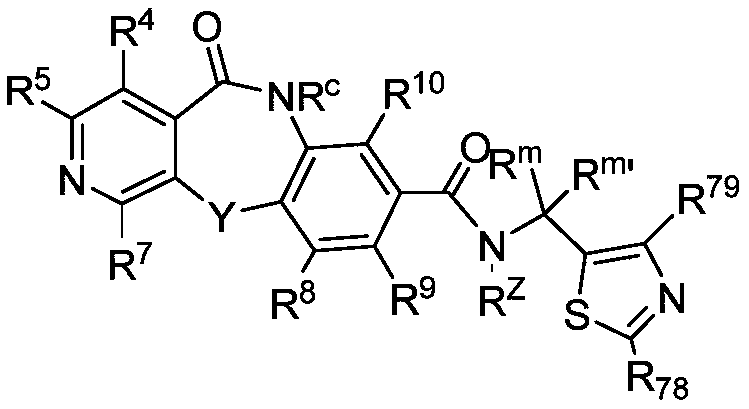
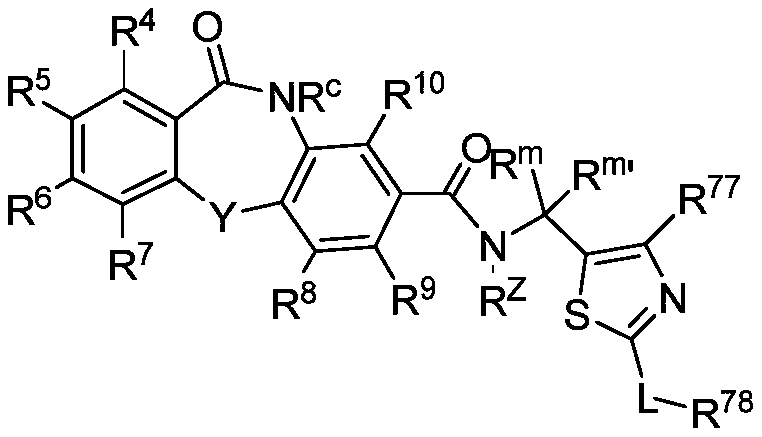
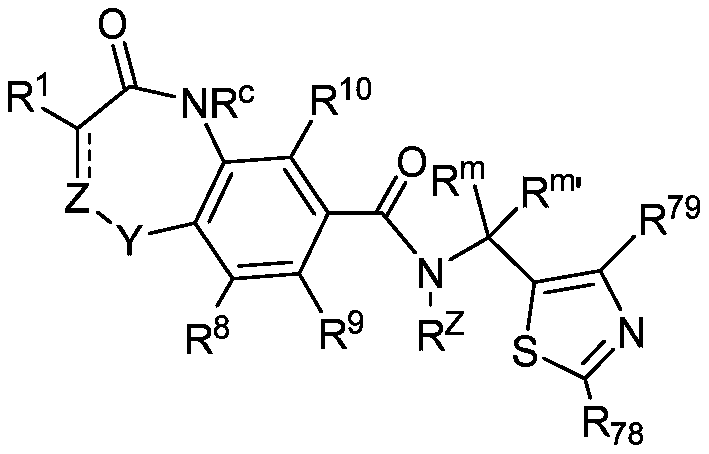
Hepatitis b core protein modulators
An ethyl and compound technology, applied in the field of hepatitis B core protein regulator, can solve the problems of poor tolerance of interferon alpha side effects and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0365] Example 1: Synthesis of 11-oxo-10,11-dihydrodibenzo[b,f][1,4]thiazepine-8-carboxylic acid 5,5- Dioxide(9)
[0366]
[0367] Synthesis of methyl 4-((2-(methoxycarbonyl)phenyl)thio)-3-nitrobenzoate (3):
[0368]
[0369] To a stirred solution of methyl 4-fluoro-3-nitrobenzoate 2 (30 g, 150.67 mmol) in DMF (300 mL) at room temperature under an inert atmosphere was added cesium carbonate (58.76 g, 180.8 mmol) and 2- Methyl mercaptobenzoate 1 (22.6 mL, 165.47 mmol); heated to 55-60°C and stirred for 2 hours. The reaction was monitored by TLC; after completion of the reaction, the reaction mixture was diluted with water (1500 mL) and the precipitated solid was filtered to obtain a crude product. The crude product was washed with water (500 mL), hexane (200 mL) and dried in vacuo to give compound 3 (48.8 g, 93%) as a yellow solid. TLC: 20% EtOAc / hexane (R f :0.4); 1 H NMR (CDCl 3 ,400MHz): δ8.85(s,1H),7.99-7.92(m,2H),7.66-7.56(m,3H),6.93(d,J=8.6Hz,1H),3.94(s,3H),...
Embodiment 2
[0388] Example 2: Synthesis of tert-butyl 4-(5-(aminomethyl)thiazol-2-yl)piperidine-1-carboxylate (18)
[0389]
[0390] Synthesis of tert-butyl 4-(5-(((tert-butyldimethylsilyl)oxy)methyl)thiazol-2-yl)-4-hydroxypiperidine-1-carboxylate (12):
[0391] To a stirred solution of 5-(((tert-butyldimethylsilyl)oxy)methyl)thiazole 10 (5 g, 21.83 mmol) in anhydrous THF (100 mL) at -78 °C under an inert atmosphere n-BuLi (1.6M, hexane solution, 22.0 mL, 1.2 mmol) was added dropwise to the solution over 15 minutes, and stirred for 2 h. At -78°C, tert-butyl 4-oxopiperidine-1-carboxylate 11 (4.8 g, 24.01 mmol) was added thereto and stirred at the same temperature for 2 h. The reaction was monitored by TLC; upon completion, the reaction mixture was quenched by saturated ammonium chloride (20 mL) and extracted with EtOAc (2 x 100 mL). The combined organic extracts were dried over sodium sulfate, filtered and concentrated in vacuo to obtain crude product. The crude product was purifie...
Embodiment 3
[0404] Example 3: Synthesis of (2-(3,3-dimethylcyclopentyl)thiazol-5-yl)methanamine hydrochloride (27)
[0405]
[0406] Synthesis of 3,3-Dimethylcyclopent-1-one(20)
[0407] To a stirred solution of cuprous iodide (12 g, 62.5 mmol) in diethyl ether (200 mL) was added methyllithium (65 mL, 104.1 mmol) dropwise over 1 h at 0 °C under an inert atmosphere. The reaction mixture was stirred at 0 °C for 2 h.
[0408] To a stirred solution of 3-methylcyclopent-2-en-1-one 19 (5 g, 52 mmol) in ether (50 mL) was added dropwise the above reaction mixture at 0 °C under an inert atmosphere. The reaction mixture was stirred at 0 °C for 2 h. The reaction was monitored by TLC; after completion of the reaction, the reaction mixture was poured into sodium sulfate hydrate (50 mL) and stirred for 30 min. The reaction mixture was filtered through celite. The filtrate was dried over sodium sulfate and concentrated in vacuo to obtain crude product. The crude product was purified by column ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com