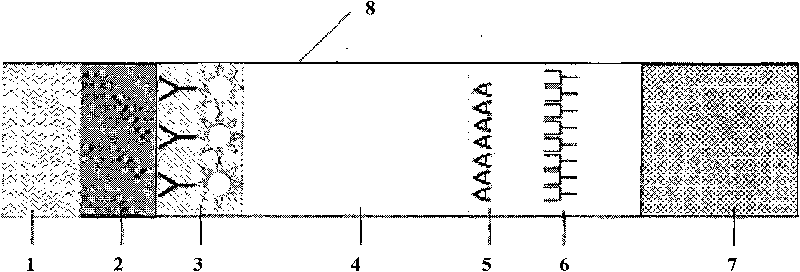
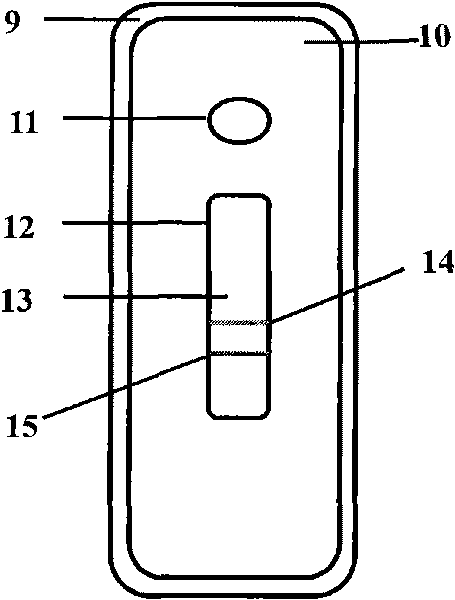
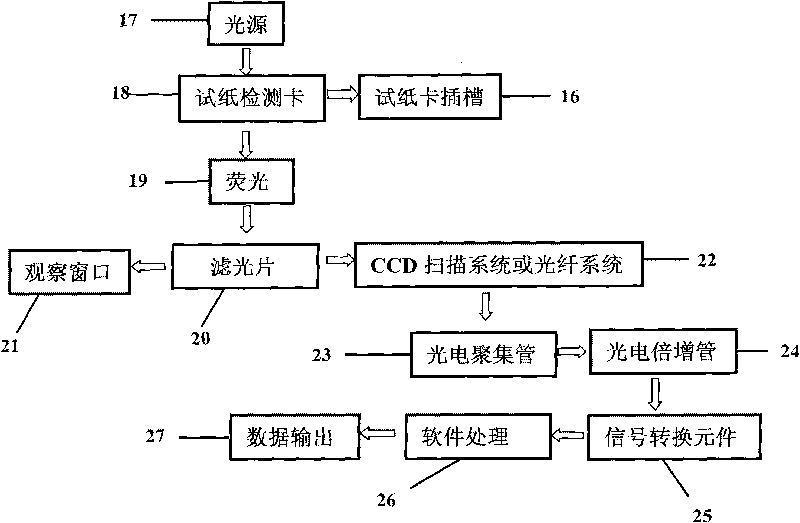
Fluorescent microsphere immunochromatographic testing card for testing five indexes of hepatitis b and method for preparing same
A technology of fluorescent microspheres and immunochromatography, applied in the field of medical testing, can solve problems such as poor anti-solution interference and easy leakage of dyes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] Example 1 Preparation and detection method of isothiocyanate fluorescent microsphere immunochromatography test strip A for detecting HBsAg
[0065] (1) Preparation of fluorescein isothiocyanate-silica-shell-core dual-structure fluorescent microspheres:
[0066] Mix 60ml of absolute ethanol, 2.5ml of ammonia water, 4ml of ethyl orthosilicate, 1ml of ultrapure water, and 1mg of fluorescein isothiocyanate, and react in a constant temperature water bath at 80°C for 6 hours, then add Na 2 SiO 3 Closed microspheres, Na 2 SiO 3 The final concentration of the fluorescent microspheres was 3%, and the size of the fluorescent microspheres was controlled by adding hydrochloric acid to adjust the pH value of the solution, and the fluorescent microspheres with the required particle size were synthesized, and the active groups (-COOH) were modified on the surface of the microspheres.
[0067] (2), preparation of fluorescent microsphere-labeled anti-HBs monoclonal antibody S1 (EDC m...
Embodiment 2
[0090] Example 2 Preparation and Detection Method of Tetramethylrhodamine Isothiocyanate Fluorescent Microspheres Immunochromatography Test Strip B for Detecting HBeAg
[0091] (1), preparation of rhodamine tetramethylisothiocyanate fluorescent microspheres
[0092] Mix 10ml of cyclohexane, 12ml of n-hexanol and 5ml of Triton X-100- uniformly in a ratio of 2:1.2:1, add an appropriate amount of water as a dispersed phase, and after ultrasonic emulsification, add 0.5mg of rhodamine tetramethylisothiocyanate, Stir for 20 minutes, add a certain amount of ammonia water until the solution is clear, add 2.5ml of tetraethyl orthosilicate and stir for 2 minutes, add 2ml of aminoethylaminopropyl polydimethylsiloxane (AEAPS), mix well, stir for 24 hours, and then The microparticles obtained by repeated washing and centrifugation are the prepared rhodamine isothiocyanate fluorescent microspheres.
[0093] (2), preparation of anti-HBe monoclonal antibody labeled with fluorescent microsphe...
Embodiment 3
[0107] Example 3 Preparation and detection method of isothiocyanate fluorescent microsphere immunochromatography test strip C for detecting anti-HBs
[0108] (1) The preparation of fluorescein isothiocyanate-silica shell-core dual-structure fluorescent microspheres is as in Example 1.
[0109] (2), preparation of fluorescent microsphere-labeled HBsAg (EDC method):
[0110] Take 1mg of fluorescent microspheres coated with fluorescein isothiocyanate and centrifuge at 1000×g for 10-15min, collect the precipitate, and adjust the concentration of the microspheres to OD with 0.01M borate buffer solution at pH 4.8 450= 0.2. Then add 90μL 50mg / mL p-ethyl-N,N-dimethylpropylcarbodiimide (EDC), 150μL 5mg / mL nitrogen hydroxysuccinimide (NHS), shake and mix well, and incubate at room temperature for 10-30min Afterwards, centrifuge at 1000×g for 5-15min, dissolve the precipitate with 0.01M pH4.8 borate buffer, and adjust the concentration of microspheres to OD 450 0.2 to 1. Add 1 to 20 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com