Hepatitis B core protein allosteric modulators
A compound, selected technology, applied in the directions of plant growth regulators, biocides, antiviral agents, etc., can solve problems such as poor tolerance to side effects of interferon alpha
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
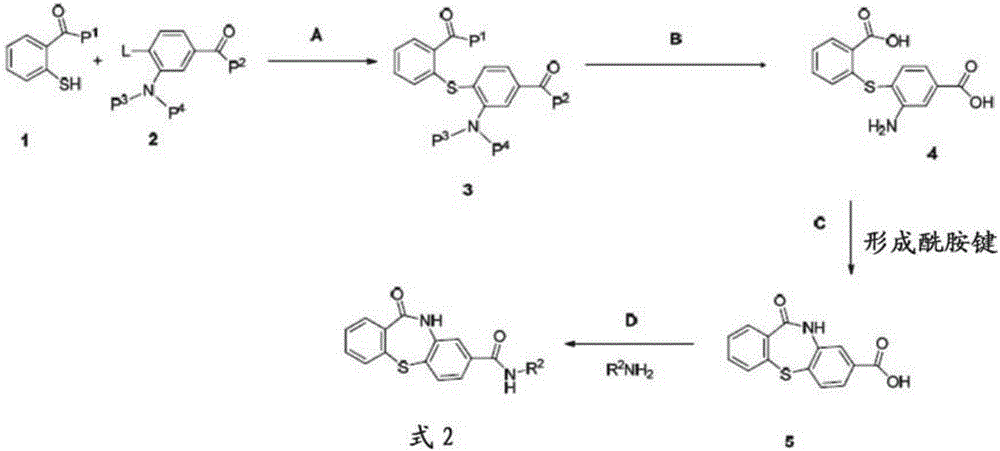
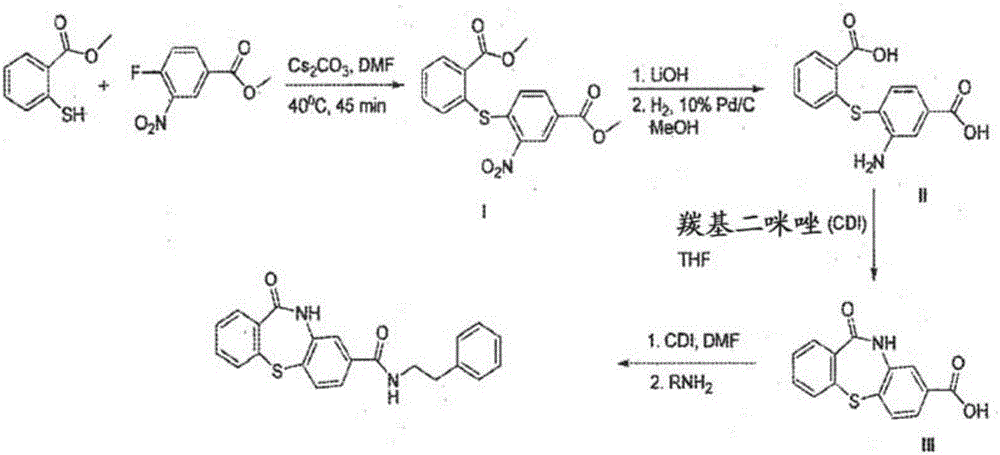
[0183] Example 1: 11-oxo-10,11-dihydrodibenzo[b,f][1,4]thiazepine -8-Formic acid (6)-common Synthesis of Intermediates
[0184]
[0185] Synthesis of methyl 4-((2-(methoxycarbonyl)phenyl)thio)-3-nitrobenzoate (3):
[0186]
[0187] To a stirred solution of methyl 4-fluoro-3-nitrobenzoate 2 (30 g, 150.67 mmol) in DMF (300 mL) was added cesium carbonate (58.76 g, 180.8 mmol) and 2- Methyl mercaptobenzoate 1 (22.6 mL, 165.47 mmol); heated to 55-60° C. and stirred for 2 h. The reaction was monitored by TLC; after the reaction was complete, the reaction mixture was diluted with water (1500 mL) and the precipitated solid was filtered to give the crude product. The crude product was washed with water (500 mL), hexane (200 mL) and dried in vacuo to give compound 3 (48.8 g, 93%) as a yellow solid. TLC: 20% EtOAc / hexane (R f :0.4); 1 H NMR (CDCl 3 , 400MHz): δ8.85(s, 1H), 7.99-7.92(m, 2H), 7.66-7.56(m, 3H), 6.93(d, J=8.6Hz, 1H), 3.94(s, 3H), 3.79 (s, 3H).
[0188] S...
Embodiment 2
[0197] Example 2: 2-Chloro-11-oxo-10,11-dihydrodibenzo[b,f][1,4]thiazepine -8-Formic acid (14)- Synthesis of common intermediates
[0198]
[0199] Synthesis of 5-chloro-2-((4-methoxybenzyl)thio)benzonitrile (9):
[0200]
[0201] To a stirred solution of 5-chloro-2-fluorobenzonitrile 7 (1 g, 6.41 mmol) in DMF (10 mL) was added cesium carbonate (2.30 g, 7.05 mmol) at room temperature under an inert atmosphere; heated to 40 °C and To this was added (4-methoxyphenyl)methanethiol 8 (1.08 g, 7.05 mmol); heated to 60° C. and stirred for 2 h. The reaction was monitored by TLC; after the reaction was complete, the reaction mixture was diluted with water (20 mL) and extracted with EtOAc (2 x 25 mL). The combined organic extracts were dried over sodium sulfate, filtered and concentrated in vacuo to give crude product. The crude product was purified by silica gel column chromatography, eluting with 3-5% EtOAc / hexanes, to afford compound 9 (1 g, 54%) as a white solid. TL...
Embodiment 3
[0217] Example 3: 3-Chloro-11-oxo-10,11-dihydrodibenzo[b,f][1,4]thiazepine -8-Formic acid (21)- Synthesis of Common Intermediates
[0218]
[0219] Synthesis of 4-chloro-2-((4-methoxybenzyl)thio)benzonitrile (16):
[0220]
[0221] To a stirred solution of 4-chloro-2-fluorobenzonitrile 15 (1 g, 6.41 mmol) in DMF (25 mL) was added cesium carbonate (2.30 g, 7.05 mmol) at room temperature under an inert atmosphere at room temperature; heated to 40 °C and (4-methoxyphenyl)methanol 8 (1.08 g, 7.05 mmol) was added thereto; heated to 60 °C and stirred for 2 h. The reaction was monitored by TLC; after the reaction was complete, the reaction mixture was diluted with water (20 mL) and extracted with EtOAc (2 x 25 mL). The combined organic extracts were dried over sodium sulfate, filtered and concentrated in vacuo to give crude product. The crude product was purified by silica gel column chromatography using 4% EtOAc / hexanes to afford compound 16 (900 mg, 48%) as a white ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com