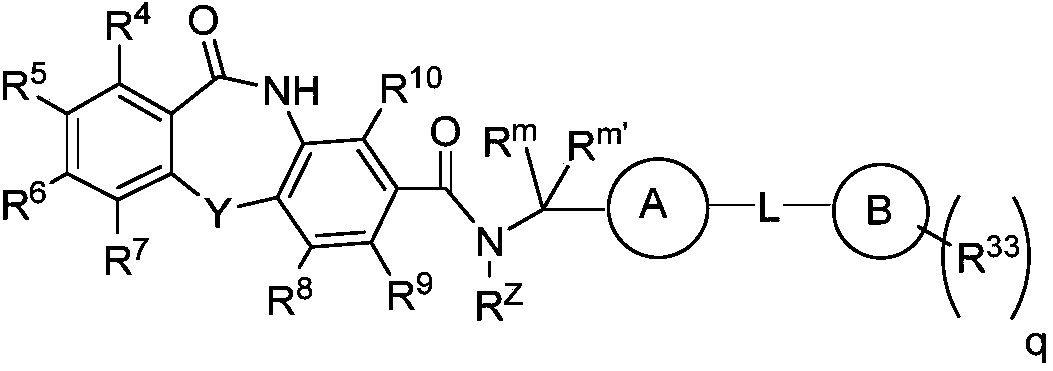
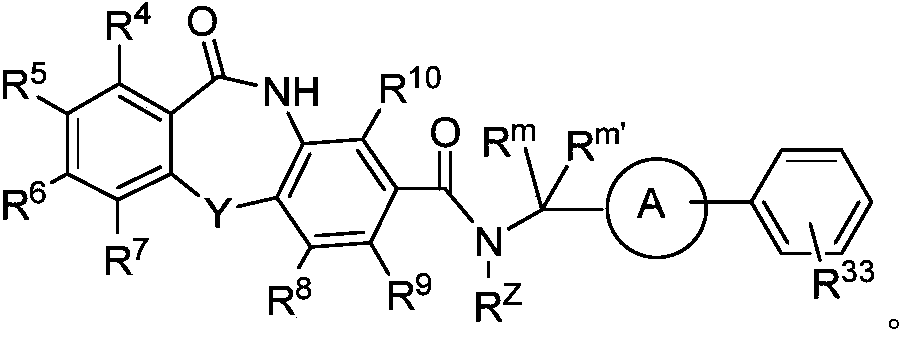
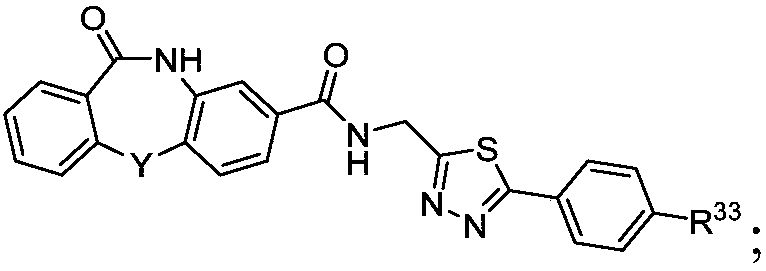
Hepatitis b core protein modulators
An ethyl and compound technology, applied in the field of hepatitis B core protein regulator, can solve the problems of poor tolerance of interferon alpha side effects and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0108] Example 1: Synthesis of 7-methyl-11-oxo-10,11-dihydrodibenzo[b,f][1,4]thiazepine-8- Formic acid (7): a common intermediate
[0109]
[0110] Synthesis of 4-fluoro-2-methyl-5-nitrobenzoic acid (3):
[0111]
[0112] To a stirred solution of 4-fluoro-2-methylbenzoic acid 2 (500 mg, 3.24 mmol) in concentrated sulfuric acid (2.5 mL) at 0 °C under an inert atmosphere was added potassium nitrate (655 mg, 6.49 mmol); warmed to room temperature and stirred for 6 hours. The reaction was monitored by TLC; after completion of the reaction, the reaction mixture was quenched with ice water (20 mL), and the precipitated solid was filtered and dried in vacuo to obtain a crude product. The crude product was chromatographed on a silica gel column using 5% MeOH / CH 2 Cl 2 Purification afforded compound 3 (300 mg, 60%) as a brown syrup. TLC: 10% MeOH / CH 2 Cl 2 (R f :0.3); 1 H-NMR (DMSO-d 6 , 500MHz): δ13.56 (br s, 1H), 8.52 (d, J=8.0Hz, 1H), 7.61 (d, J=12.5Hz, 1H), 2.63 (...
Embodiment 2
[0125] Example 2: Synthesis of 2-chloro-11-oxo-10,11-dihydrodibenzo[b,f][1,4]thiazepine-8-methan Acid (16): a common intermediate
[0126]
[0127] Synthesis of 5-chloro-2-((4-methoxybenzyl)thio)benzonitrile (10):
[0128]
[0129] To a stirred solution of 5-chloro-2-fluorobenzonitrile 8 (1.0 g, 6.41 mmol) in DMF (10 mL) under an inert atmosphere was added cesium carbonate (2.30 g, 7.05 mmol) at room temperature; heated to 40 °C And (4-methoxyphenyl)methanol 9 (1.08 g, 7.05 mmol) was added thereto; heated to 60° C. and stirred for 2 hours. The reaction was monitored by TLC; after completion of the reaction, the reaction mixture was diluted with water (20 mL) and extracted with EtOAc (2 x 25 mL). The combined organic extracts were dried over sodium sulfate, filtered and concentrated in vacuo to give crude product. The crude product was purified by silica gel column chromatography using 3-5% EtOAc / hexanes to afford compound 10 (1 g, 54%) as a white solid. TLC: 10% E...
Embodiment 3
[0145] Example 3: Synthesis of 3-chloro-11-oxo-10,11-dihydrodibenzo[b,f][1,4]thiazepine-8-methan Acid (23): a common intermediate
[0146]
[0147] Synthesis of 4-chloro-2-((4-methoxybenzyl)thio)benzonitrile (18):
[0148]
[0149] To a stirred solution of 4-chloro-2-fluorobenzonitrile 17 (1 g, 6.41 mmol) in DMF (25 mL) under an inert atmosphere was added cesium carbonate (2.30 g, 7.05 mmol) at room temperature; heated to 40 °C and To this was added (4-methoxyphenyl)methanethiol 9 (1.08 g, 7.05 mmol); heated to 60° C. and stirred for 2 hours. The reaction was monitored by TLC; after completion of the reaction, the reaction mixture was diluted with water (20 mL) and extracted with EtOAc (2 x 25 mL). The combined organic extracts were dried over sodium sulfate, filtered and concentrated in vacuo to give crude product. The crude product was purified by silica gel column chromatography using 4% EtOAc / hexanes to afford compound 18 (900 mg, 48%) as a white solid. TLC: 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com