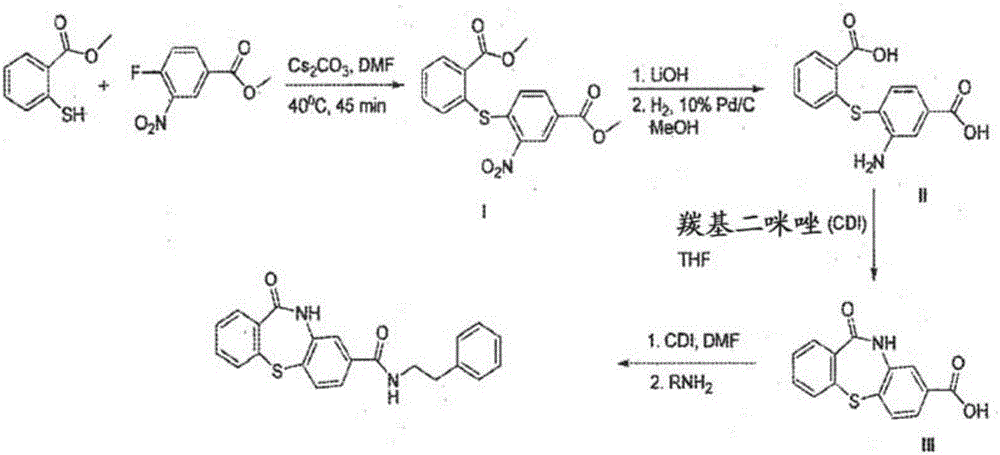
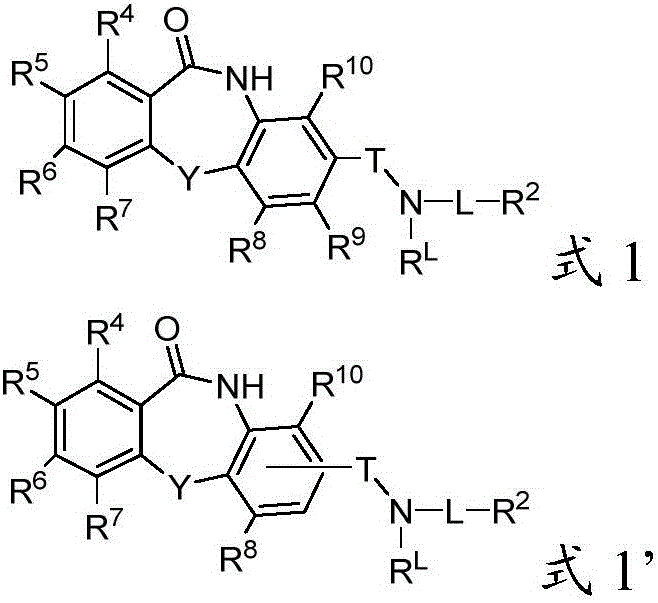
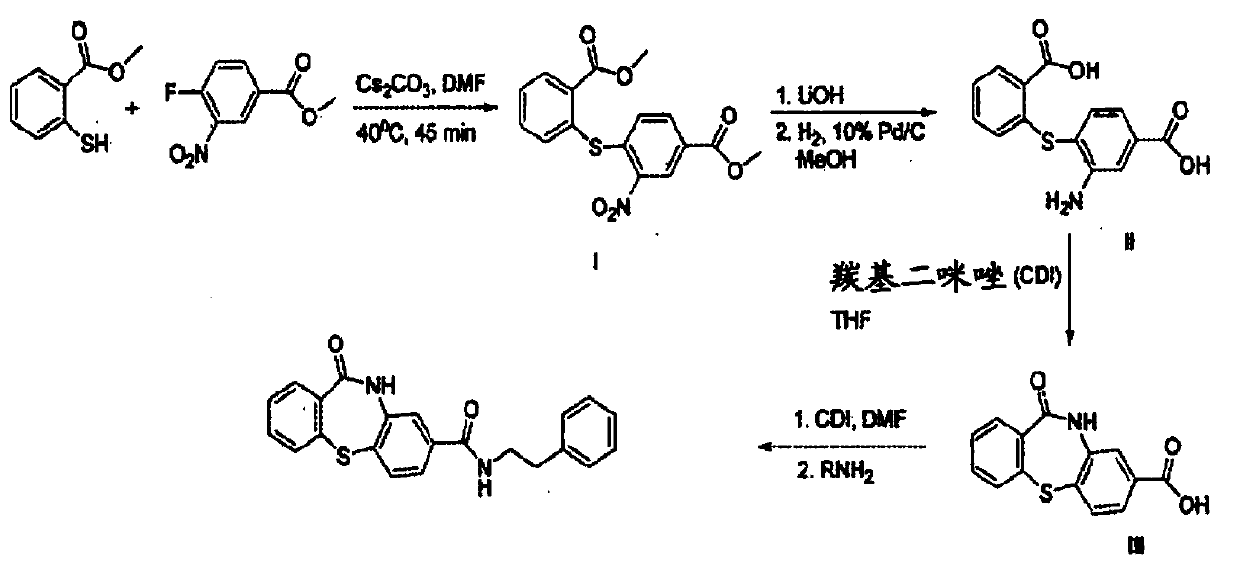
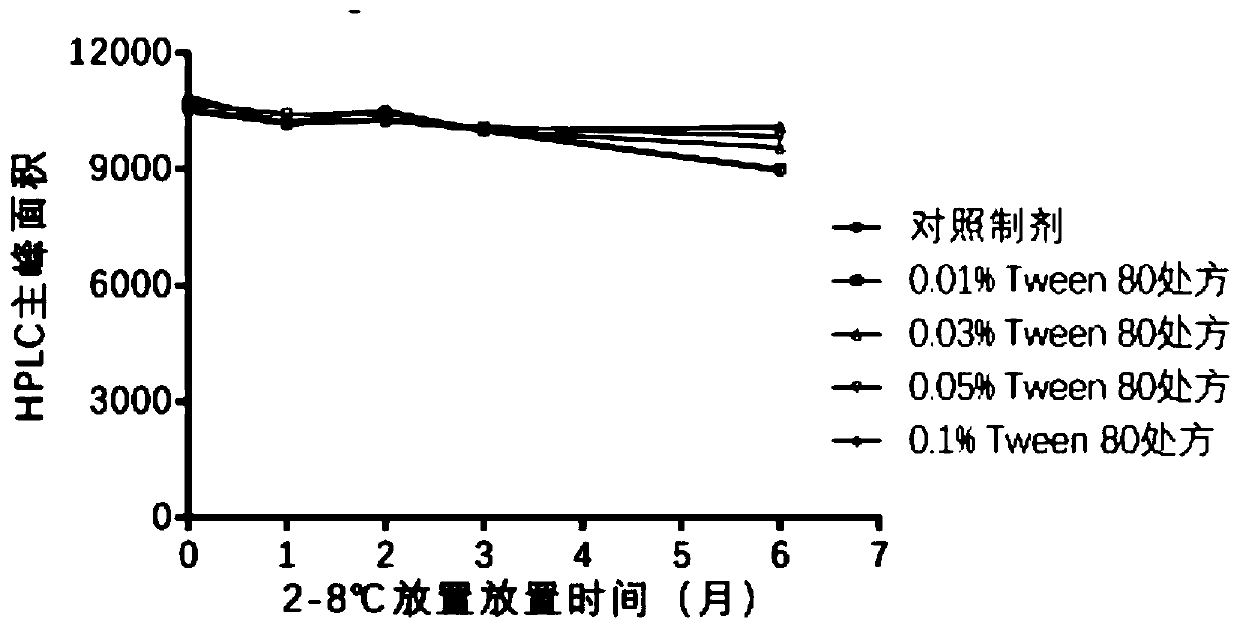
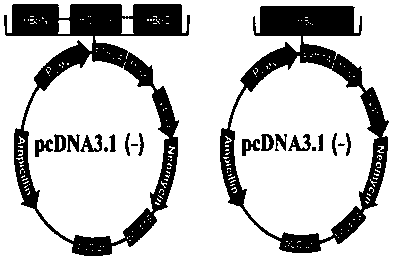
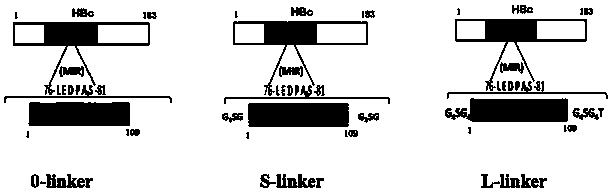
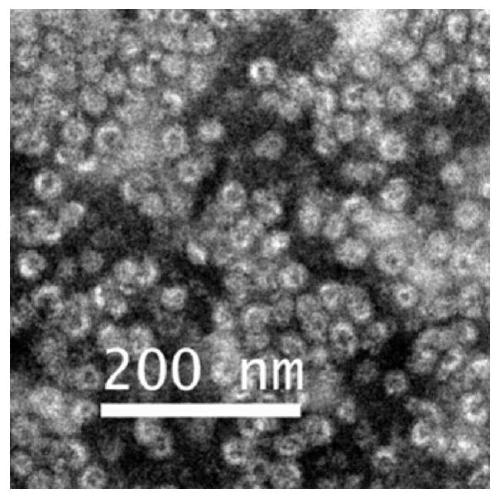
Patents
Literature
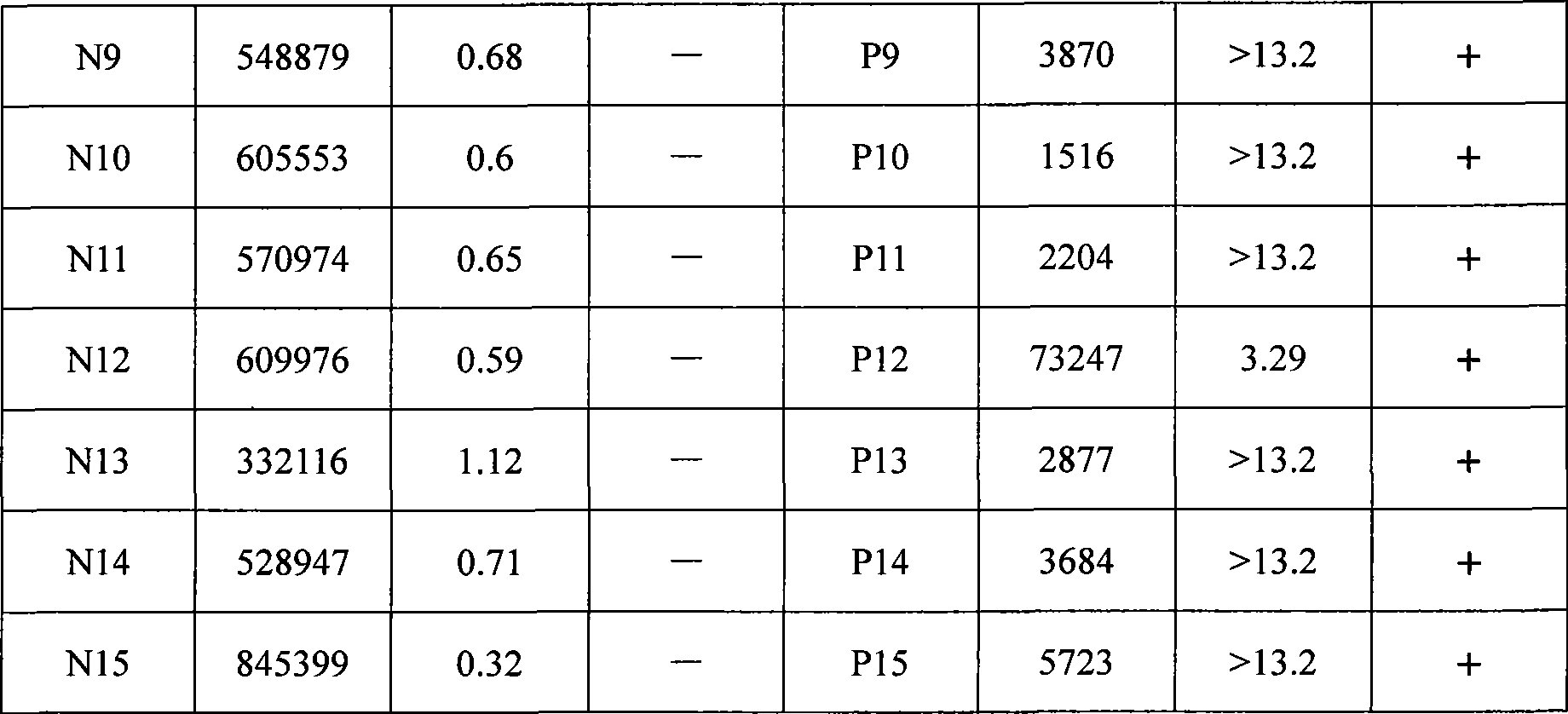
48 results about "Hepatitis b core" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
HLA binding peptides and their uses
InactiveUS7252829B1Effective combinationTumor rejection antigen precursorsSsRNA viruses positive-senseImmunodeficiency virusBinding peptide
The present invention provides the means and methods for selecting immunogenic peptides and the immunogenic peptide compositions capable of specifically binding glycoproteins encoded by HLA alleles and inducing T cell activation in T cells restricted by the allele. The peptides are useful to elicit an immune response against a desired antigen. The immunogenic peptide compositions of the present invention comprise immunogenic peptides having an HLA binding motif, where the peptide is from a target antigen. Target antigens of the present invention include prostate specific antigen (PSA), hepatitis B core and surface antigens (HBVc, HBVs) hepatitis C antigens, Epstein-Barr virus antigens, melanoma antigens (e.g., MAGE-1), human immunodeficiency virus (HIV) antigens, human papilloma virus (HPV) antigens, Lassa virus, mycobacterium tuberculosis (MT), p53, CEA, trypanosome surface antigen (TSA) and Her2 / neu. An example of an immunogenic peptide of the present invention corresponds to a peptide less than about 15 amino acids in length that comprises an HLA-A2.1 binding motif, where the peptide comprises the p53 sequence SMPPPGTRV.
Owner:OSE PHARMA INT
Fluorescent microsphere immunochromatographic testing card for testing five indexes of hepatitis b and method for preparing same
InactiveCN101726596ASimple and fast operationHigh sensitivityBiological testingLuminescent compositionsCelluloseHepatitis B virus
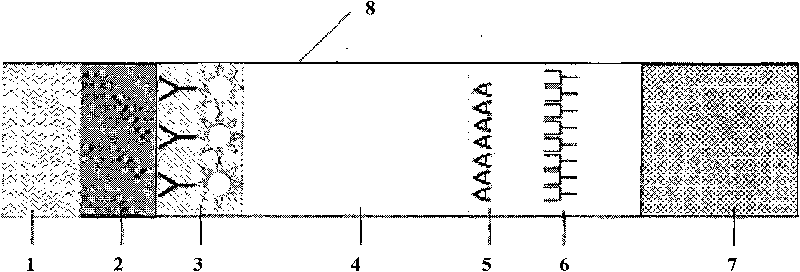
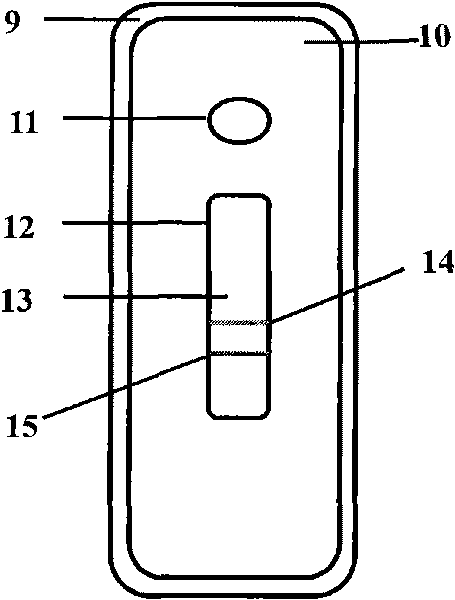
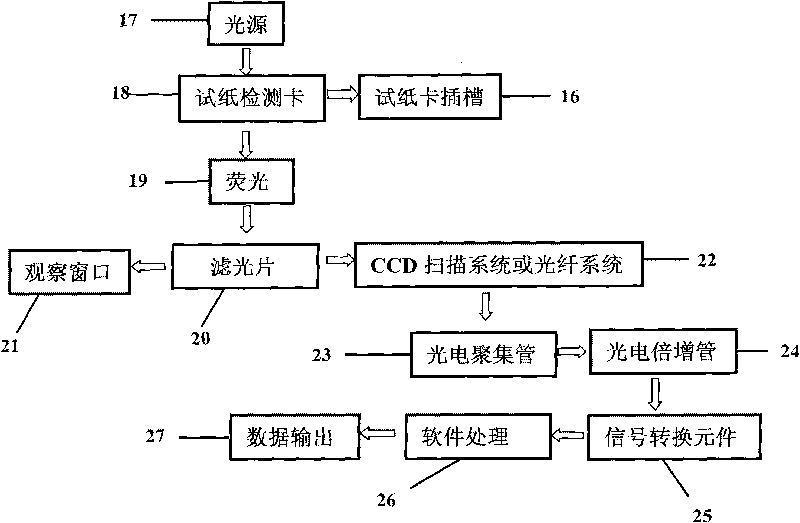
The invention discloses a fluorescent microsphere immunochromatographic testing card for testing five indexes of hepatitis b and a method for preparing the same. The testing card comprises a hepatitis b surface antigen test paper strip, a hepatitis b e surface antigen test paper strip, a hepatitis b surface antibody test paper strip, a hepatitis b e surface antibody test paper strip, and a hepatitis b core antibody test paper strip. Each test paper strip is formed by overlapping and bonding filter paper, a sample pad, a glass fiber film spray-coated with fluorescent microspheres, a cellulose nitrate film and water absorption paper on a bottom plate by glue in sequence, wherein the cellulose nitrate film is coated with antigens serving as a testing area and anti-rabbit antibodies serving as a quality control area; and during a test, after emitted fluorescent light passes a filter, the emitted spectrum is collected, accumulated and multiplied by the CCD scanning technology and then converted into a numerical signal, the numerical signal is multiplied by a correction factor, and the strength of the corrected fluorescent light is substituted in a standard curve of a fluorescence analyzer, so that the concentrations of the five indexes of hepatitis b of the sample can be automatically worked out. The test of hepatitis b viruses by the testing card has the characteristics of specificity, sensitivity, simpleness and accuracy.
Owner:WUXI ZODOLABS BIOTECH
HLA binding peptides and their uses
InactiveUS20090012004A1Effective combinationSsRNA viruses positive-senseTumor rejection antigen precursorsImmunodeficiency virusBinding peptide
The present invention provides the means and methods for selecting immunogenic peptides and the immunogenic peptide compositions capable of specifically binding glycoproteins encoded by HLA alleles and inducing T cell activation in T cells restricted by the allele. The peptides are useful to elicit an immune response against a desired antigen. The immunogenic peptide compositions of the present invention comprise immunogenic peptides having an HLA binding motif, where the peptide is from a target antigen. Target antigens of the present invention include prostate specific antigen (PSA), hepatitis B core and surface antigens (HBVc, HBVs) hepatitis C antigens, Epstein-Barr virus antigens, melanoma antigens (e.g., MAGE-1), human immunodeficiency virus (HIV) antigens, human papilloma virus (HPV) antigens, Lassa virus, mycobacterium tuberculosis (MT), p53, CEA, trypanosome surface antigen (TSA) and Her2 / neu.
Owner:OSE PHARMA INT
Chemoluminescence immunoassay measuring kit and preparation method thereof for hepatitis B core antibody magnetic particles
InactiveCN101545906ASimple structureLow priceChemiluminescene/bioluminescenceAntigenHepatitis b core
The invention provides a chemoluminescence immunoassay measuring kit and a preparation method thereof for hepatitis B core antibody magnetic particles. The kit comprises a hepatitis B core antibody calibration sample, magnetic particles coated by a hepatitis B core antigen, an enzyme marker, a chemoluminescence substrate and a concentrated washing solution. The invention effectively utilizes a magnetic particle solid separation system and a chemoluminescence immunoassay technology to detect the hepatitis B core antibody. The kit has the advantages of simple and fast detection, high sensitivity, strong specificity, stability, and the like, can well satisfy the requirement of clinic diagnosis and is suitable to be effectively generalized and applied in industry.
Owner:CHEMCLIN DIAGNOSTICS CO LTD
Therapeutic hepatitis B vaccine
ActiveCN102462840AInhibition of replicationDigestive systemAntiviralsActive componentHepatitis B Surface Antigens
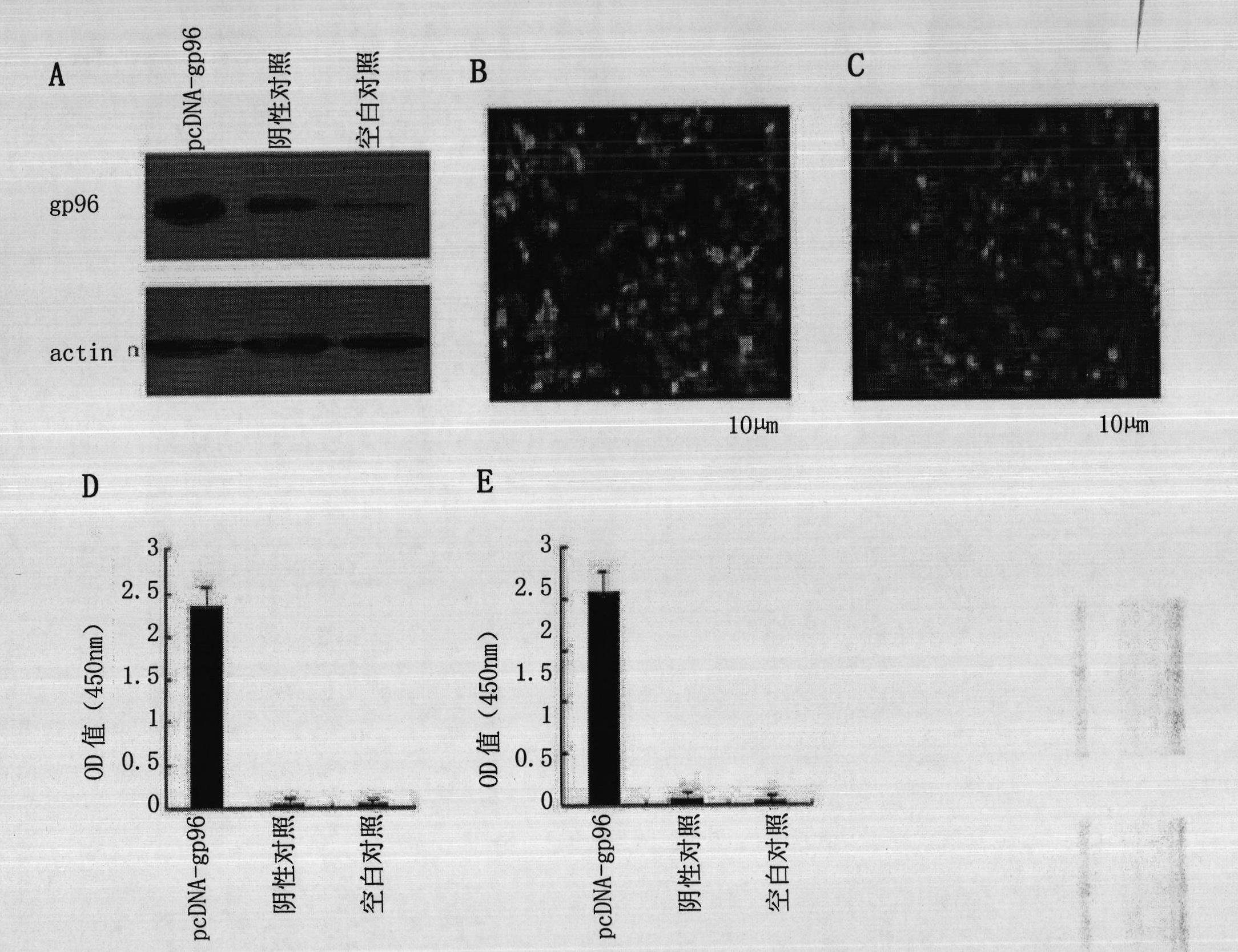
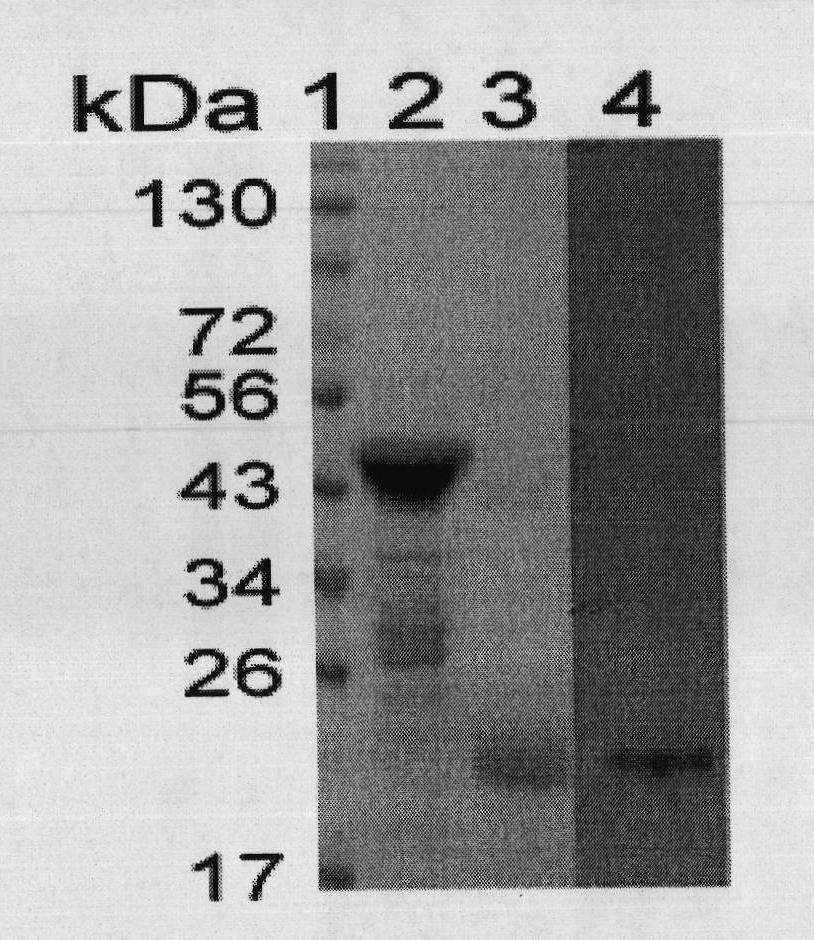
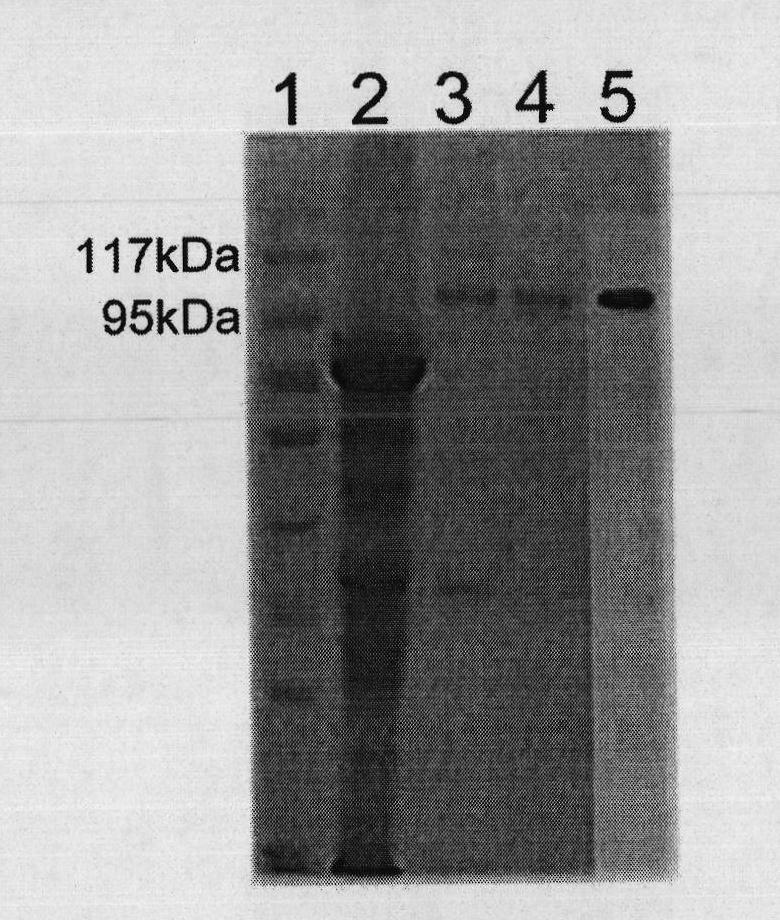
The invention discloses a therapeutic hepatitis B vaccine. Active components of the therapeutic hepatitis B vaccine comprise a protein gp96, a hepatitis B surface antigen and a hepatitis B core protein. The protein gp96 has a sequence shown in the sequence 1 of the sequence table. The hepatitis B surface antigen has a sequence shown in the sequence 5 of the sequence table. The hepatitis B core protein has a sequence shown in the sequence 3 of the sequence table. The active components also comprise a plasmid pcDNA-gp96 containing a coding gene of the protein gp96, a plasmid pcDNA-HB containing a coding gene of the hepatitis B surface antigen and a plasmid pcDNA-HBc containing a coding gene of the hepatitis B core protein. The therapeutic hepatitis B vaccine provided by the invention can effectively inhibit hepatitis B virus (HBV) replication, can eliminate viruses infecting the liver and has a very important value to hepatitis B prevention and treatment.
Owner:北京热休生物技术有限公司
Fusion protein of divisive ranilla luciferase and HBC (hepatitis B core) and application of fusion protein
InactiveCN106188310AInhibition of replicationVirus peptidesBiological testingBiologyHepatitis b core
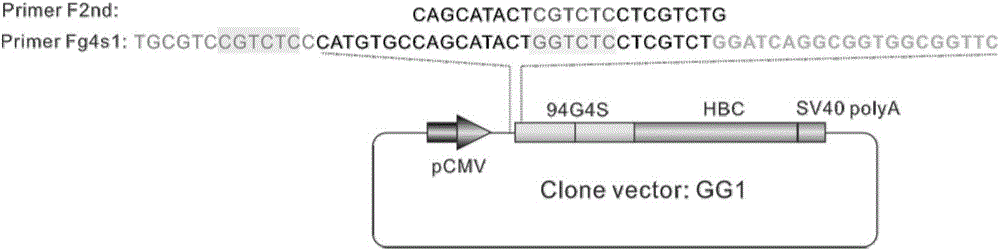
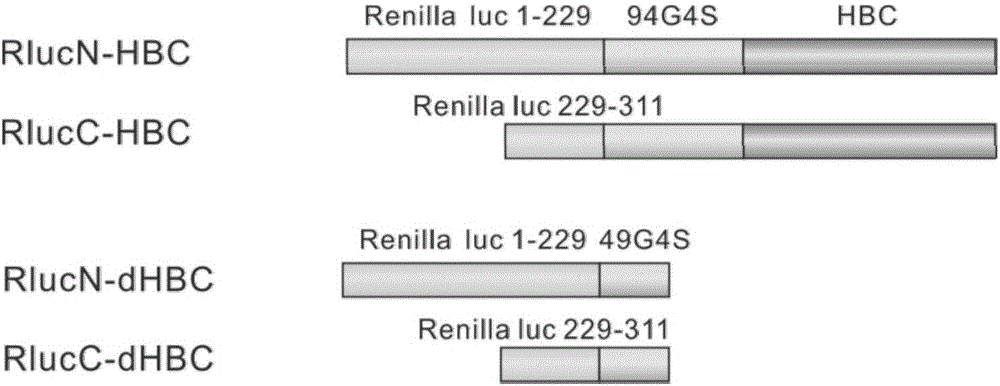
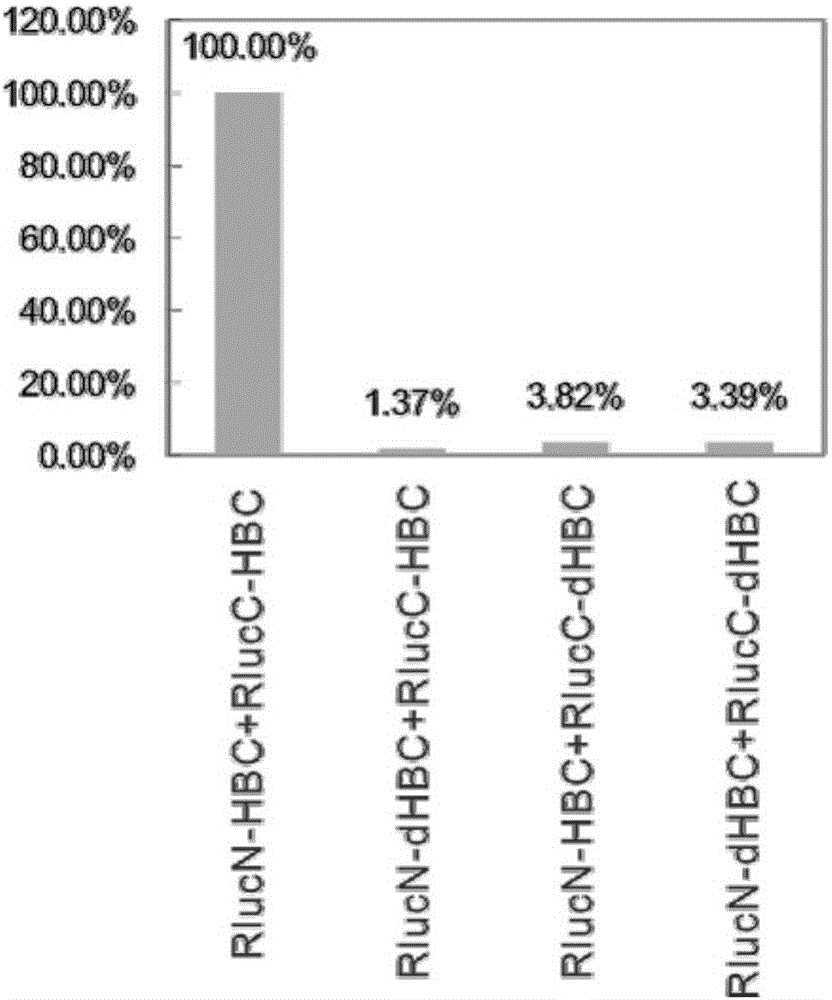
The invention discloses fusion protein of divisive ranilla luciferase and HBC (hepatitis B core). The fusion protein is obtained by joint connection of 1 to 229 amino acids of the N-terminal of the ranilla luciferase and core protein of hepatitis B virus (HBV), or obtained by joint connection of 229 to 311 amino acids of the N-terminal of the ranilla luciferase and the core protein of the HBV, the sequence of the amino acids is as shown in SEQ (sequence) ID (identity) No: 1 and No: 3 respectively, and nucleotide sequence encoding the two fusion protein is as shown in SEQ ID No: 2 and No: 4 respectively. The invention further discloses a carrier containing the nucleotide sequence as is shown in the SEQ ID No: 2 and No: 4. and discloses an application about whether the fusion protein as is shown in the SEQ ID No: 1 or the No: 3 is formed or not in indication of core protein dimers of the HBV. An importation foundation is laid for establishing cell models for drug screening in formation of the anti-HBC dimers.
Owner:CHONGQING MEDICAL UNIVERSITY
Enzyme linked immunity diagnose reagent kit for HB core antigen detecting in two sandwich method and application thereof
The disclosed dual-sandwich enzyme-linked immunologic diagnosis agent box for hepatitis-B core antibody comprises: a micro-porous reaction plate composed by the hepatitis-B core antigen recombinant with purity more than 90% and 5*104~5*105u / mg titer, and the enzyme compound composed by the hepatitis-B core antigen recombinant marked by HRP. This invention has high specificity and sensitivity and convenient to qualitative or semi-qualitative detection.
Owner:SHENYANG HUIMIN BIOTECH CO LTD
Application of lindley eupatorium herb in preparing anti-hepatitis B virus medicaments
The invention discloses an application of lindley eupatorium herb in preparing anti-hepatitis B virus medicaments. Lindley eupatorium herb ethanol extract, lindley eupatorium herb flavone part, lindley eupatorium herb hemiterpene part, lindley eupatorium herb 70% ethanol water elution part and chain diterpene are prepared from lindley eupatorium herb and are subjected to hepatitis B virus (HBV) testing, and testing results indicate that the four extracts and the chain diterpene have the effect of remarkably inhibiting hepatitis B surface antigen (HBsAg) and hepatitis B core antigen (HBeAg), and have better drug activities than those of clinical medicament lamivudine (3TC) for treating hepatitis B. The lindley eupatorium herb can be used for preparing medicaments for resisting hepatitis B virus.
Owner:SUZHOU UNIV
Recombinant fusion proteins of hepatitis B core proteins and tuberculosis antigen or antigen fragments and application thereof
ActiveCN101891825AEvaluate immunogenicityAntibacterial agentsBacterial antigen ingredientsAntigenMycobacterial antigen
The invention relates to one or more types of recombinant fusion proteins formed by hepatitis B core proteins and mycobacterium tuberculosis antigens or antigen fragments. The tuberculosis antigens or antigen fragments are inserted in the same or different permission sites of hepatitis B core proteins in a mode of single antigen or antigen fragment or various types of antigens or antigen fragments which are combined together. The recombinant fusion proteins may comprise a plurality of cofactors. In addition, the invention also relates to the type of the fusion of the tuberculosis antigen or antigen fragments and cofactors and hepatitis B core proteins, nucleic acid and amino acid sequences of coded corresponding recombinant fusion proteins, a method for preparing the recombinant fusion proteins and application of the recombinant fusion proteins in the preparation of medicaments and vaccines for preventing and / or treating tuberculosis.
Owner:INST OF BIOENG ACAD OF MILITARY MEDICAL SCI OF THE CHINESE
CXCL13 (chemokine(C-X-C motif) ligand 13) DNA vaccine and application thereof
InactiveCN105797147AWith preventionHas anti-lung cancer effectCancer antigen ingredientsWhole-cell/virus/DNA/RNA ingredientsTumor targetLymphatic Spread
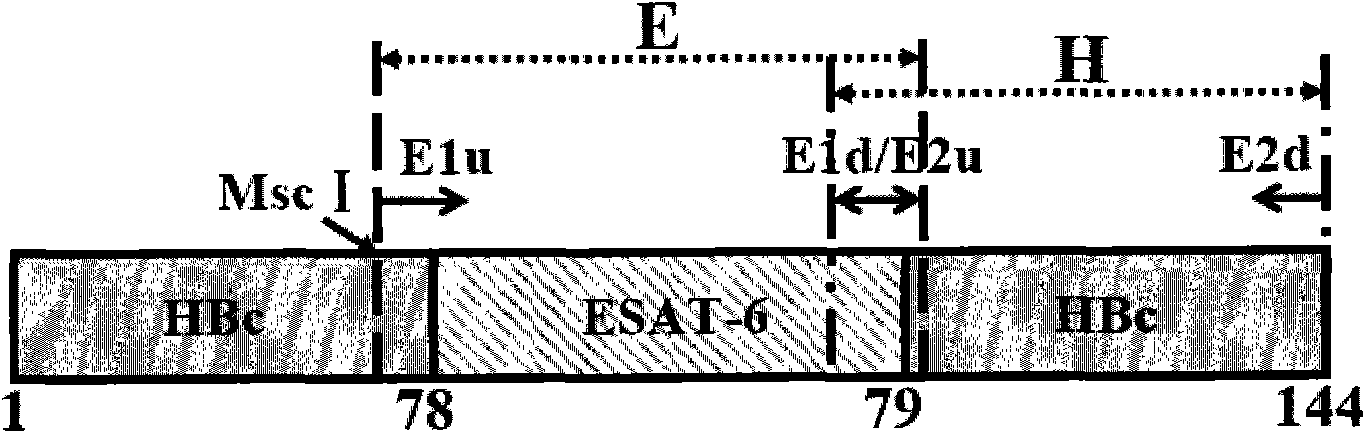
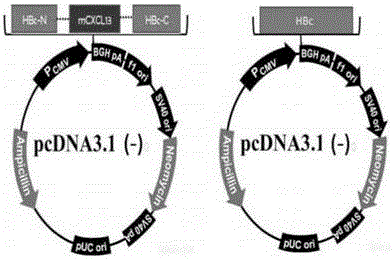
The invention provides a CXCL13 (chemokine(C-X-C motif) ligand 13) DNA vaccine and an application thereof. According to the CXCL13 DNA vaccine, overall-length cDNA of CXCL13 and HBc (Hepatitis B core) are connected, and a vector is pcDNA 3.1(-). A tumor targeted drug prepared from the CXCL13 DNA vaccine generates stronger humoral immunity response by stimulating an active immunity system of an organism and can inhibit p-AKT, p-ERK and EMT signal pathways, thereby inhibiting tumor cell growth as well as invasion and metastasis and realizing the anti-tumor effect.
Owner:INST OF ZOOLOGY CHINESE ACAD OF SCI
Gene engineering vaccine used for preventing pig cysticercosis and its preparation method
InactiveCN1634596AImprove the immunityEnhancing Antigen Presentation CapabilityPeptide/protein ingredientsGenetic material ingredientsProtective antigenPlasmid dna
A gene engineering vaccine for preventing pig cysticercosis and its preparation process are disclosed. The process includes the steps: making the hepatitis B virus core protein as carrier, inserting cysticercus cellulosae protective antigen gene in multiple locations between the first amino acid to No. 183 amino acid hepatitis B core protein, cloning to procaryon expression plasmid or eucaryon expression plasmid, transforming host bacteria, expressing by inducing aim gene and acquiring aim protein or column purified eucaryon expression plasmid DNA therefore, subunit protein vaccine or nucleic acid vaccine for preventing pig cysticercosis are obtained.
Owner:中国人民解放军南京军区联勤部军事医学研究所
Connective tissue growth factor chimeric vaccine for treating liver fibrosis and application of connective tissue growth factor chimeric vaccine
ActiveCN104740628ADegree of inhibitory activationReduce processDigestive systemAntibody medical ingredientsHepatitis B virus core AntigenCTGF
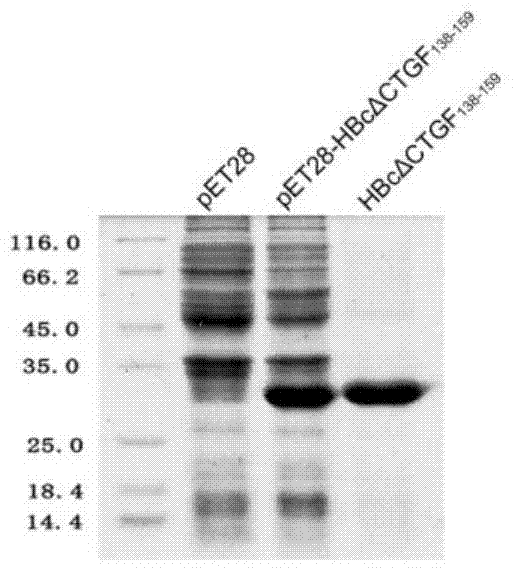
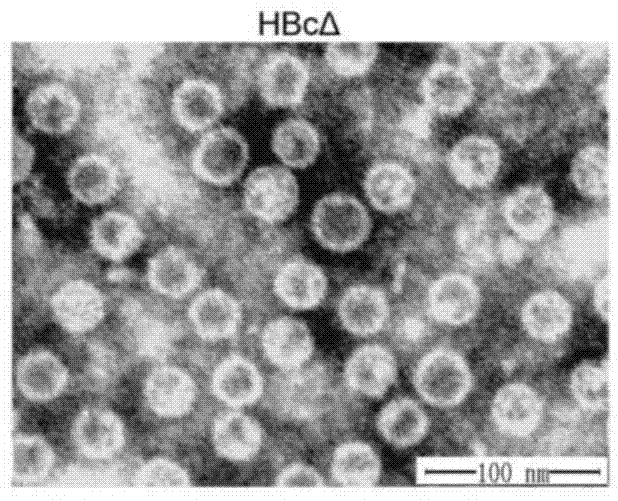
The invention discloses a CTGF (connective tissue growth factor) chimeric vaccine for treating liver fibrosis. The antigenic epitope of the CTGF is inserted into c / e1B cell epitope of hepatitis b virus core antigen to form the CTGF chimeric vaccine which can be assembled into hepatitis B core sample particles. Under the non-adjuvant assistance, the chimeric vaccine can stimulate a body to generate high-valence anti-CTGF neutral antibody. A mouse which is immunized by the chimeric vaccine is obviously relieved in fibrosis degree generated after carbon tetrachloride induction. According to the experimental verification, the CTGF chimeric vaccine can be used for obviously restraining the activation intensity of hepatic stellate cells in the mouse liver, and also can be used for stimulating liver cells to multiply and restraining liver apoptosis. Besides, the TGF-beta 1 and PDGF level in the immunized mouse is obviously reduced, so that the process of liver fibrosis can be relieved in a facilitated manner. According to the results, the CTGF chimeric vaccine can be used for successfully restraining the carbon-tetrachloride-induced mouse liver fibrosis. Therefore, the CTGF chimeric vaccine is expected to be developed into effective means for treating the liver fibrosis.
Owner:THE FIRST AFFILIATED HOSPITAL OF MEDICAL COLLEGE OF XIAN JIAOTONG UNIV
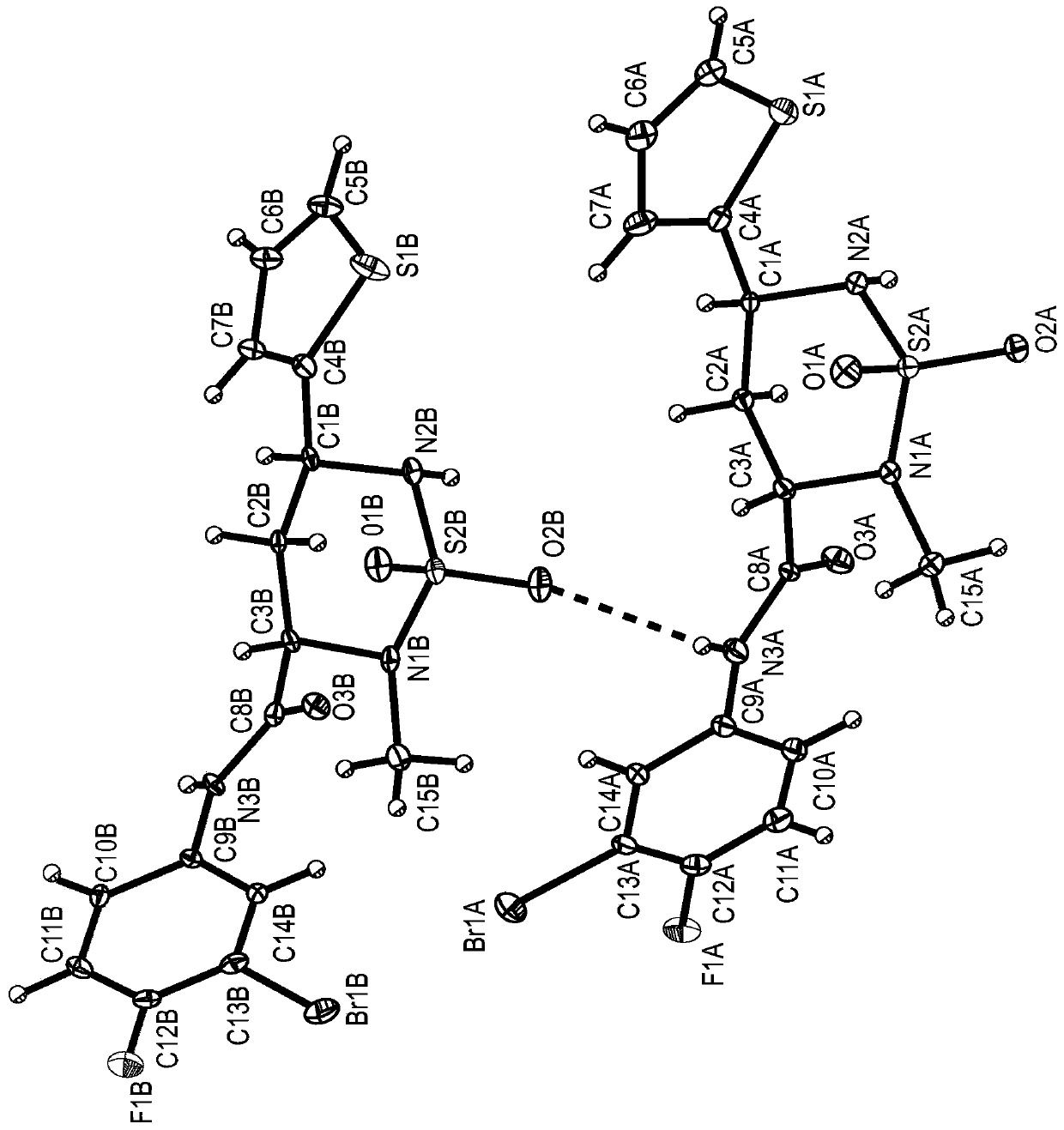
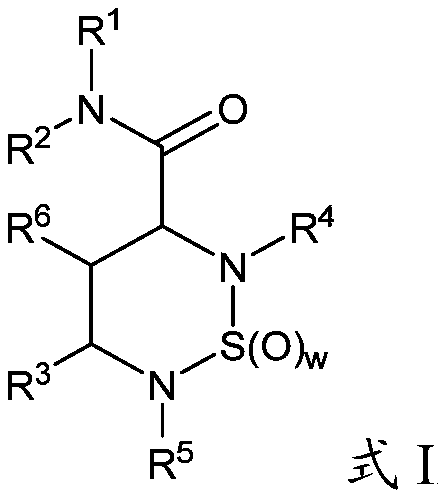
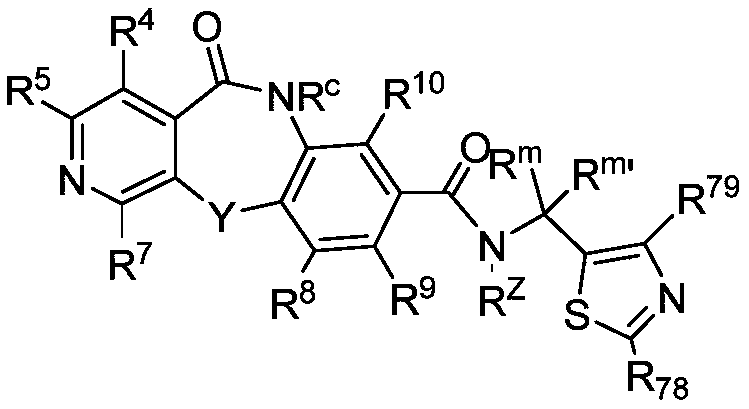
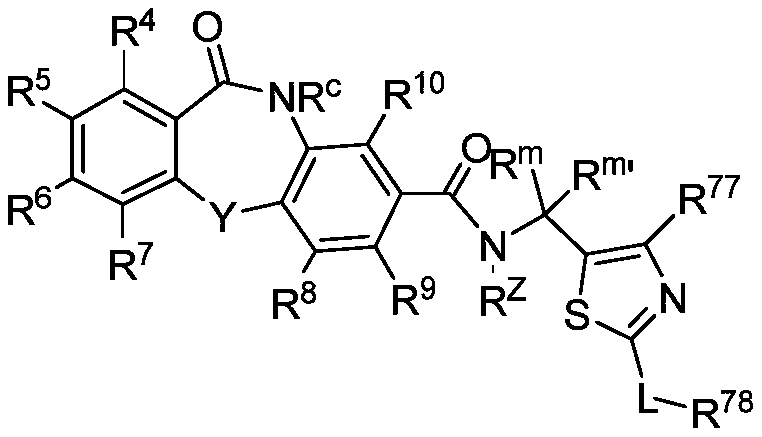
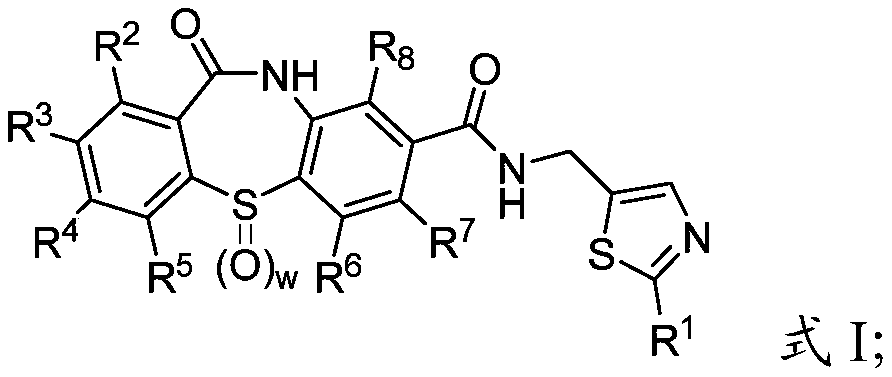
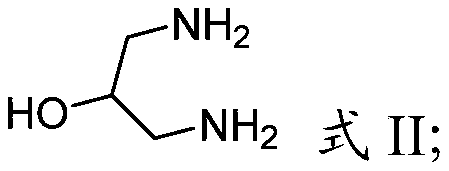
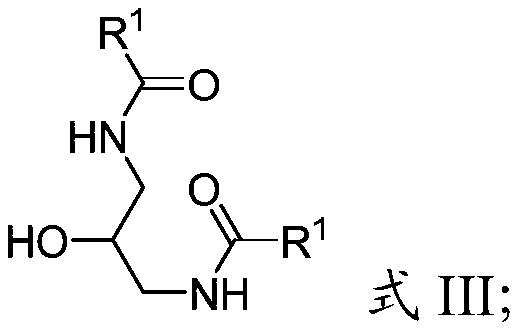
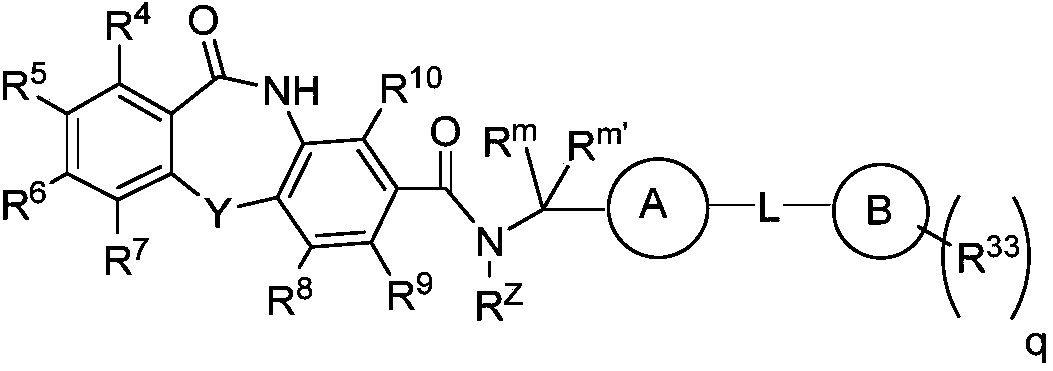
Cyclic sulfamide compounds and methods of using same
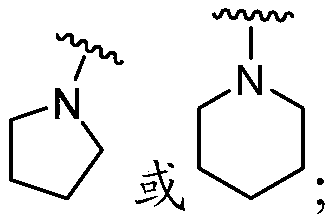
The present disclosure provides, in part, cyclic sulfamide compounds, and pharmaceutical compositions thereof, useful as modulators of Hepatitis B (HBV) core protein, and methods of treating HepatitisB (HBV) infection.
Owner:ASSEMBLY BIOSCI
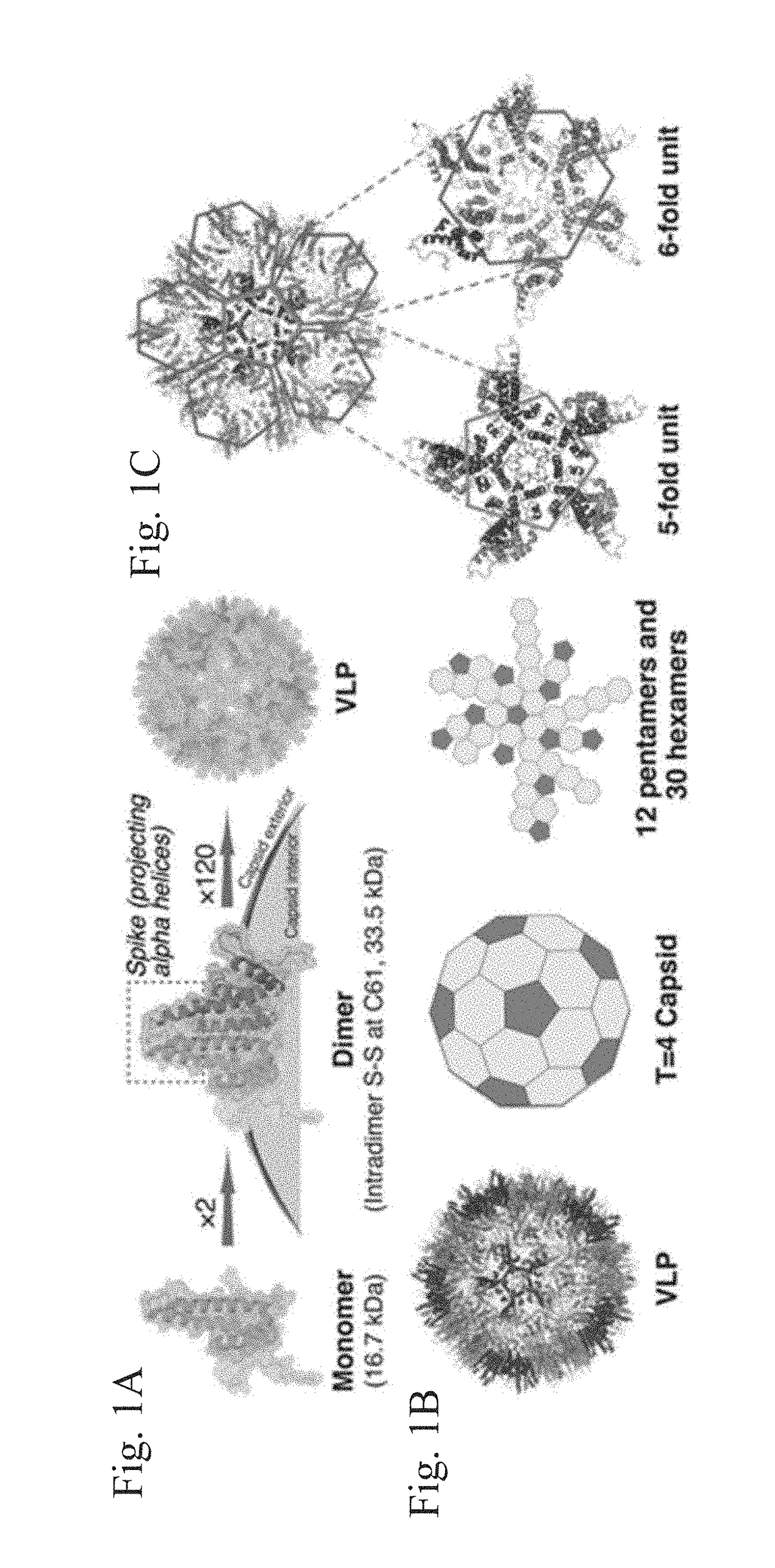
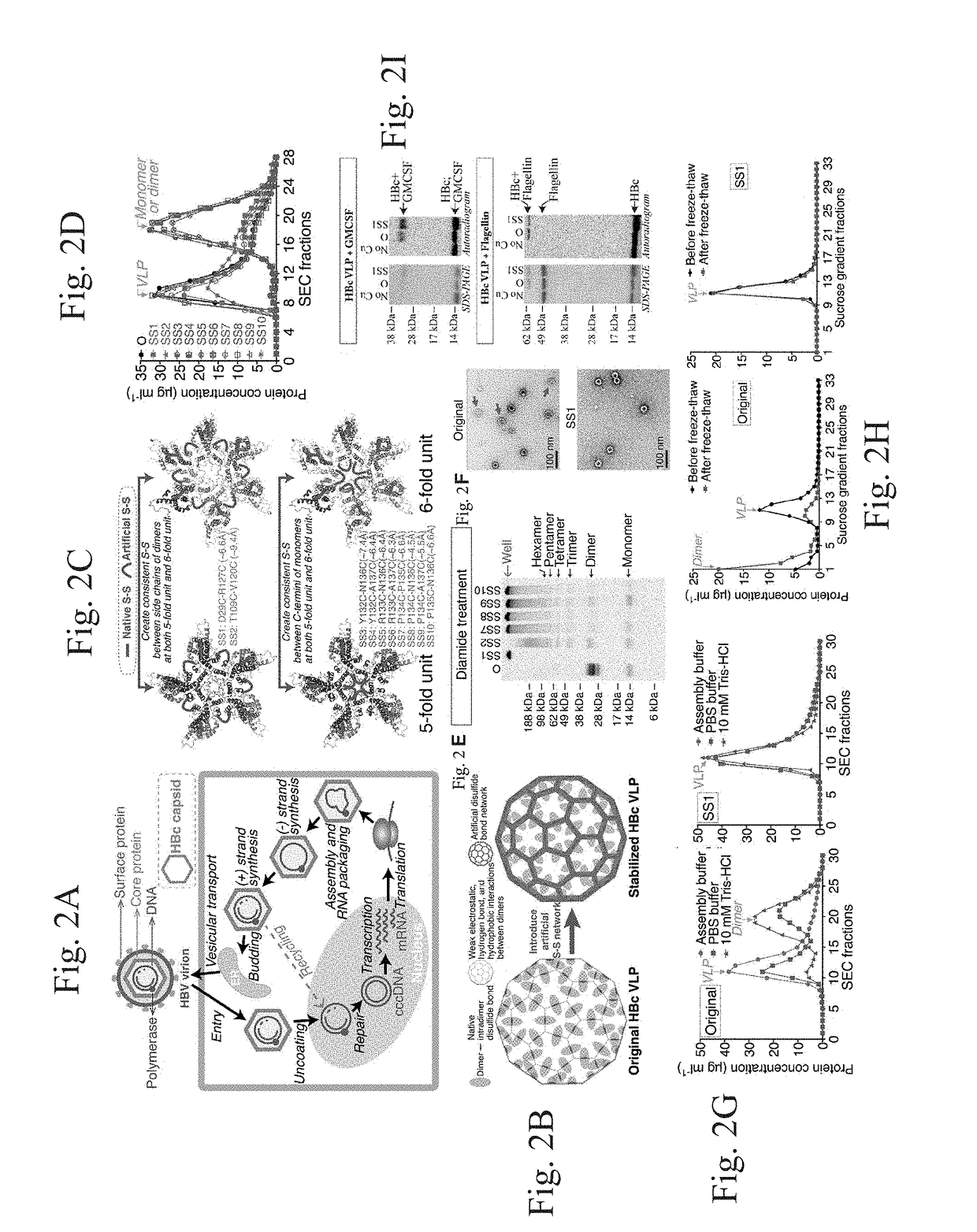
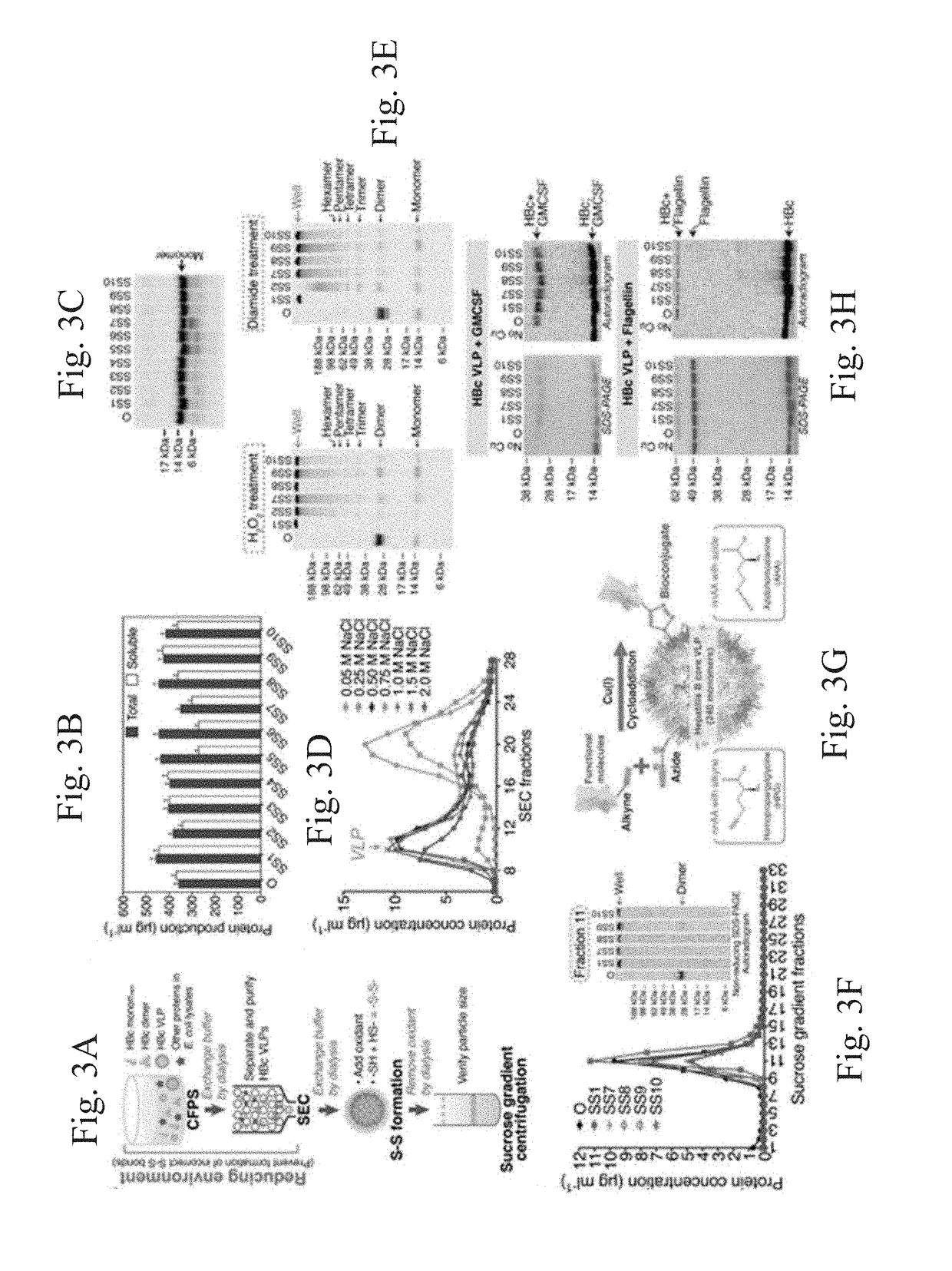
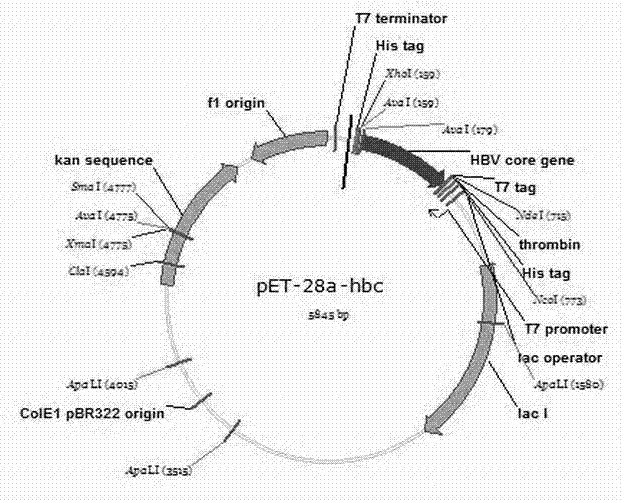
Stabilized hepatitis B core polypeptides
ActiveUS9896483B2Improve stability and utilizationSsRNA viruses positive-senseVirus peptidesHepatitis b coreChemistry
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Recombinant plasmid DNA vaccine composition for treating Hepatitis B
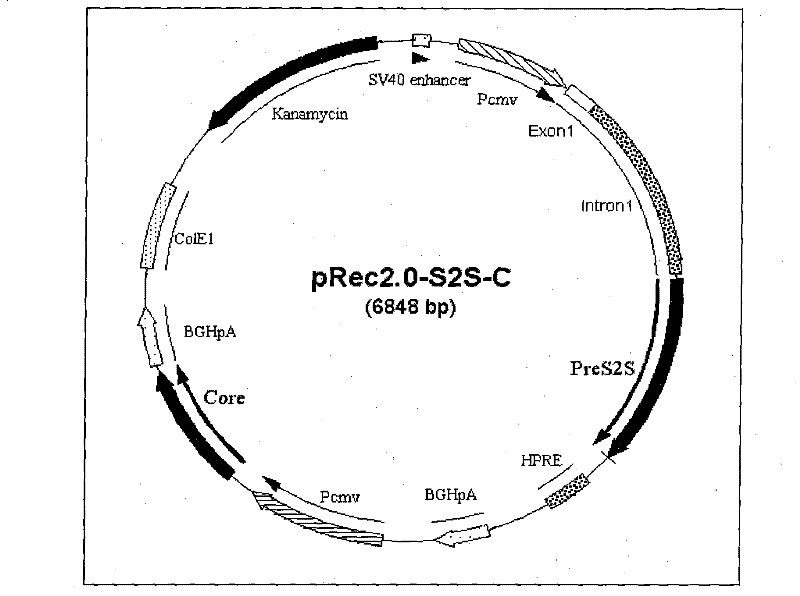
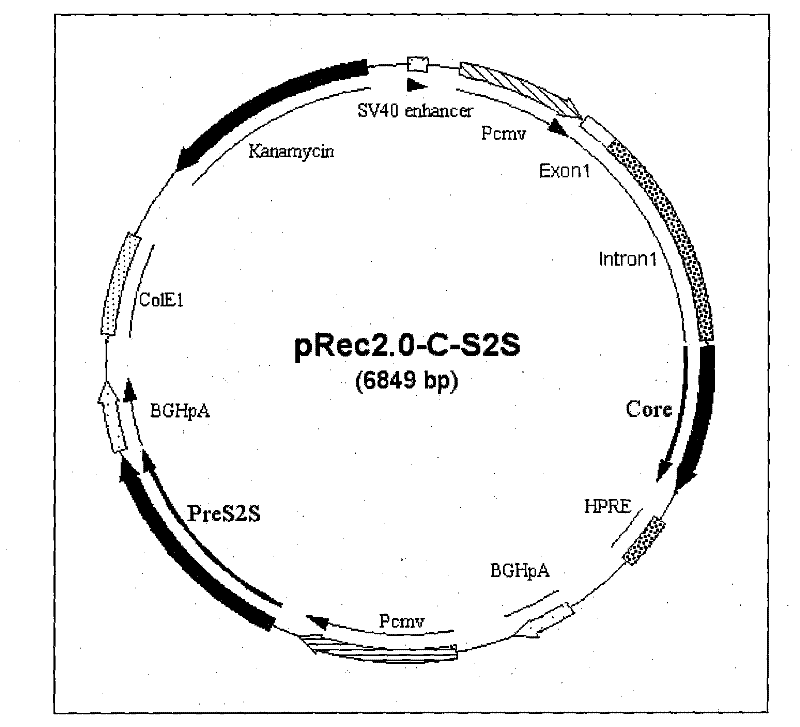
The invention relates to a recombinant plasmid DNA vaccine composition for treating Hepatitis B. The composition contains a recombinant vaccine plasmid which simultaneously carries Hepatitis B middle protein S2S coding gene and Hepatitis B Core protein coding gene as well as a recombinant adjuvant plasmid, wherein the adjuvant plasmid is the adjuvant plasmid of recombinant interleukin 2 or recombinant human interferon gamma.
Owner:BEIJING KAWIN TECH SHARE HLDG
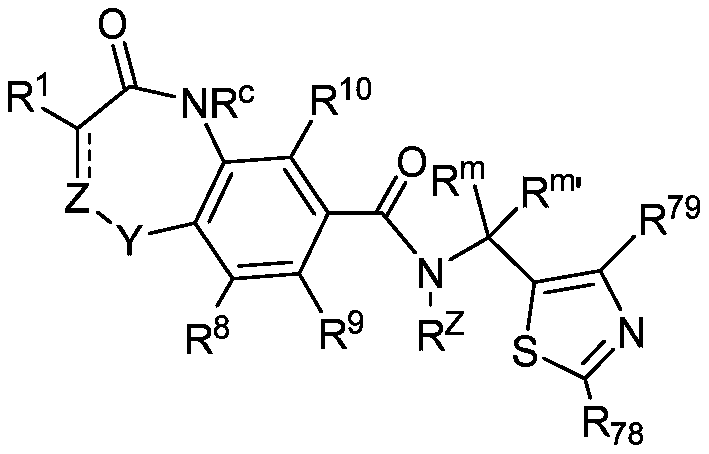
Hepatitis b core protein modulators
InactiveCN109937201AOrganic active ingredientsOrganic chemistryHepatitis B immunizationHepatitis B virus
Owner:ASSEMBLY BIOSCI
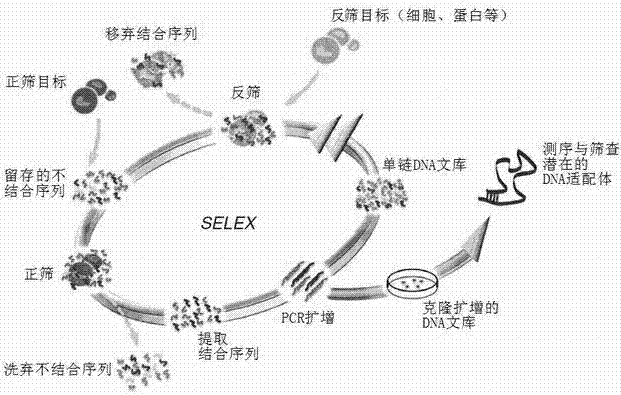
Aptamer sequence of hepatitis B virus (HBV) core antigen and application of nucleic aptamer sequence
ActiveCN102649961AMicrobiological testing/measurementGenetic material ingredientsHepatitis B virus core AntigenAptamer
The invention belongs to the field of molecular immunity and relates to an aptamer sequence for a hepatitis B virus (HBV) core antigen and an application of the nucleic aptamer sequence. According to the invention, a gene-recombination plasmid is adopted to express the core antigen, and an aptamer specially bound with the core antigen is screened, so as to prepare a characteristic sequence CATTT with the special binding HBV core antigen. The characteristic sequence is the base of the special binding of the aptamer and the HBc (hepatitis B core) antigen, can be used for drug design and the preparation of drugs and other products; and the aptamer with the characteristic sequence can be used as a probe or therapeutic target resisting to HBV to design and prepare drugs or agents resisting to HBV.
Owner:AFFILIATED HUSN HOSPITAL OF FUDAN UNIV
Process for making hepatitis b core protein modulators
PendingCN110612300AOrganic active ingredientsOrganic compound preparationHepatitis B immunizationHepatitis B virus
Owner:ASSEMBLY BIOSCI
Hepatitis b core protein modulators
InactiveCN108348529AOrganic active ingredientsOrganic chemistryHepatitis B immunizationHepatitis B virus
Owner:ASSEMBLY BIOSCI +1
Nanoparticles with AIAE (aggregation-induced absorption enhancement) phenomenon as well as synthesis and application of nanoparticles
ActiveCN108226048AThe synthesis method is simpleUniform sizeFluorescence/phosphorescenceWAS PROTEINDepolymerization
The invention discloses nanoparticles with an AIAE (aggregation-induced absorption enhancement) phenomenon as well as synthesis and an application of the nanoparticles and relates to nanoparticles. The nanoparticles with the AIAE phenomenon comprise inner cores and outer layers, wherein the inner cores are croconaine analogues, the outer layers are protein particles, the inner cores are loaded ininner cavities of the outer layers, and composite nanostructures with the croconaine analogues as the inner cores and the protein particles as the outer layers are formed. Hepatitis B core protein particles are diluted and added to a depolymerization buffer solution to be stirred; dye is uniformly stirred in DMSO, and croconaine analogue dye is obtained; the croconaine analogue dye is added to anobtained mixture and stirred, and a mixed liquid is obtained; the mixed liquid is added to a dialysis bag, dialysis is performed in an assembling buffer solution 1, and a magnetic stirring reaction isperformed; the mixed liquid is added to the dialysis bag, dialysis is performed in an assembling buffer solution 2, and a magnetic stirring reaction is performed to obtain medicine-carrying protein nanoparticles; the obtained medicine-carrying protein nanoparticles are subjected to <125>I labeling, and the nanoparticles with the AIAE phenomenon are obtained.
Owner:XIAMEN UNIV
Anti-hbc quantitative detection method and uses thereof in monitoring and controlling disease progression of chronic hepatitis b patient and in predicting therapeutic effect
ActiveUS20150118674A1Good treatment effectPositive correlation with therapeutic response rateMicrobiological testing/measurementDigestive systemTreatment effectChronic hepatitis
The present invention relates to a method for quantitative detection of anti-HBc and its use in monitoring disease progression of chronic hepatitis B patients and predicting therapeutic effects. By quantitative detection of antibodies against hepatitis B core protein (Anti-HBc), it is able to monitor disease progression of chronic hepatitis B patients, effectively predict therapeutic effects in chronic hepatitis B patients who accept a therapy against hepatitis B virus (especially, a therapy based on interferon and a therapy based on nucleoside / nucleotide analogue anti-HBV drug), and thus guide the patients to reasonably choose drugs.
Owner:XIAMEN INNOVAX BIOTECH +1
Pharmaceutical composition for treating hepatitis B, and preparation method and application thereof
PendingCN111420043AMaintain antigen stabilitySimple compositionViral antigen ingredientsDigestive systemAntigenAdjuvant
Owner:JIANGSU THERAVAC BIO PHARMA CO LTD
cxcl13 DNA vaccine and its application
InactiveCN105797147BHigh antibody titerStrong neutralizationCancer antigen ingredientsWhole-cell/virus/DNA/RNA ingredientsTumor targetLymphatic Spread
The invention provides a CXCL13 (chemokine(C-X-C motif) ligand 13) DNA vaccine and an application thereof. According to the CXCL13 DNA vaccine, overall-length cDNA of CXCL13 and HBc (Hepatitis B core) are connected, and a vector is pcDNA 3.1(-). A tumor targeted drug prepared from the CXCL13 DNA vaccine generates stronger humoral immunity response by stimulating an active immunity system of an organism and can inhibit p-AKT, p-ERK and EMT signal pathways, thereby inhibiting tumor cell growth as well as invasion and metastasis and realizing the anti-tumor effect.
Owner:INST OF ZOOLOGY CHINESE ACAD OF SCI
Nanoparticles with Aggregation-Induced Light Absorption Enhancement and Synthesis Method
ActiveCN108226048BThe synthesis method is simpleUniform sizeFluorescence/phosphorescenceWAS PROTEINDepolymerization
Owner:XIAMEN UNIV
Gene engineering vaccine used for preventing pig cysticercosis and its preparation method
InactiveCN100389831CImprove the immunityEnhancing Antigen Presentation CapabilityPeptide/protein ingredientsGenetic material ingredientsProtective antigenPlasmid dna
A gene engineering vaccine for preventing pig cysticercosis and its preparation process are disclosed. The process includes the steps: making the hepatitis B virus core protein as carrier, inserting cysticercus cellulosae protective antigen gene in multiple locations between the first amino acid to No. 183 amino acid hepatitis B core protein, cloning to procaryon expression plasmid or eucaryon expression plasmid, transforming host bacteria, expressing by inducing aim gene and acquiring aim protein or column purified eucaryon expression plasmid DNA therefore, subunit protein vaccine or nucleic acid vaccine for preventing pig cysticercosis are obtained.
Owner:中国人民解放军南京军区联勤部军事医学研究所
Recombinant fusion proteins of hepatitis B core proteins and tuberculosis antigen or antigen fragments and application thereof
ActiveCN101891825BEvaluate immunogenicityAntibacterial agentsBacterial antigen ingredientsAntigenMycobacterial antigen
The invention relates to one or more types of recombinant fusion proteins formed by hepatitis B core proteins and mycobacterium tuberculosis antigens or antigen fragments. The tuberculosis antigens or antigen fragments are inserted in the same or different permission sites of hepatitis B core proteins in a mode of single antigen or antigen fragment or various types of antigens or antigen fragments which are combined together. The recombinant fusion proteins may comprise a plurality of cofactors. In addition, the invention also relates to the type of the fusion of the tuberculosis antigen or antigen fragments and cofactors and hepatitis B core proteins, nucleic acid and amino acid sequences of coded corresponding recombinant fusion proteins, a method for preparing the recombinant fusion proteins and application of the recombinant fusion proteins in the preparation of medicaments and vaccines for preventing and / or treating tuberculosis.
Owner:INST OF BIOENG ACAD OF MILITARY MEDICAL SCI OF THE CHINESE
Special-effect oral liquid for treating hepatitis B
InactiveCN105688030ALiver function returns to normalNormal liver functionAmphibian material medical ingredientsPharmaceutical delivery mechanismDiseaseHepatitis B virus surface
The invention discloses a special-effect oral liquid for treating hepatitis B; the special-effect oral liquid is prepared by decoction and concentration of capillary artemisia, fructus gardeniae, rheum officinale, cortex phellodendri, rhizoma coptidis, dandelion, radix scrophulariae, fructus forsythiae, radix rehmanniae recen, toad venom, brucea javanica, herba lysimachiae, sowthistle tasselflower herb, cortex cinnamomi, rhizoma acori graminei, rhizoma chuanxiong, radix salviae miltiorrhizae, radix curcumae, caulis spatholobi, hawthorn, Chinese yam, radix astragali, jujube, radix morindae officinalis, rhizoma curculiginis, fructus psoraleae, kernels, rhizoma cibotii, radix polygoni multiflori, longan aril, Chinese wolfberry, fructus ligustri lucidi, radix ophiopogonis, bitter apricot kernel, lotus seed, ganoderma lucidum, radix saposhnikoviae, radix angelicae tuhuo, radix puerariae, radix bupleuri, rhizoma cimicifugae, mung beans, pericarpium citri reticulatae, and endothelium corneum gigeriae galli. The pure traditional Chinese medicine preparation has stronger functions of cleaning hepatitis B viruses, regulating immunity, repairing damaged liver cells, improving hepatic microcirculation, and promoting hepatitis B virus surface antibodies to be turned into positive and hepatitis B surface antigens, hepatitis B e antigens, hepatitis B e antibodies and hepatitis B core antibodies to be turned into negative, has the advantages of exact curative effect, short treatment course and quick acting, and allows the disease not to relapse after the disease is cured.
Owner:仲从开
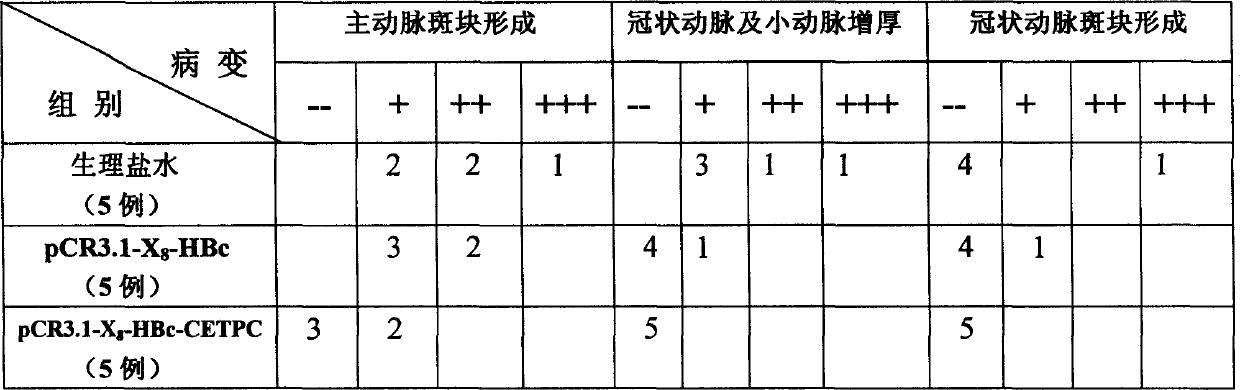
Bacterin of nucleic acid for anti atherosclerosis and preparation method
InactiveCN1562349ASuppress immune responseReduce formationPeptide/protein ingredientsAntibody medical ingredientsCholesterylester transfer proteinAtheroma
A nucleic acid vaccine for preventing and treating atherosolerosis and its preparing process are disclosed. The core protein of hepatitis B is used as its immune carrier to intensify the immunogenicity of CETPC. The body immunized by said vaccine can generate CETPC antibody to suppress the activity of cholesteryl ester transfer protein, so decreasing the generation of atherosclerosis spots. It can also act on the formed atherosclerosis sports to relay them.
Owner:CHINA PHARM UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com