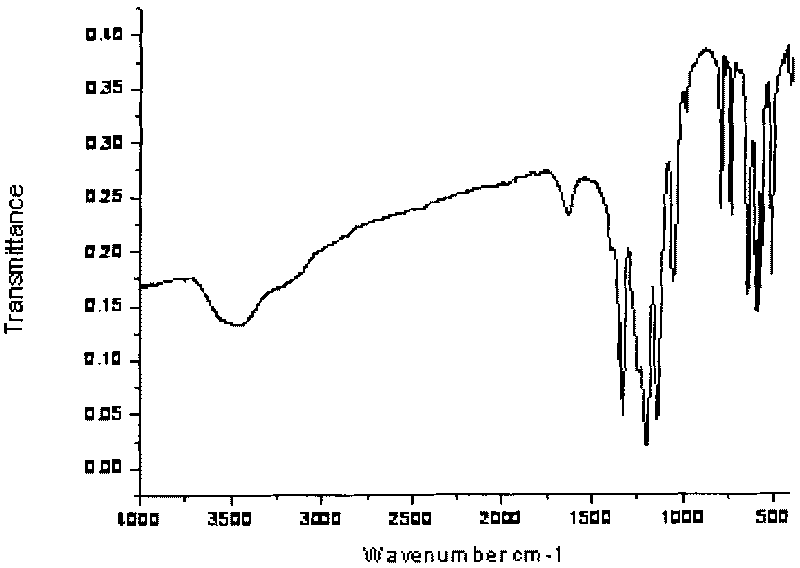
Method for synthesizing perfluoroalkyl sulfimide alkali metal salt and ionic liquid synthesized by same
A technology of perfluoroalkyl sulfonimide and alkali metal salt, applied in the field of organic fluorine chemical synthesis, can solve the problem of rare ionic liquid, meet the requirements of high current charge and discharge, speed up the reaction and improve the yield Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-12
[0058] Embodiment 1-12 relates to the preparation of M[PFSI]
Embodiment 1
[0059] Example 1: Preparation of potassium bis(trifluoromethylsulfonyl)imide (K[TFSI]).
[0060] The synthesis reaction scheme is as follows:
[0061]
[0062] 26.4 grams (0.14mol) trifluoromethanesulfonamide potassium salt, 13.4 grams (0.097mol) anhydrous potassium carbonate are placed in the three-necked flask of 250 milliliters, add 150 milliliters of acetonitriles as solvent, then add 24.0 grams (0.14mol) trifluoromethanesulfonyl chloride, stirred at room temperature for 8 hours.
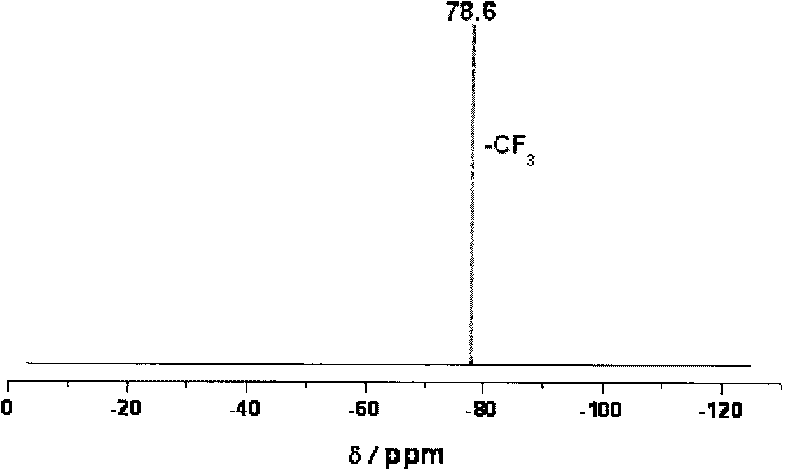
[0063] The solvent was distilled off under reduced pressure to obtain a white solid, which was added with 150 ml of tetrahydrofuran, stirred at room temperature for 0.5 hour, and the insoluble matter was removed by filtration. The filtrate was distilled under reduced pressure, and the remaining solid was recrystallized with n-butanol. After filtration and drying, 39.2 g (0.12 mol) of K[TFSI] solid was obtained, with a yield of 88%. 19 F NMR (acetonitrile-d 3 , CCl 3 F, 376.5MHz): δ=-78.6p...
Embodiment 2
[0064] Example 2: Preparation of lithium bis(trifluoromethylsulfonyl)imide (Li[TFSI]).
[0065] The synthesis reaction scheme is as follows:
[0066] K[N(SO 2 CF 3 ) 2 ]+LiClO 4 →Li[N(SO 2 CF 3 ) 2 ]+KClO 4
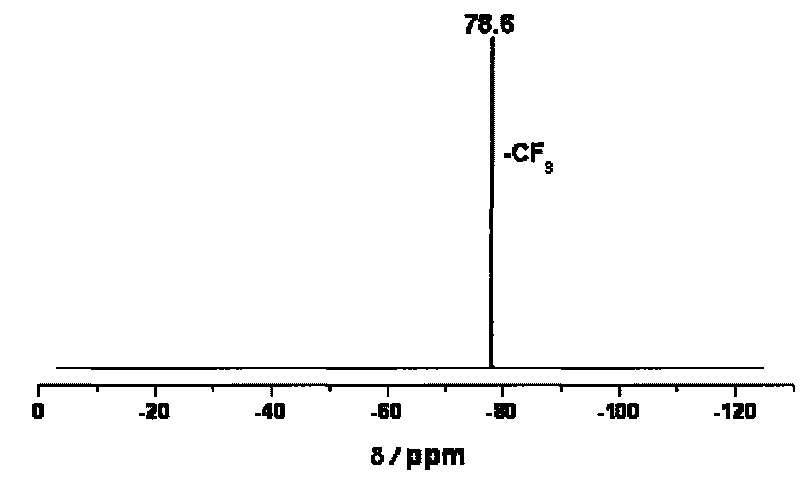
[0067]In a vacuum glove box, 31.9 g (0.1 mol) of K[TSFI] was dissolved in 50 ml of acetonitrile solvent, and then equimolar lithium perchlorate was added in portions. After stirring the reaction at room temperature for 20 hours, insoluble potassium perchlorate was removed by filtration. Concentrate the filtrate to about 20 mL, add an equal volume of CH 2 Cl 2 Perform recrystallization. Filtration, CH 2 Cl 2 After washing and vacuum drying, 27.3 g (0.095 mol) of white solid powder Li[TFSI] was obtained. 19 F NMR (acetonitrile-d 3 , CCl 3 F, 376.5MHz): δ=-78.8ppm(s).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com