Recombinant plasmid DNA vaccine composition for treating Hepatitis B
A technology of DNA vaccines and recombinant plasmids, which is applied in the field of biomedicine and can solve problems such as the unsatisfactory immune effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
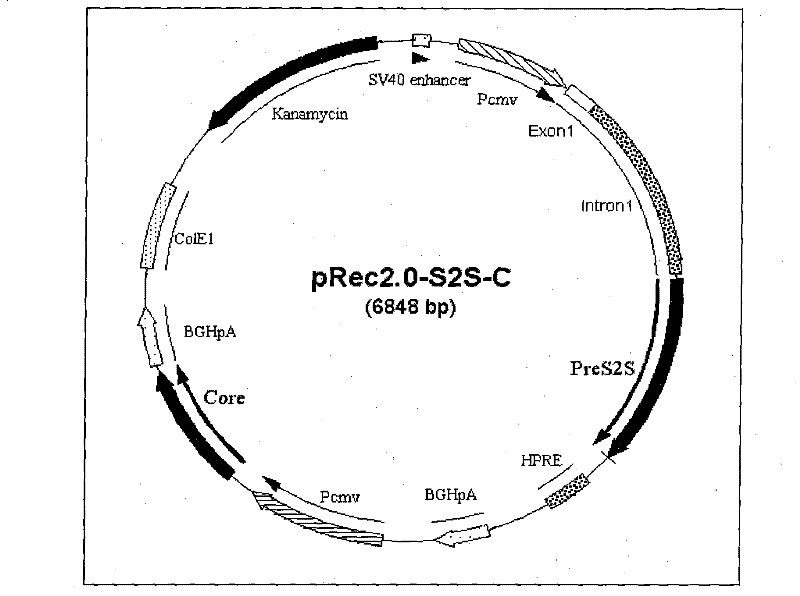
[0076] Example 1 Construction of recombinant plasmid pRec2.0-preS2S-C (pS2S-C)
[0077] (1) First construct the recombinant plasmid vector backbone pOE-EKS:
[0078] Using pDRVISV1.0 as a template, Primer1 and Primer2 as primers, among which primer 2 is a primer phosphorylated at the 5' end, amplified to obtain a replicon region (Ori) with a size of 748bp, and introducing EcoRI, KpnI and SwaI restriction sites at the same time point;
[0079] Primer1: 5'-GGAATTCGGGGTACCATTTAAATTTGAACGTTCGCAAtATGTGAGCAAAAGGCCAGC-3'
[0080] Primer2: 5'-CGGCGCGCGCCGAAAACGACGATTGCGAACGTTCAACCCGTAGAAAAAGATCAAAGG-3'
[0081] Using pDRVISV1.0 as a template, using Primer3 and Primer4 as primers, among which primer 3 is a primer phosphorylated at the 5' end, amplified to obtain a Kanna resistance marker gene (Kan) with a size of 1056bp, and introducing an EcoRI restriction site ;
[0082] Primer3: 5'-tcgtcgttttcggcgcgcgccgTTGAACGTTCGCAAtTCAAGTCAGCGTAATGCTC-3'
[0083] Primer4: 5'-GGAATTCGGCGCGCG...
Embodiment 2
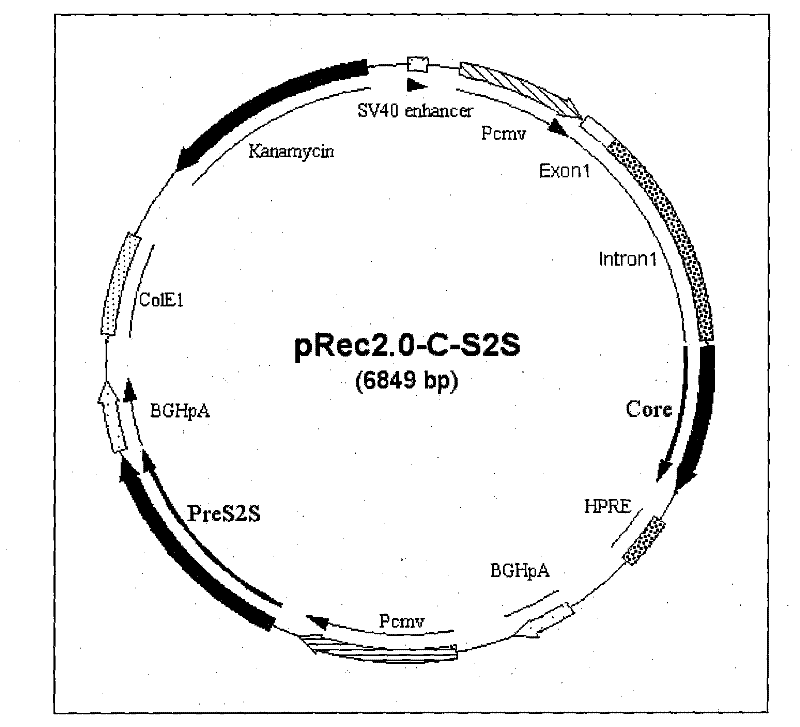
[0103] Example 2 Construction of recombinant plasmid pRec2.0-C-preS2S (pC-S2S)
[0104] Using the pRec2.0-PreS2S constructed in Example 1 as a template, using Primer11 and Primer12 as primers, amplify the PreS2S gene with a size of 873bp, and pass HindIII+XbaI double digestion and HindIII+XbaI double digestion vector pcDNA3 .1 connect, construct and obtain pcDNA3.1-PreS2S;
[0105] Primer11: 5'-CCCAAGCTTGCCGCCACCATGCAGTGGAACTC-3'
[0106] Primer12: 5'-GCTCTAGAATCAGATGTAAACCCAC-3'
[0107] The PreS2S expression cassette was excised from the plasmid pcDNA3.1-PreS2S by BglII+PvuII double enzyme digestion, and cloned into pRec2.0-C which was digested by BglII+EcoRV double enzymes, and the recombinant plasmid pRec2.0-C-PreS2S (pC -S2S).
Embodiment 3
[0108] Example 3 Containing the core protein gene of hepatitis B and not containing the Core protein gene to the humoral and cellular immunity of healthy Balb / c mice
[0109] 6-8-week-old Balb / c female mice were purchased from the Animal Breeding Center of the Chinese Academy of Medical Sciences and kept in clean grade. 24 mice were divided into 4 groups, 3 immunized groups were given pRec2.0 empty vector group, recombinant plasmid DNA vaccine pRec2.0-S2S group, recombinant plasmid DNA vaccine pRec2.0-S2S-C (pS2S-C) , recombinant plasmid DNA vaccine pRec2.0-C-S2S (pC-S2S) for immunization, the dose is 50 μg / monkey, and the immunization procedure is to immunize once in the 0th, 2nd, and 4th weeks, and the rats are killed in the 6th week to get serum and separate lymphocytes.
[0110] Humoral immunity test: take mouse serum in strict accordance with the instructions of Beijing Yuande Biotechnology Co., Ltd. kit.
[0111] 1. Cellular immunity detection: mouse lymphocyte prepara...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com