Pharmaceutical composition for treating hepatitis B, and preparation method and application thereof
A technology of hepatitis B and hepatitis B virus, applied in the field of biomedicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] The preparation of each component of embodiment 1 therapeutic hepatitis B pharmaceutical composition
[0040] 1. Preparation of HBsAg stock solution:
[0041] The amino acid sequence of the HBsAg protein is shown in SEQ ID NO.1.
[0042] The HBsAg protein is prepared by HBsAg gene recombinant yeast cells, and the yeast cell types include Hansenula, Saccharomyces and Pichia, preferably Hansenula. For the specific preparation steps, refer to the Chinese patent application CN108330145A, the HBsAg gene recombinant Hansenula cells are fermented and cultured, and the cells are harvested. The HBsAg stock solution was obtained by bacteria-breaking treatment, silica gel adsorption, column chromatography and TFF purification.
[0043] 2. Preparation of HBcAg stock solution:
[0044] The amino acid sequence of the HBcAg protein is shown in SEQ ID NO.2.
[0045] The HBcAg protein is prepared by HBcAg gene recombinant yeast cells, and the yeast cell types include Hansenula, Sacc...
Embodiment 2
[0050] The preparation of embodiment 2 therapeutic hepatitis B pharmaceutical composition
[0051] 1. PBS buffer solution: Na 2 HPO 4 12H 2 O 2.0g, NaH 2 PO 4 2H 2 O 2.4g, NaCl 9g, add water to dissolve and dilute to 1000ml, adjust the pH value to 7.20 with hydrochloric acid or sodium hydroxide;
[0052] 2. 0.5% polysorbate 80 (Tween 80) solution: weigh 5.0 g of Tween 80 and dilute to 1000 ml with PBS buffer solution;
[0053] 3. HBsAg solution: get the HBsAg stock solution (prepared in Example 1), add 0.5% Tween 80 solution, and dilute the concentration of Tween 80 to 0.05% with PBS buffer solution;
[0054] 4. HBcAg solution: take the HBcAg stock solution (prepared in Example 1), add 0.5% Tween 80 solution, and dilute the concentration of Tween 80 to 0.05% with PBS buffer solution;
[0055] 5. CpG solution: take CpG (prepared in Example 1) and dissolve and dilute it with PBS buffer solution to obtain a CpG stock solution, add 0.5% Tween 80 solution, and dilute the c...
Embodiment 3
[0057] The screening test of embodiment 3 therapeutic hepatitis B pharmaceutical composition prescription
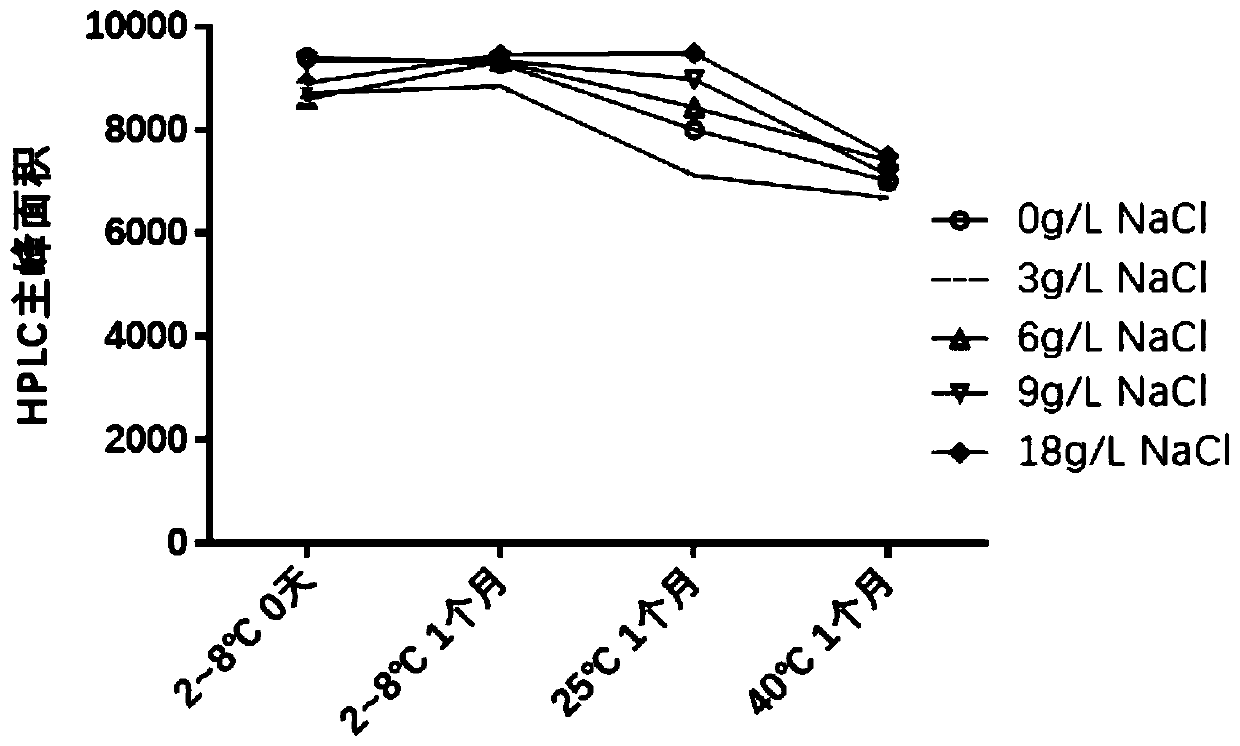
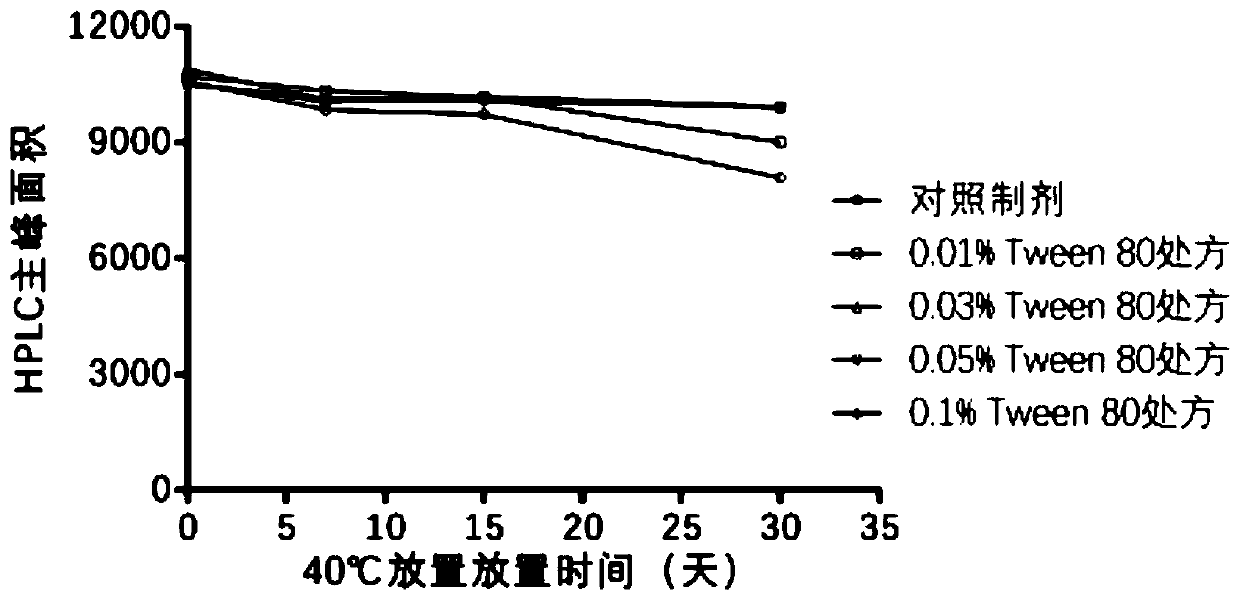
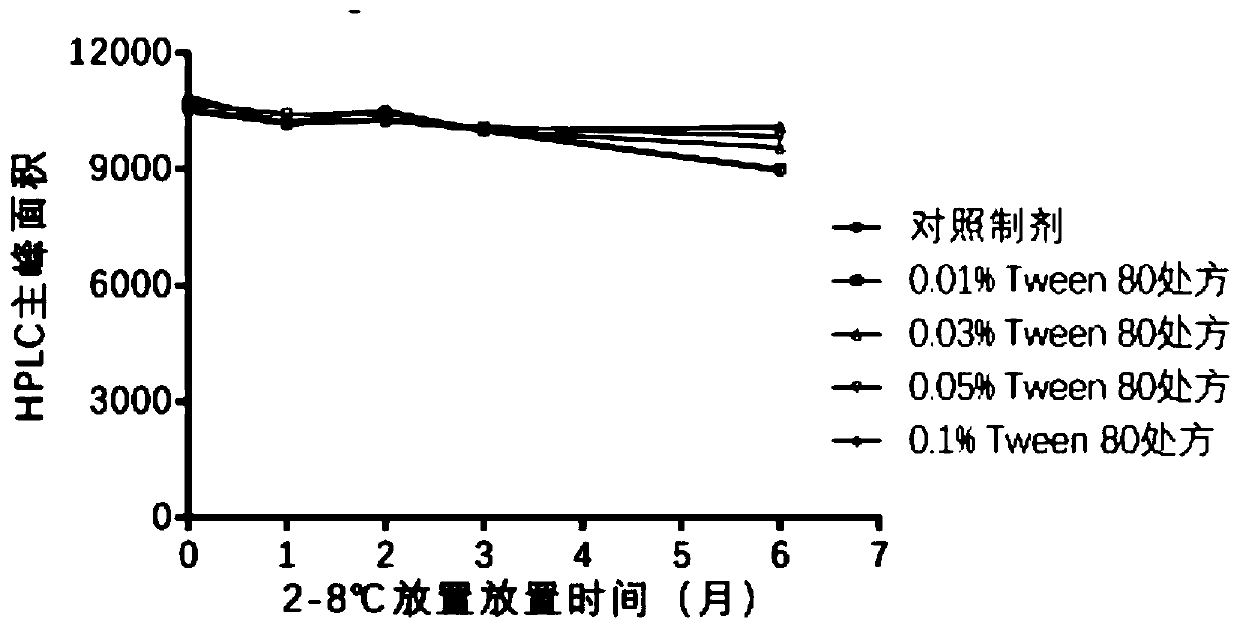
[0058] Among the three components of HBsAg, HBcAg and CpG, HBsAg was most affected by various factors and had the worst stability. Therefore, HBsAg is used as an index to screen prescriptions.
[0059] HBsAg mainly exists in the form of virus-like particles (hereinafter referred to as VLP), and the area of the main peak detected by HPLC represents the amount of VLP in the solution. Therefore, a decrease in the area of the main peak represents a decrease in the amount of HBsAg in the VLP form in solution.
[0060] 1) Screening of buffer system
[0061] Solution preparation:
[0062] 1.0.5% polysorbate 80 (Tween 80) solution is made by embodiment 2;
[0063] 2. Histidine buffer solution: histidine 1.55g, histidine hydrochloride 2.095g, add water to dissolve and dilute to 1000ml.
[0064] 3. HBsAg solution (PBS buffer solution): Take the HBsAg stock solution, add...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com