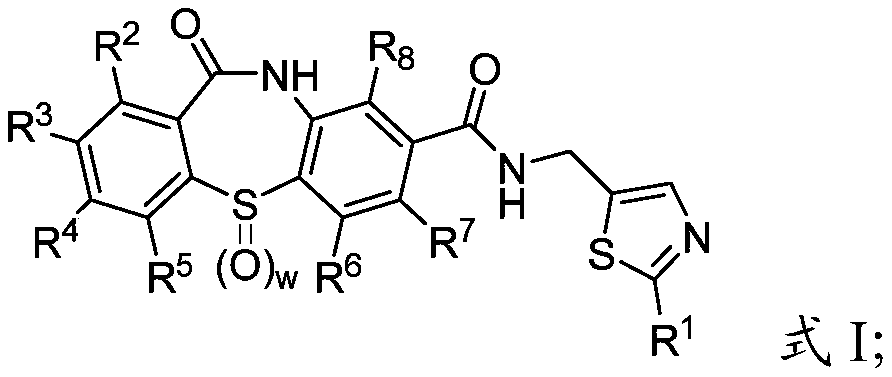
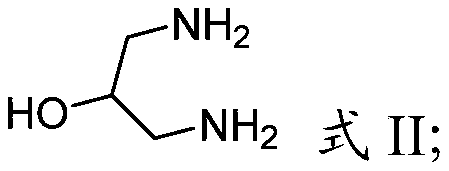
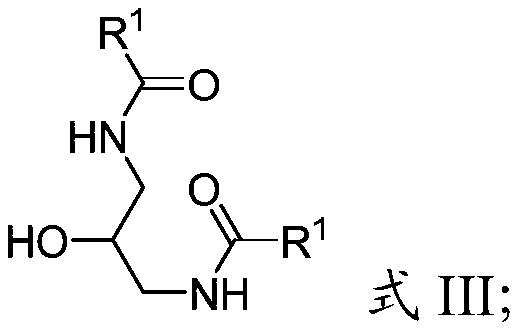
Process for making hepatitis b core protein modulators
A technology of acylating agent and compound, which is applied in the field of preparing hepatitis B core protein regulator, and can solve the problems such as poor tolerance of side effects of interferon alpha
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0121] Example 1: Synthesis of (2-(trifluoromethyl)thiazol-5-yl)methanamine
[0122]
[0123] Step 1: Preparation of 1,3-bis(trifluoroacetylamino)-2-propanol (1-2):
[0124] The title compound was prepared as follows according to the method described in the Supporting Information section of Org. Lett. 2008, 10, 2935-2938. At 0°C, a solution of trifluoroacetic anhydride (7kg, 4.66mol) in 15L of anhydrous acetonitrile was added dropwise to 1,3-diaminopropan-2-ol (1kg, 1.11mol) and triethylamine (3.38 kg; 3.33 mol) in a solution in anhydrous acetonitrile (13.3 L). After 2 hours, the reaction mixture was warmed to room temperature and stirred for 14 hours. The volatiles were removed and the residue was dissolved in ethyl acetate (20 L) and washed sequentially with saturated aqueous sodium bicarbonate, saturated ammonium chloride and brine. The organic layer was dried over anhydrous sodium sulfate, filtered and concentrated to give a solid. The solid was suspended in petrole...
Embodiment 2
[0131] Example 2: Synthesis of (2-ethylthiazol-5-yl)methanamine hydrochloride (2-7) via an alternative route:
[0132]
[0133] Step 1: Synthesis of ethyl 2-chloro-3-oxopropionate (2-2):
[0134] 2-Chloroacetate ethyl 2-1 (5 g, 40.98 mmol) and ethyl formate (3.03 g, 40.98 mmol) in diisopropyl To a solution in ether (100 mL) was added potassium tert-butoxide (5.49 g, 45.08 mmol) in portions; warmed to room temperature and stirred for 24 hours. The reaction was monitored by TLC; upon completion of the reaction, the pH of the reaction mixture was adjusted to about 6 using 5N HCl. The obtained solid was filtered, washed with diethyl ether (200 mL), and dried in vacuo to give compound 2-2 (6 g) as a light brown syrup. TLC: 30% EtOAc / hexane (R f : 0.2); LC-MS: 21.49%+75.58%; 149.0 (M + -1); (column; X-Select C-18, (50×3.0mm, 3.5μm); RT 0.56min, 0.77min.5Mm NH 4 OAc in water: ACN 0.8 mL / min).
[0135] Step 2: Synthesis of ethyl 2-ethylthiazole-5-carboxylate (2-3):
[0136] ...
Embodiment 3
[0145] Example 3: 11-oxo-10,11-dihydrodibenzo[b,f][1,4]thiazepine -Synthesis of 8-formic acid 5,5-dioxide (3-7):
[0146]
[0147] Step 1: Preparation of methyl 4-((2-(methoxycarbonyl)phenyl)thio)-3-nitrobenzoate (3-3):
[0148] Add methyl 4-fluoro-3-nitrobenzoate (300 g, 1.0 equiv) and methyl thiosalicylate (278.7 g, 1.1 equiv) in DMF (1.8 L) under stirring at 0 to 5° C. Cs was added batchwise to the solution in 2 CO 3 (589 g, 1.2 equiv). The reaction mixture was stirred at 0 to 5°C for 30 minutes, warmed to room temperature over 2 hours and stirred at room temperature for 2 hours. The reaction mixture was cooled to 10-15°C, diluted with water (26V) and stirred for 30 minutes. The solid was collected by filtration, washed with water (20V) and n-heptane (10V), and dried under reduced pressure at a temperature below 50°C. The dried solid was suspended in n-heptane (10V) at 90-95°C to form a slurry and cooled to 35-40°C. The solid was collected by filtration, washed w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com