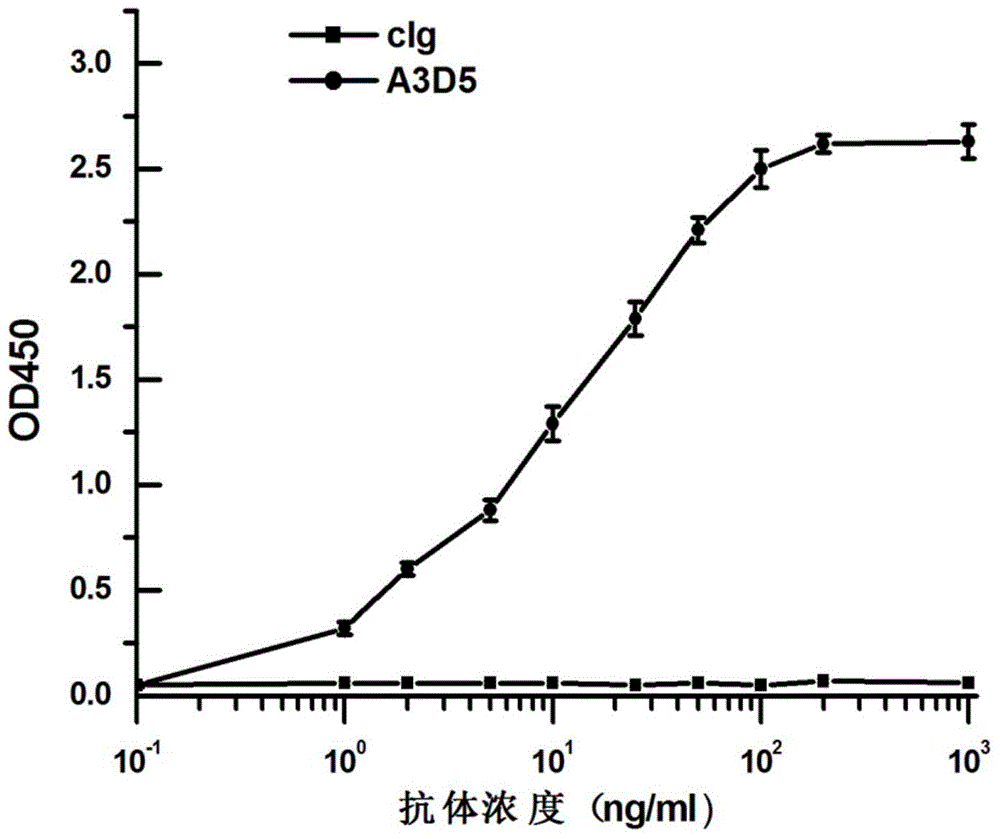
Anti-hepatitis B virus surface antigen completely humanized human antibody and use thereof
InactiveCN105061591ALow immunogenicityAvoid infectionImmunoglobulins against virusesAntiviralsHumanized antibodyFhit gene
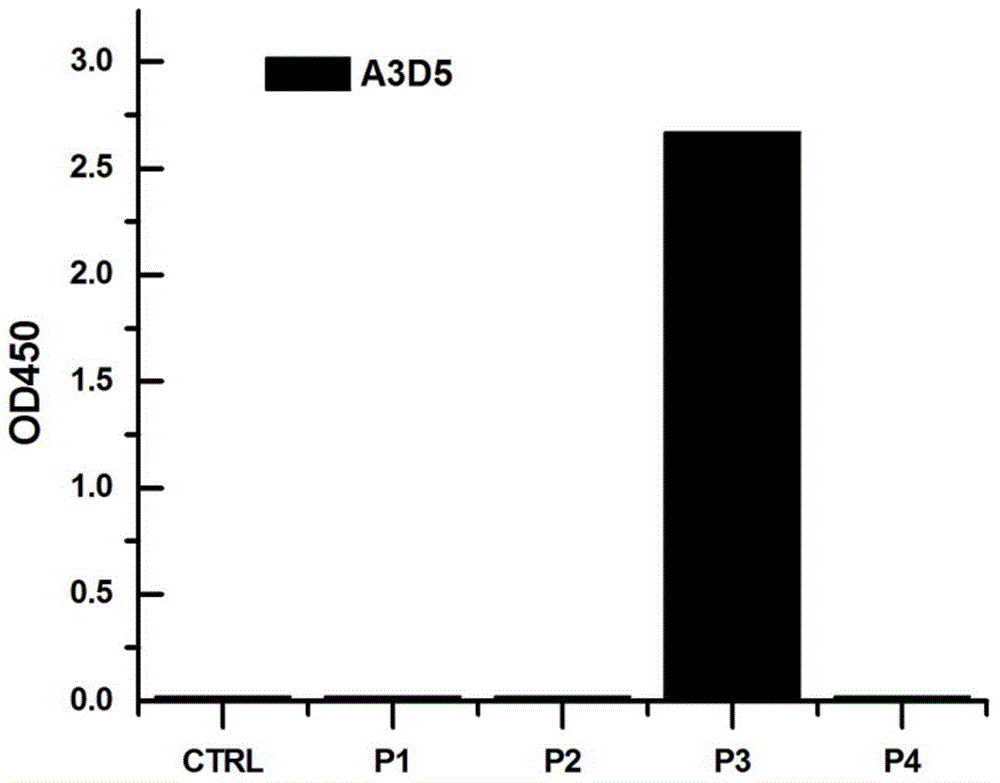
The invention provides an anti-hepatitis B virus surface antigen completely humanized human antibody A3D5 and a gene for encoding the antibody. Experiments show that the antibody A3D5 can specifically bind to an HBsAg protein, and has good HBV neutralization activity in order to possibly prevent HBV infection related hepatitis, hepatic cirrhosis and liver cancer. The antibody is a completely humanized human antibody obtained through cloning HBsAg specific memory B cells in the peripheral blood of hepatitis B vaccine inoculated volunteer, has lower immunogenicity than murine, chimeric and humanized antibodies, and can be used to prepare hepatitis B virus related hepatopathy prevention or treatment drugs or diagnose reagents.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Hepatitis B virus vaccine synergistic protein and its gene
InactiveCN1948493AImproving immunogenicityImprove immunityBacteriaPeptide/protein ingredientsSequence analysisHepatitis B immunization
This invention relates to a hepatitis B virus vaccine synergic protein and its genes, and utilizes gene engineering principle to express the method and application of the protein. CDNA of the protein is gained by screening from human liver CDNA expression bank, after sequence analysis ,using the way of gene engineering to clone to prokaryotic cell, eukaryotic cell(animals, plants) to express protein by coding of the CDNA ,for instance ,cloning CDNA to prokaryotic expression vector and gaining recombination protein by expression depuration in Bacterium coli. Combination of the protein and hepatitis B virus vaccine can improve vaccine immune effectiveness, reinforcing the immunity of hepatitis B virus infectors, increasing antibody titer. Protein of this invention can be used as adjuvant to hepatitis B vaccine.
Owner:FUDAN UNIV
Culture medium additive for recombinant protein expression of humanized HEK293 cell line, recombinant hepatitis B vaccine and expression method
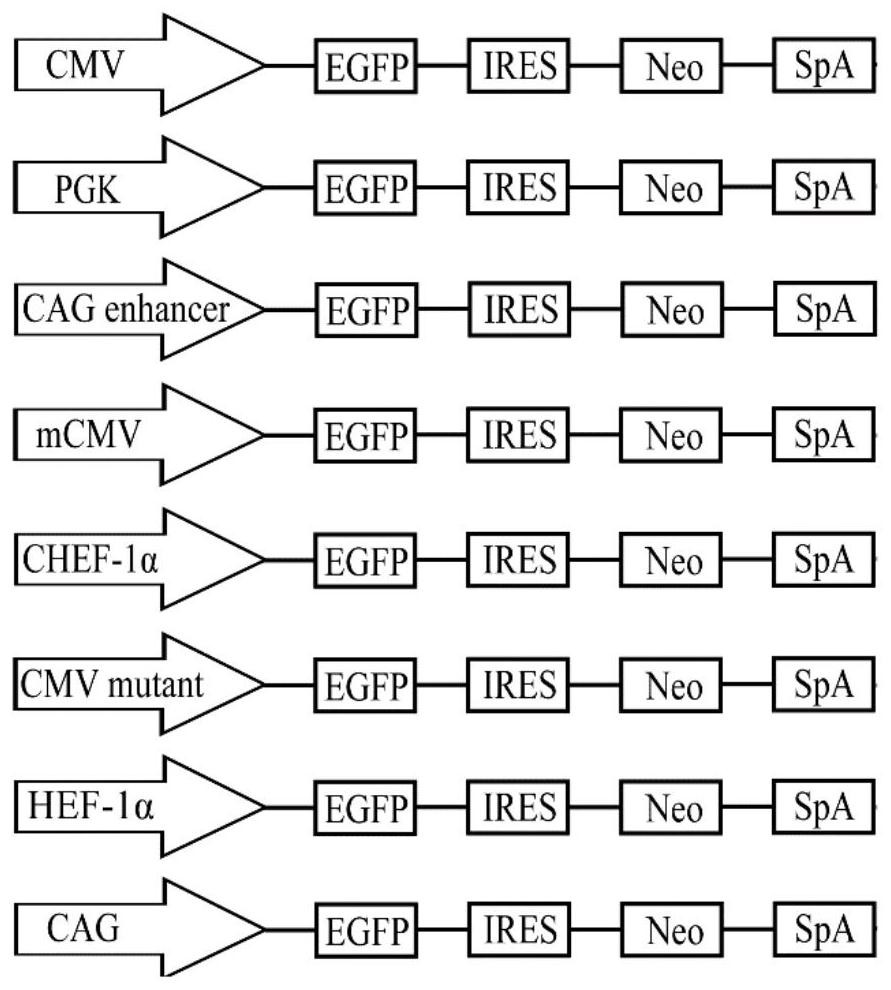
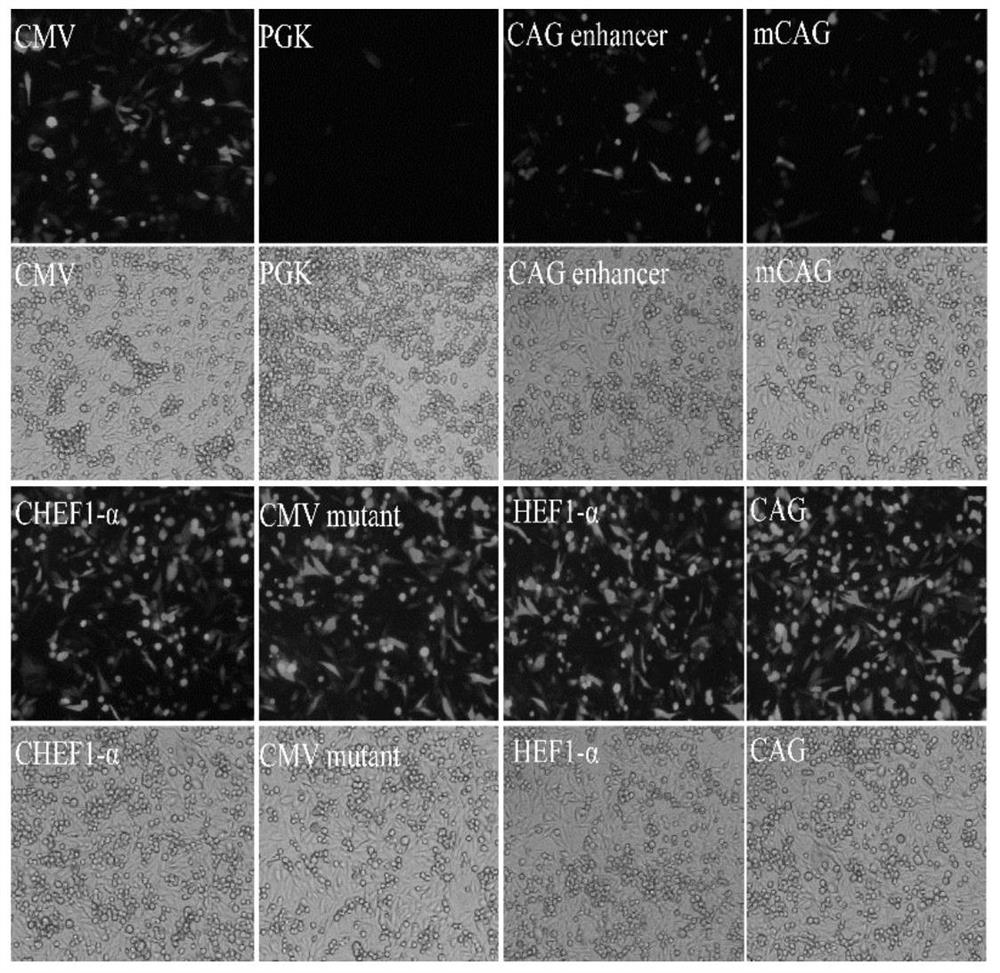
The invention belongs to the technical field of gene recombinant vaccines and particularly relates to a culture medium additive for recombinant protein expression of a humanized HEK293 cell line, a recombinant hepatitis B vaccine and an expression method. According to the culture medium additive for the recombinant protein expression of the humanized HEK293 cell line, provided by the invention, sodium butyrate and hydrocinnamic acid are added in a suspended serum-free culture process of HEK293 cells transfected with a recombinant expression vector, preferably, the addition amount of the sodium butyrate is 1.0-3.0 mol / L, the addition amount of the hydrocinnamic acid is 0.2-1.0 mol / L, synergy is achieved, and the expression level of a recombinant protein is increased. Tests show that through carrying out culturing under a low-temperature condition by utilizing the culture medium additive provided by the invention, the expression level of a hepatitis B surface antigen (HBsAg) target gene in an HEK293 cell is improved.
Owner:河南普诺易生物制品研究院有限公司 +1
Fusion protein for preparing hepatitis B vaccine and its carrier
InactiveCN101037476APeptide/protein ingredientsAntibody medical ingredientsAntibody hepatitisBio engineering
The invention discloses a fusion protein of antibody Hepatitis B vaccine belonging to the bio-engineering field. It has two functional domains which composed of preS2 of Hepatitis B surface antigen in N-terminal and S protein, and IgG1 Fc in rats. The invention also discloses the vector containing the gene coding the said fusion protein. The invention also discloses a antibody Hepatitis B vaccine. The constructed antibody Hepatitis B vaccine may induce a strong humoral-mediated immune response and a cell-mediated immune response which has a better effect than the traditional vaccine.
Owner:FUDAN UNIV
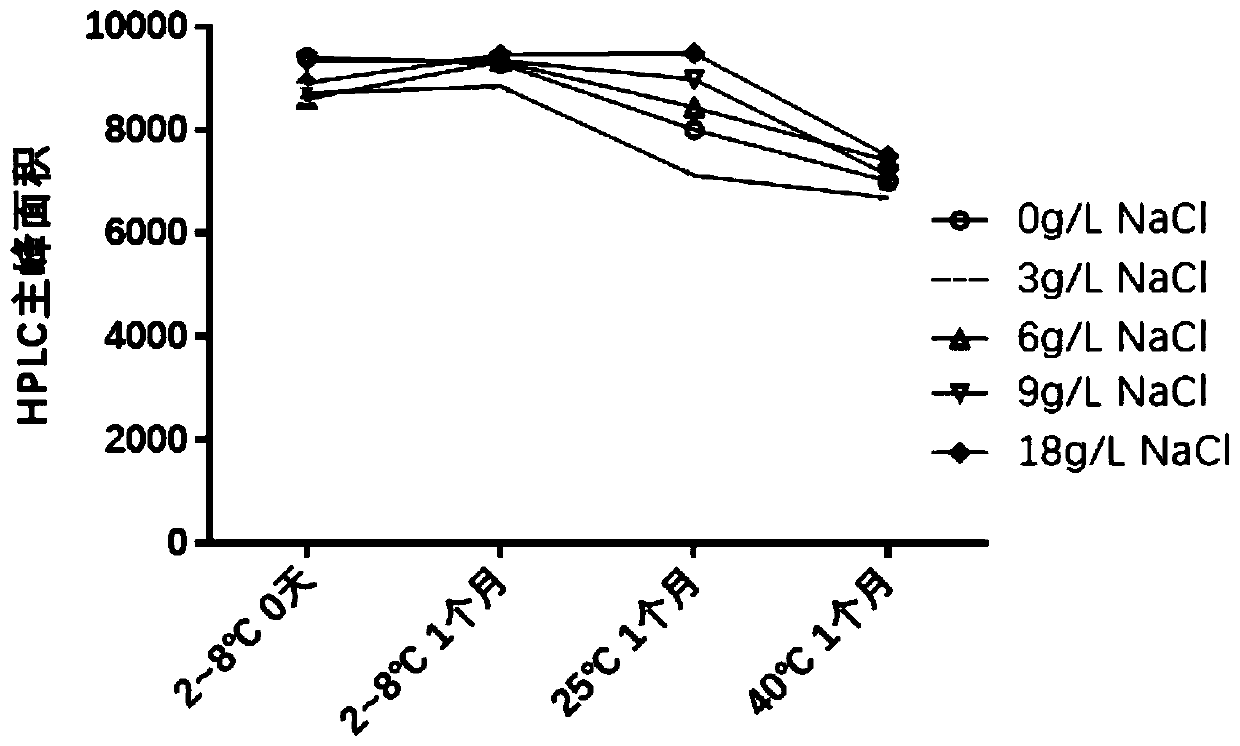
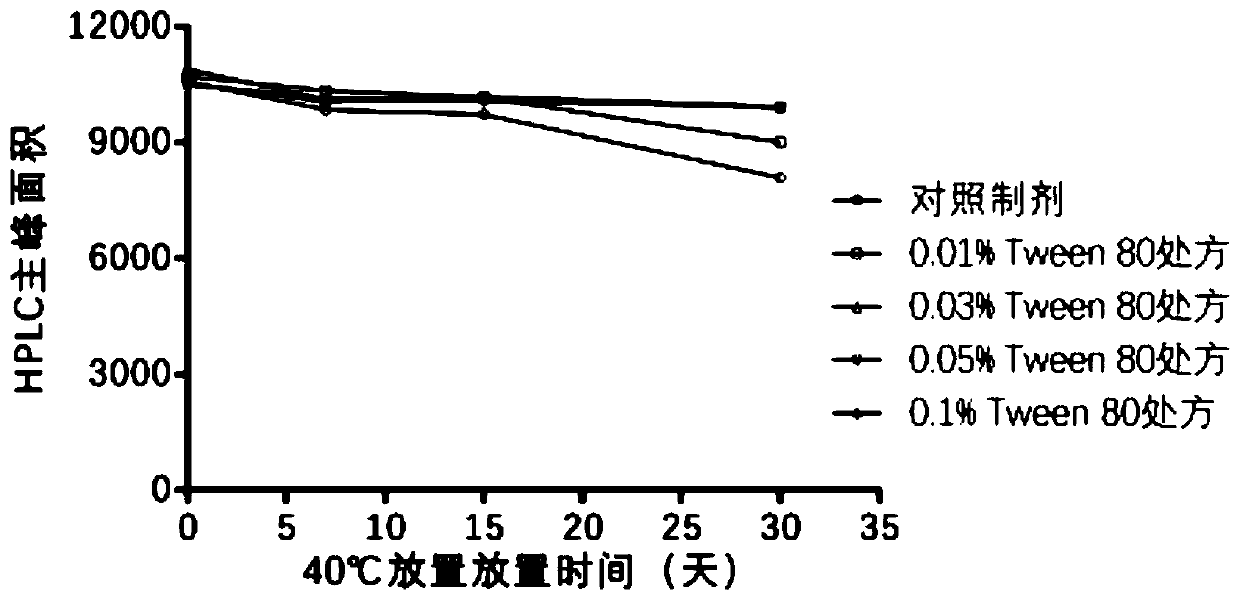
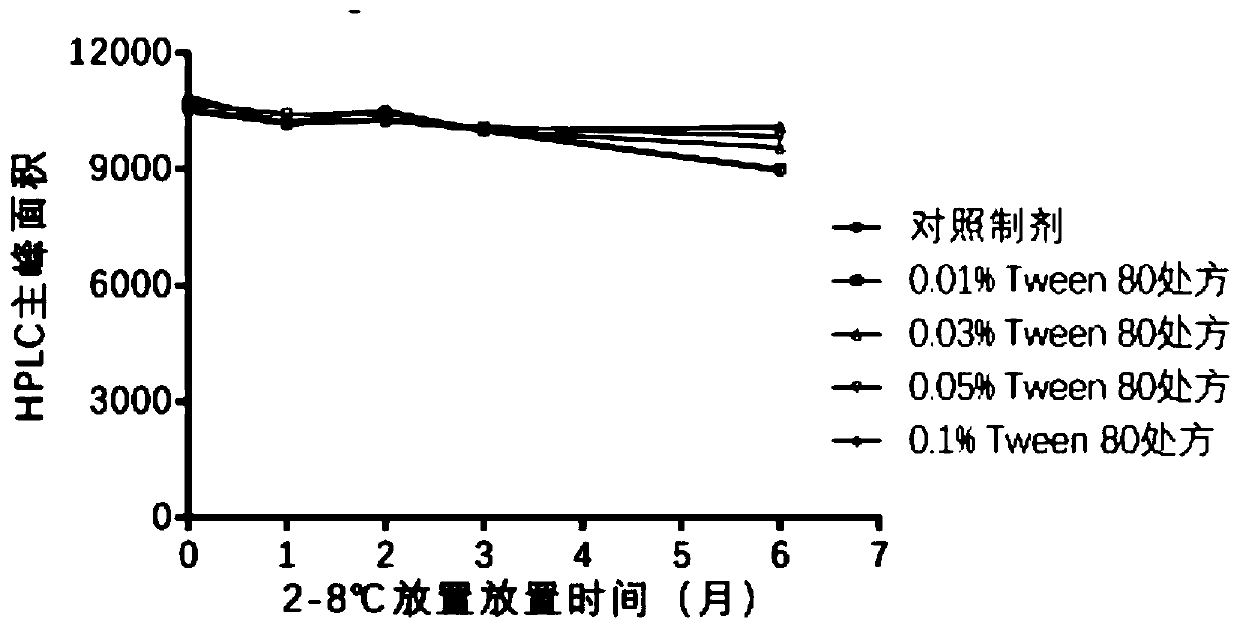
Analytical method of hepatitis B vaccine
PendingCN111840540AGuaranteed validityImprove inspection efficiencyViral antigen ingredientsDigestive systemAdjuvantA hepatitis b vaccine
The invention provides an analytical method of a hepatitis B vaccine. The analytical method comprises the following steps: (a) repeatedly freezing and thawing a hepatitis B vaccine sample at-15 DEG Cor below for at least one time, (b) mixing the freeze-thawed hepatitis B vaccine sample with hydrochloric acid in a volume ratio of 1: (1-2), and incubating at 37 DEG C for 1-3 hours, and (c) finally,adding a protective agent Triton100, and carrying out water bath for 1-3 hours at 37 DEG C to finish analysis. According to the present invention, the effective components in the hepatitis B vaccinecan be effectively separated from the adjuvant, such that the effective components can be accurately monitored through the immunoassay kit. Due to the fact that the analysis rate of previous analysisliquid is low, an existing method for determining the activity of the hepatitis B vaccine is an animal method, animal experiments can be effectively replaced after a new analysis mode is invented, andanimal cost is saved.
Owner:华北制药金坦生物技术股份有限公司
Hepatitis B vaccine
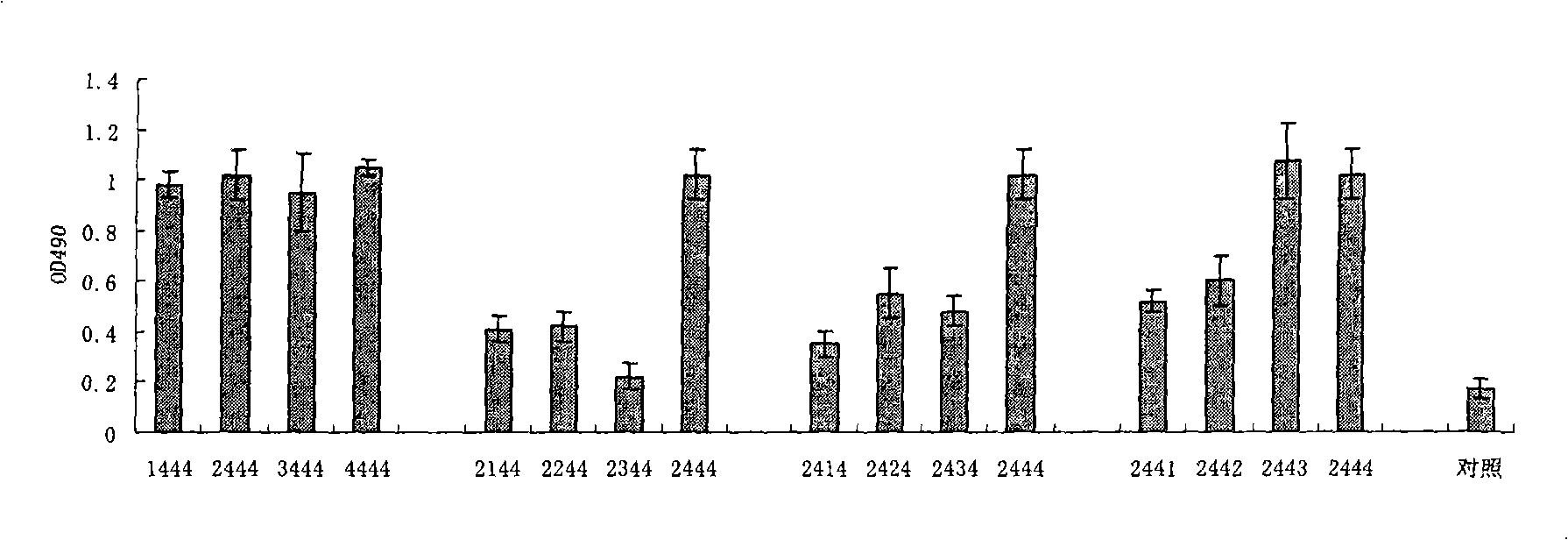
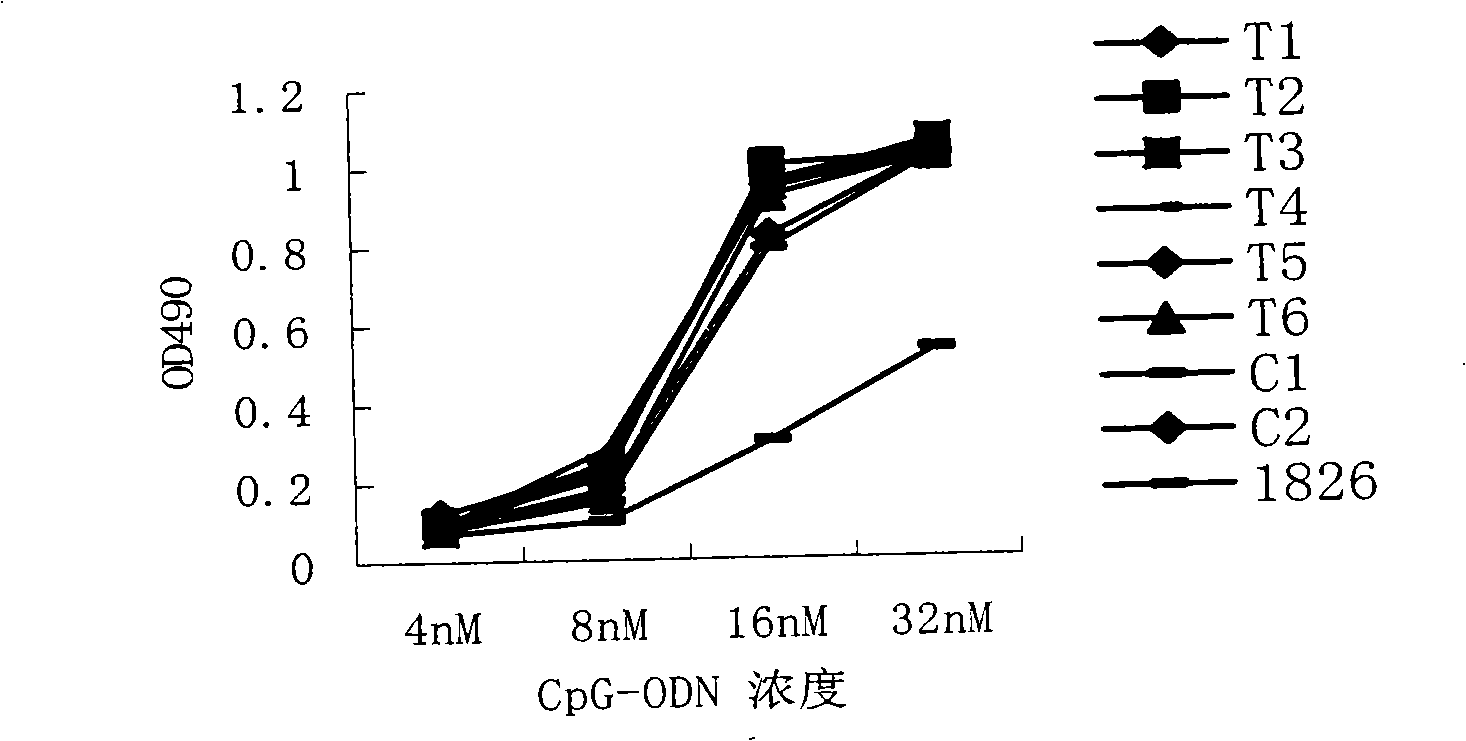
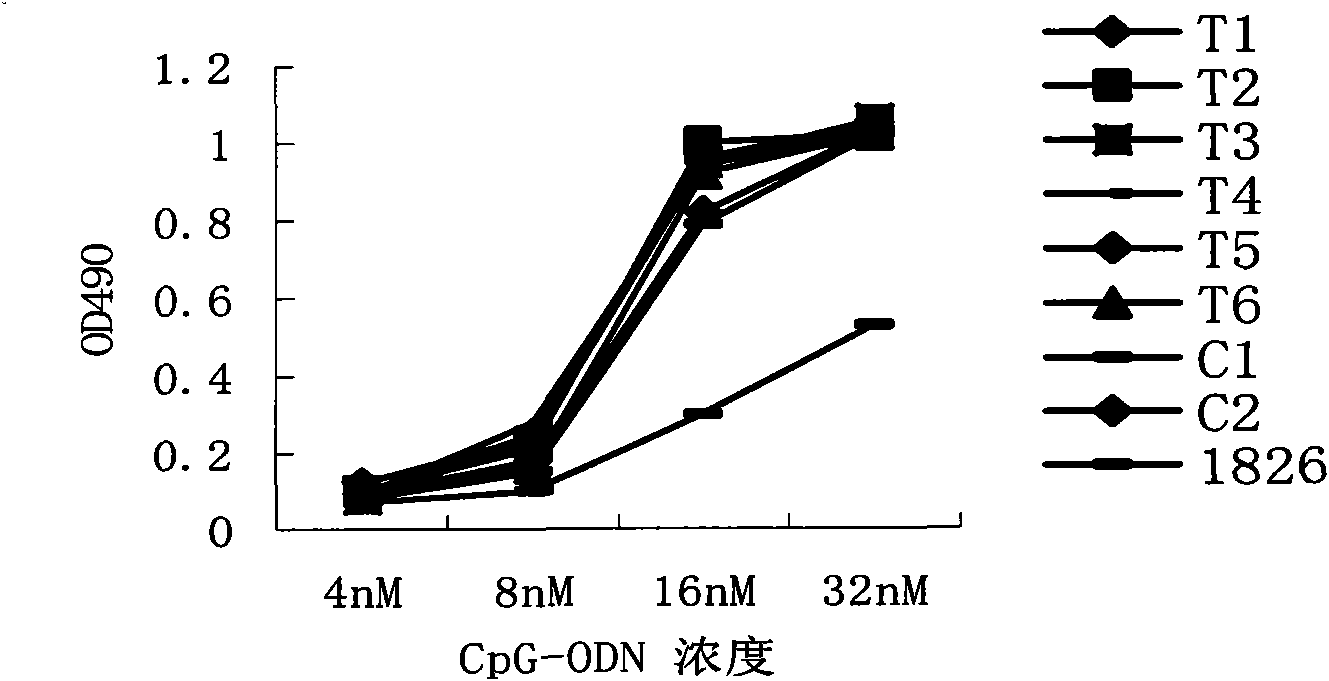
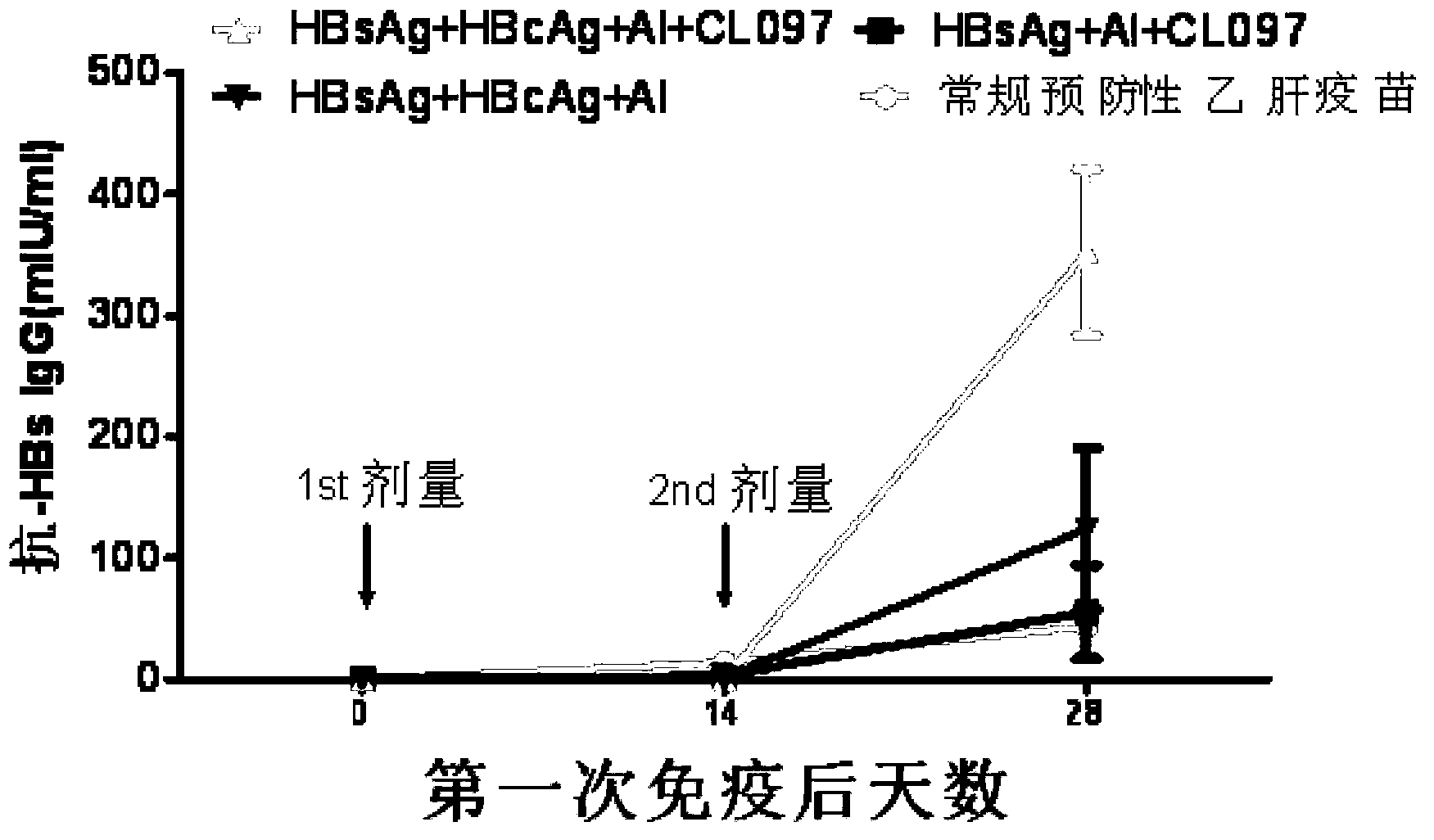
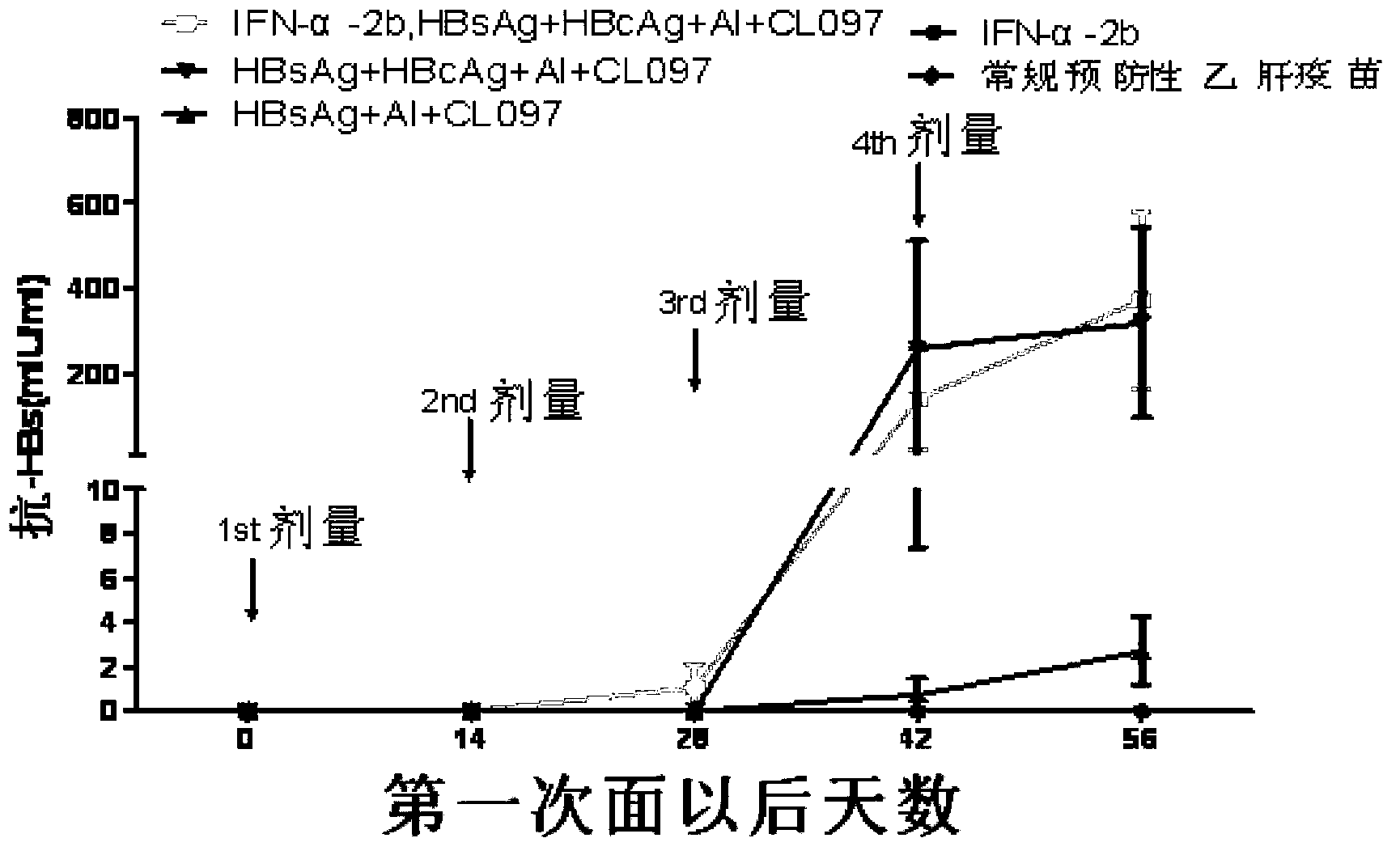
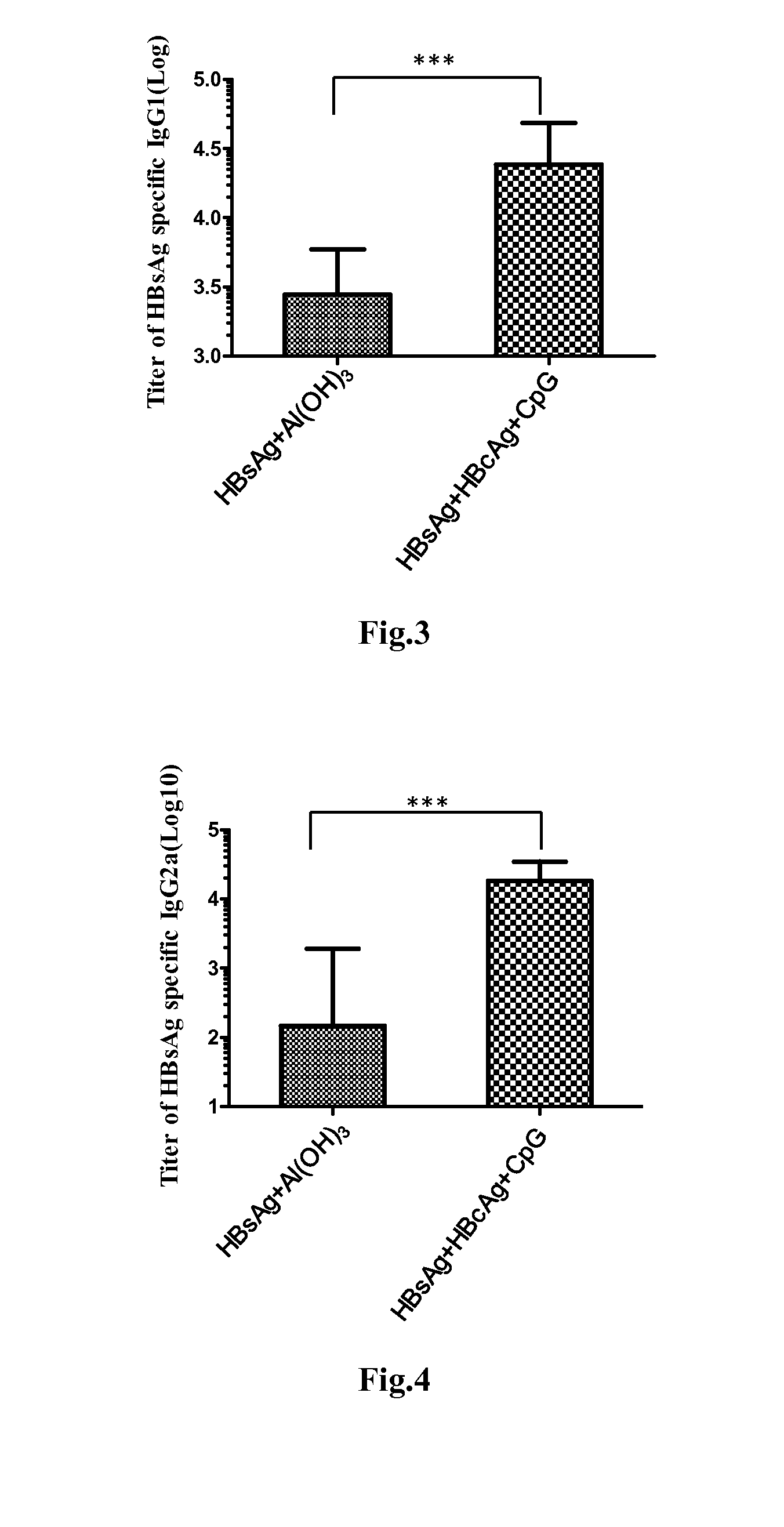
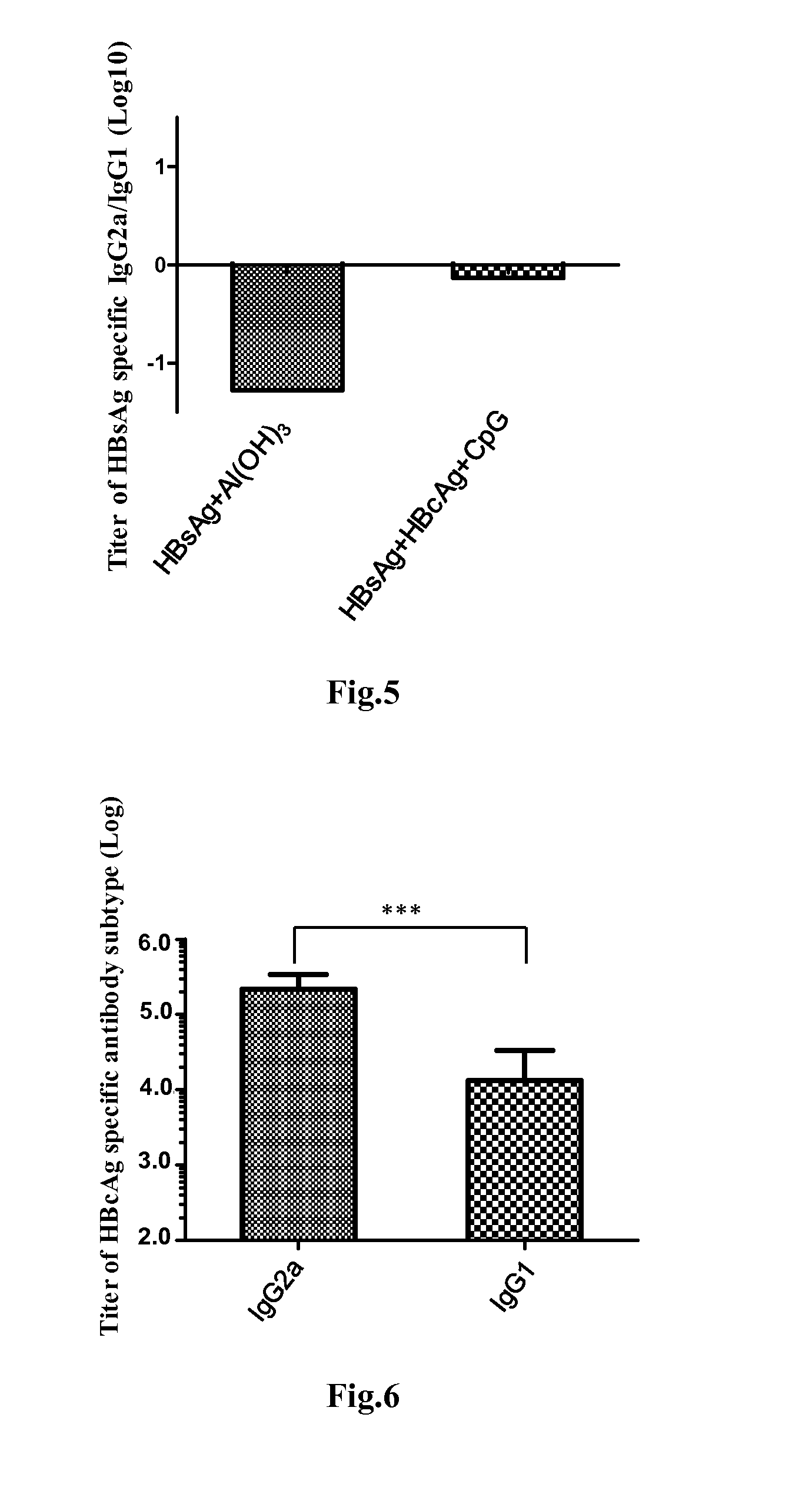
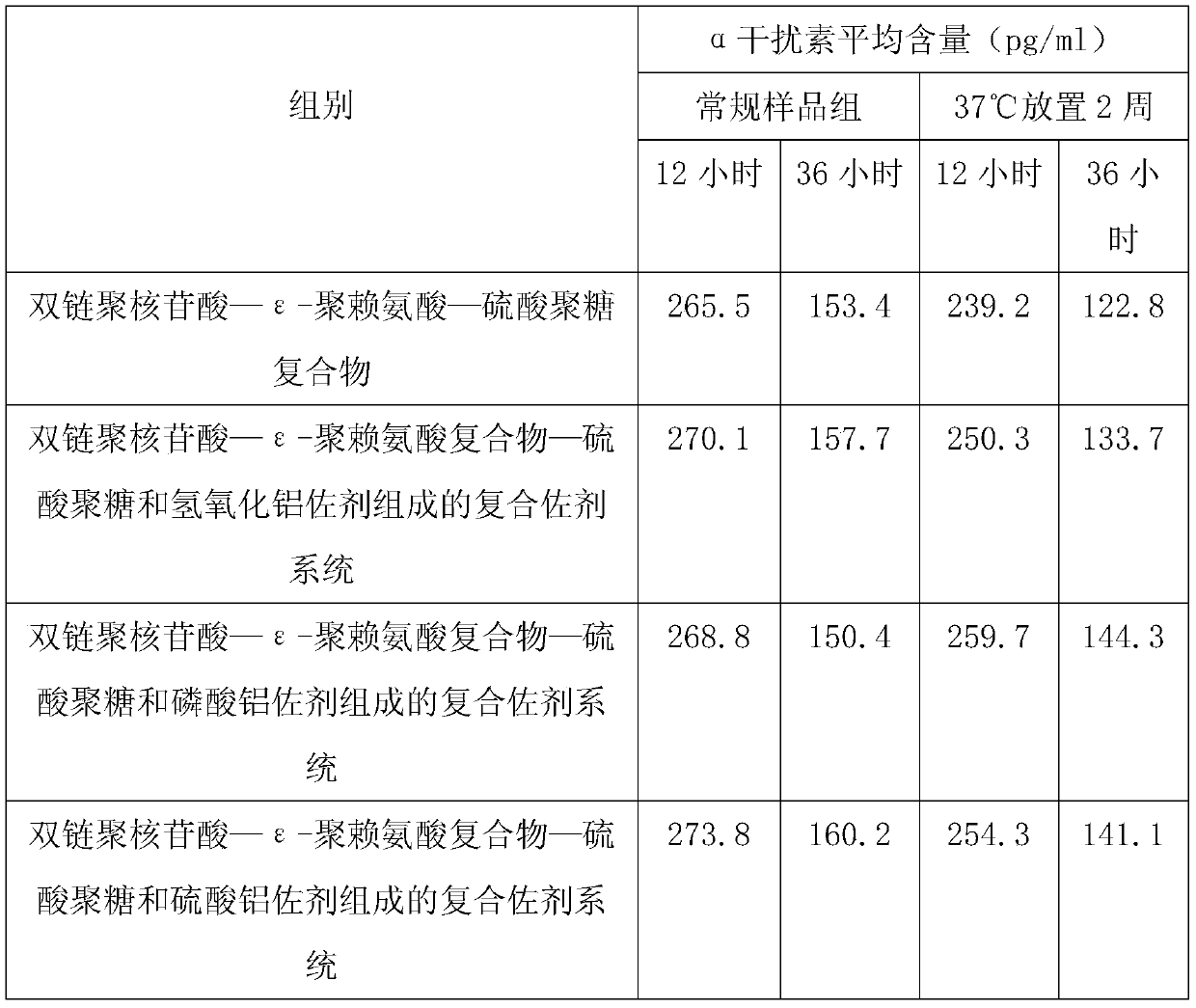
Disclosed is a composition comprising: i) HBsAg, a fragment thereof, a variant thereof, or the mixture of at least two of them, ii) HBcAg1-X, a fragment thereof, a variant of the antigen or the antigen fragment, or the mixture of at least two of them, wherein X is an integer of from 149 to 183, iii) CpG-ODN, 21 bases long, which is a phosphorothioate oligonucleotide and includes two or more copies of 5′-NTCGTT-3′ motifs. The use of the composition in the treatment of HBV-infection and HBV-induced diseases, and the therapy methods of HBV-infection and HBV-induced diseases are also disclosed.
Owner:JIANGSU THERAVAC BIO PHARMA CO LTD
Gene VII type newcastle disease marker vaccine strain as well as preparation method and application thereof
ActiveCN112111467AEliminate distractionsAchieving Differential DiagnosisSsRNA viruses negative-senseViral antigen ingredientsAvian paramyxovirusGenotype
The invention discloses a gene VII type Newcastle disease marker vaccine strain as well as a preparation method and application thereof. A reverse genetic manipulation technology is utilized, a wholegenome transcription plasmid of a serum type 2 avian paramyxovirus Y2 strain is used as a skeleton, extracellular domains of F and HN genes of the serum type 2 avian paramyxovirus Y2 strain are respectively replaced with extracellular domains of F and HN genes of a gene VII Newcastle disease virus strain, and a recombinant chimeric virus is obtained through virus rescue. The vaccine strain is preserved in China Center for Type Culture Collection (CCTCC), and the preservation number is CCTCC NO: 202052. The chimeric virus can be used for prevention and control of the main epidemic gene VII Newcastle disease in China. Meanwhile, the chimeric virus does not contain a Newcastle disease virus NP protein, and virus aiming at a Newcastle disease antibody NP protein cannot be generated after chickens are immunized. The rY2-HB vaccine strain immunized chicken flocks are subjected to antibody detection by a conventional Newcastle disease virus NP antibody ELISA detection method, NP antibody generated by clinical wild strains can be specifically detected, vaccine interference generated by antibody strains is eliminated, and monitoring and purification of Newcastle disease are facilitated.
Owner:INST OF ANIMAL SCI & VETERINARY HUBEI ACADEMY OF AGRI SCI
Hepatitis b vaccine
Disclosed is a composition comprising: i) HBsAg, a fragment thereof, a variant thereof, or the mixture of at least two of them, ii) HBcAg1-X, a fragment thereof, a variant of the antigen or the antigen fragment, or the mixture of at least two of them, wherein X is an integer of from 149 to 183, iii) CpG-ODN, 21 bases long, which is a phosphorothioate oligonucleotide and includes two or more copies of 5′-NTCGTT-3′ motifs. The use of the composition in the treatment of HBV-infection and HBV-induced diseases, and the therapy methods of HBV-infection and HBV-induced diseases are also disclosed.
Owner:JIANGSU THERAVAC BIO PHARMA
Vaccine composite adjuvant system and applications thereof in antigens
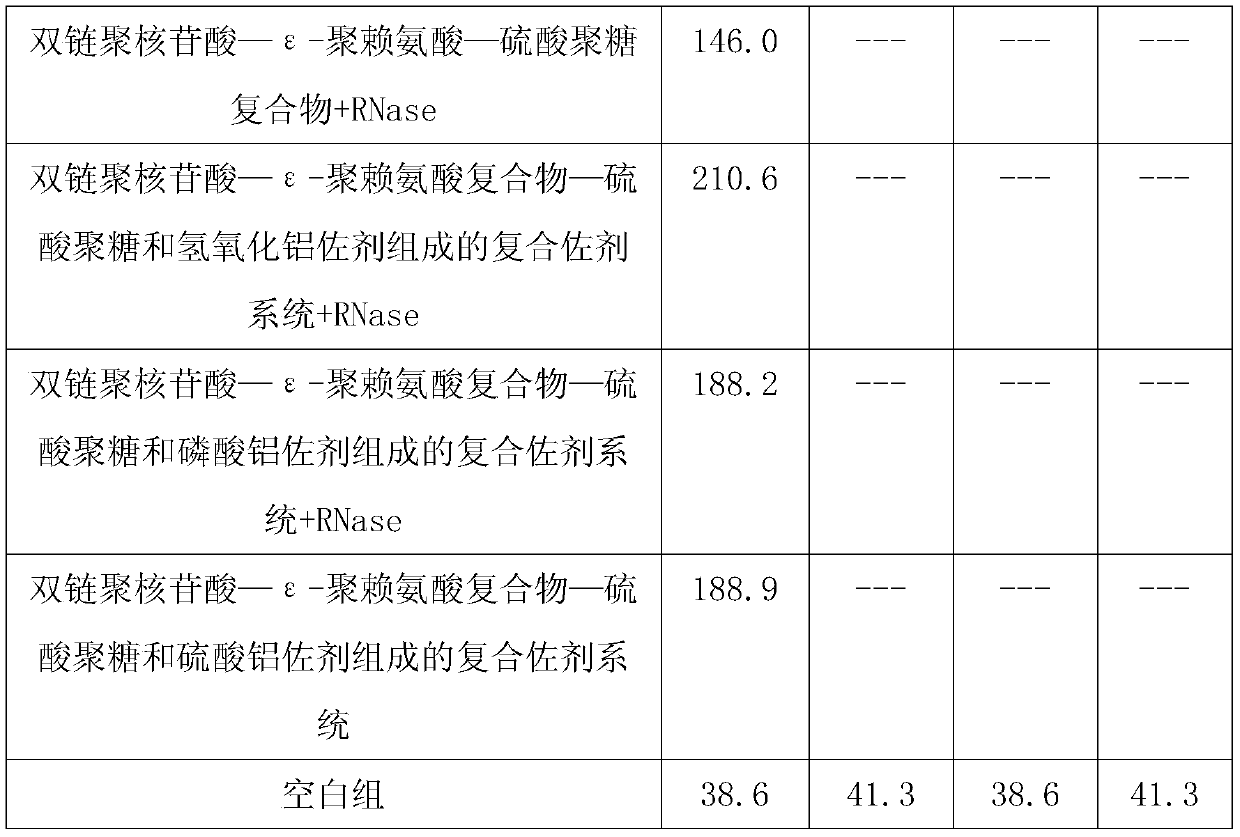
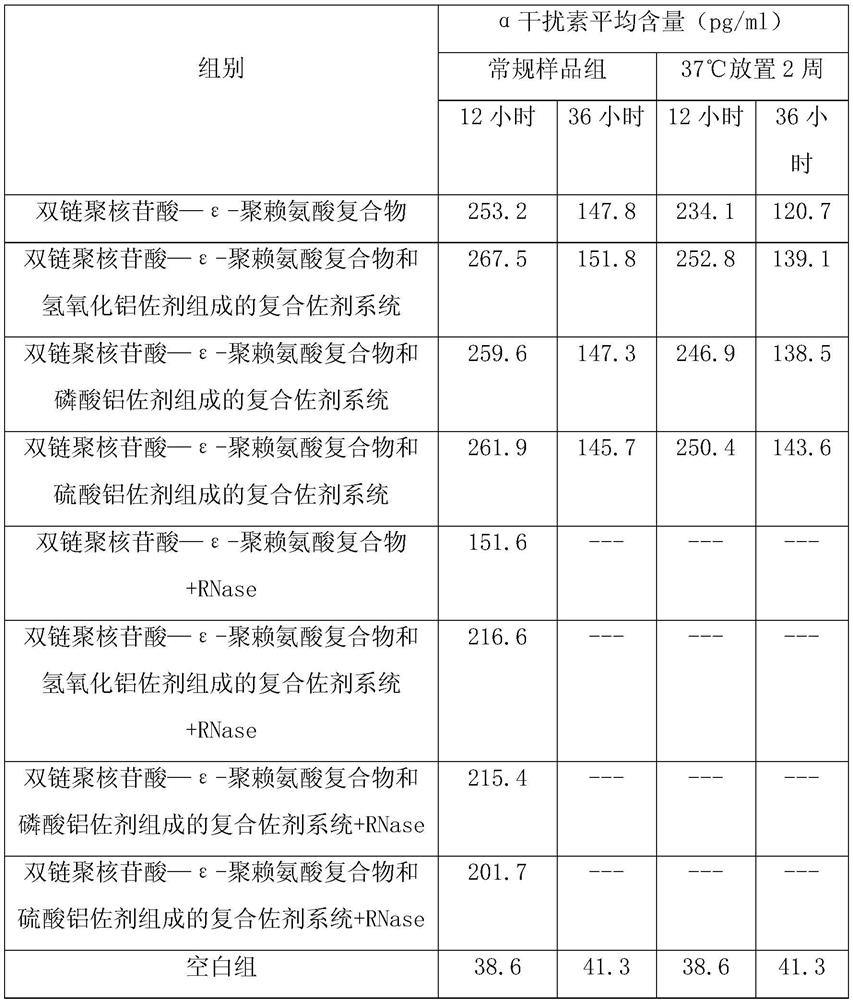
ActiveCN109701011AStrong hydrolysis abilityImprove stabilityImmunological disordersAntibody medical ingredientsSolventDouble stranded
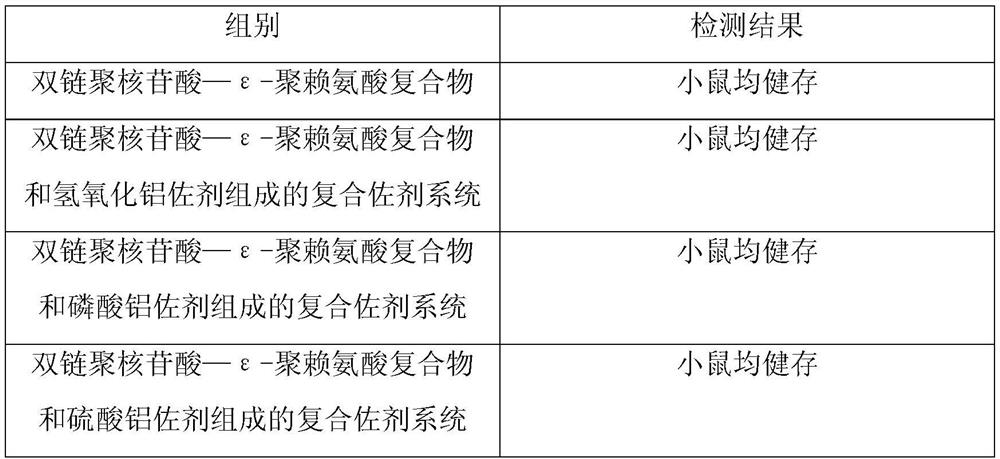
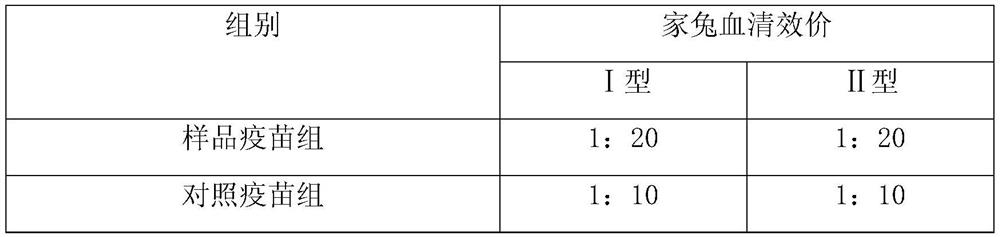
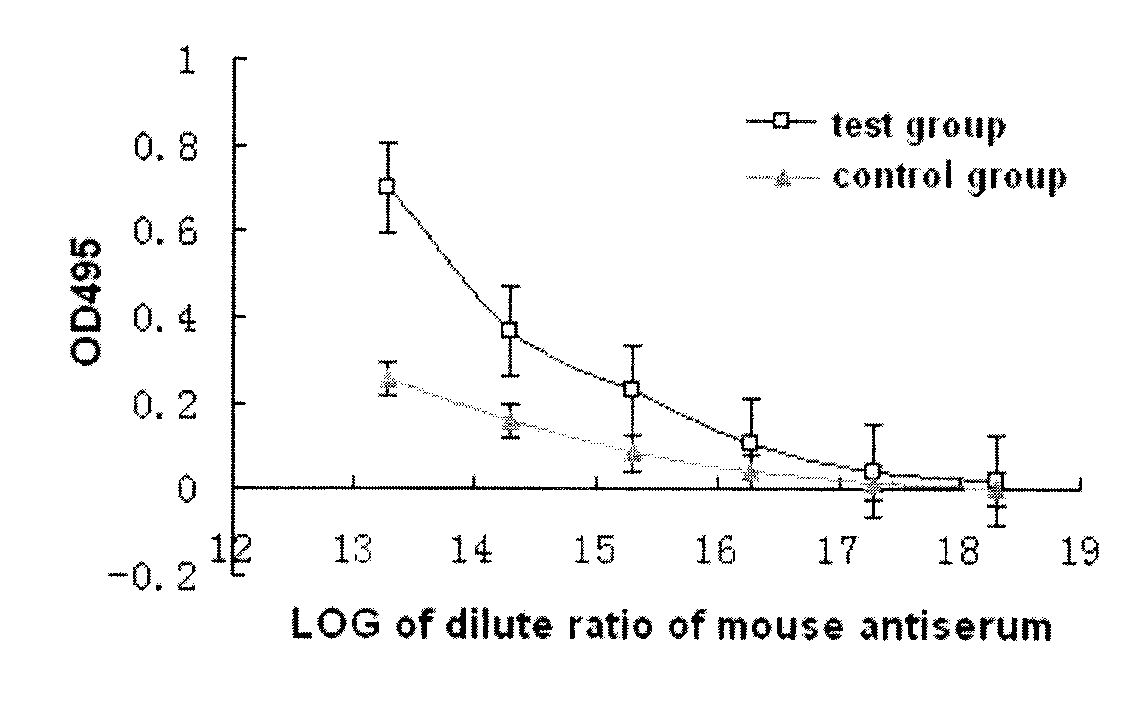
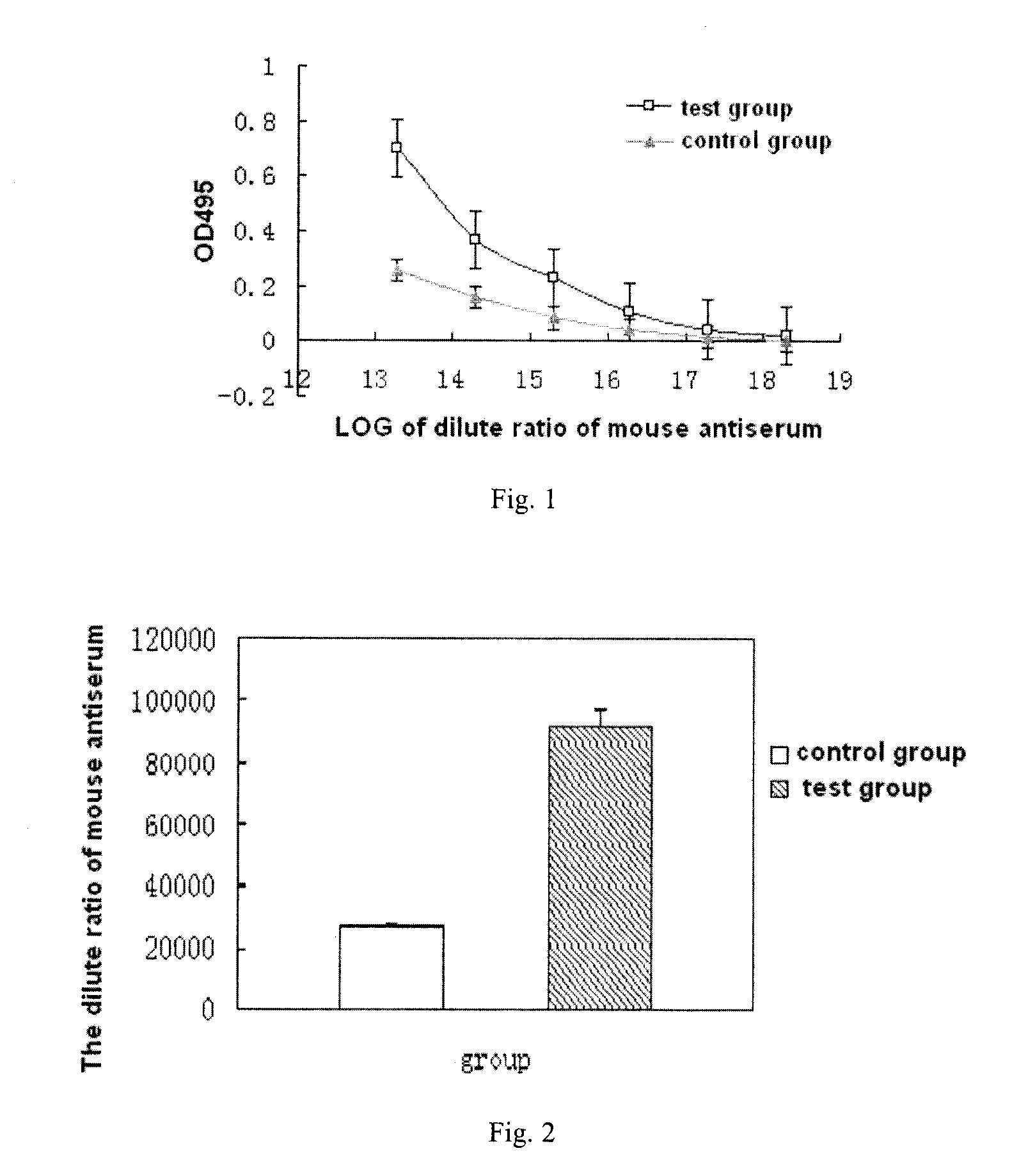
The present invention provides a vaccine composite adjuvant system and applications thereof in antigens. The system is composed of an aluminum adjuvant, a double-stranded polynucleotide-epsilon-polylysine-sulfuric acid glycan compound and an aqueous solvent, wherein the clinical use concentration of the aluminum adjuvant is 0.1-10.0 mg / ml, and the clinical use concentration of the double-strandedpolynucleotide-epsilon-polylysine-sulfuric acid glycan compound is 100-10000 [mu]g / ml by double-stranded polynucleotide. The formed compound adjuvant system has good anti-RNase hydrolysis ability, stability, safety and immune stimulating activity, and can significantly improve immunogenicity of vaccine when combined with different forms of vaccines such as hepatitis B vaccine and inactivated rabies vaccine.
Owner:辽科生物(沈阳)有限公司
Hepatitis B vaccine agonist composition and application thereof
ActiveCN105194666AImprove antigen presentation functionRaise the level of immune responseAntiviralsAntibody medical ingredientsNucleotideBiotin
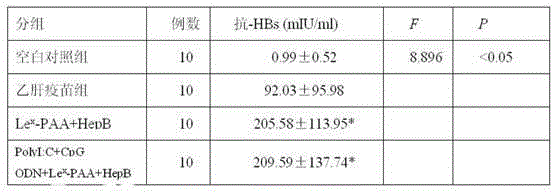
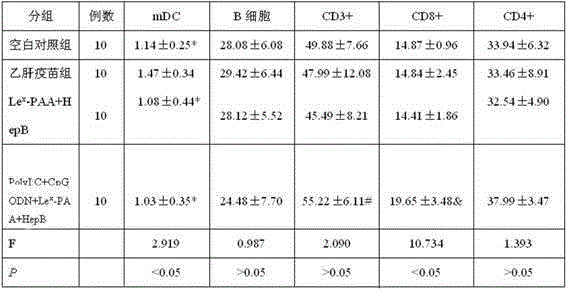
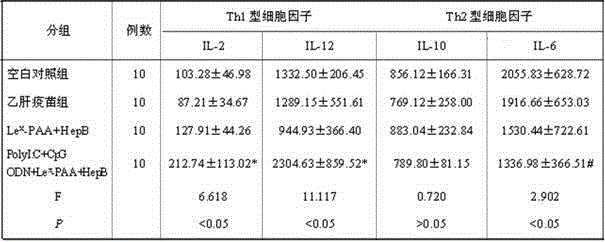
The invention discloses a hepatitis B vaccine agonist composition and application thereof, and relates to a hepatitis B vaccine. The hepatitis B vaccine agonist composition comprises Lewisx-Polyacrylamide-biotin, polyinosinic acid-polycytidylic acid, and oligodeoxynucleotide, wherein the component of the Lewisx-Polyacrylamide-biotin is 30 kd polyacrylamide containing 5 mol% of biotin and 20 mol% of a carbohydrate; the sequence of the oligodeoxynucleotide is 5'-tcgacgttcgtcgttcgtcgttc-3', and the whole process is subjected to phosphorothioate modification. Through agonists of three receptors DC-SIGN, TLR3 and TLR9, high expression of corresponding acceptors are stimulated, so that the antigen presenting function of DC is improved, and Th1 immune response of a body is promoted.
Owner:SHANXI MEDICAL UNIV
Pharmaceutical composition for treating hepatitis B, and preparation method and application thereof
PendingCN111420043AMaintain antigen stabilitySimple compositionViral antigen ingredientsDigestive systemAntigenAdjuvant
Owner:JIANGSU THERAVAC BIO PHARMA CO LTD
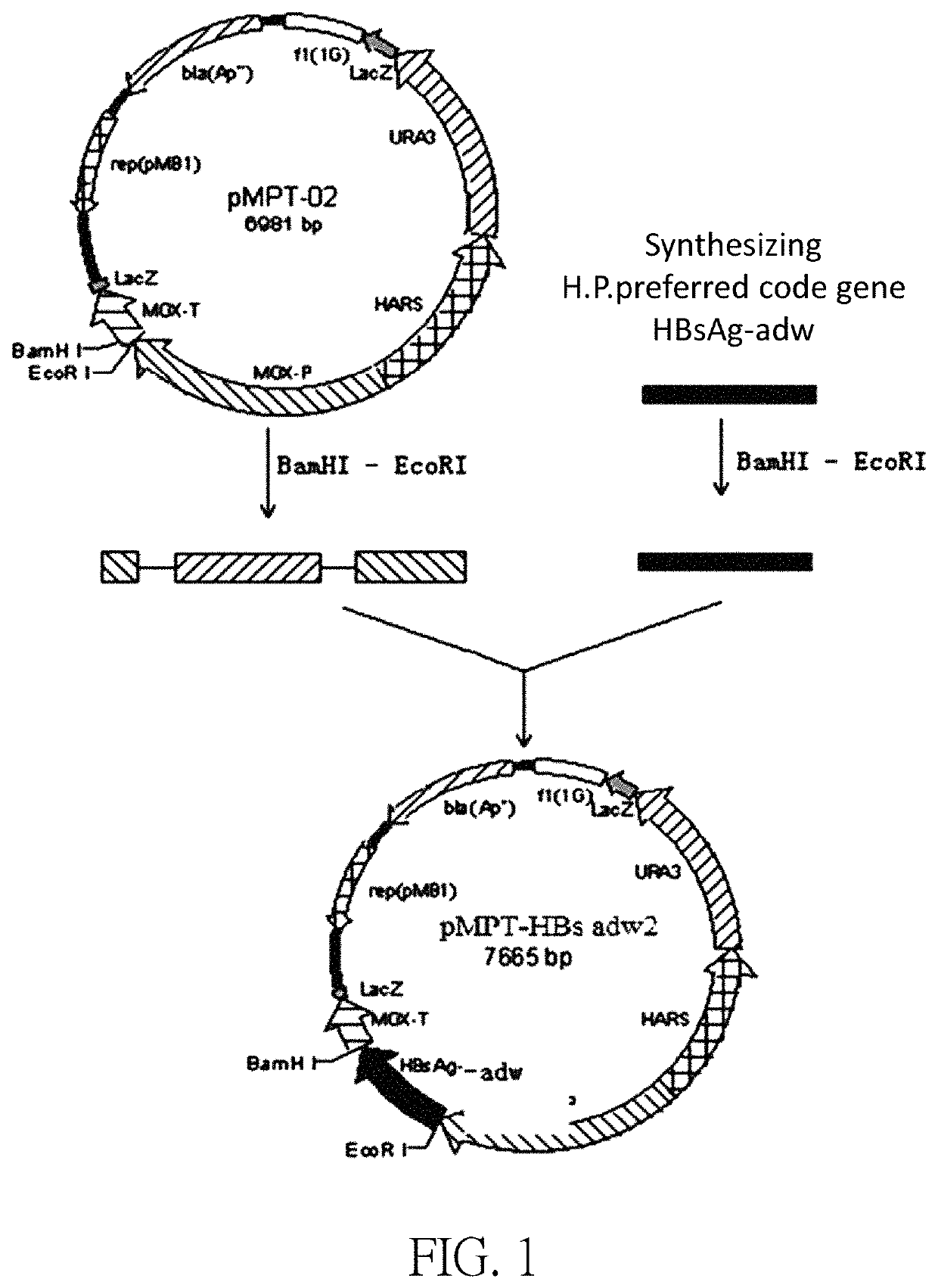
Recombinant Hansenula polymorpha-based high dosage hepatitis B vaccine
ActiveUS10821174B2High yieldQuality improvementViral antigen ingredientsMicroorganismsHepatitis B immunizationHansenula mrakii
Provided is a recombinant Hansenula polymorpha-based high dosage hepatitis B vaccine, an HBsAg pure stock solution yield of a recombinant Hansenula polymorpha fermentation broth used for producing the hepatitis B vaccine being 300 mg / L-400 mg / L.
Owner:TIANJIN HEMU JIANMIN BIOTECHNOLOGY CO LTD
Medicine composition, kit and application thereof
ActiveCN100586474CBreak toleranceProtective functionPeptide/protein ingredientsViral antigen ingredientsMedicineImmune tolerance
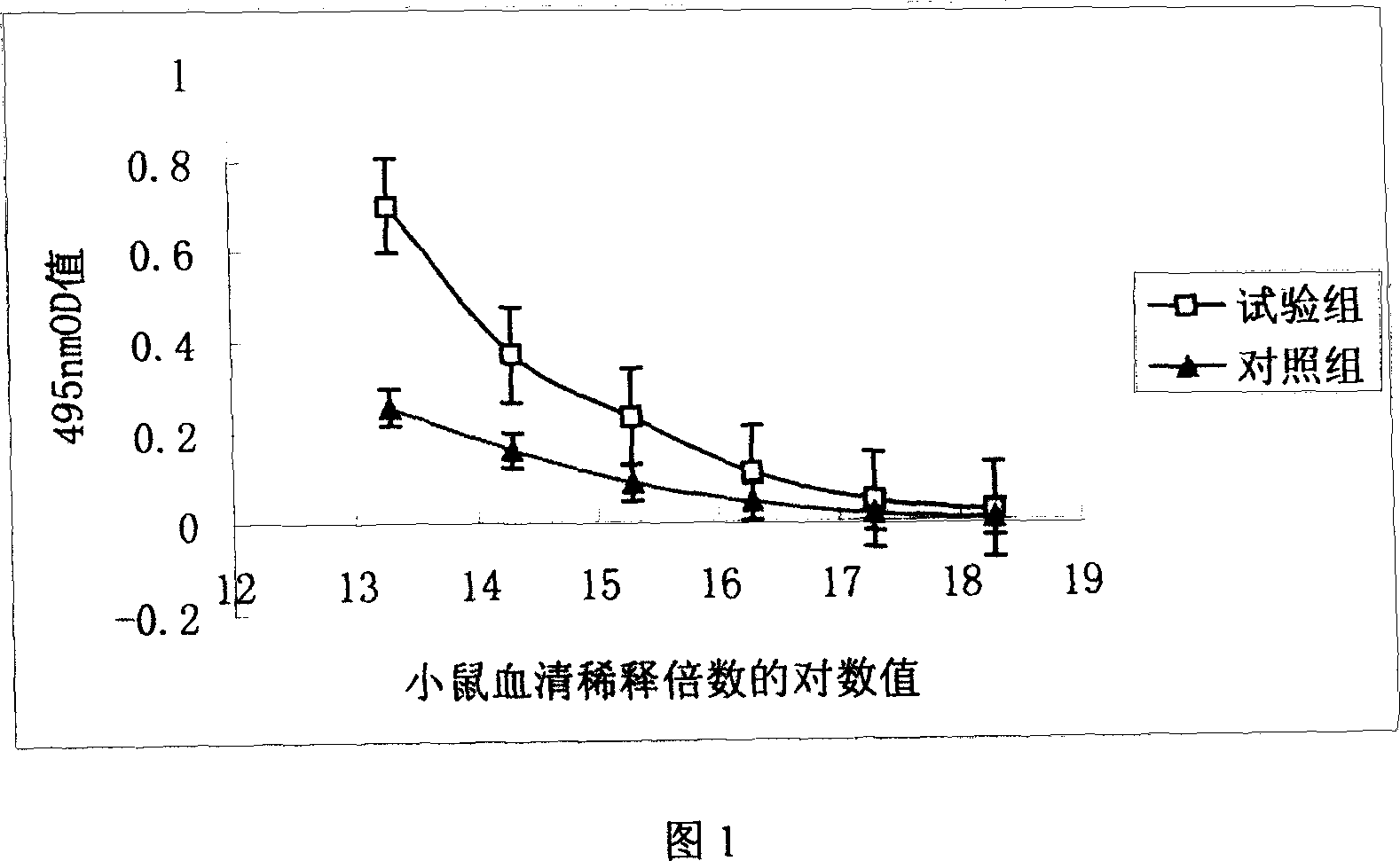
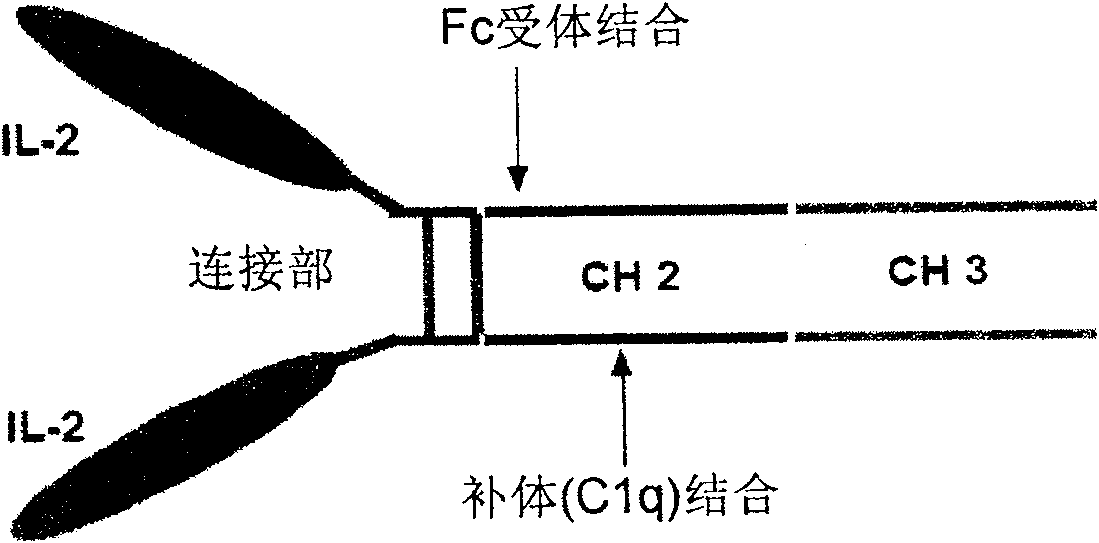
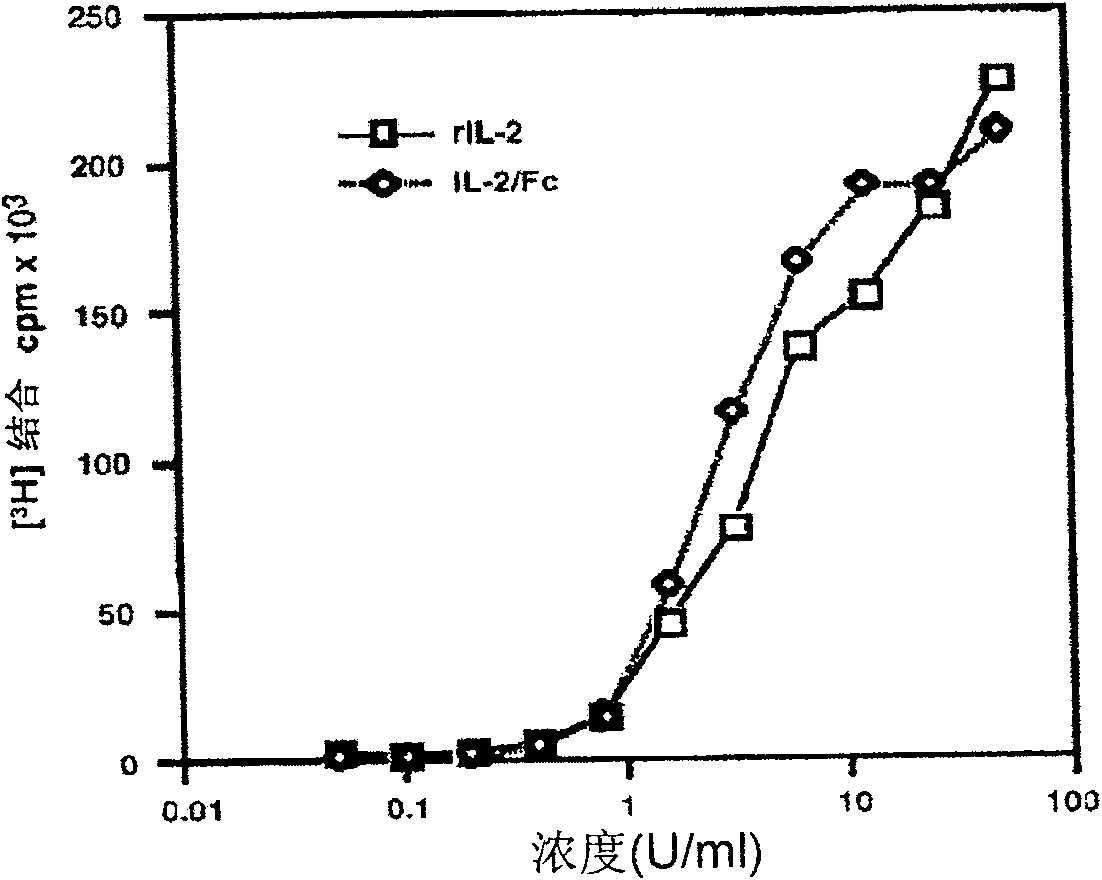
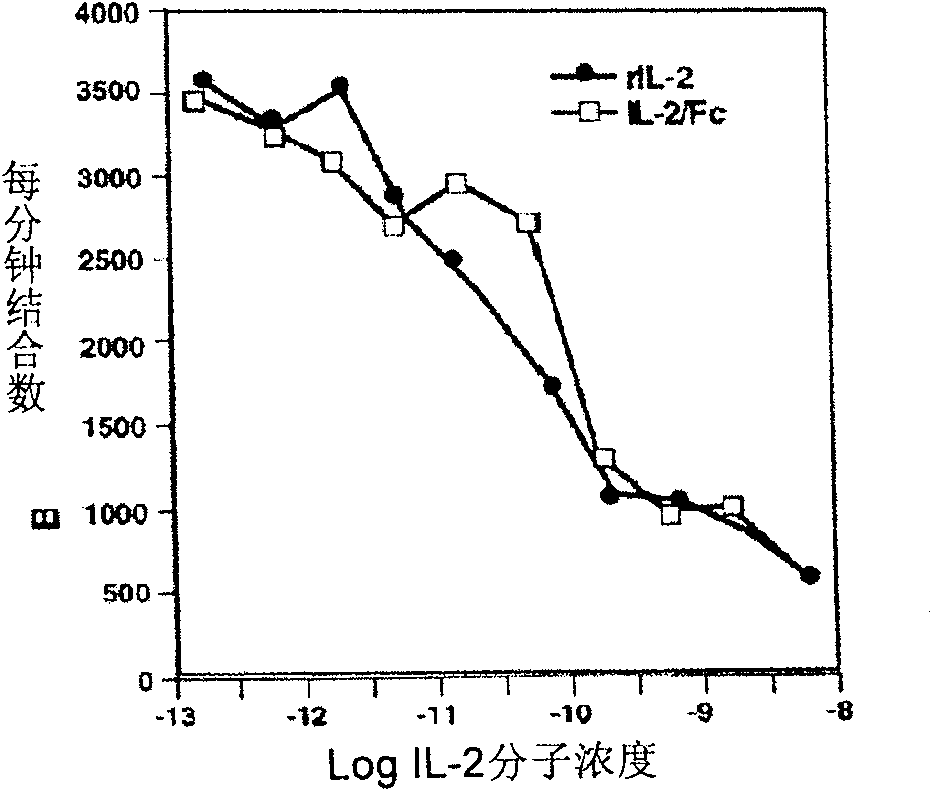
The present invention is a kind of medicine composition comprising cell factor fusion protein (IL-2 / Fc) and compound Chinese medicine 861, their medicine composition preparation and kit, and their application as immunity potentiator for strengthening HB vaccine immune response and breaking the immune tolerance of HBV.
Owner:江苏百英生物科技有限公司
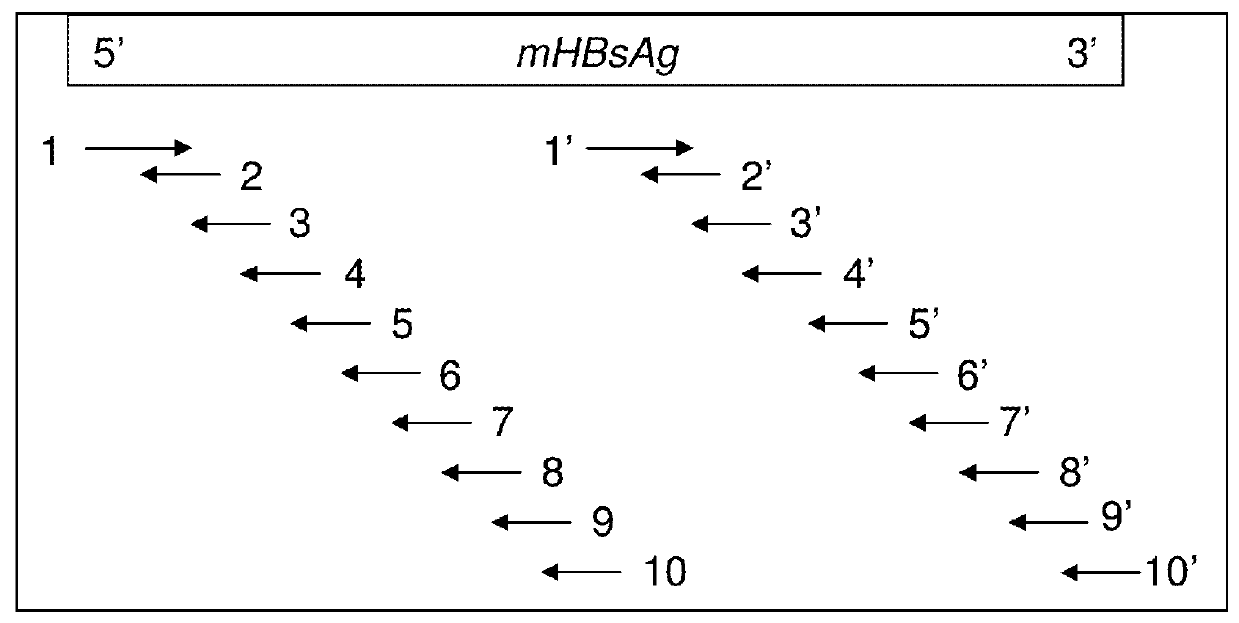
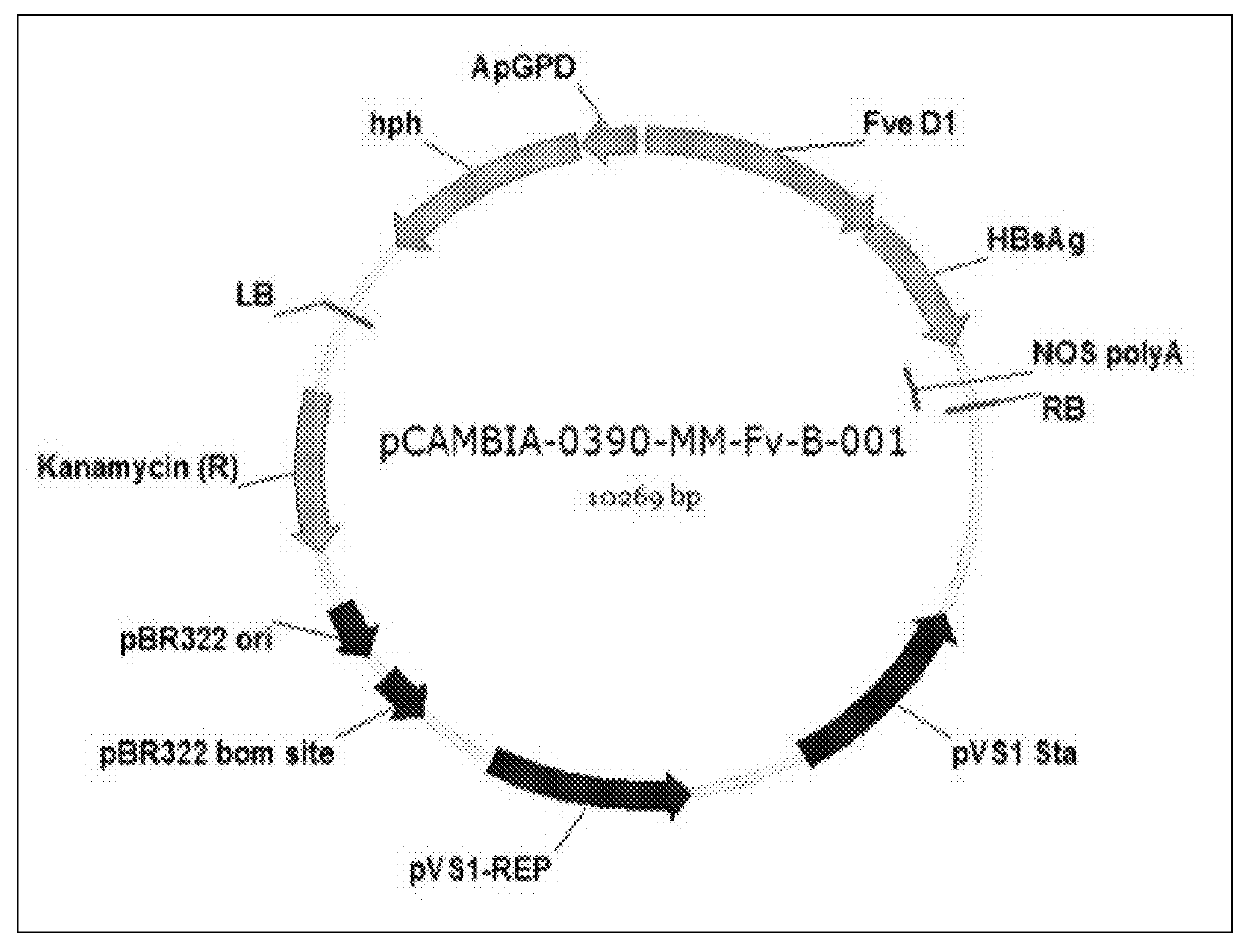
Recombinant polynucleotide and a transgenic flammulina velutipes carrying the same
The invention provides a recombinant polynucleotide comprising a truncated glyceraldehyde-3-phosphate dehydrogenase (gpd) promoter and a modified HBV S protein gene and a transgenic Flammulina velutipes carrying the recombinant polynucleotide. The invention surprisingly found that after administering the transgenic Flammulina velutipes to a subject, the subject can successfully generate an antibody against HBV. Therefore, the transgenic Flammulina velutipes can be used as a vaccine against HBV.
Owner:MYCOMAGIC BIOTECH
Bispecific antibody against hepatitis B surface protein and use thereof
ActiveCN105061590BAvoid infectionCombined Application ExcellenceImmunoglobulins against virusesAntiviralsHumanized antibodyImmunogenicity
The invention provides a bispecific antibody A3B5-BsAb for a hepatitis B surface protein. The bispecific antibody is formed by combining an antibody A3D5 with an antibody B5H6. The bispecific antibody has better HBV virus neutralization ability and HBsAg release inhibition ability than single use of the antibody A3D5 and the antibody B5H6, and has strong synergistic effects, so the bispecific antibody is likely to prevent HBV infection related hepatitis, hepatic cirrhosis and liver cancer. The bispecific antibody is a completely humanized antibody obtained through cloning HBsAg specific memory B cells in the peripheral blood of hepatitis B vaccine inoculated volunteer, has lower immunogenicity than murine, chimeric and humanized antibodies, and can be used to prepare hepatitis B virus related hepatopathy prevention or treatment drugs or diagnose reagents.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Application of virus immunotherapy drug compound in preparation of drugs for treating HBV infection
InactiveCN110859961AEnhance immune responseAvoid unresponsiveness andOrganic active ingredientsPeptide/protein ingredientsDiseasePharmaceutical Substances
The invention belongs to the field of biological medicines, and relates to a novel virus immunotherapy drug compound, in particular to an application of the virus immunotherapy medicine compound in preparation of a medicine for treating HBV infection, the virus immunotherapy medicine compound is composed of an antiviral medicine, an immunomodulatory medicine and a recombinant hepatitis B vaccine,virus replication is inhibited through the antiviral medicine, and then immunization is conducted in the mode of adding the immunomodulatory agent and the vaccine. An effective immune memory protection reaction is established for organisms, strong antibody protection and cellular immunity are generated, virus recurrence is prevented, a purpose of removing viruses is even achieved, and HBV reinfection is prevented. Compared with an existing antiviral immune medicine, the novel virus immunotherapy drug compound is safe and effective and can be used for clinically treating virus infection diseases.
Owner:FUDAN UNIV
Application of 3'-deoxyadenosine to preparation of immunologic adjuvant for synergizing broad-spectrum vaccine
The invention belongs to the field of biotechnology, and particularly relates to an immunologic adjuvant and a preparation method thereof. The invention provides a synergistic effect of innate immune synergizing molecular 3'-deoxyadenosine or a mixture of 3'-deoxyadenosine and MHC epitope peptide or covalent conjugates on vaccines and antigens. The immunologic adjuvant can effectively enhance the immune effect of various vaccines (antigens), such as rabies vaccines, Hepatitis B vaccines, tuberculosis vaccines, various antigens and the like, obviously increase the titer of protective antibodies in animal bodies and the number and the functions of immune effector cells, and simultaneously accelerate the formation of the antibodies and cells obviously. The preparation method for the immunologic adjuvant is easy to implement, and can be used for developing high-efficient and quick synergistic vaccines. Therefore, the preparation method has excellent application prospect, and will bring huge social effect and economic benefits.
Owner:FUDAN UNIV
Dendritic cell obtained by antigen pulsing
It is intended to provide a dendritic cell efficacious in preventing and treating diseases, mainly hepatitis B. A dendritic cell capable of presenting an HBs antigen, which is obtained by a method involving the step of pulsing a dendritic cell with the HBs antigen, is efficacious in preventing and treating hepatitis B. As the HBs antigen, use can be made of an HBs antigen contained in an HB vaccine. A vaccine-pulsed dendritic cell, which is obtained by a method involving the step of pulsing a dendritic cell with a vaccine, is usable not only for patients but also for healthy subjects. As the vaccine to be used in the pulsing, various types of vaccines can be employed.
Owner:博傲西腾医疗科技(上海)有限公司 +1
Vaccine compound adjuvant system and its application in antigen
ActiveCN109701010BStrong hydrolysis abilityImprove stabilityAntiviralsImmunological disordersAdjuvantNucleotide
The invention provides a vaccine compound adjuvant system and its application in antigen. It is composed of aluminum adjuvant, double-stranded polynucleotide-ε-polylysine complex and aqueous solvent, wherein the clinical use concentration of aluminum adjuvant is 0.1-10.0mg / ml, double-stranded polynucleotide- The clinical use concentration of ε‑polylysine complex is 100‑10000 μg / ml in terms of double-stranded polynucleotide. The compound adjuvant system formed has good anti-RNase hydrolysis ability, stability, safety and immunostimulatory activity, and combined application with different forms of vaccines such as hepatitis B vaccine and inactivated rabies vaccine can significantly improve the immunogenicity of the vaccine .
Owner:北京加益维科生物科技有限公司
Enhancin of hepatitis b virus vaccine and its gene
ActiveUS20100055135A1Improving immunogenicityEnhance immune responsePeptide/protein ingredientsLibrary screeningCDNA librarySAA protein
This invention relates to a HB vaccine enhancing protein, its gene, gene engineering method for expressing this protein, and the application of this method. The cDNA of this protein, which is screened out from human liver cDNA library, is sequenced and then cloned into prokaryotic or eukaryotic (animal or plant) cell for expression of protein coded by the cDNA (for example, cloning into prokaryotic expression carrier and expression in E. coli) and purification of the protein. The protein obtained, when used with HB vaccine, can significantly increase the effect of the vaccine, the immune power of HBV carrier, and the titer of antibody. The protein can be used as an adjutant to HB vaccine.
Owner:FUDAN UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com