Gene engineering vaccine used for preventing pig cysticercosis and its preparation method
A genetically engineered vaccine, porcine cysticercosis technology, applied in the field of genetic engineering vaccine for preventing porcine cysticercosis and its preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
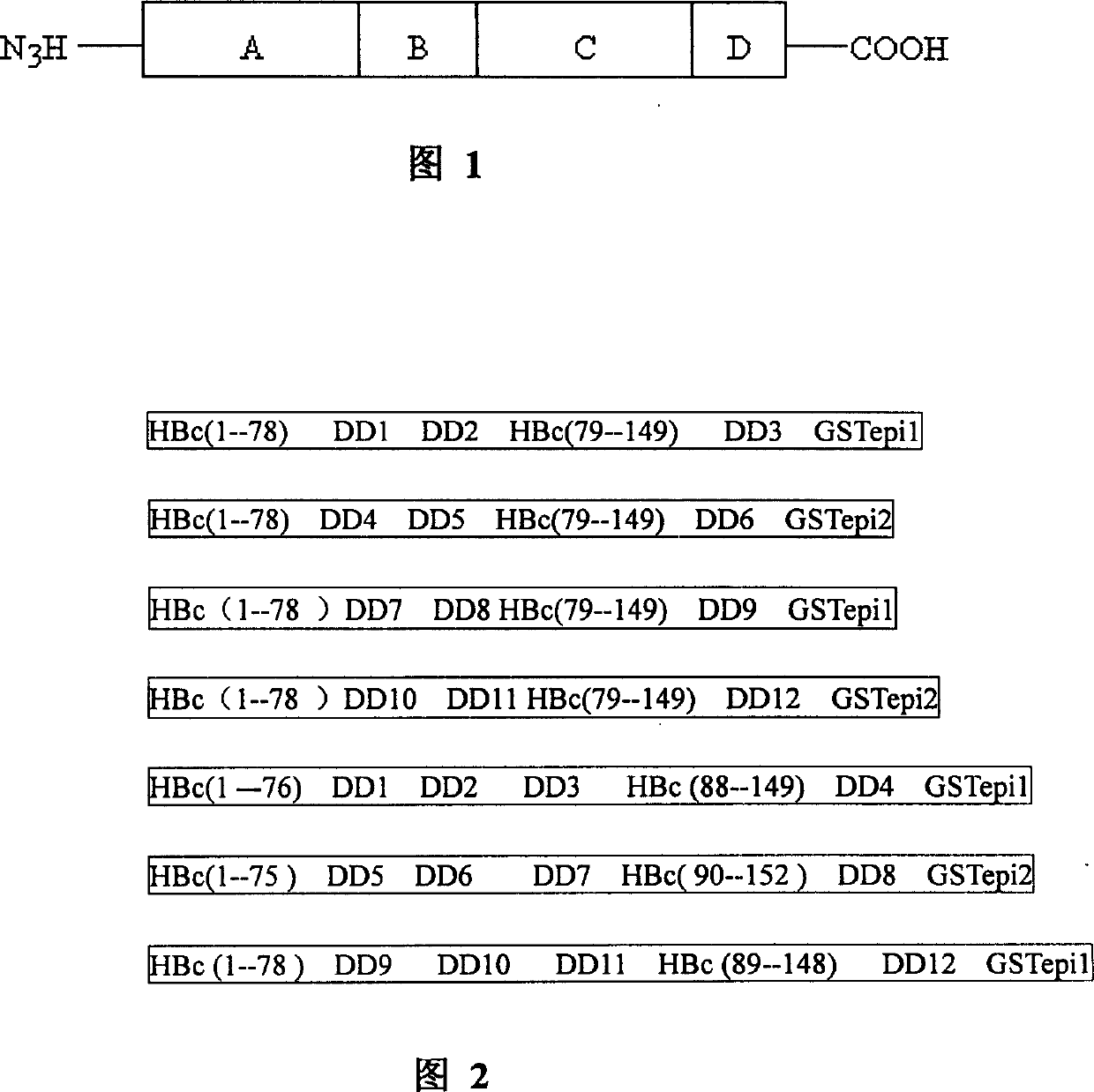
[0040] The preparation method of the genetic engineering vaccine of the present invention takes the hepatitis B virus core protein as a carrier, utilizes molecular biology techniques and methods, and multiple positions between the 1st amino acid to the 183rd amino acid of the hepatitis B virus core protein (HBc) gene Insert the protective antigen gene of cysticercus porcine into the prokaryotic expression plasmid (the prokaryotic expression plasmid can be: pET28a, pET18, pET32, pQE32), transform the host bacteria, and construct engineering bacteria. Induced expression, obtaining a large amount of target protein and purifying it, can obtain the subunit protein vaccine for preventing porcine cysticercosis; or, using the core protein gene of hepatitis B virus as a carrier, using molecular biology techniques and methods, in the core of hepatitis B virus Cysticercus porcine protective antigen genes are inserted at multiple positions between the 1st amino acid to the 183rd amino acid...
Embodiment 1
[0047] As shown in Figure 1, using molecular biology techniques and methods, the epitopes KETc1(n1):APMSTPSATSVRG and KETc12(n2):GNLLLSCLG were inserted between the 78 and 79 amino acid sequences of HBc149, and the epitope GK-1(n3) : GYYYPSDPNTFYAPPYSA was fused to the 149th amino acid to construct the prokaryotic expression plasmid pET28a-Δc-3n. After the correct sequence was proved by SDS-PAGE and sequencing, the engineered bacteria were cultivated on a large scale and expressed, and the subunit protein was obtained after purification Vaccine, this recombinant protein is named as PCCE, and it is quantified by ultraviolet spectrophotometer.
[0048] Mice were immunized by intramuscular injection, supplemented with Freund's incomplete adjuvant, boosted once 3 weeks later, and then boosted again 4 weeks later, a total of three injections. The sera of the mice in the test group and the control group were collected at 1, 3, 5, 7, and 9 weeks after immunization, and the anti-PCCE ...
Embodiment 2
[0051] On the basis of the above example, using molecular biology techniques and methods, the signal peptide gene sequence of human IL-2 was added to the 5' end of the PCCE protein gene sequence, and cloned into the eukaryotic expression plasmid pVAX3.0 to construct a nucleic acid Vaccine pVAX-S-Δc-3n. After the sequencing is correct, a large number of engineered bacteria are cultivated, the plasmid is extracted, purified through a column, and quantified by an ultraviolet spectrophotometer to obtain a nucleic acid vaccine.
[0052] The one-month-old piglets were immunized with nucleic acid vaccine, and boosted once three weeks later. Two weeks after the booster immunization, 20,000 fresh infectious tapeworm eggs were administered to each pig. Three months later, the pigs were killed by bloodletting. The pathological changes of muscle tissue were dissected to calculate the protection rate.
[0053] The experimental results showed that the relative protection rate of nucleic ac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com