Cyclic sulfamide compounds and methods of using same
A compound and formyl technology, applied to cyclic sulfonamide compounds and their application fields, can solve problems such as poor side effect tolerance of interferon alpha
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
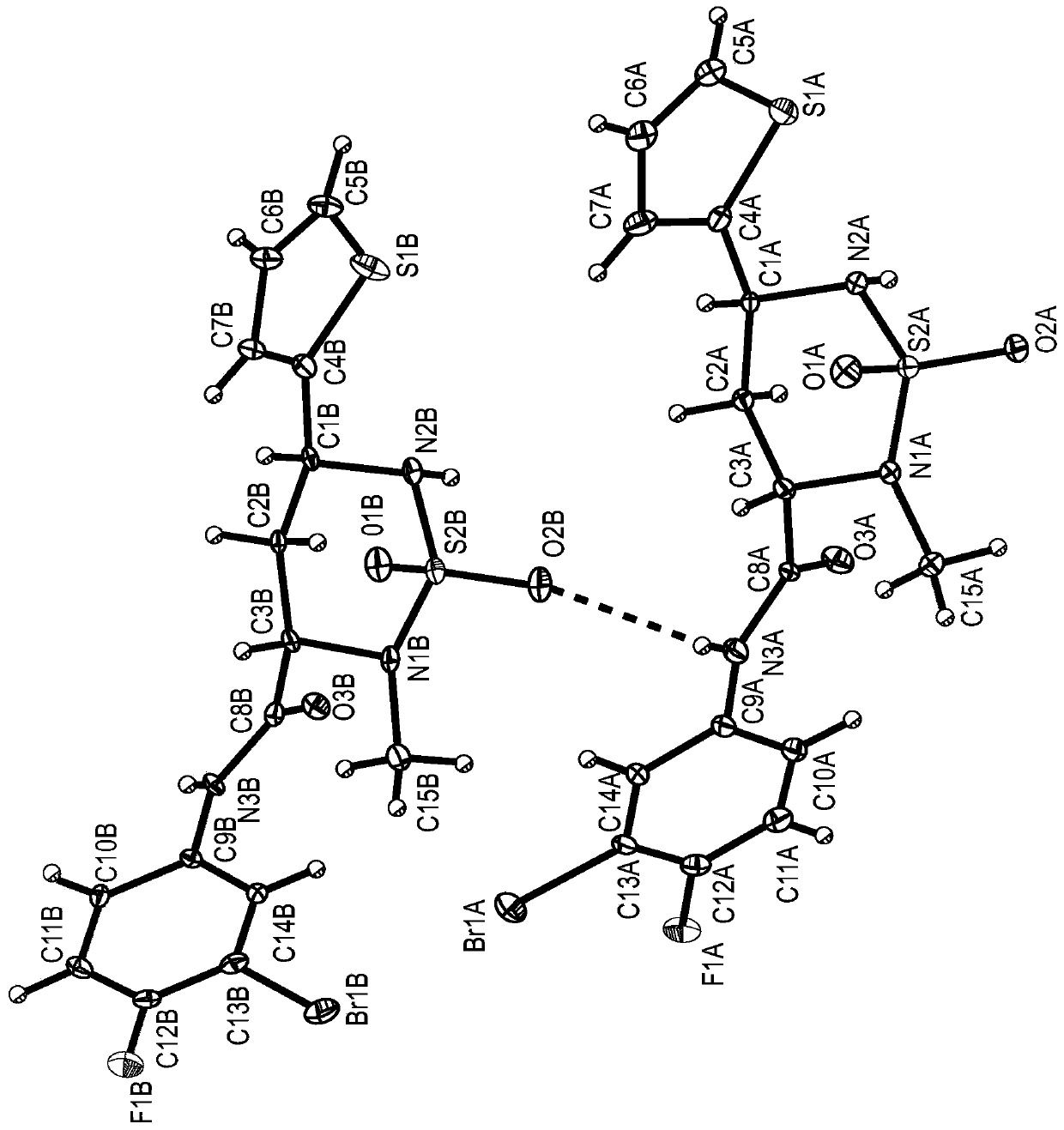
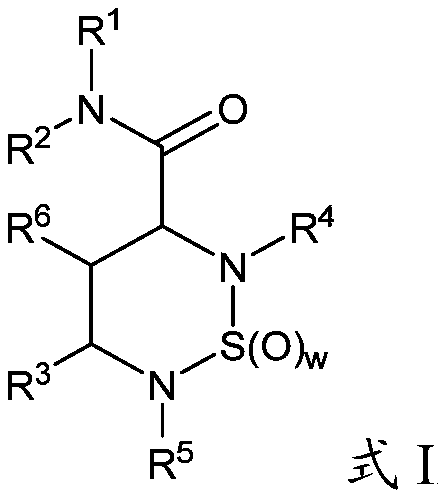
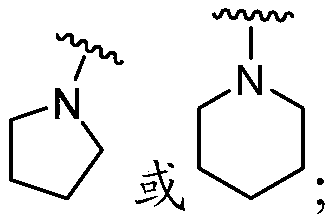
[0379]The compounds described herein can be prepared in a variety of ways based on the teachings contained herein and synthetic procedures known in the art. In the description of the synthetic methods described hereinafter, it is understood that, unless otherwise mentioned, all suggested reaction conditions, including solvent, reaction atmosphere, reaction temperature, duration of experiments and selection of work-up steps, can be chosen as Standard conditions for this reaction. Those skilled in the art of organic synthesis will appreciate that the functionality present in each part of the molecule should be compatible with the proposed reagents and reactions. Substituents which are incompatible with the reaction conditions will be apparent to those skilled in the art and alternatives are thus indicated. Starting materials used in the examples are either commercially available or readily prepared by standard methods from known materials. At least some of the compounds identi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com