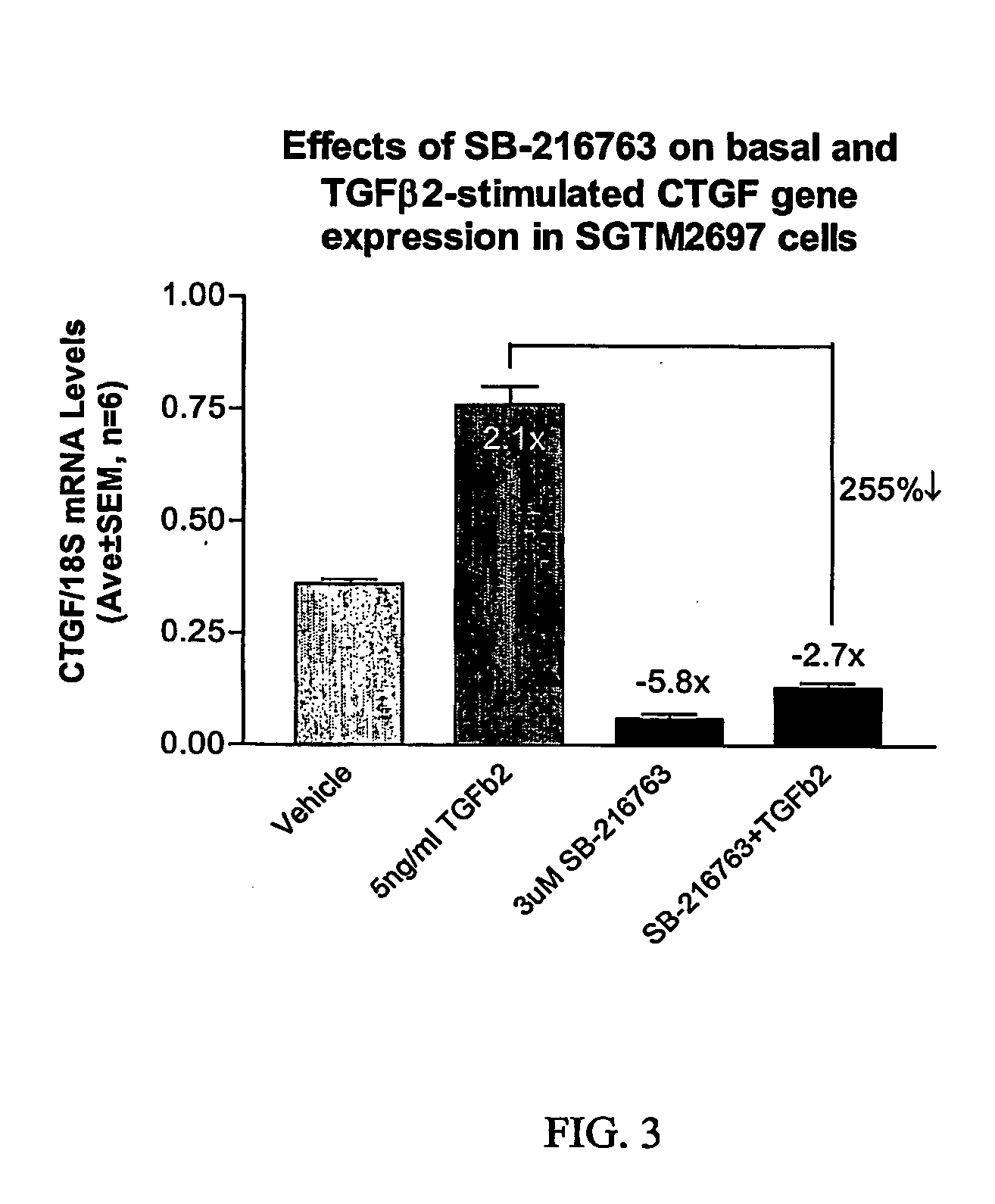
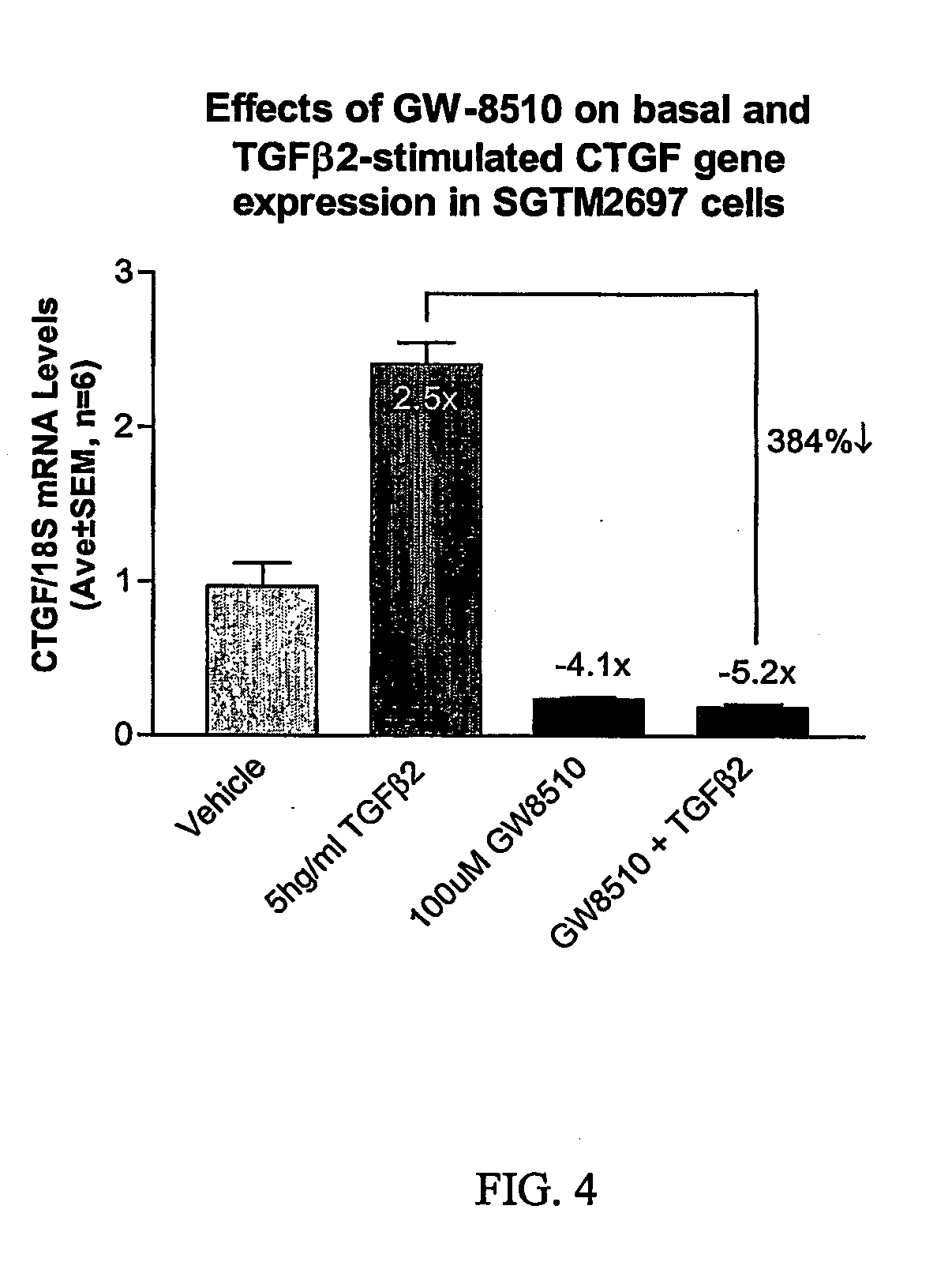
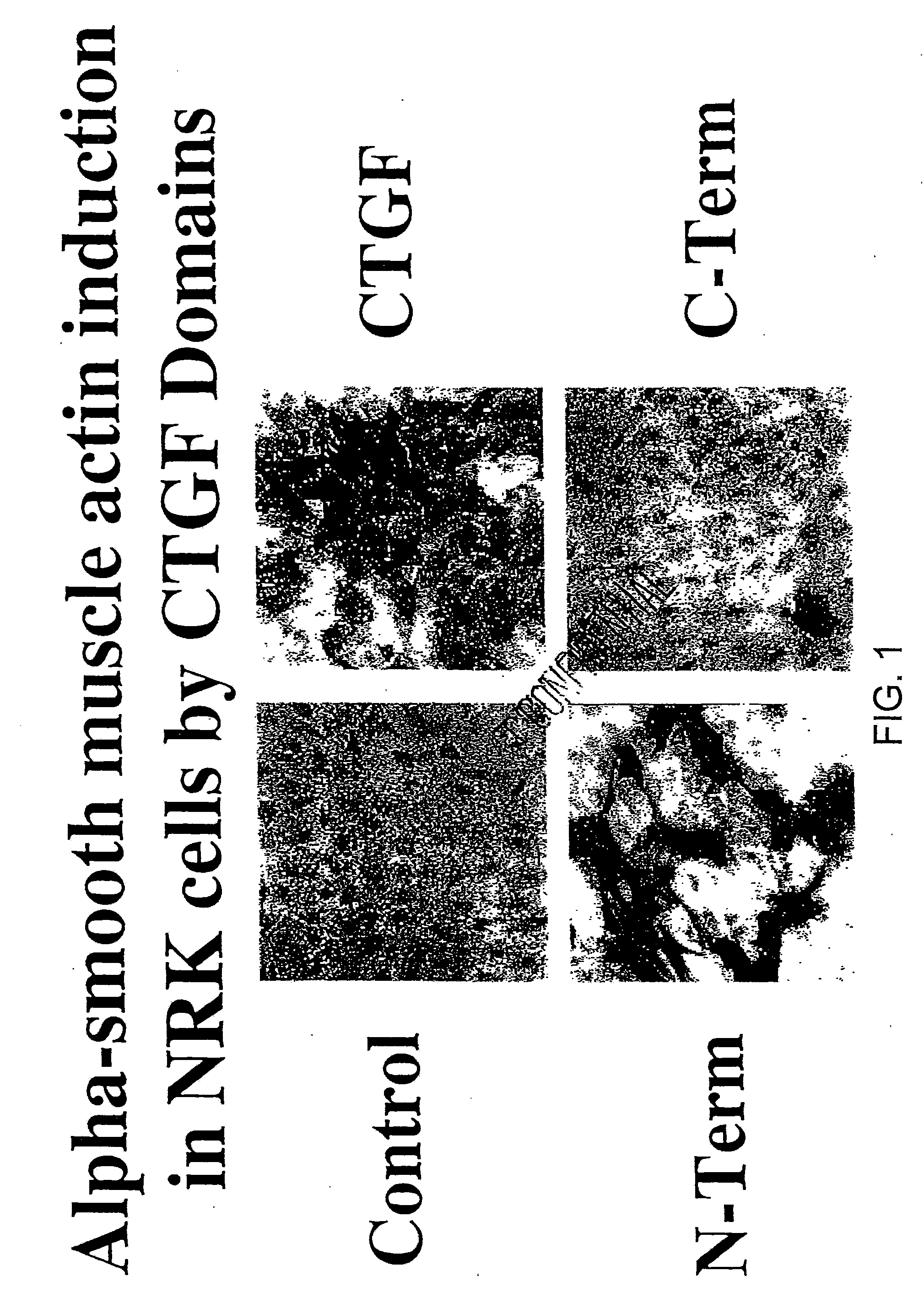
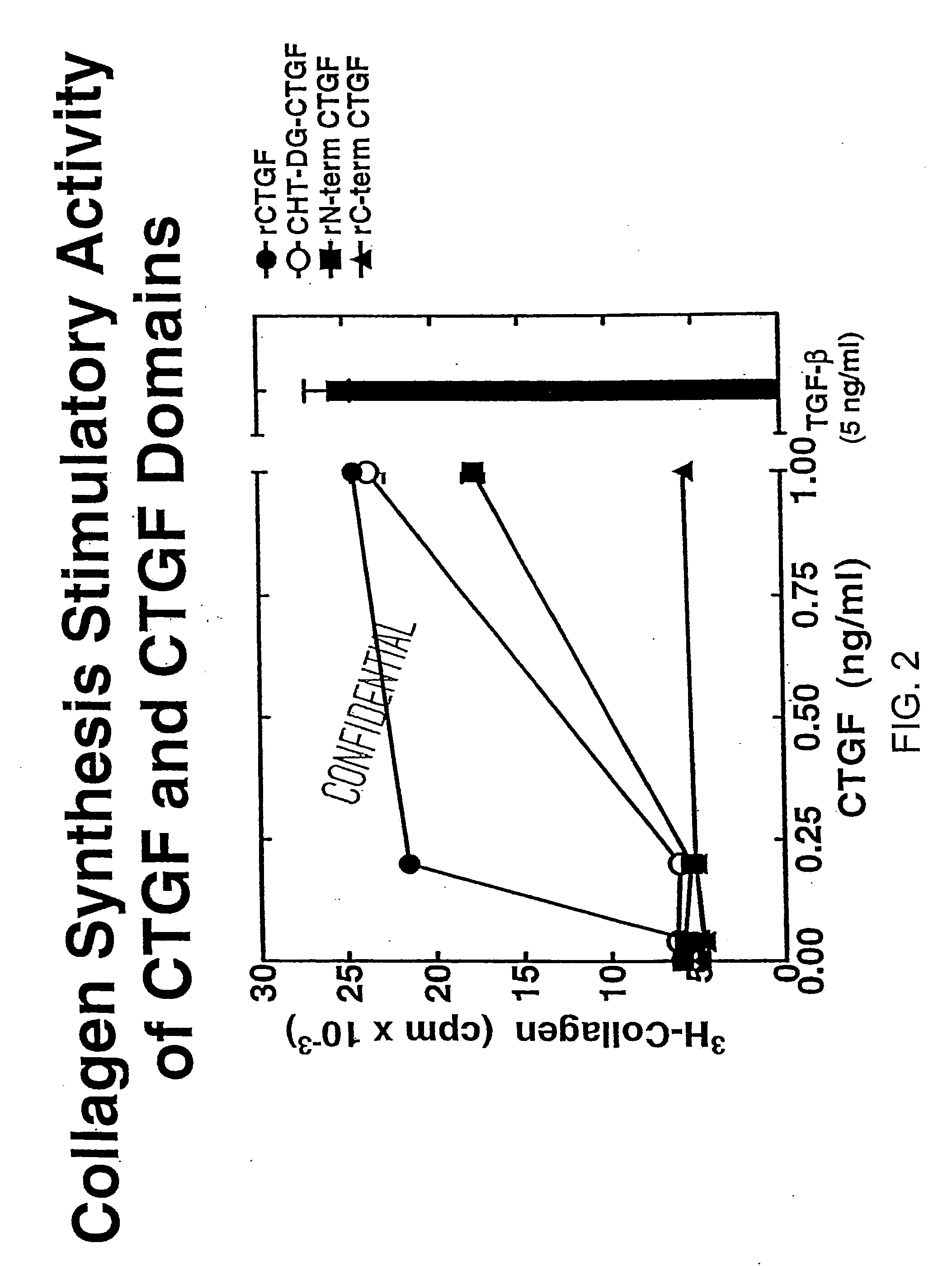
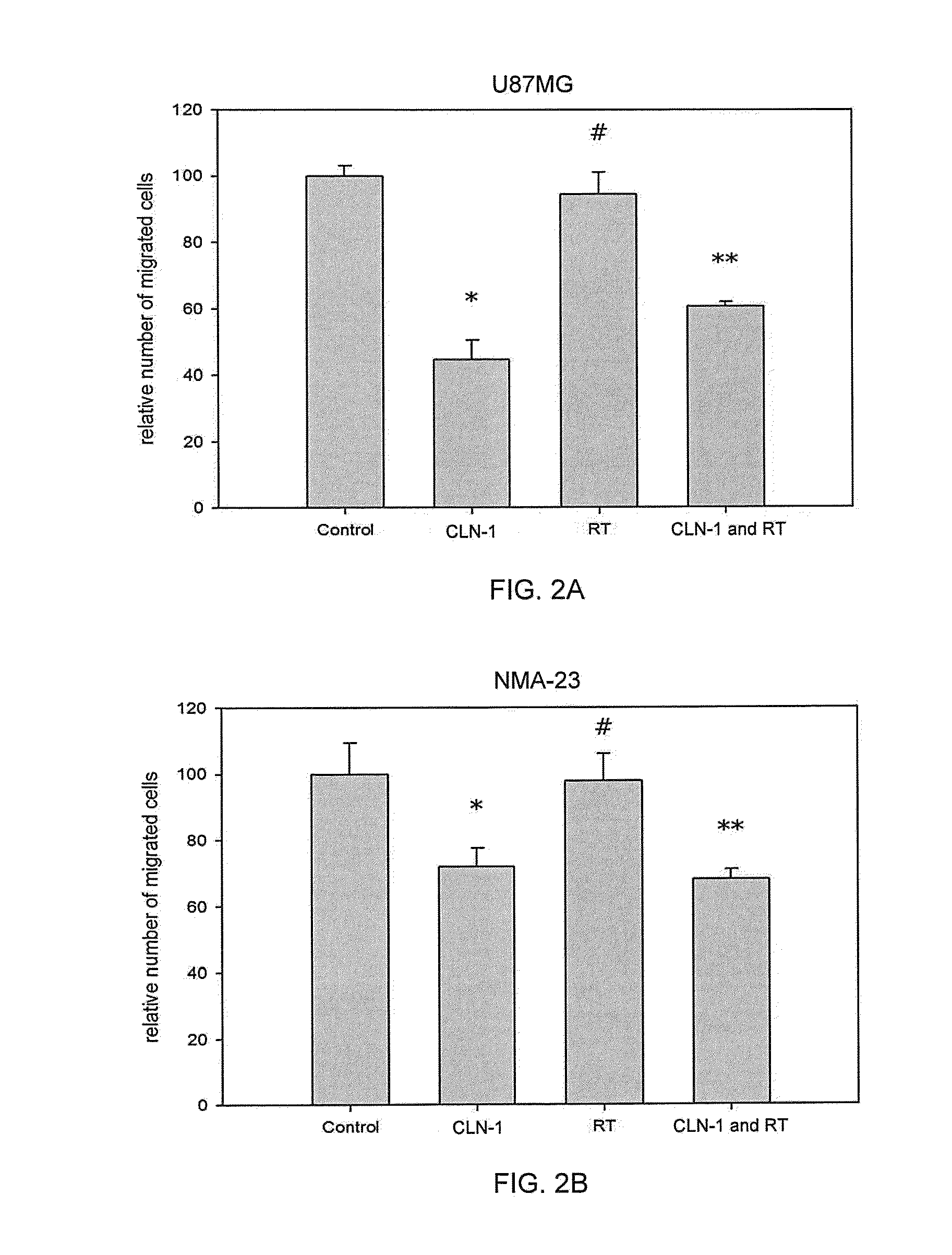
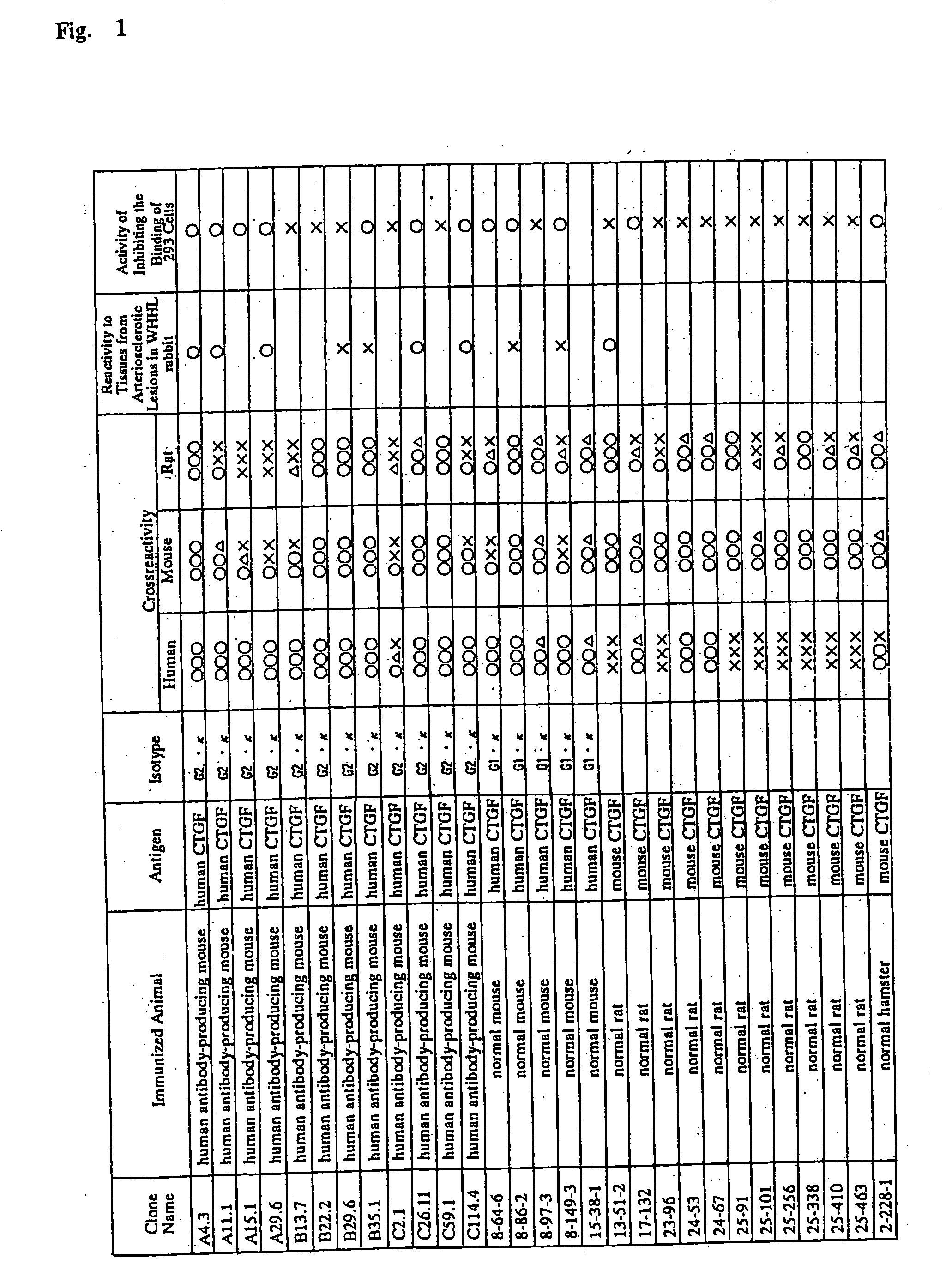
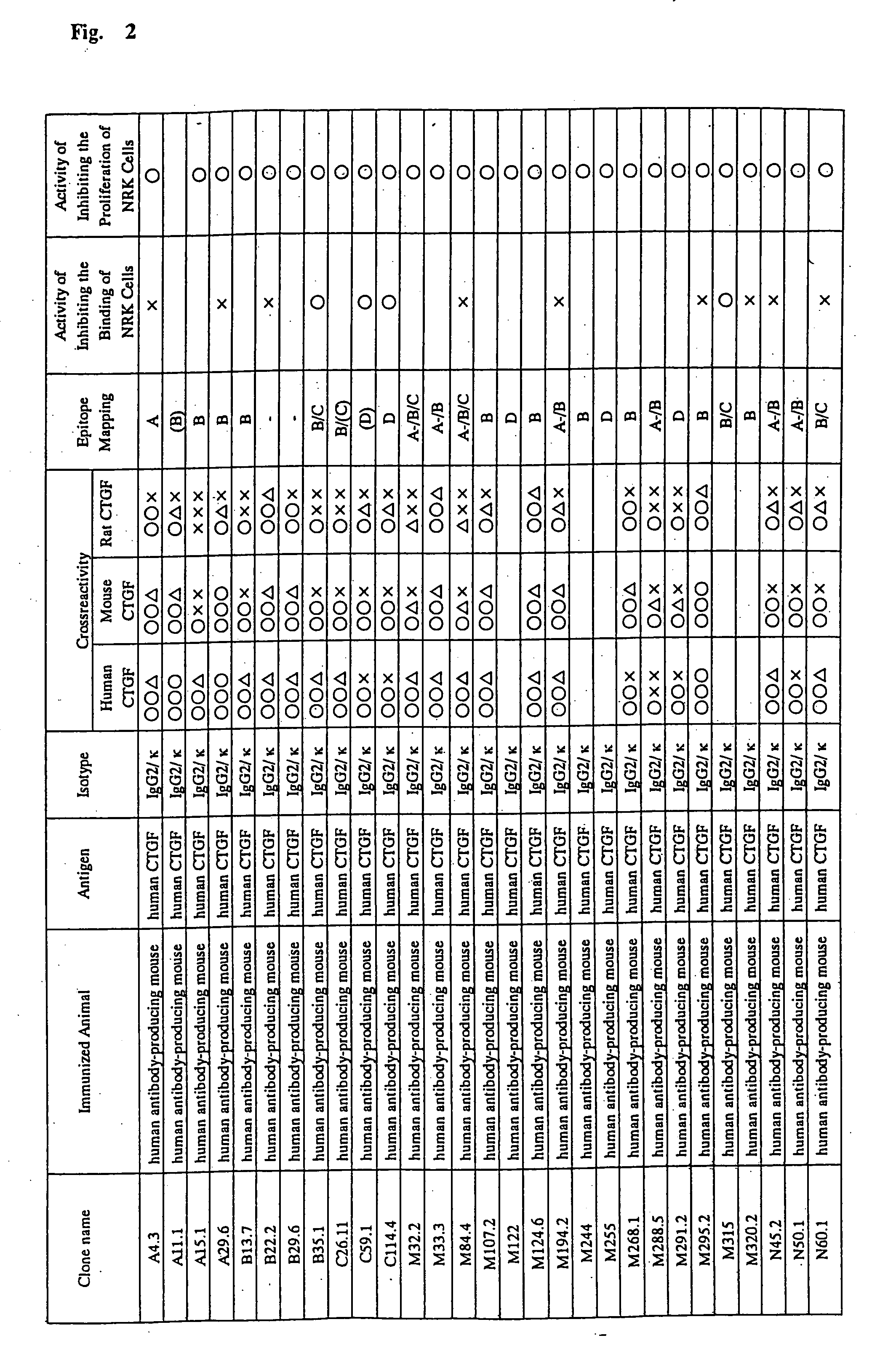
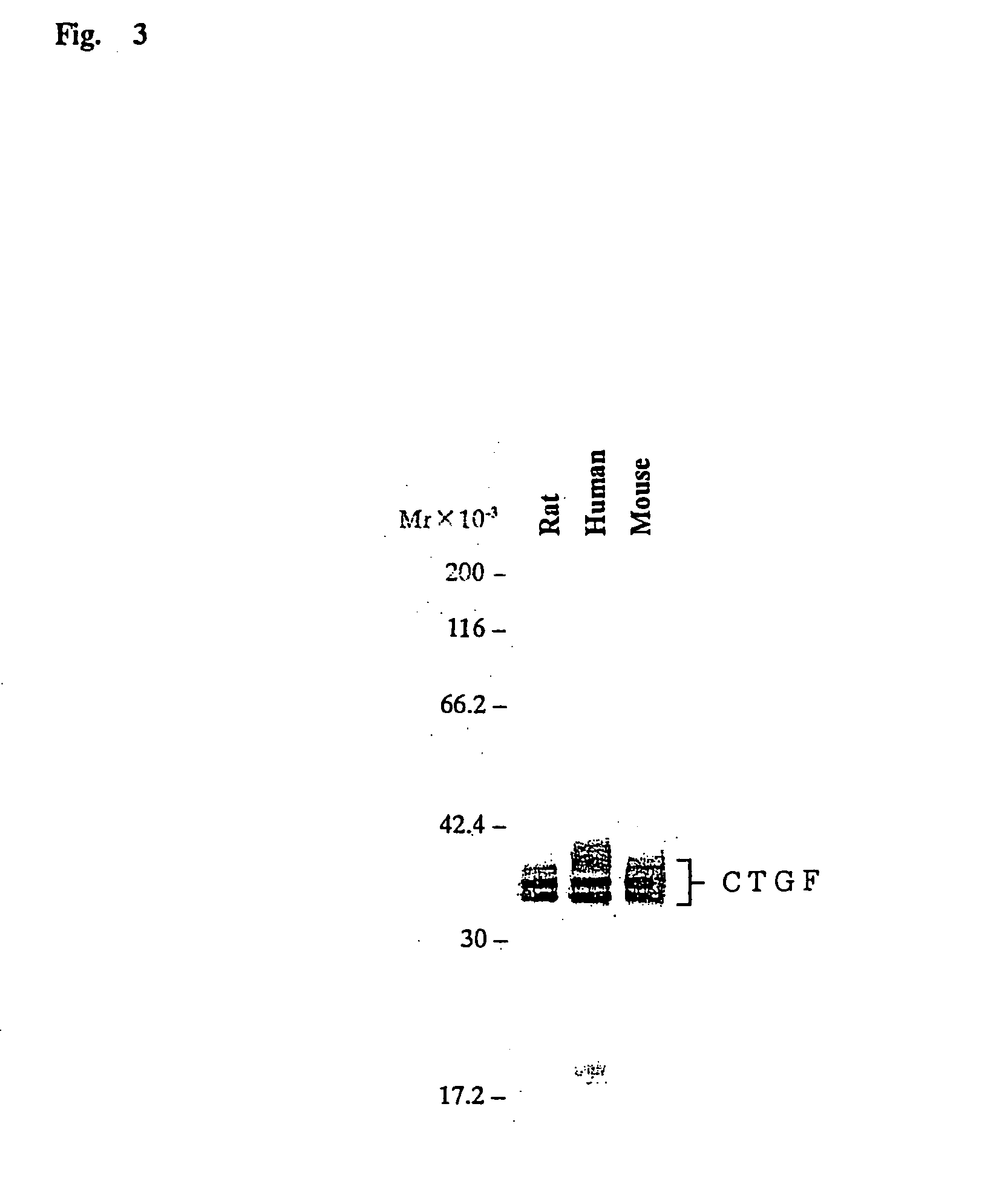
Patents
Literature
130 results about "CTGF" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
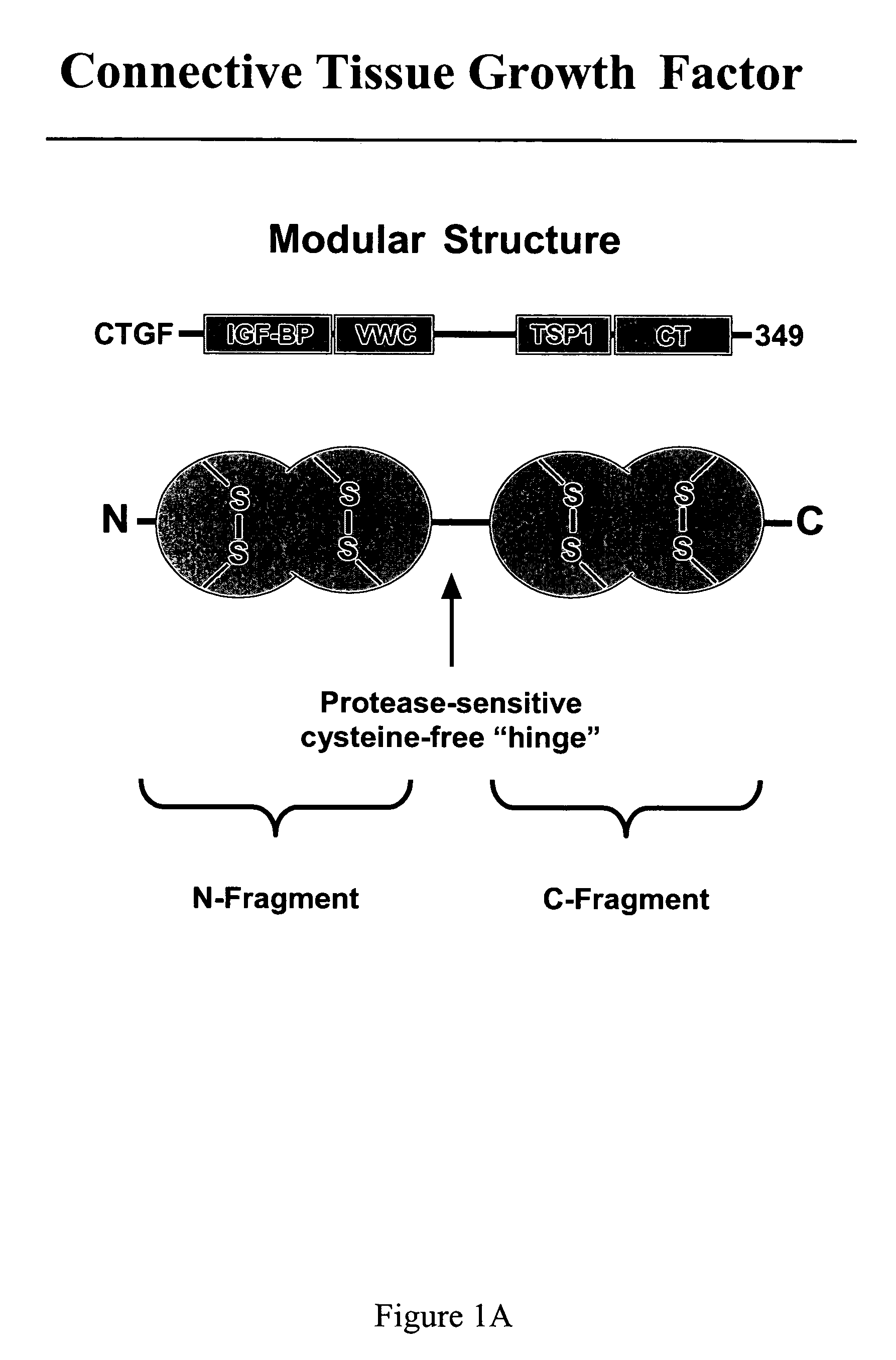
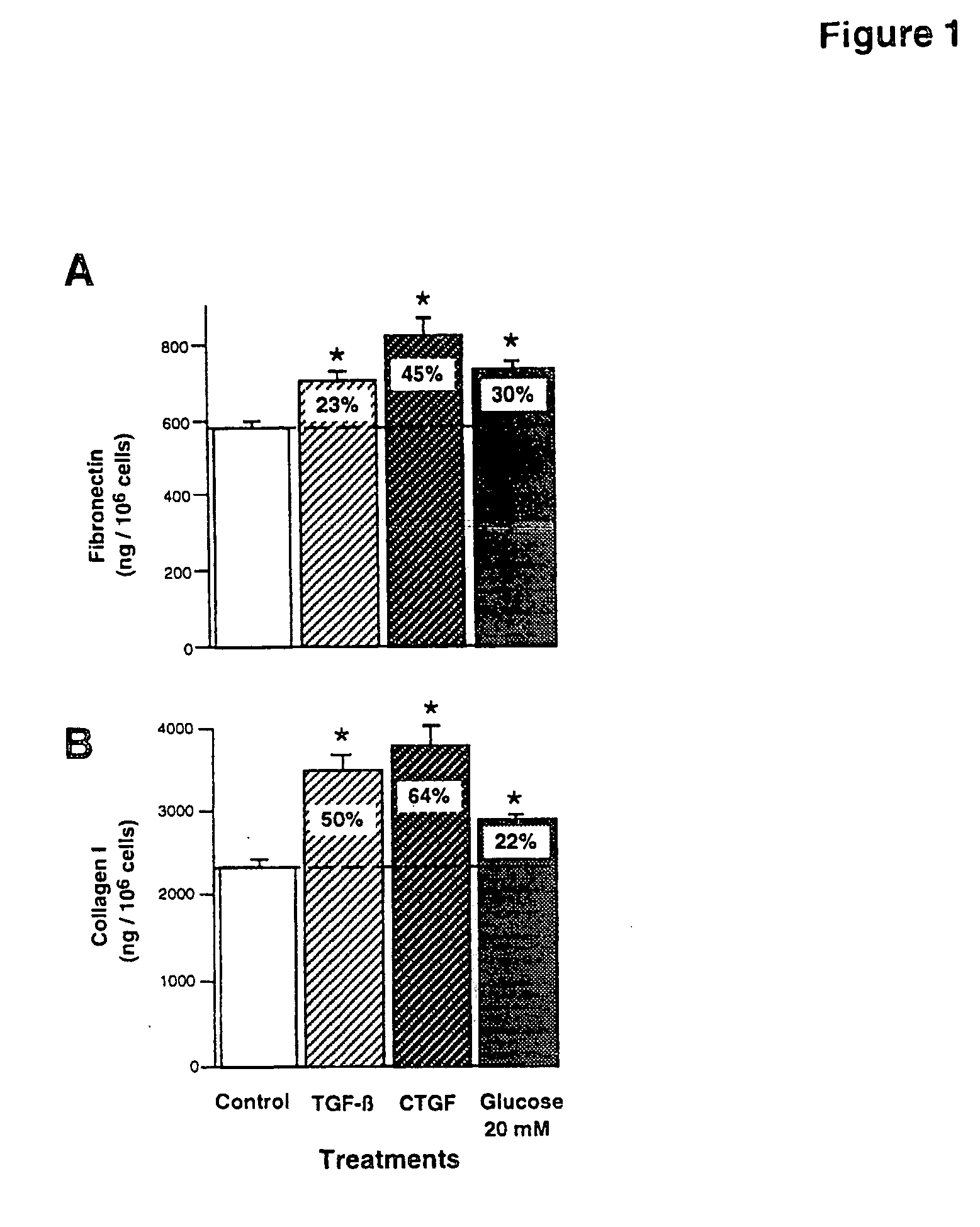
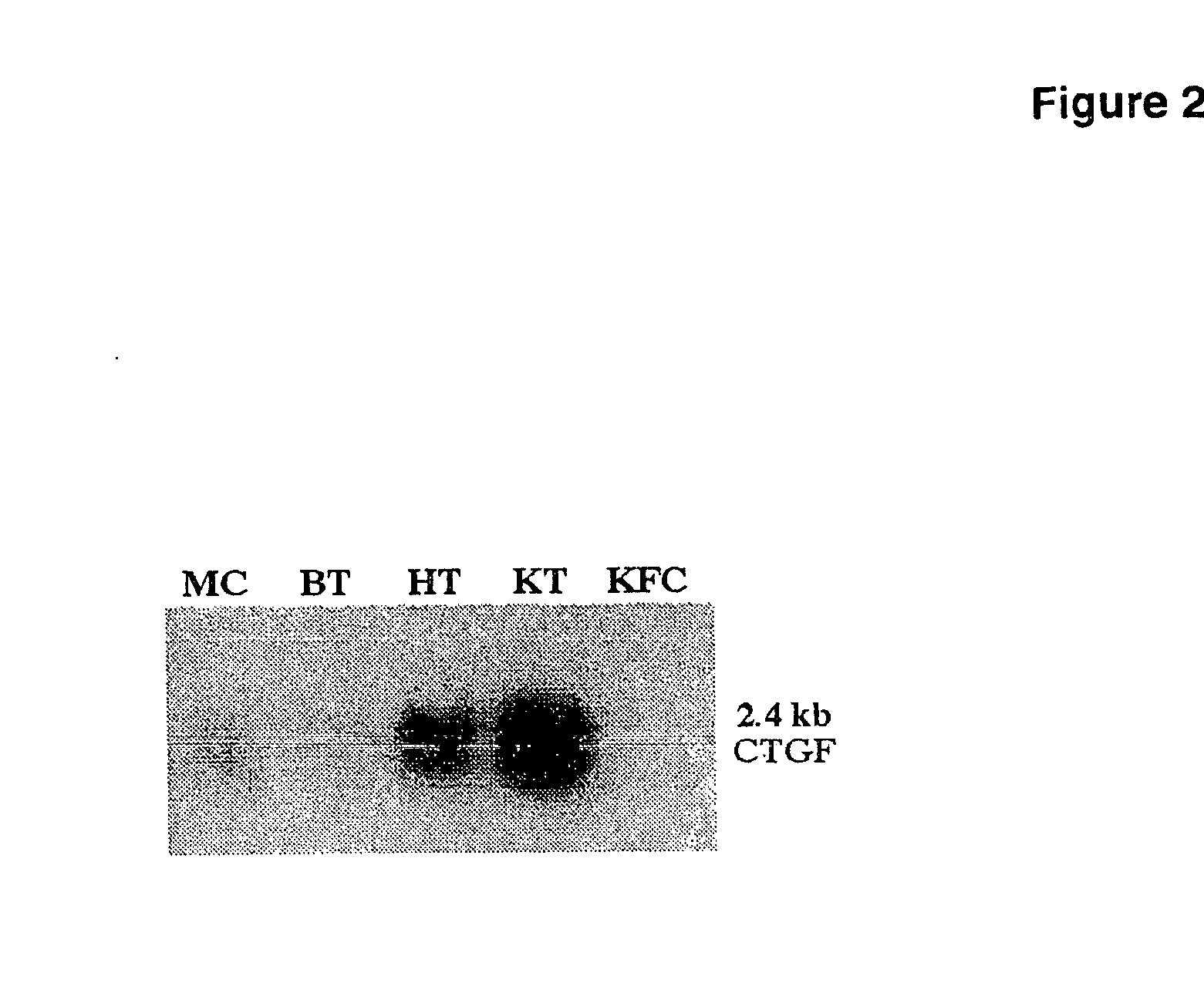
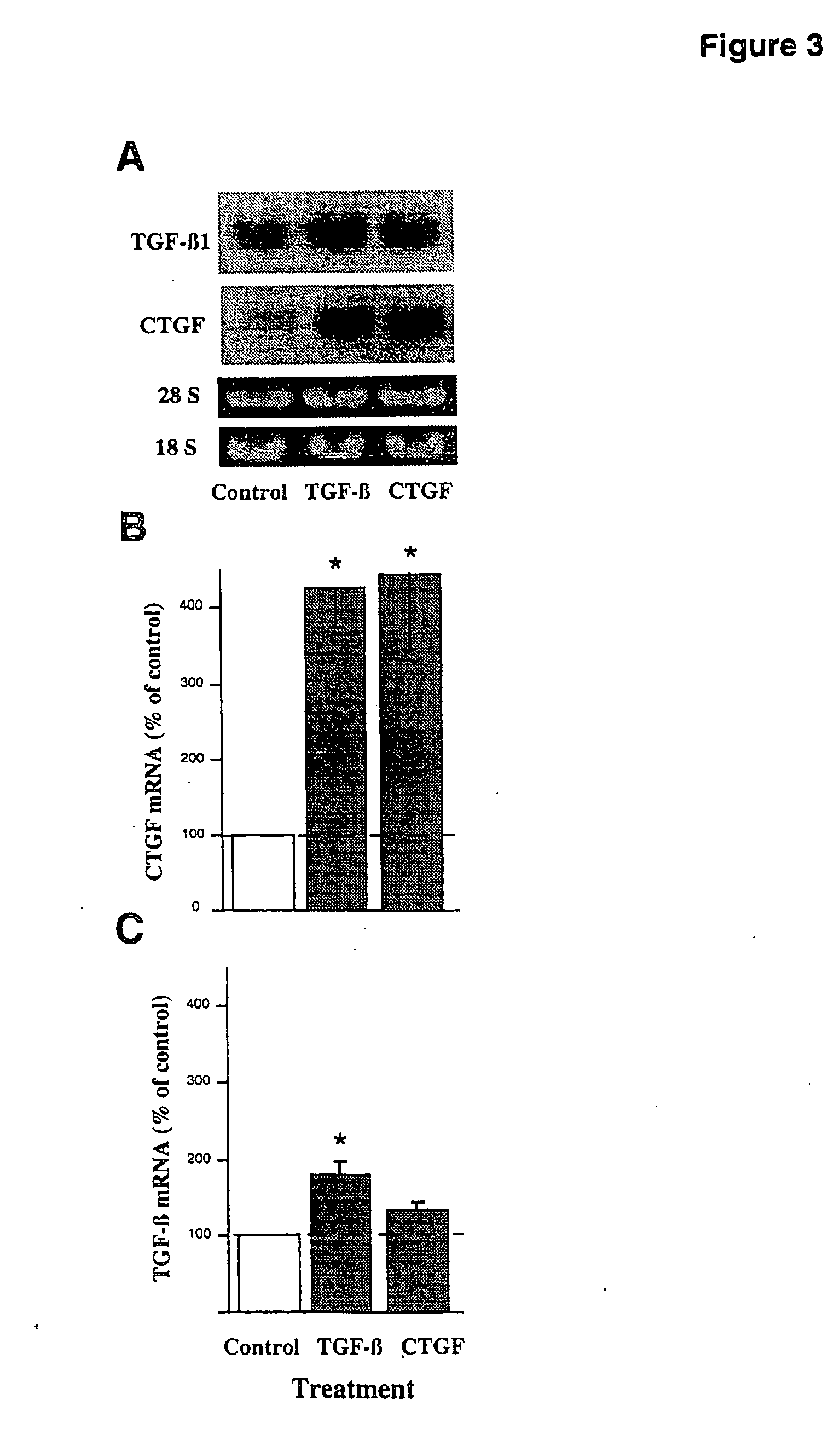
CTGF, also known as CCN2 or connective tissue growth factor, is a matricellular protein of the CCN family of extracellular matrix-associated heparin-binding proteins (see also CCN intercellular signaling protein). CTGF has important roles in many biological processes, including cell adhesion, migration, proliferation, angiogenesis, skeletal development, and tissue wound repair, and is critically involved in fibrotic disease and several forms of cancers.
Antisense oligonucleotides optimized for kidney targeting
ActiveUS20050113326A1Growth inhibitionPreventing delaying onsetSugar derivativesSpecial deliveryConnective tissue fiberCTGF
The present invention provides antisense compounds and methods for modulating the expression of target genes expressed in the kidney. In particular, this invention provides antisense oligonucleotide compounds optimized for targeting nucleic acid molecules expressed in the kidney. Such compounds are shown herein to efficiently modulate the expression of target genes SGLT2 and connective tissue growth factor (CTGF) in the kidney.
Owner:IONIS PHARMA INC
Method of examining polycystic kidney disease and method of screening for therapeutic agent of the disease
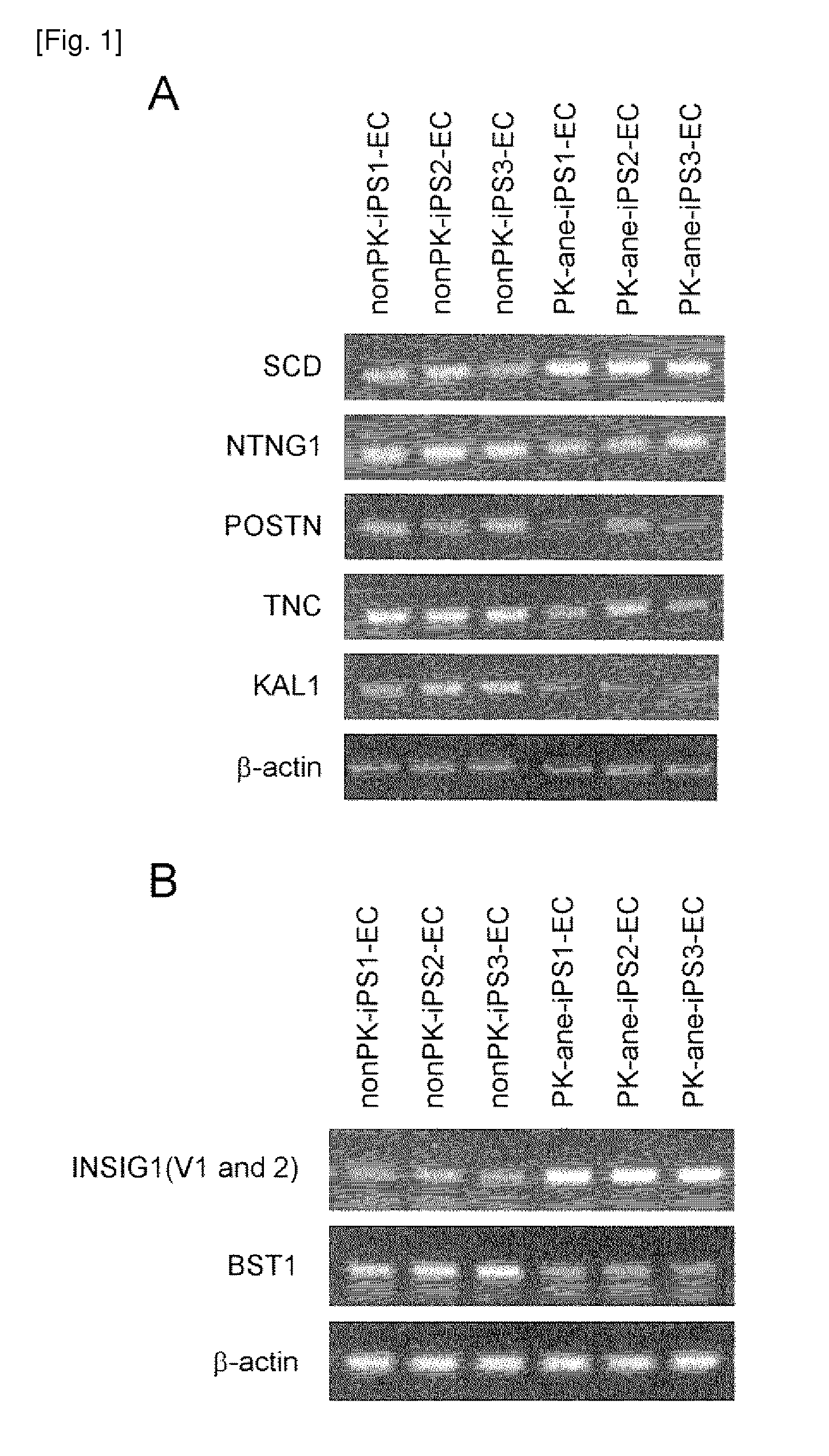
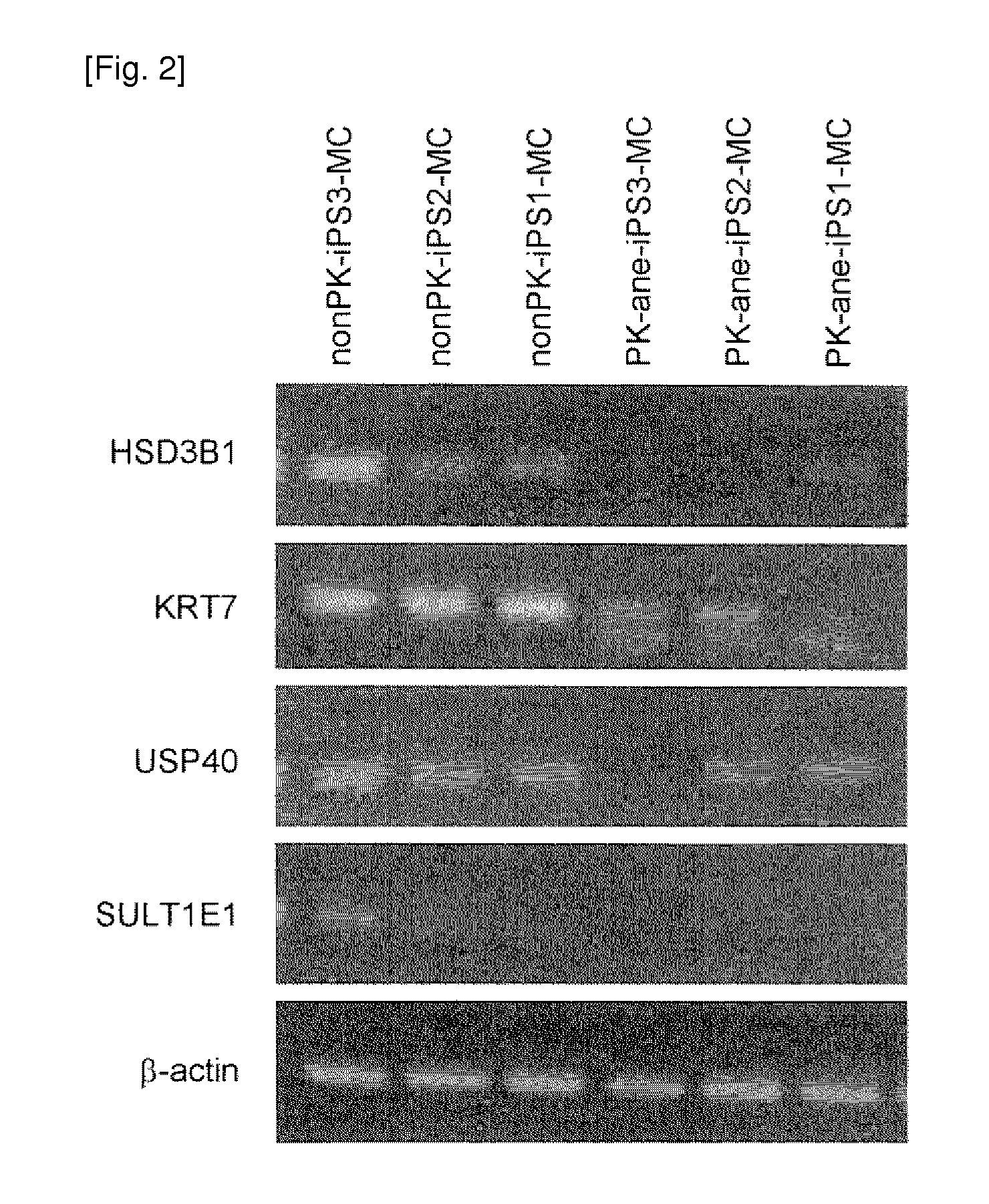
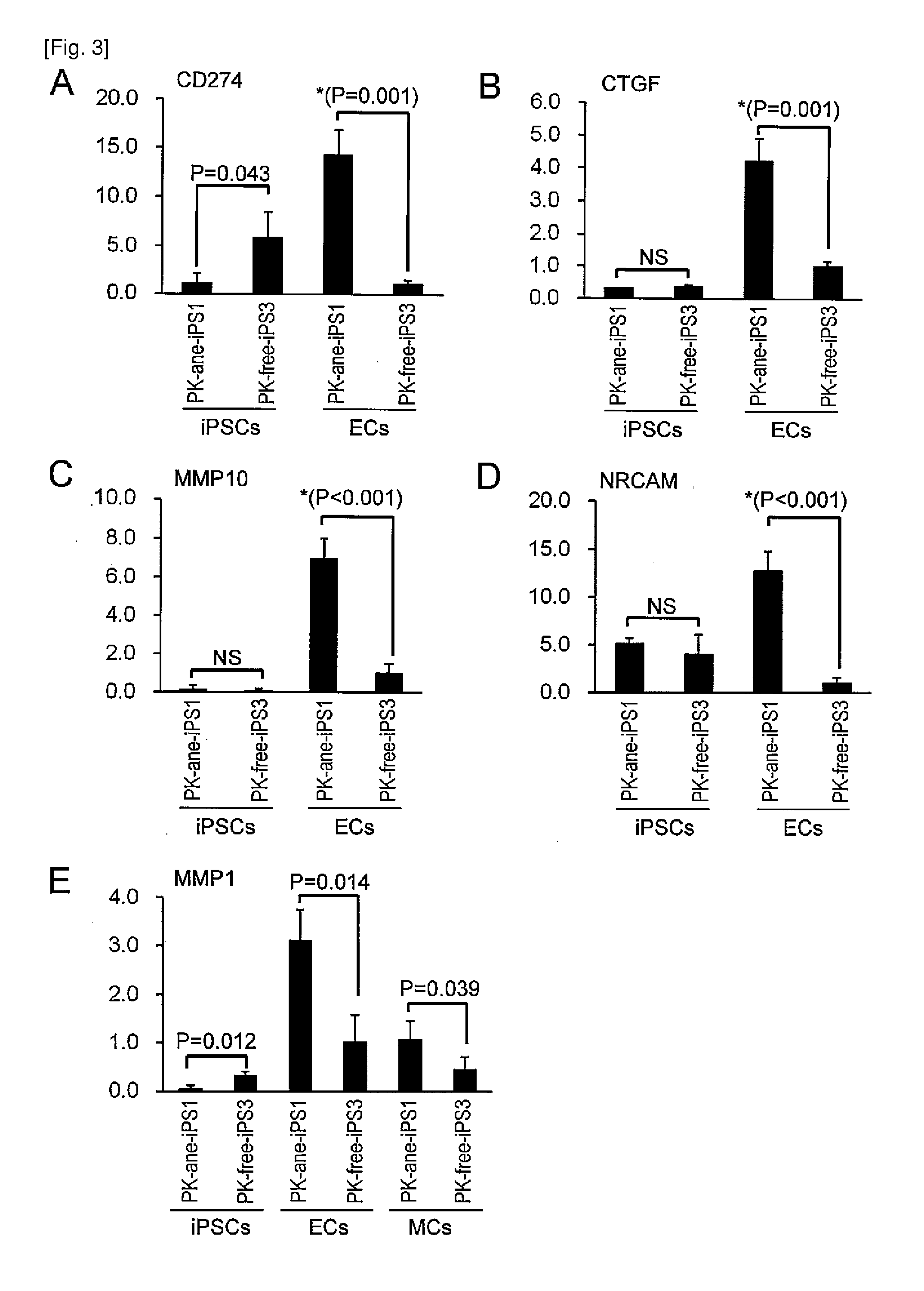
The present invention provides a method of examining polycystic kidney disease or a complication of polycystic kidney disease using a gene(s) selected from the group consisting of NTNG1, POSTN, TNC, KAL1, BST1, ACAT2, INSIG1, SCD, HSD3B1, KRT7, USP40, SULT1E1, BMP6, CD274, CTGF, E2F7, EDN1, FAM43A, FRMD3, MMP10, MYEOV, NR2F1, NRCAM, PDCK1, PLXNA2, SLC30A3, SNAI1, SPOCD1, MMP1, TFPI2, HMGA2, KRTAP4-7, KRTAP4-8, KRTAP4-9, MYPN, RPPH1, and SIAE, and a method of screening for a therapeutic agent or a preventive agent therefore, and further vascular endothelial cells or vascular mural cells obtained via differentiation induction from iPS cells formed from a somatic cell of a subject suffered from polycystic kidney disease and having cerebral aneurysm as a complication.
Owner:KYOTO UNIV
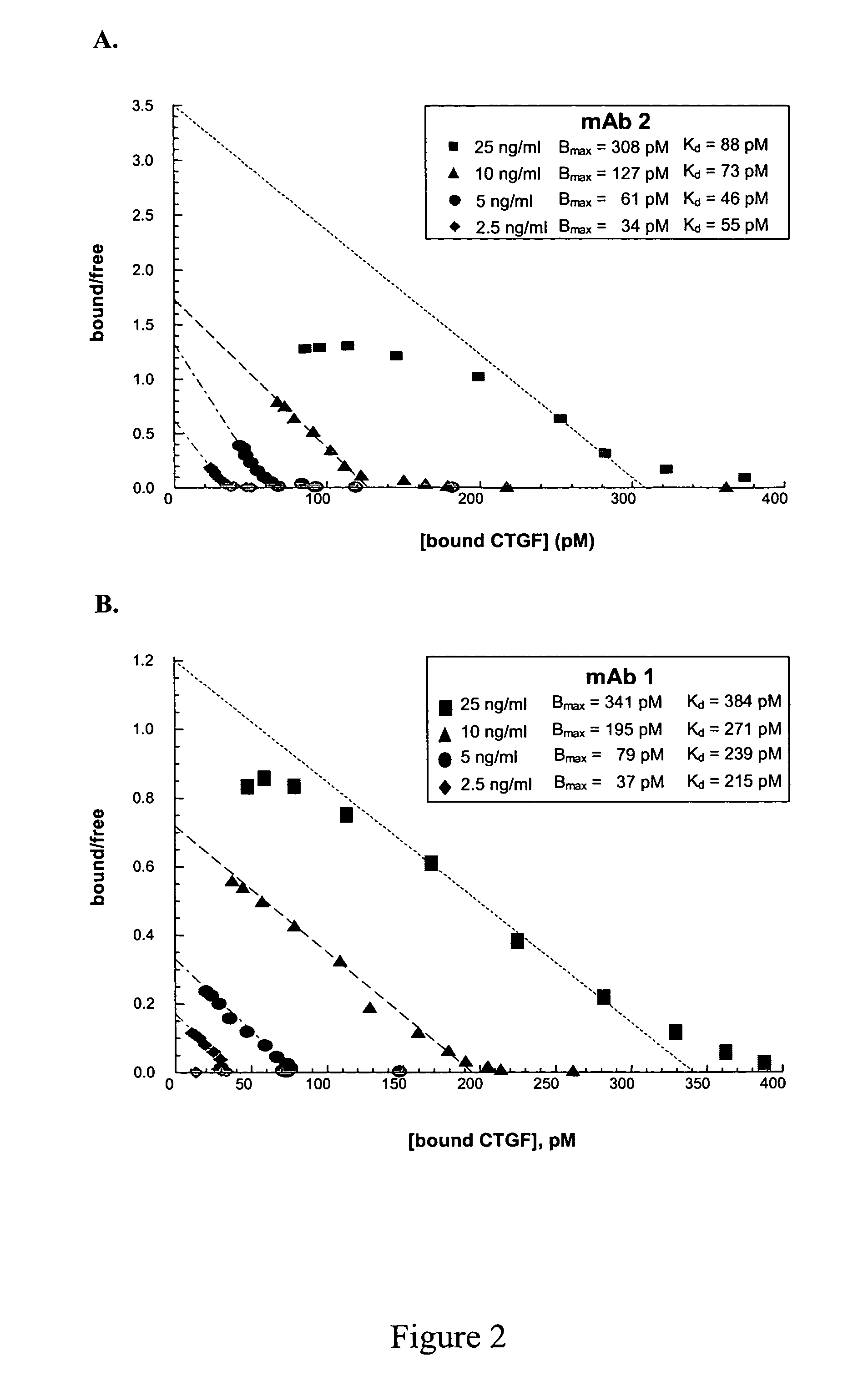
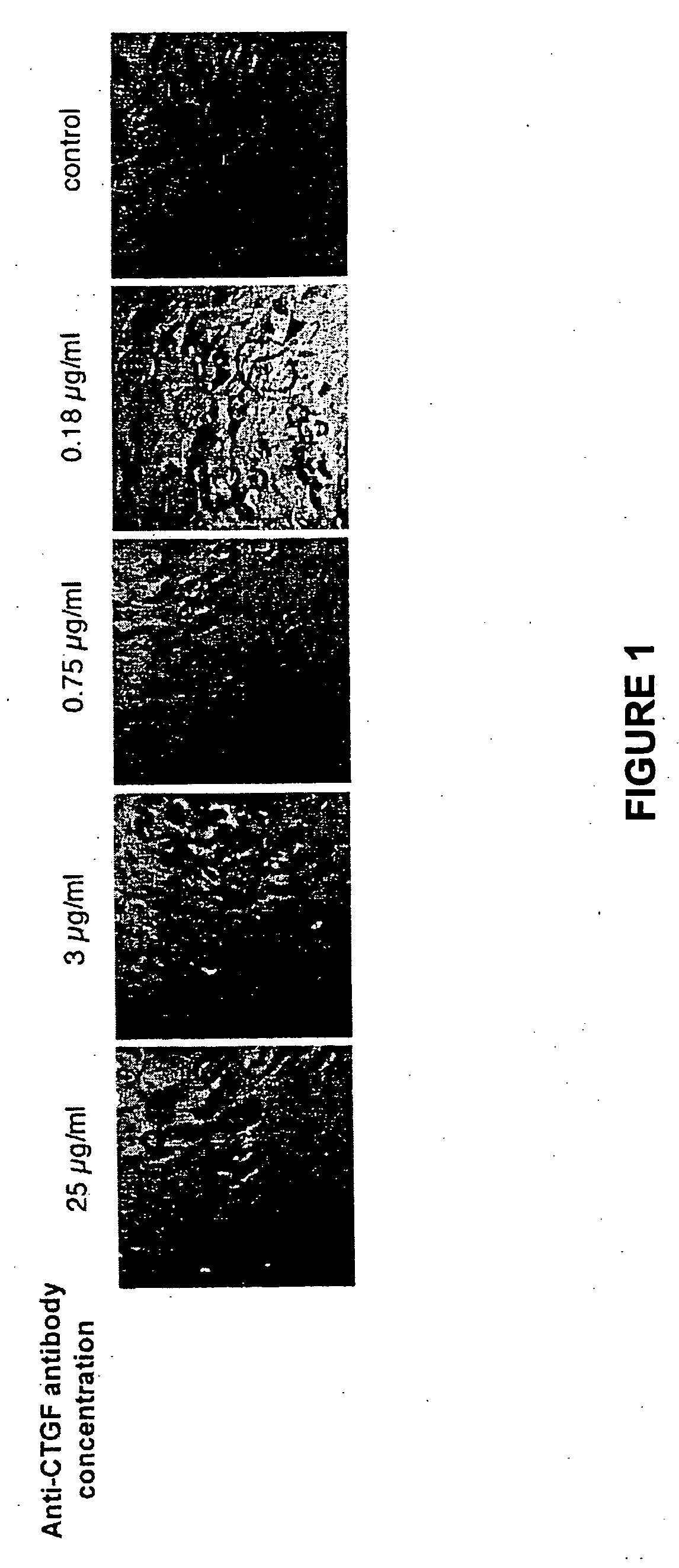
Connective tissue growth factor antibodies
The present invention relates to antibodies that bind to CTGF. The antibodies are particularly directed to regions of CTGF involved in biological activities associated with fibrosis. The invention also relates to methods of using the antibodies to treat disorders associated with CTGF including localized and systemic fibrotic disorders including those of the lung, liver, heart, skin, and kidney.
Owner:FIBROGEN INC
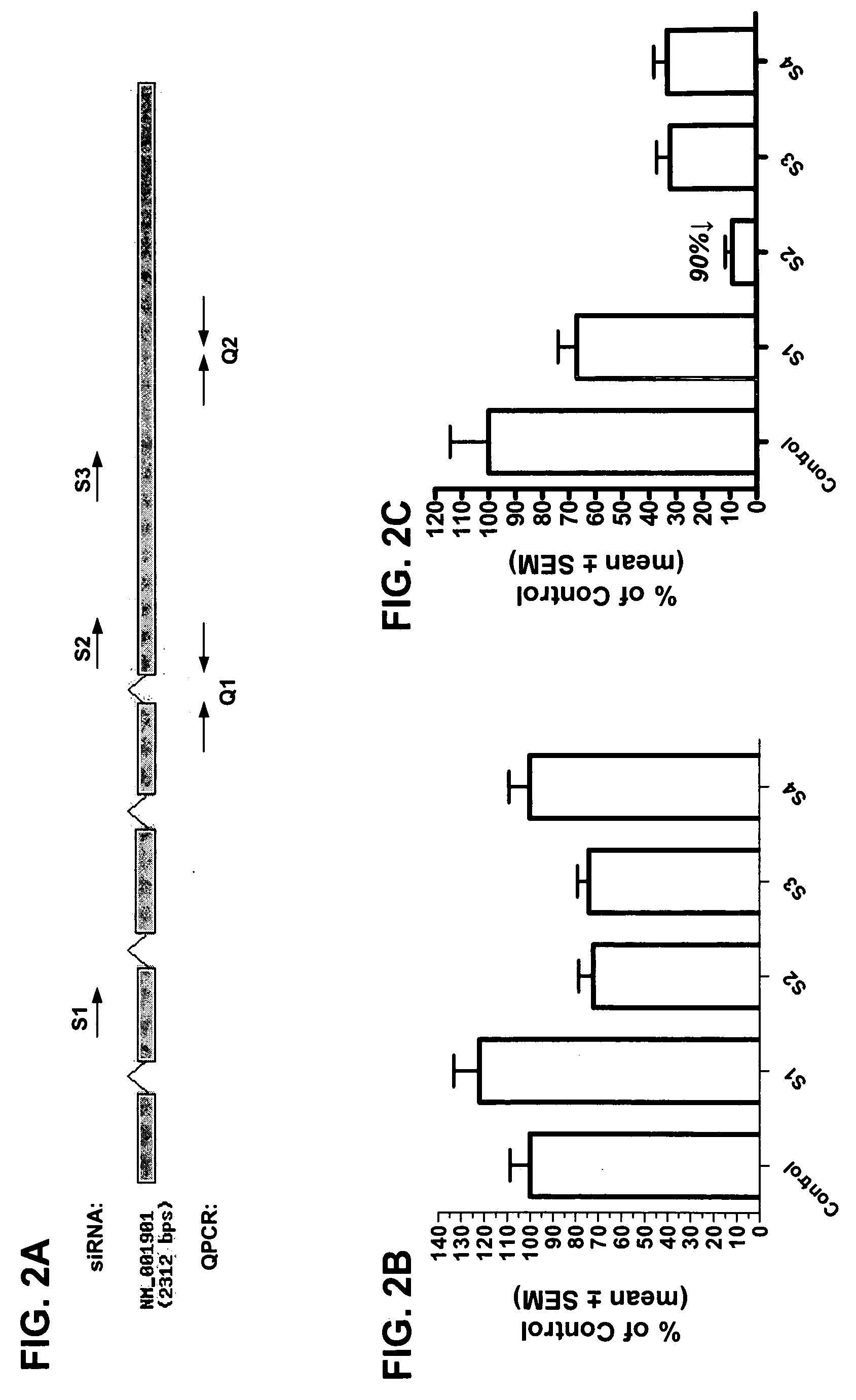
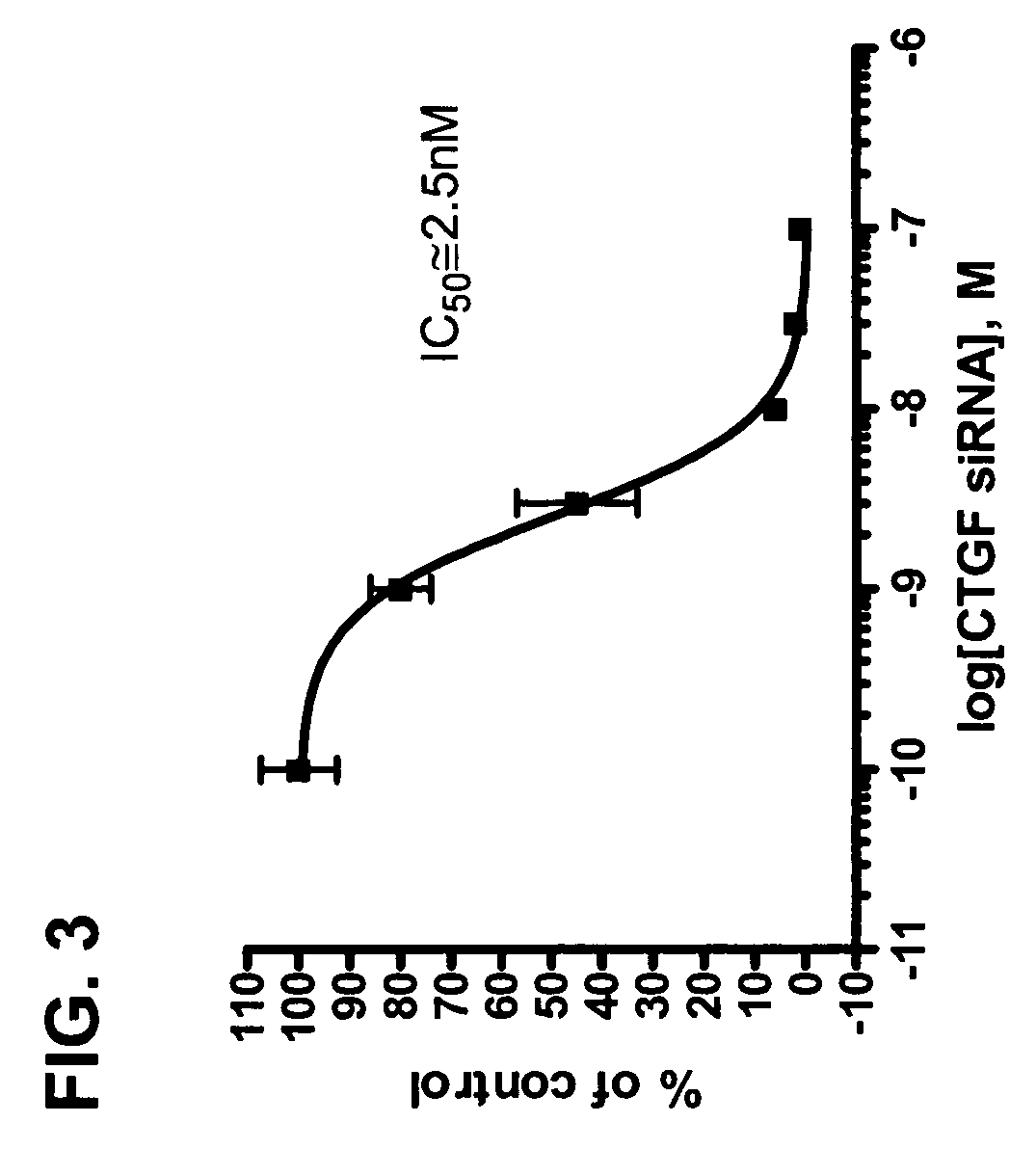
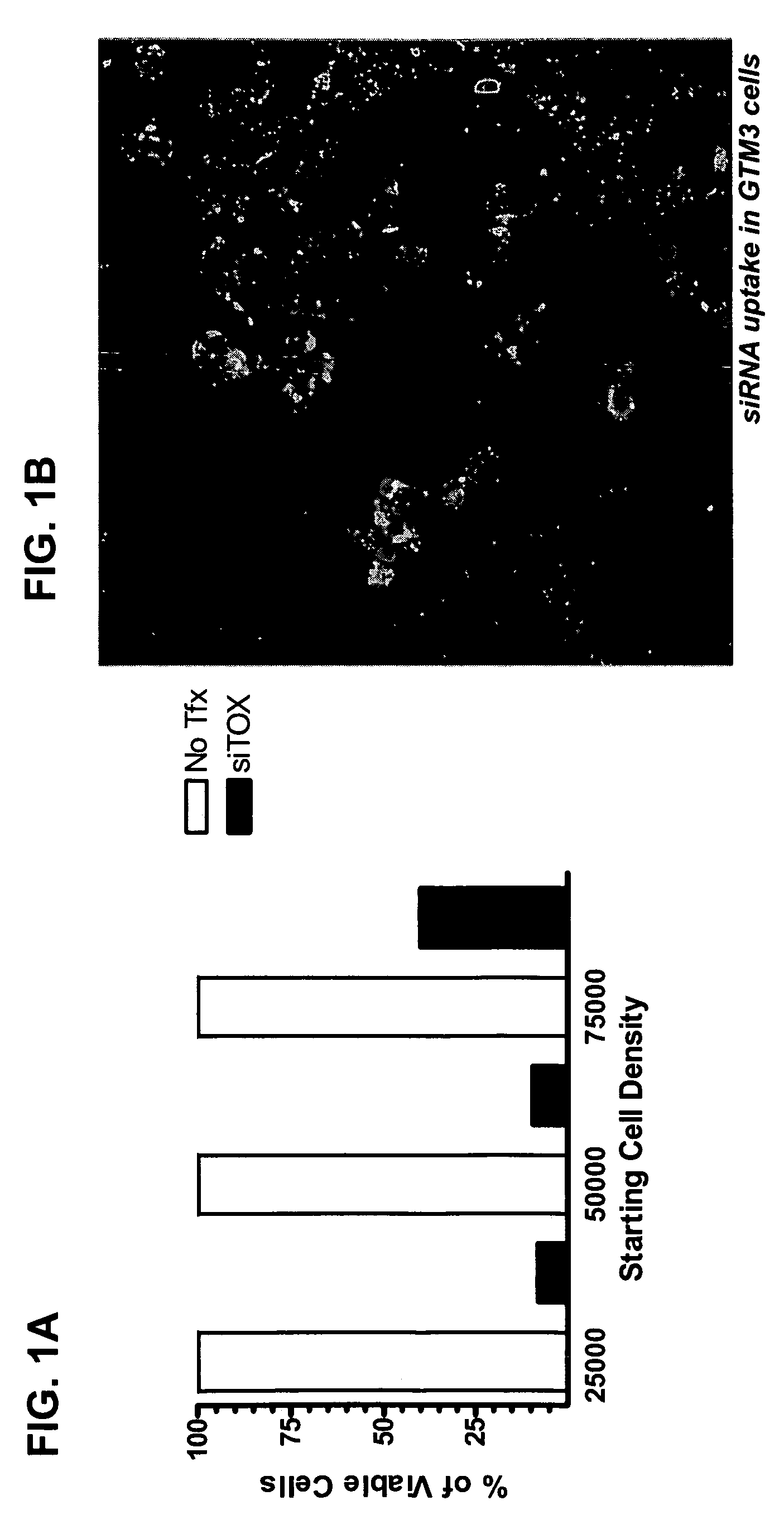
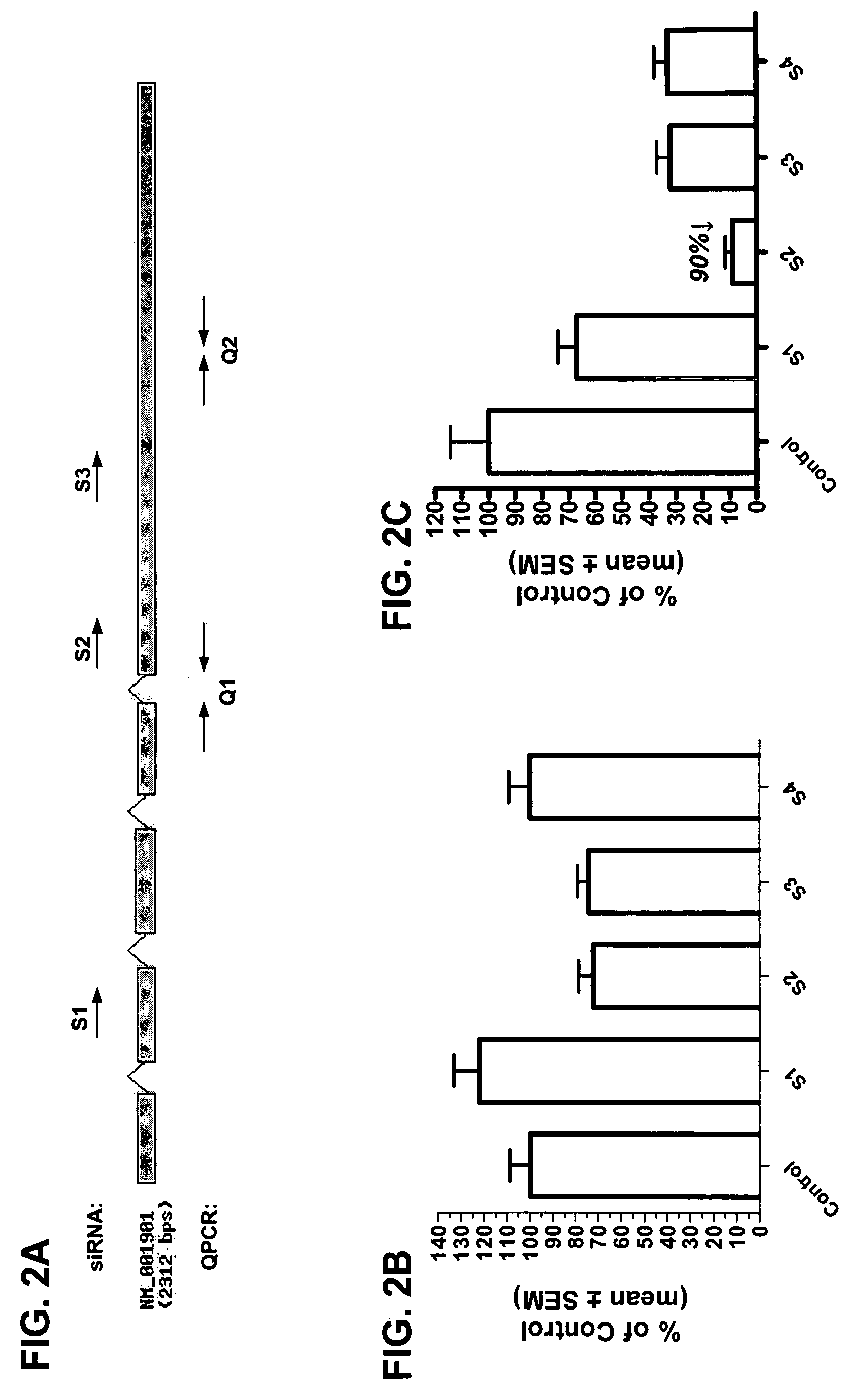
RNAi inhibition of CTGF for treatment of ocular disorders
RNA interference is provided for inhibition of connective tissue growth factor mRNA expression in ocular disorders involving CTGF expression. Ocular disorders involving aberrant CTGF expression include glaucoma, macular degeneration, diabetic retinopathy, choroidal neovascularization, proliferative vitreoretinopathy and wound healing. Such disorders are treated by administering interfering RNAs of the present invention.
Owner:NOVARTIS AG
Alfavbeta3 and alfavbet6 integrin antagonists as antifibrotic agents
InactiveUS20070117849A1Prevent and mitigate and even reverse developmentEasy to prepareBiocideDigestive systemIntestinal structureCTGF
This invention relates to inhibition of αv integrins, especially αvβ3 and αvβ6 integrins, by specific antagonists, preferably non-peptidic antagonists, related compounds and compounds with comparable specificity, that downregulate fibrogenesis by inhibiting cell migration and production of pro-fibrogenic molecules (e.g., collagens, TIMP-1) and cytokines (e.g., CTGF) by activated hepatic stellate cells / myofibroblasts, activated epithelia and endothelia. These antagonists alone or in combination with other agents can effectively prevent, mitigate or even reverse development of advanced fibrosis, such as fibrosis / cirrhosis of the liver and fibrosis of other organs, such as lungs, kidneys, intestine, pancreas, skin and arteries.
Owner:MERCK PATENT GMBH
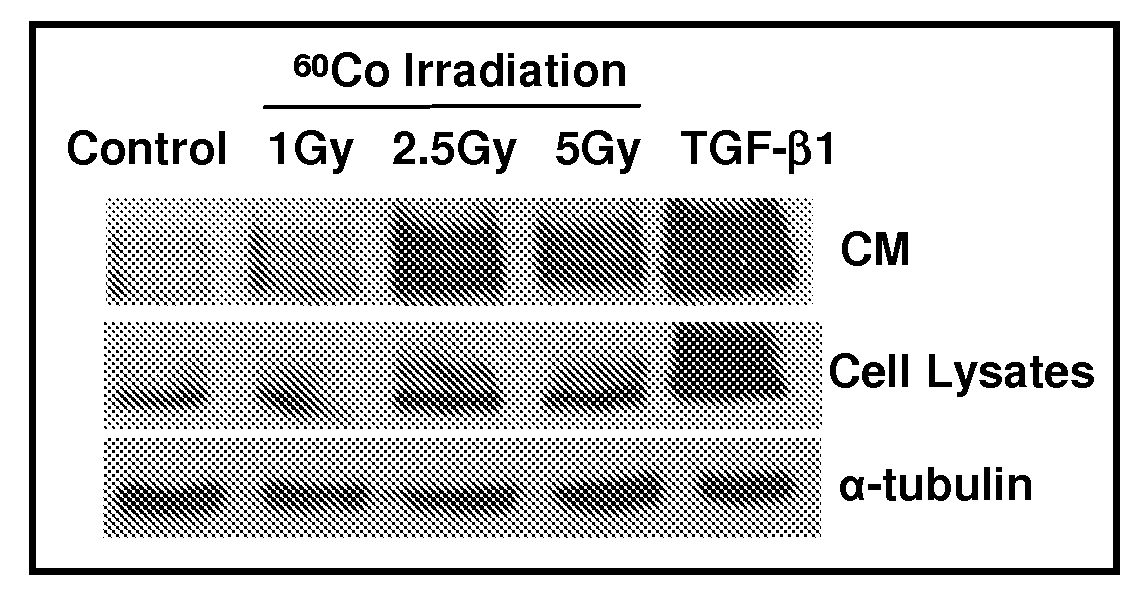
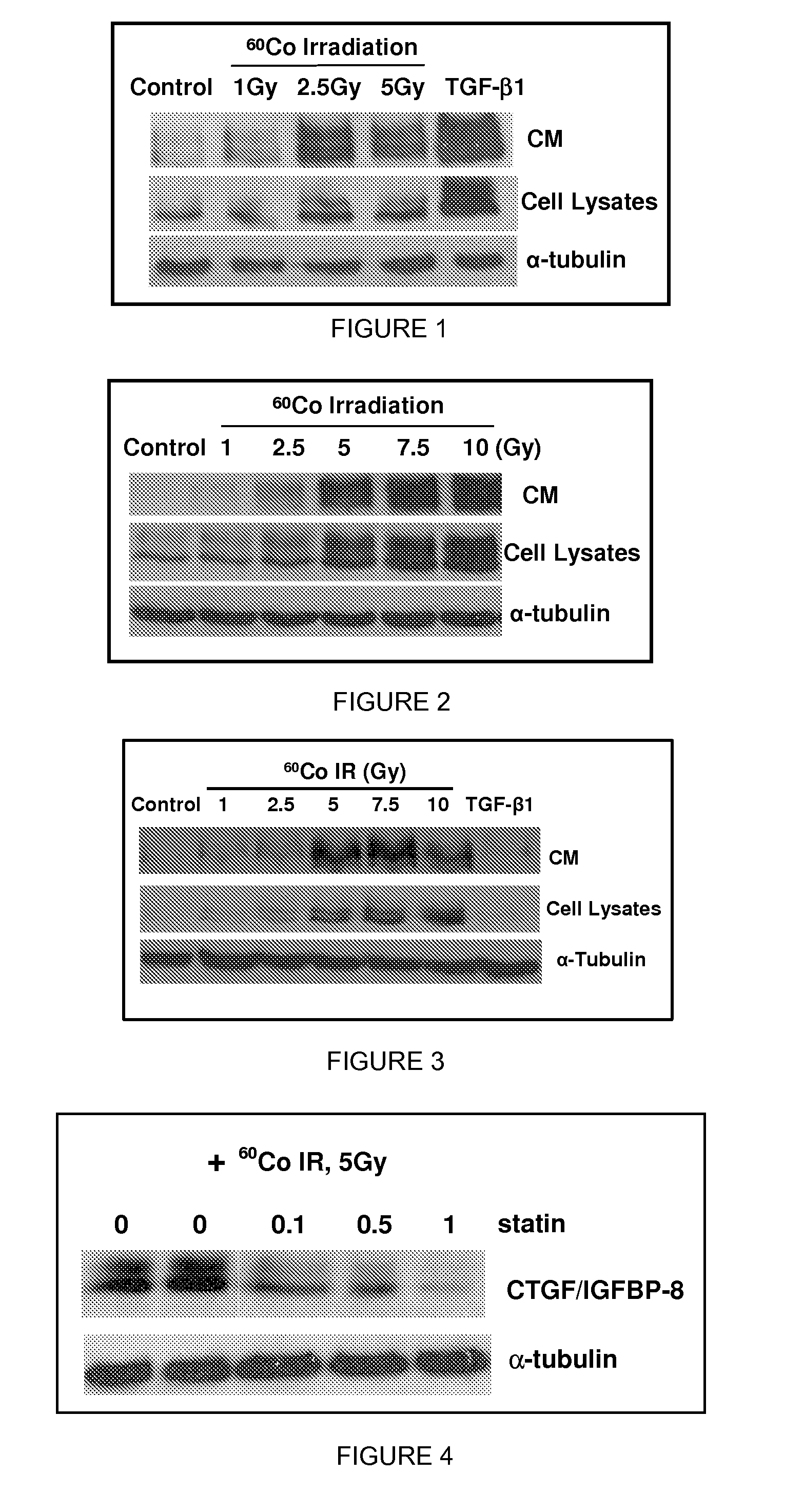
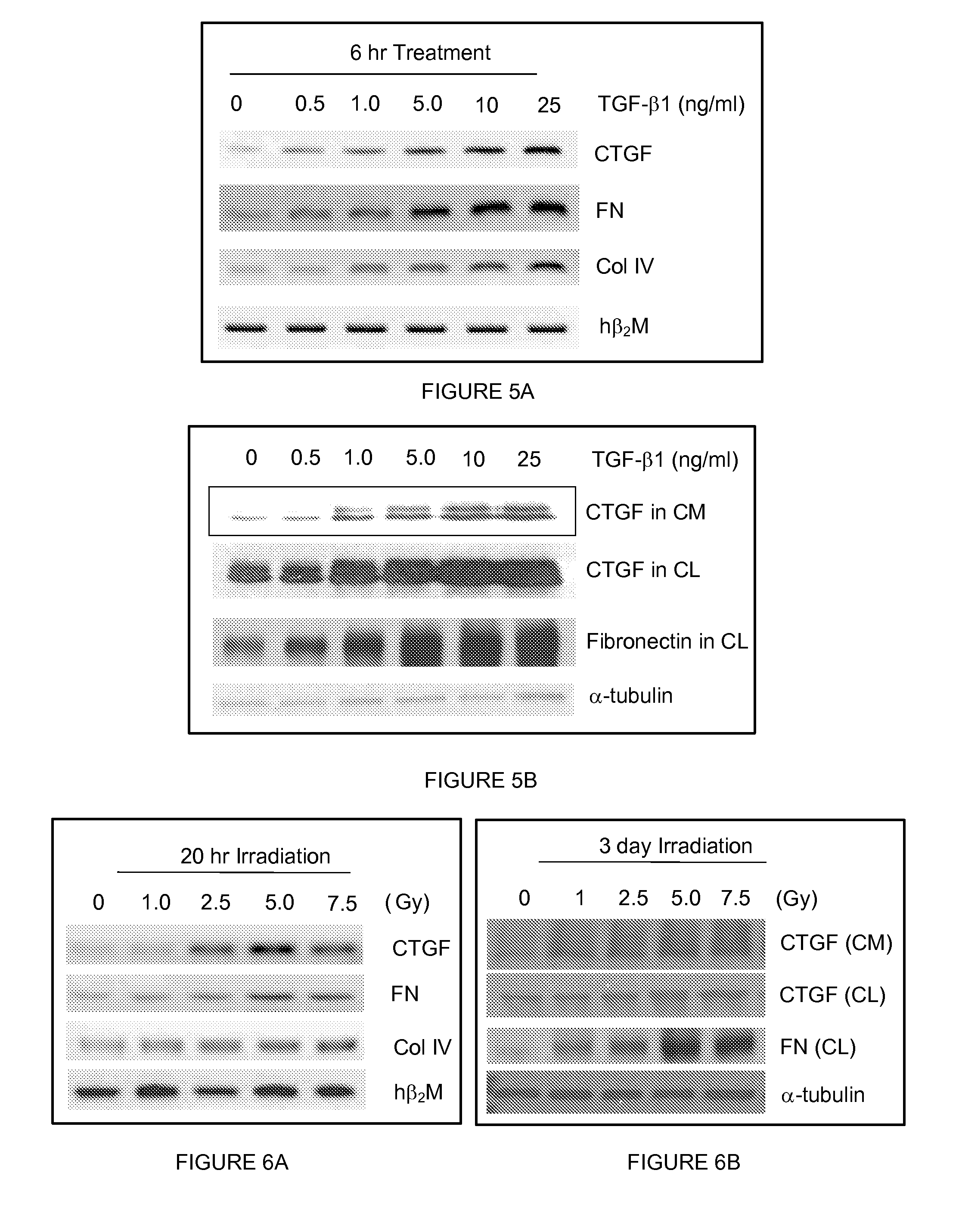
Methods and compositions for treatment or prevention of radiation-induced fibrosis
InactiveUS20090069623A1Treating and preventing RIFInhibitory activityBiocidePeptide/protein ingredientsDiseaseRadiation induced fibrosis
The present invention comprises methods and compositions for the treatment or prevention of radiation-induced fibrosis. Methods and compositions for the inhibition of CTGF are disclosed herein. Methods and compositions for treatment of neoplastic disease are disclosed herein. Inhibition of CTGF in humans or animals that have been exposed to ionizing radiation results in treatment or prevention of radiation-induced fibrosis.
Owner:VIRGINIA COMMONWEALTH UNIV INTPROP FOUND INC
Backbone modifications to modulate oligonucleotide targeting in vivo
The present invention provides antisense compounds and methods for modulating the expression of target genes expressed in the kidney. In particular, this invention provides antisense oligonucleotide compounds optimized for targeting nucleic acid molecules expressed in the kidney. Such compounds are shown herein to efficiently modulate the expression of target genes PTEN, SGLT2 and connective tissue growth factor (CTGF) in the kidney.
Owner:IONIS PHARMA INC
Methods and compositions for the treatment of polycystic diseases
InactiveUS20080193443A1DiagnosisEarly diagnosisGenetic material ingredientsImmunoglobulins against growth factorsDiseaseCTGF
This invention provides compositions and methods to diagnose and treat polycystic disorders by inhibiting the biological activity of a gene now correlated with appearance of this disorder. By way of illustrative only, the Connective Tissue Growth Factor (CTGF) gene is an example of such a gene. Also provided by this invention are compositions and methods to treat or ameliorate abnormal cystic lesions and diseases associated with the formation of cysts in tissue. The methods and compositions treat and ameliorate pathological cyst formation in tissue by inhibiting or augmenting gene expression or the biological activity of its gene expression product or its receptor.
Owner:GENZYME CORP
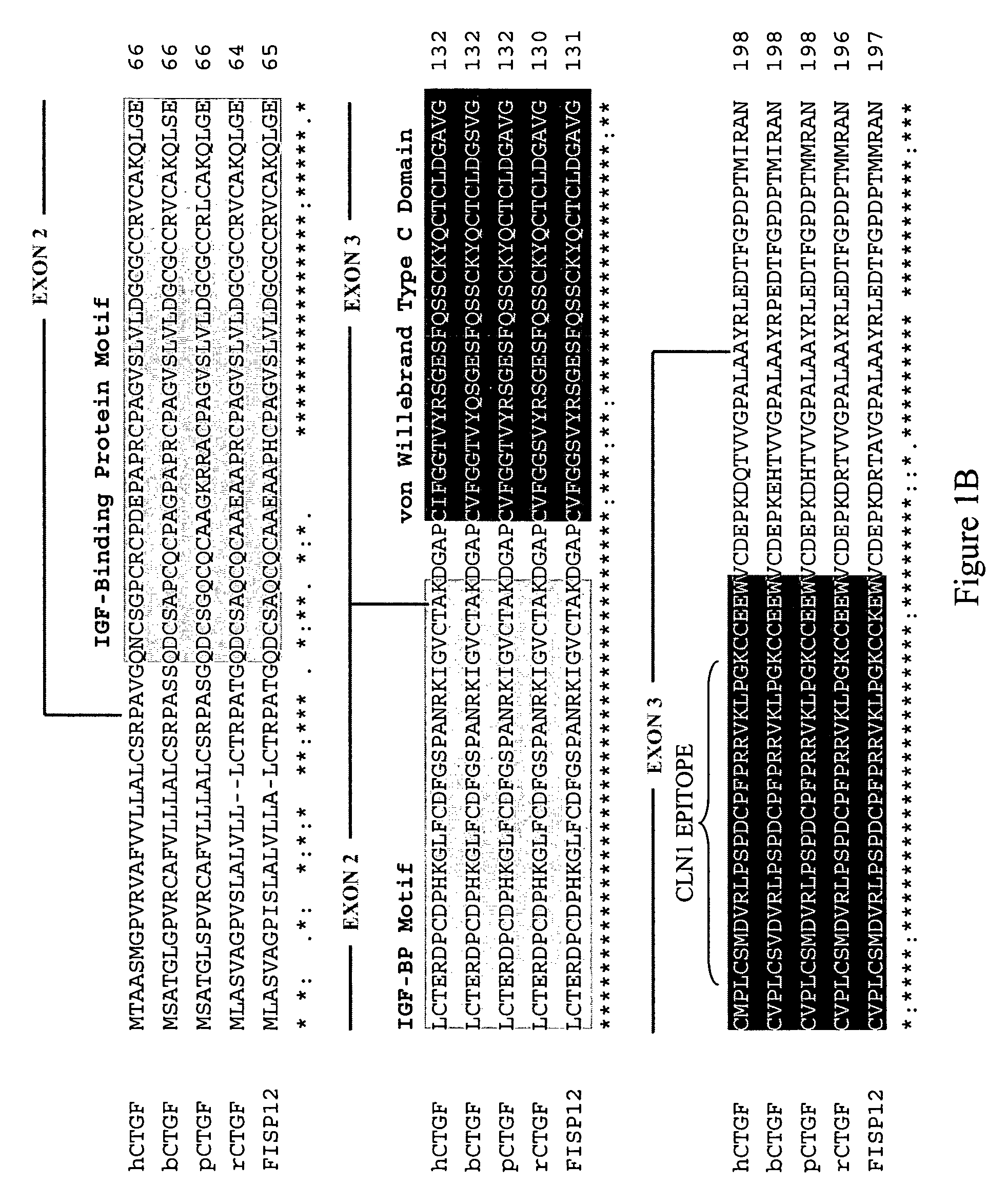
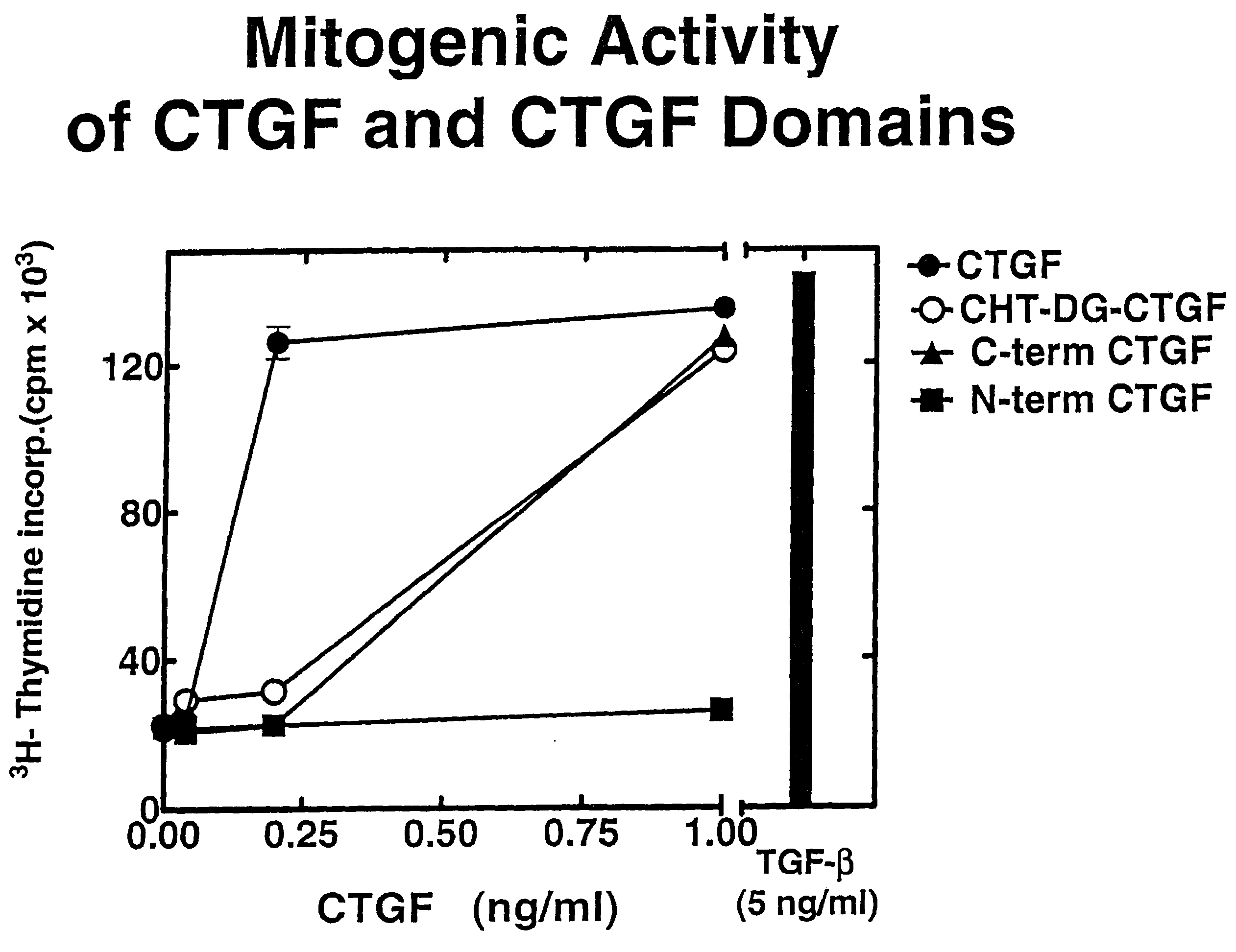
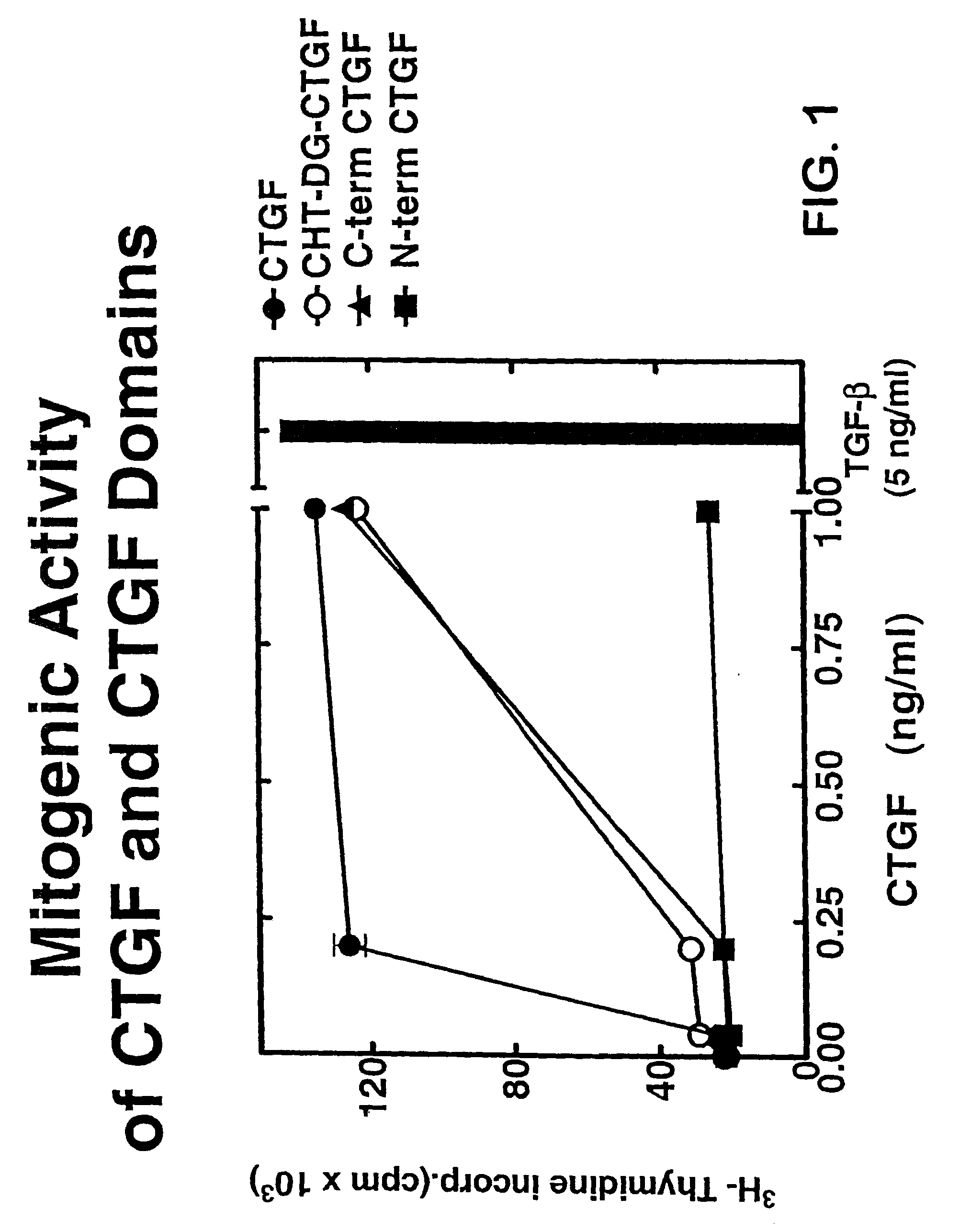
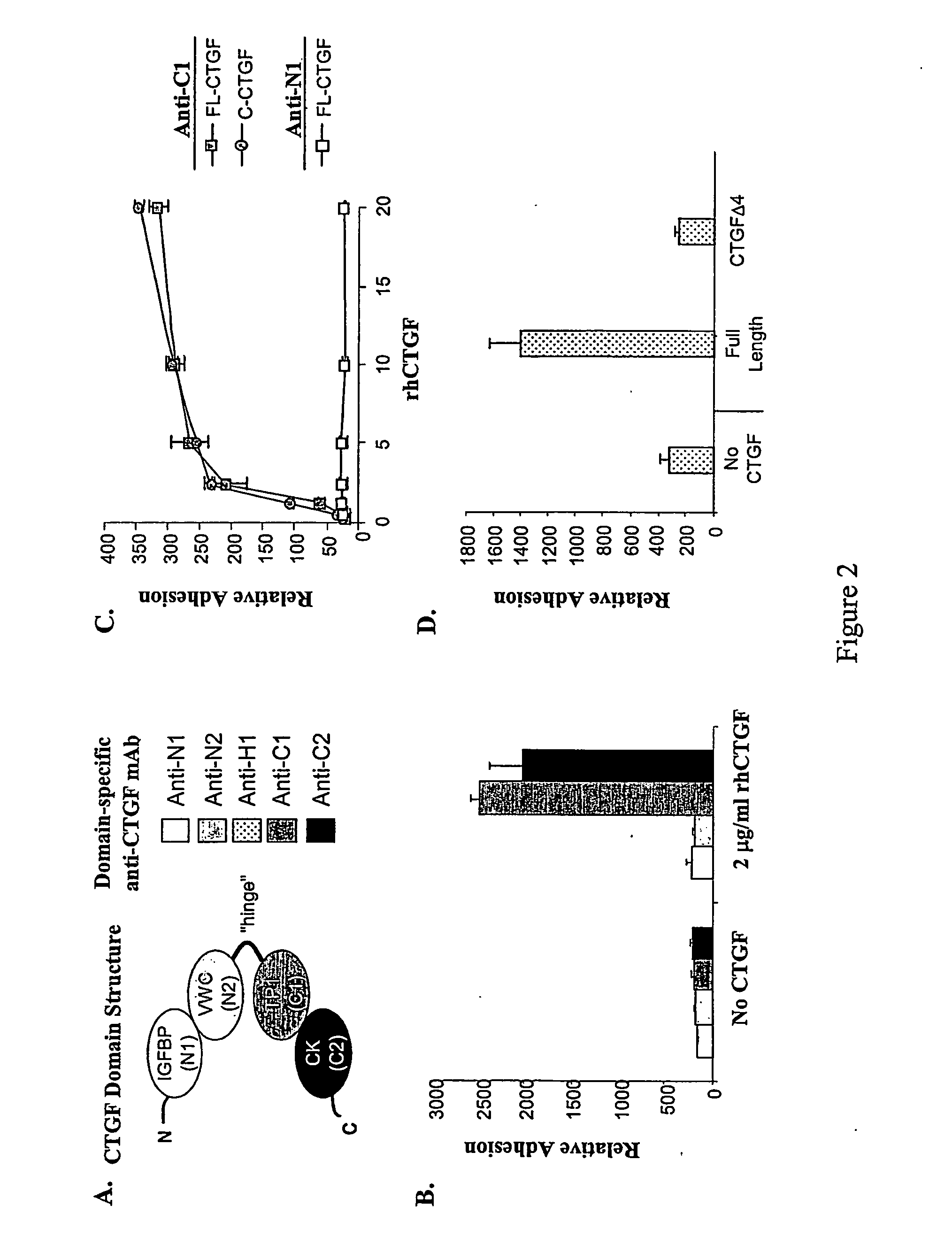
Connective tissue growth factor fragments and methods and uses thereof
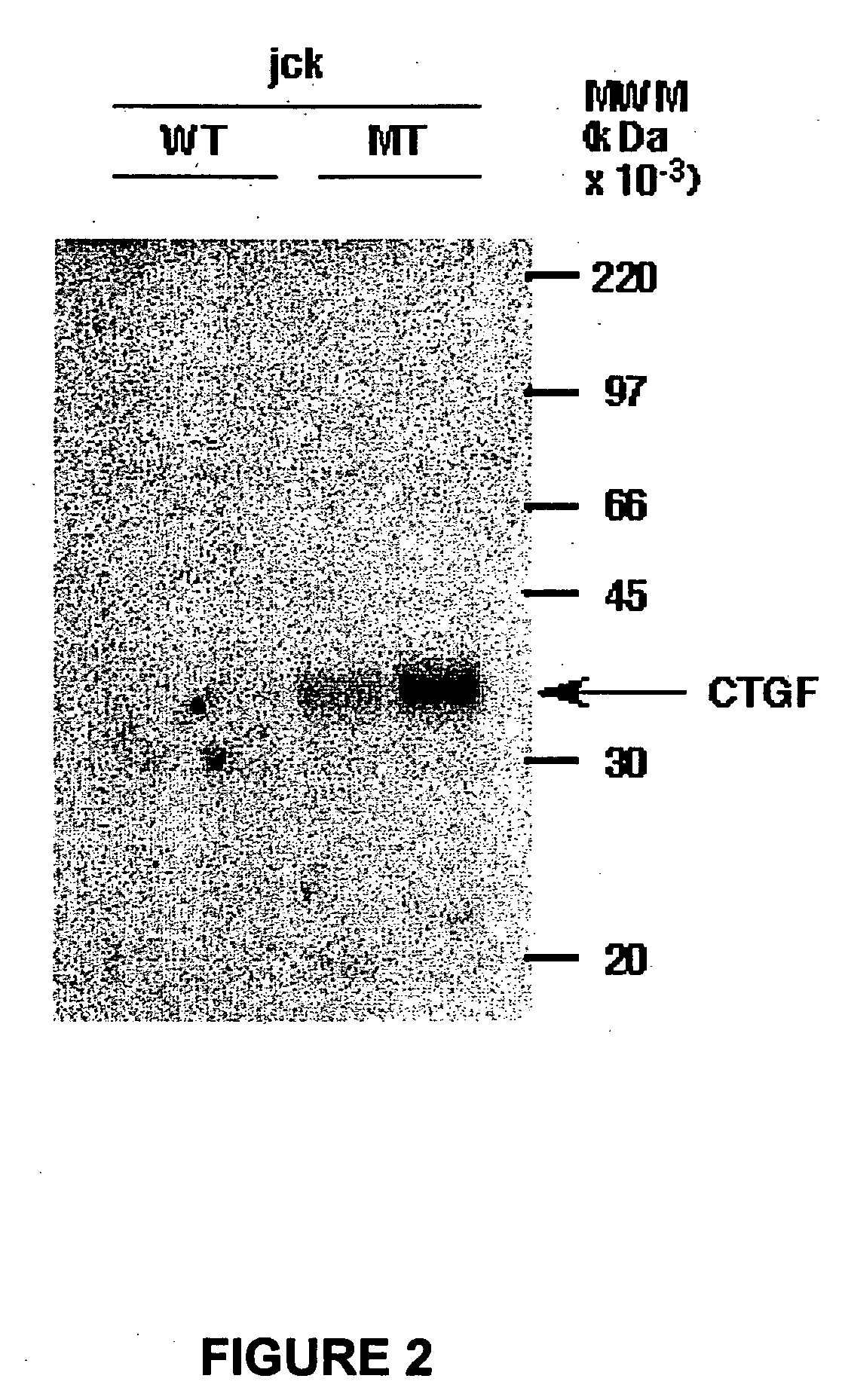
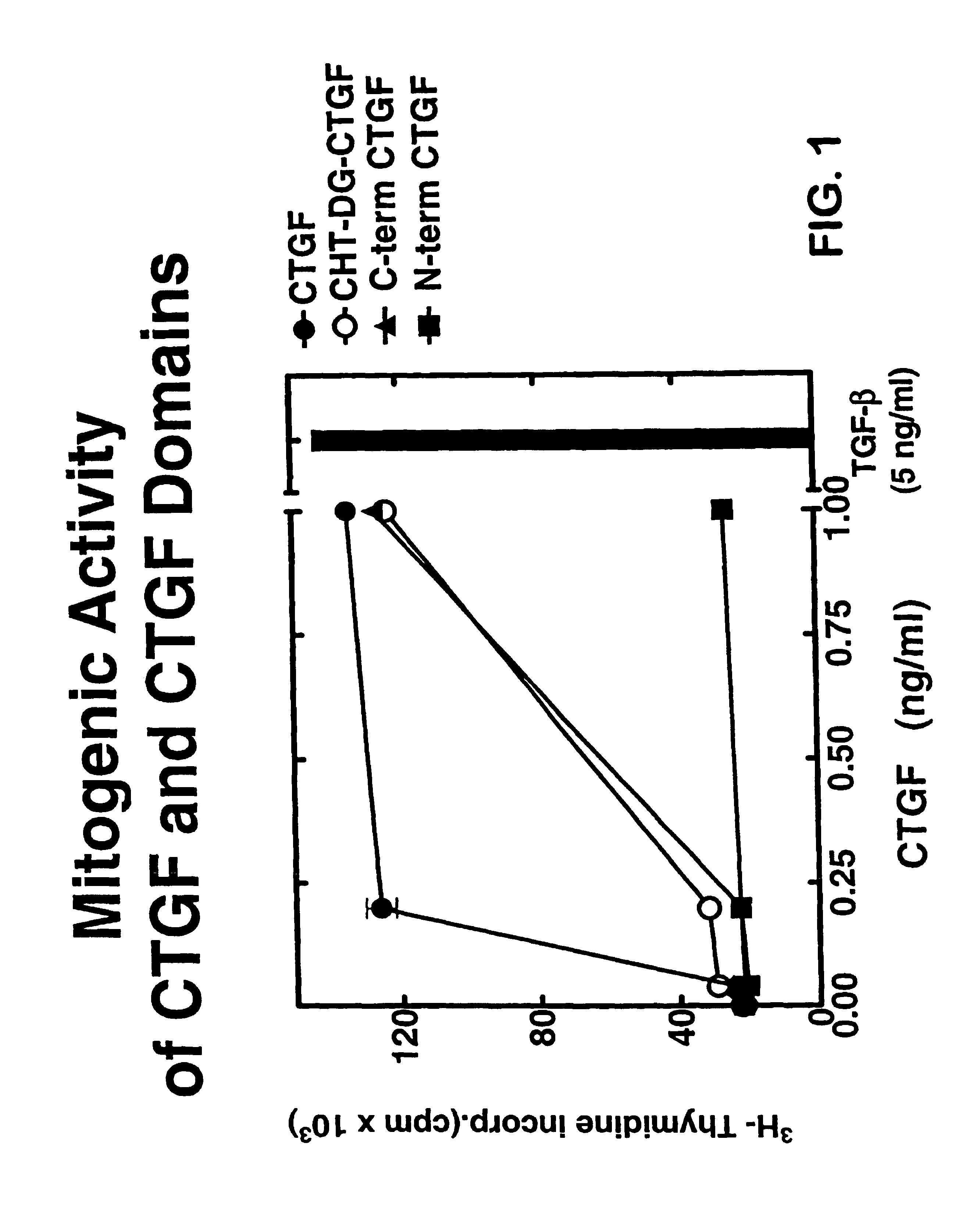
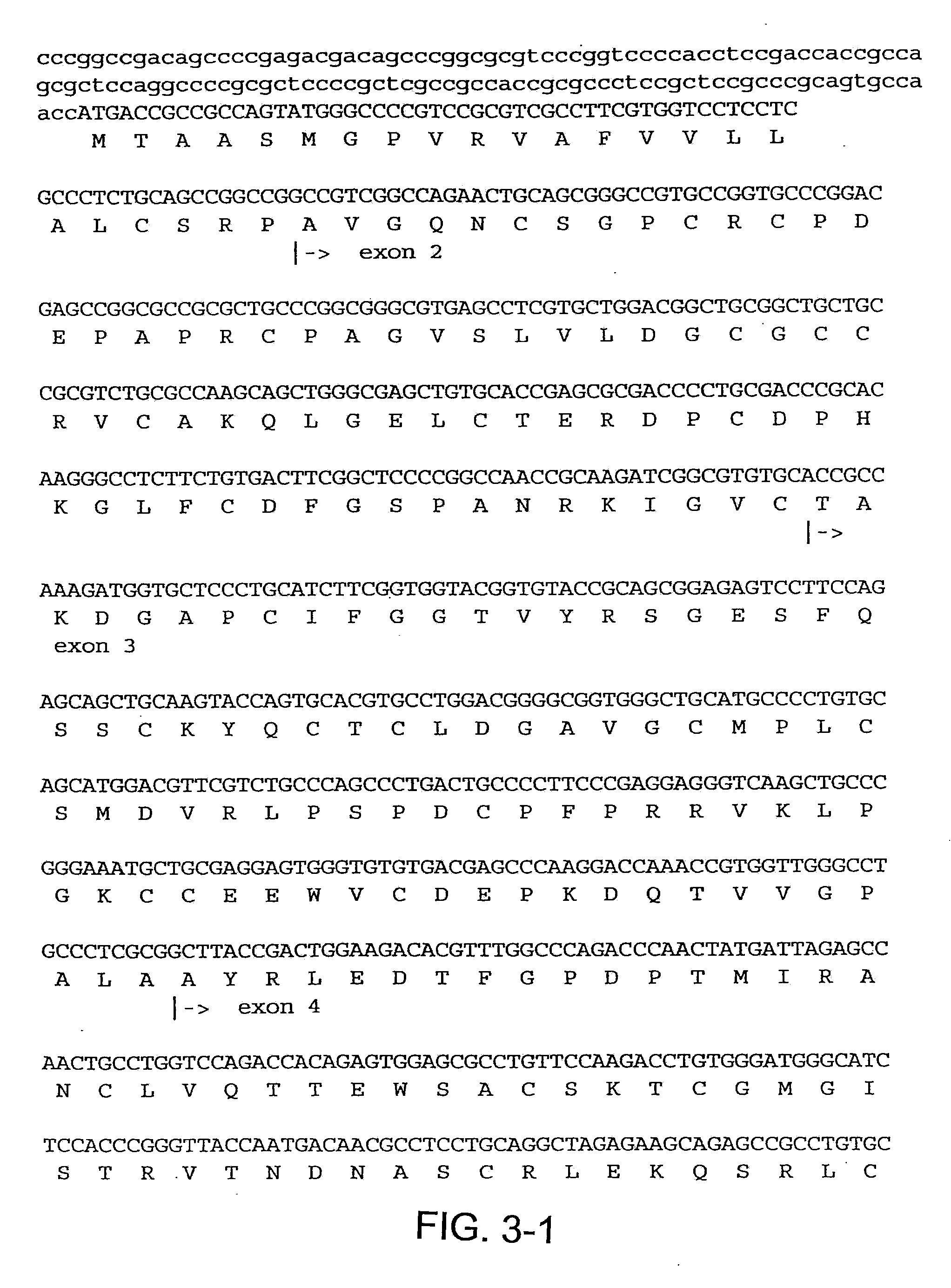
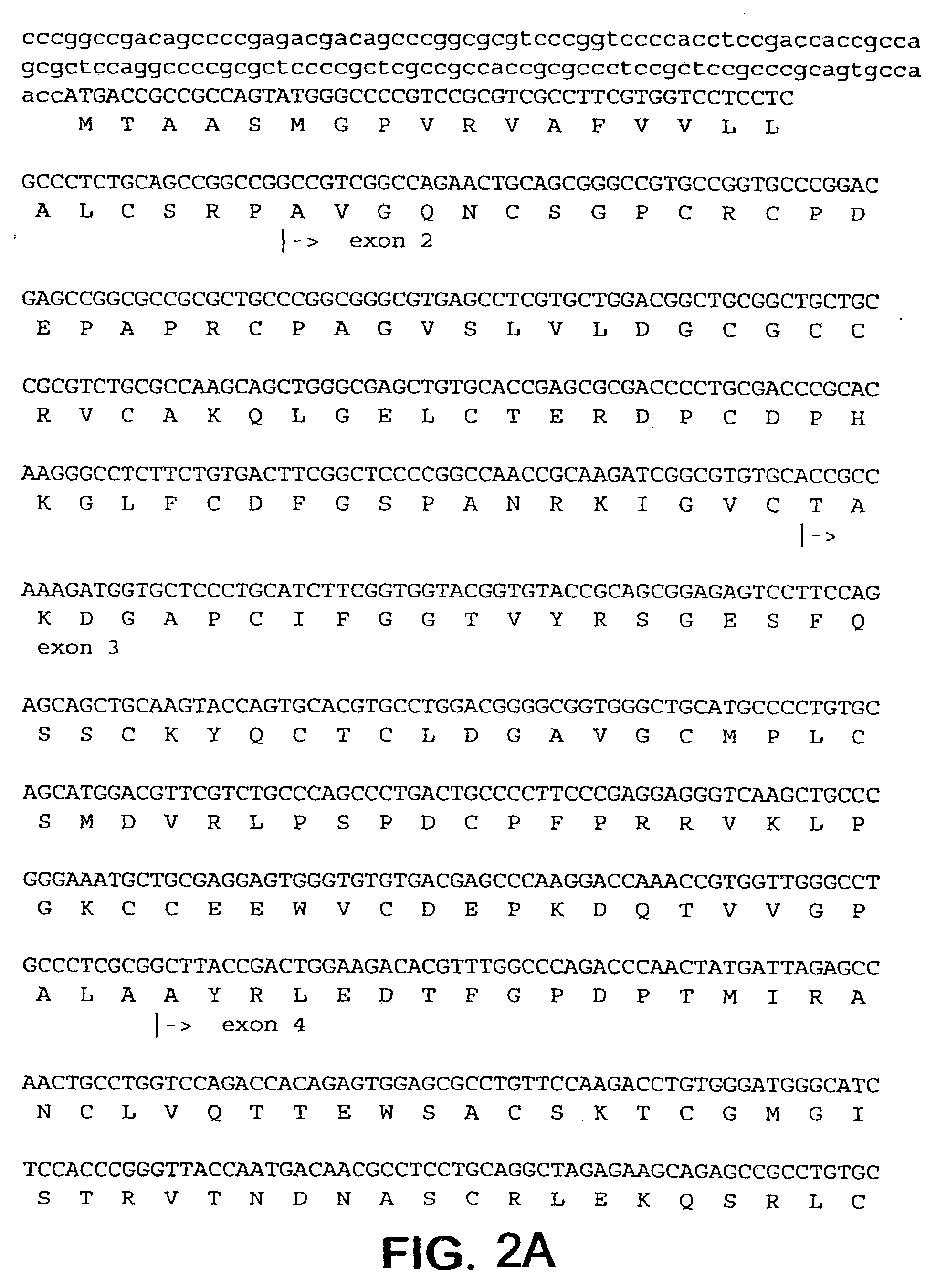
InactiveUS7115390B1Inhibit and suppress mitogenic activityIncrease mitogenic activityPeptide/protein ingredientsGenetic material ingredientsCTGFExon
The present invention is directed to CTGF fragments comprising at least exon 4 or exon 5 of CTGF and having mitogenic activity. The present invention is further directed to methods using said CTGF fragments to identify compositions which modulate the mitogenic activity of said CTGF fragments and to the compositions so identified.
Owner:MIAMI UNIVERISTY OF +1
Treatments for cancer
ActiveUS20080206256A1Microbiological testing/measurementGenetic material ingredientsAbnormal tissue growthLymphatic Spread
The present invention provides methods for reducing tumor survival, expansion, and metastasis. In particular, the invention provides methods for reducing pancreatic tumor survival, expansion, and metastasis. The invention also provides agents for use in the methods, particularly agents that reduce the level or activity of connective tissue growth factor (CTGF), and methods for identifying such agents.
Owner:FIBROGEN INC
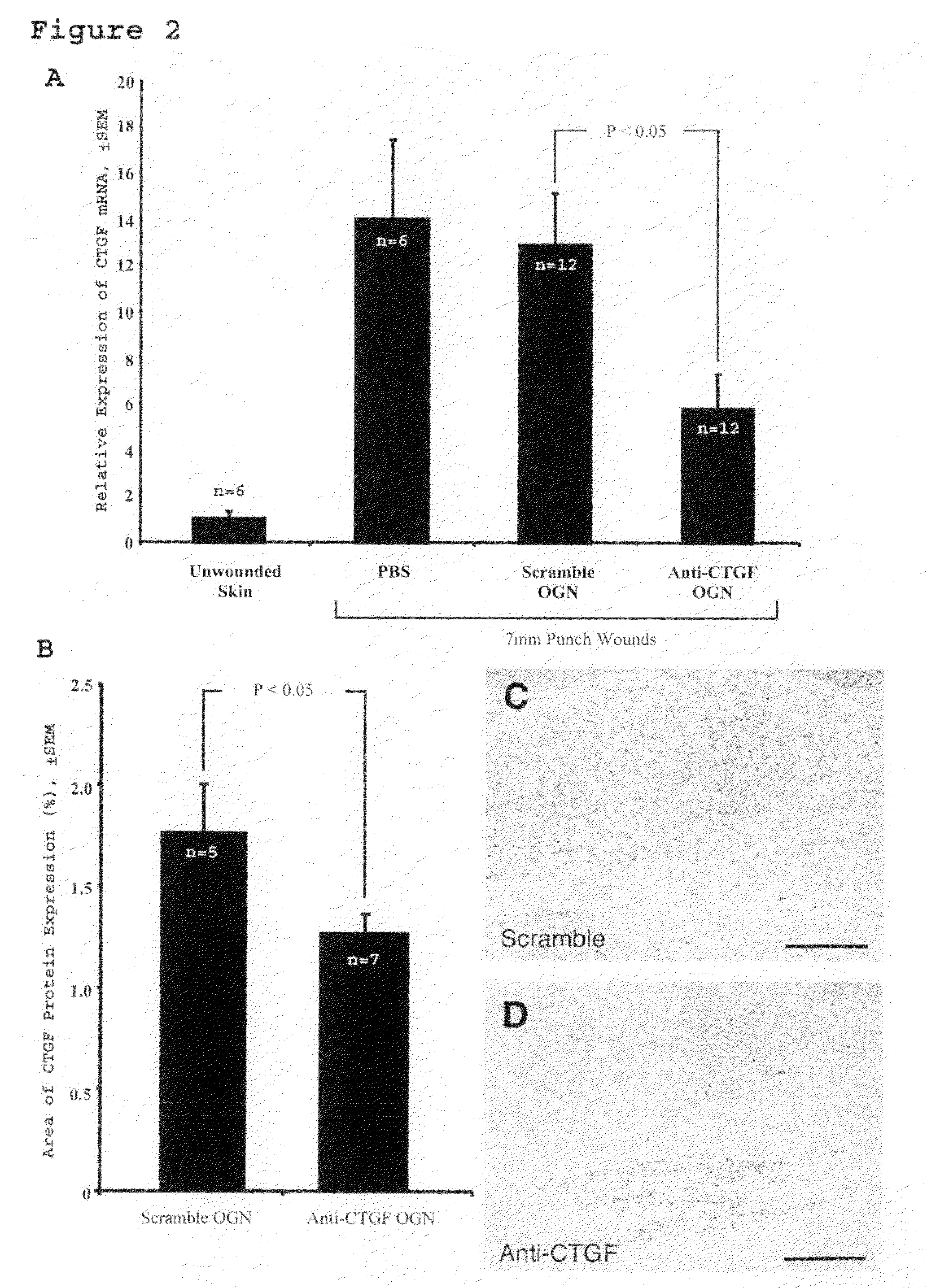
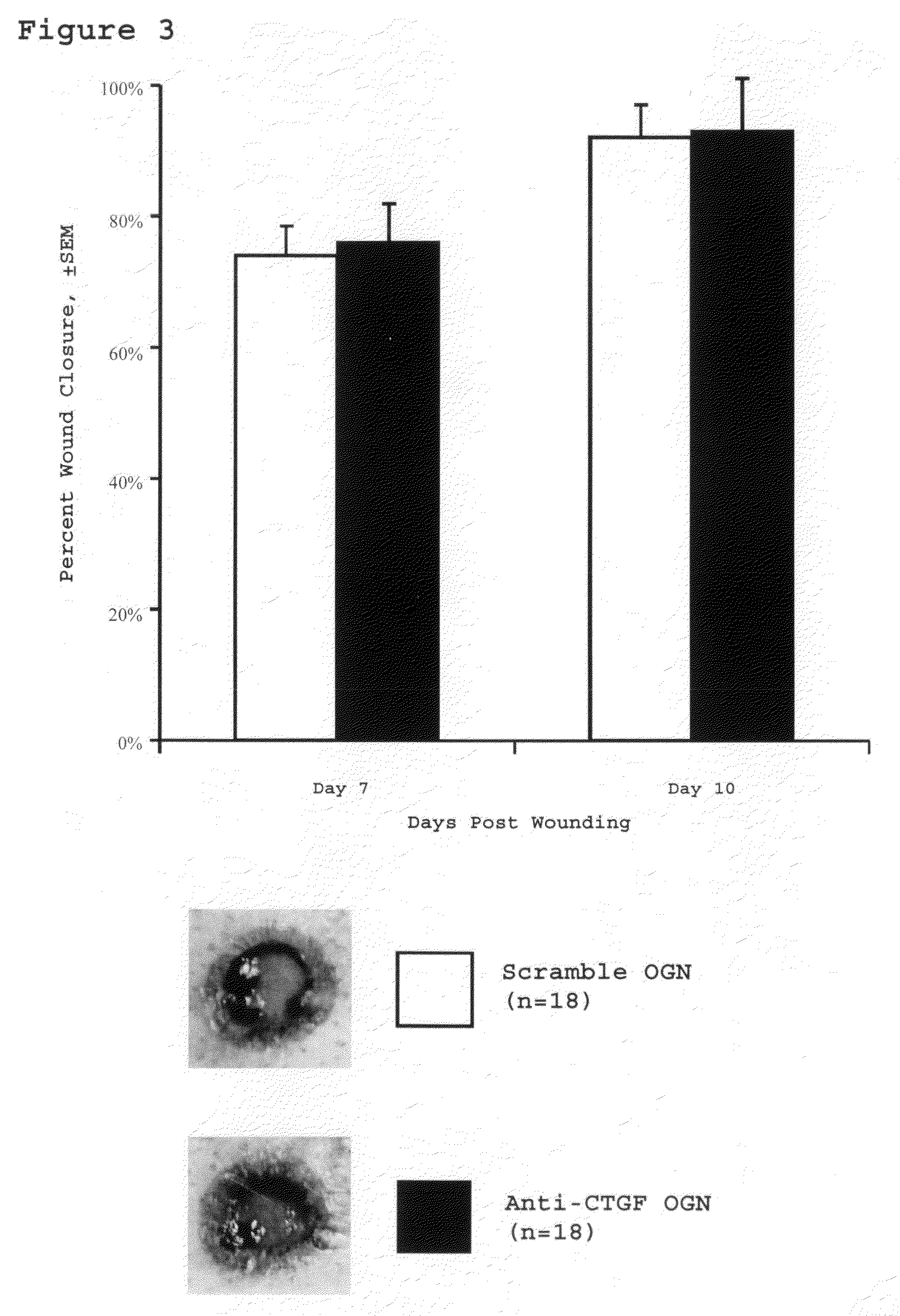
Method for reducing scarring during wound healing using antisense compounds directed to ctgf
InactiveUS20110054004A1Reduce hypertrophic scarringInhibit expressionOrganic active ingredientsDermatological disorderWound healingCTGF
This invention provides a method for reducing hypertropic scarring resulting from dermal wound healing in a subject in need which comprises administering to the subject an antisense oligonucleotide which inhibits expression of connective tissue growth factor (CTGF) in an amount effective to inhibit expression of CTGF and thereby reduce hypertrophic scarring.
Owner:NORTHWESTERN UNIV +1
RNAi inhibition of CTGF for treatment of ocular disorders
Owner:NOVARTIS AG
Antibodies that bind to a portion of the VWC domain of connective tissue growth factor
The present invention relates to antibodies that bind to a portion of the Von Willebrand's factor (VWC) domain of connective tissue growth factor (CTGF). The antibodies are particularly directed to regions of CTGF involved in biological activities associated with fibrosis. The invention also relates to methods of using the antibodies to treat disorders associated with CTGF including localized and systemic fibrotic disorders including those of the lung, liver, heart, skin, and kidney.
Owner:FIBROGEN INC
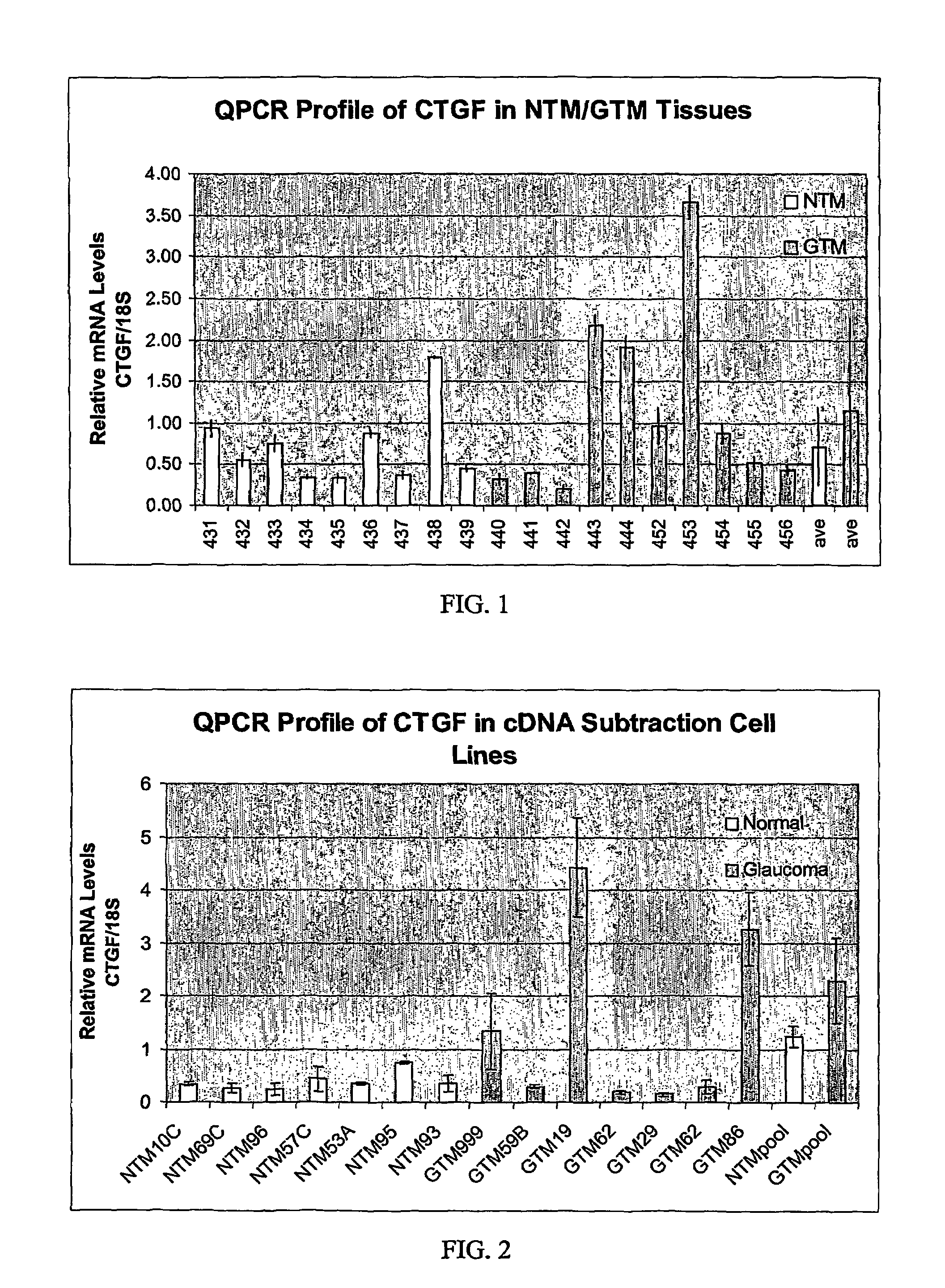
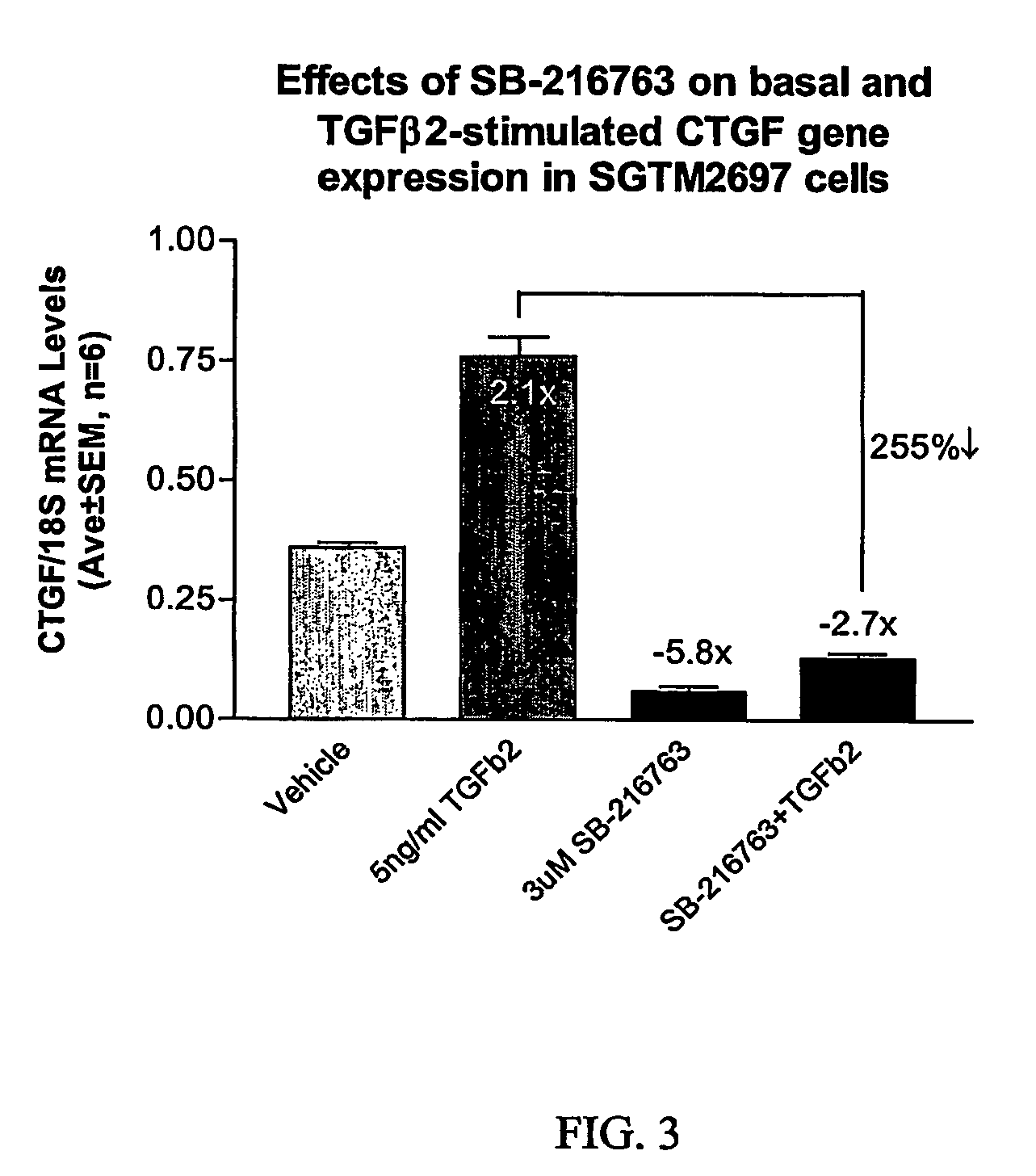
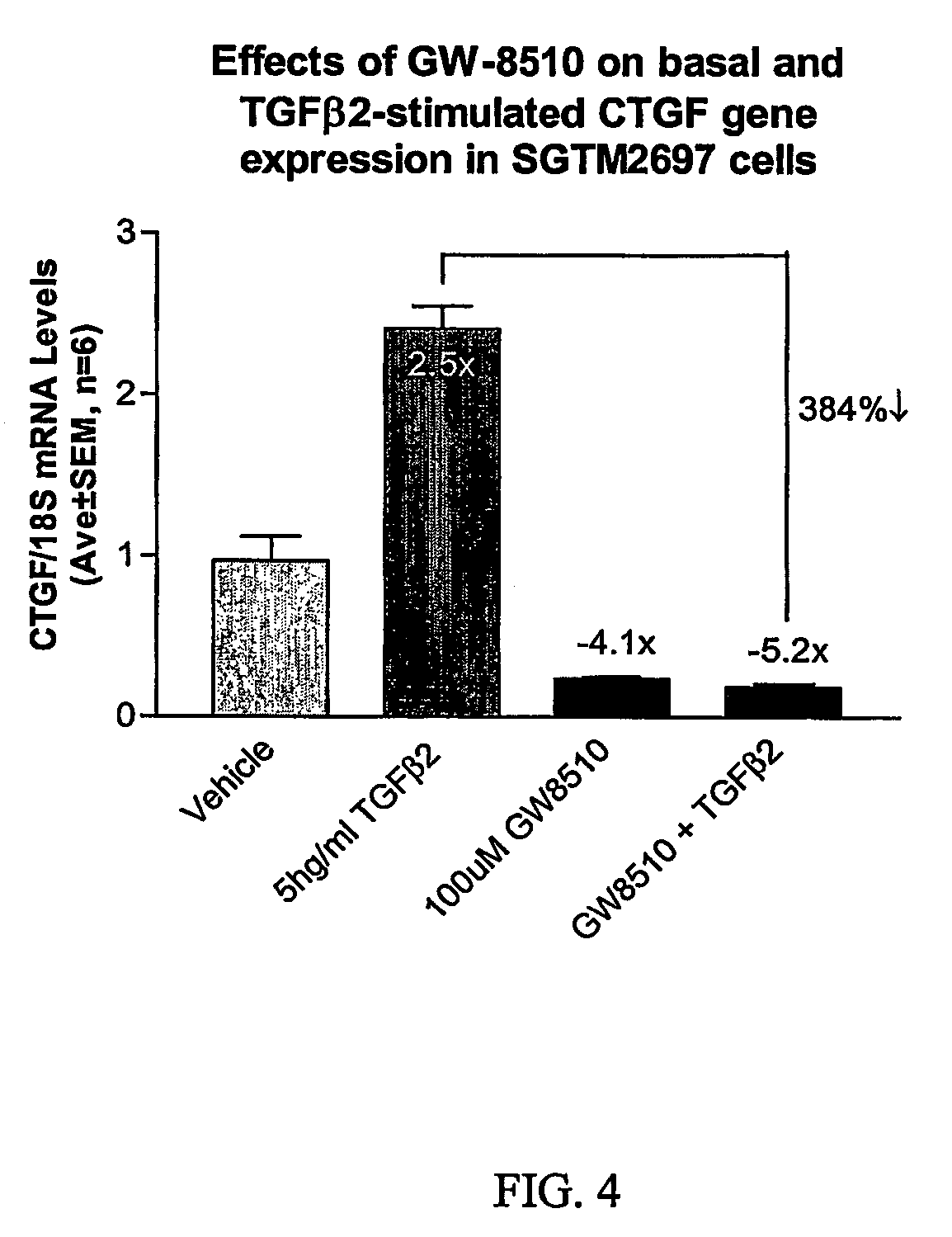
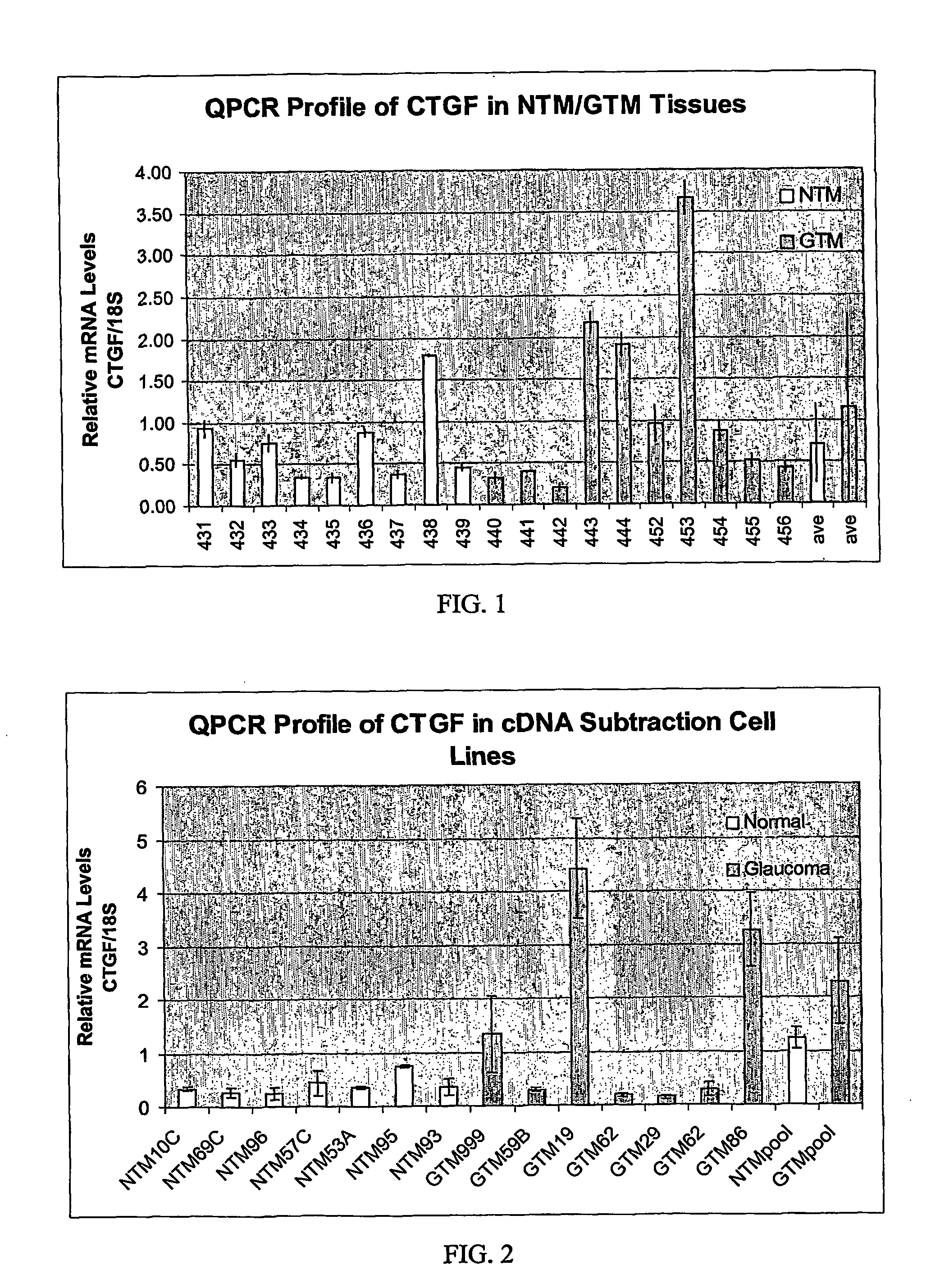
Agents which regulate, inhibit, or modulate the activity and/or expression of connective tissue growth factor (CTGF) as a unique means to both lower intraocular pressure and treat glaucomatous retinopathies/optic neuropathies
InactiveUS7351407B2Inhibit expressionLower eye pressureBiocideSenses disorderCTGFLow intraocular pressure
The present invention provides a method for lowering intraocular pressure and providing neuroprotection to a patient in need thereof by administering a therapeutically effective amount of at least one non-nucleotide or non-protein agent that inhibits expression and / or signaling of connective tissue growth factor (CTGF).
Owner:NOVARTIS AG
Agents which regulate, inhibit, or modulate the activity and/or expression of connective tissue growth factor (ctgf) as a unique means to both lower intraocular pressure and treat glaucomatous retinopathies/optic neuropathies
InactiveUS20050234075A1Lower eye pressureInhibit expressionBiocideSenses disorderConnective tissue fiberCTGF
The present invention provides a method for lowering intraocular pressure and providing neuroprotection to a patient in need thereof by administering a therapeutically effective amount of at least one non-nucleotide or non-protein agent that inhibits expression and / or signaling of connective tissue growth factor (CTGF).
Owner:NOVARTIS AG
Connective Tissue Growth Factor Fragments and Methods and Uses Thereof
InactiveUS20100190838A1High activityOrganic active ingredientsSenses disorderDiseaseCell-Extracellular Matrix
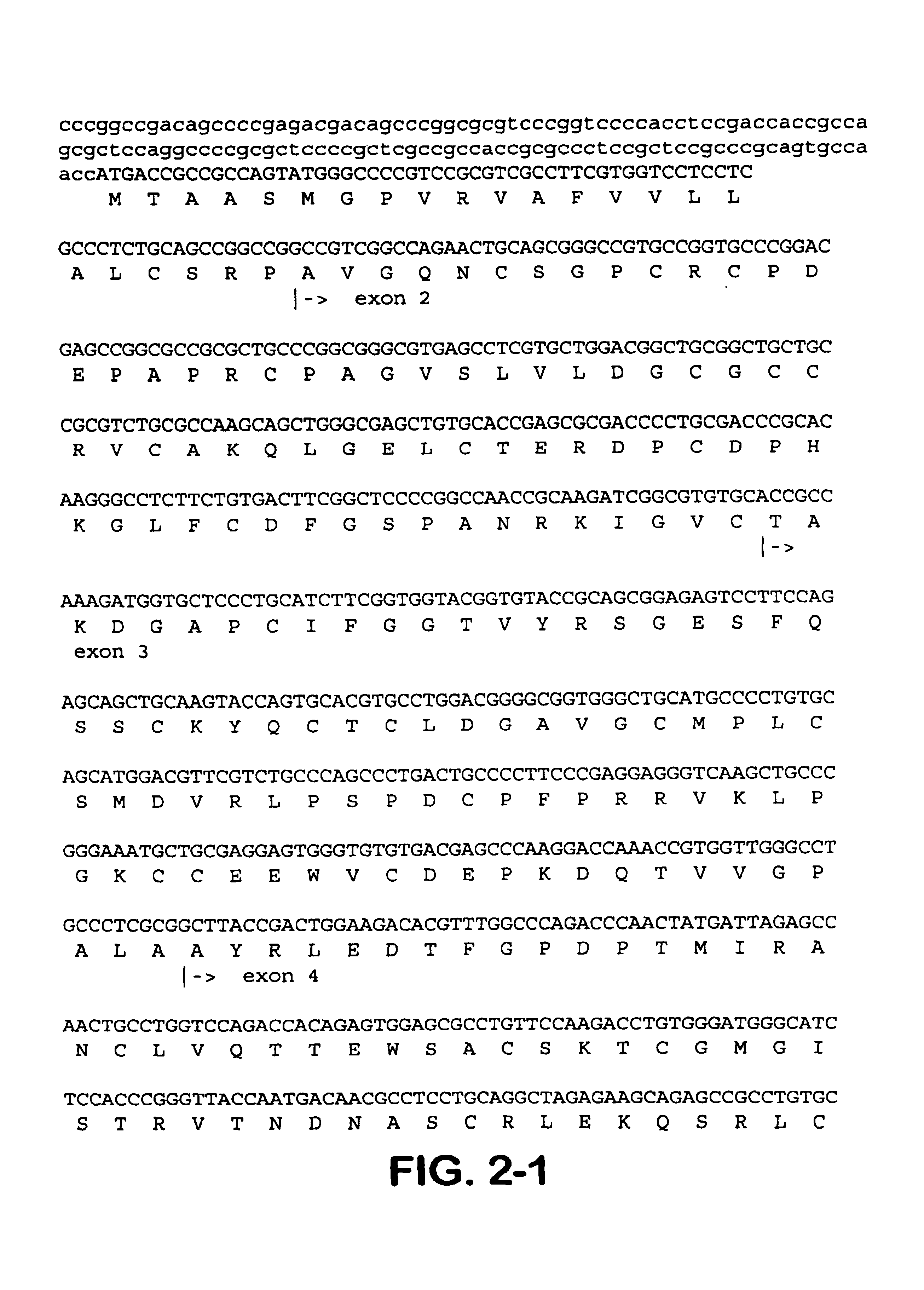
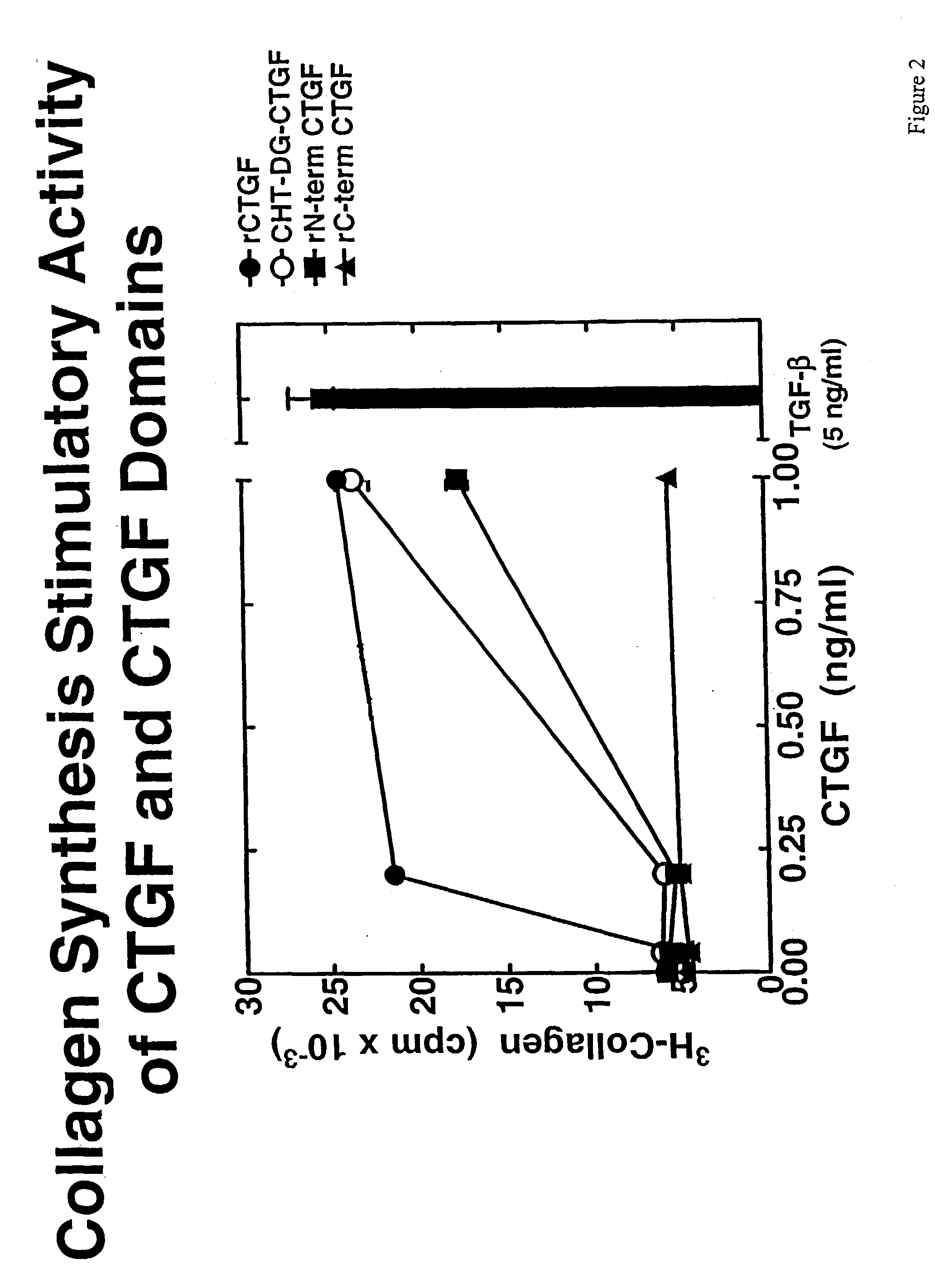
The present invention is directed to CTGF fragments comprising at least exon 2 or exon 3 of CTGF and having the ability to induce extracellular matrix synthesis, in particular, collagen synthesis and myofibroblast differentiation. The present invention is further directed to methods using said CTGF fragments to identify compositions which modulate the activity of said CTGF fragments and to the compositions so identified. The invention also relates to methods of treating CTGF-associated disorders and diseases associated with the overproduction of the extracellular matrix.
Owner:UNIV OF MIAMI
Antibodies directed to fragments of connective tissue growth factor (CTGF) polypeptide and methods and uses thereof
InactiveUS7718177B2High activityInhibit and suppress activityOrganic active ingredientsSenses disorderDiseaseCell-Extracellular Matrix
The present invention is directed to CTGF fragments comprising at least exon 2 or exon 3 of CTGF and having the ability to induce extracellular matrix synthesis, in particular, collagen synthesis and myofibroblast differentiation. The present invention is further directed to methods using said CTGF fragments to identify compositions which modulate the activity of said CTGF fragments and to the compositions so identified. The invention also relates to methods of treating CTGF-associated disorders and diseases associated with the overproduction of the extracellular matrix.
Owner:UNIV OF MIAMI
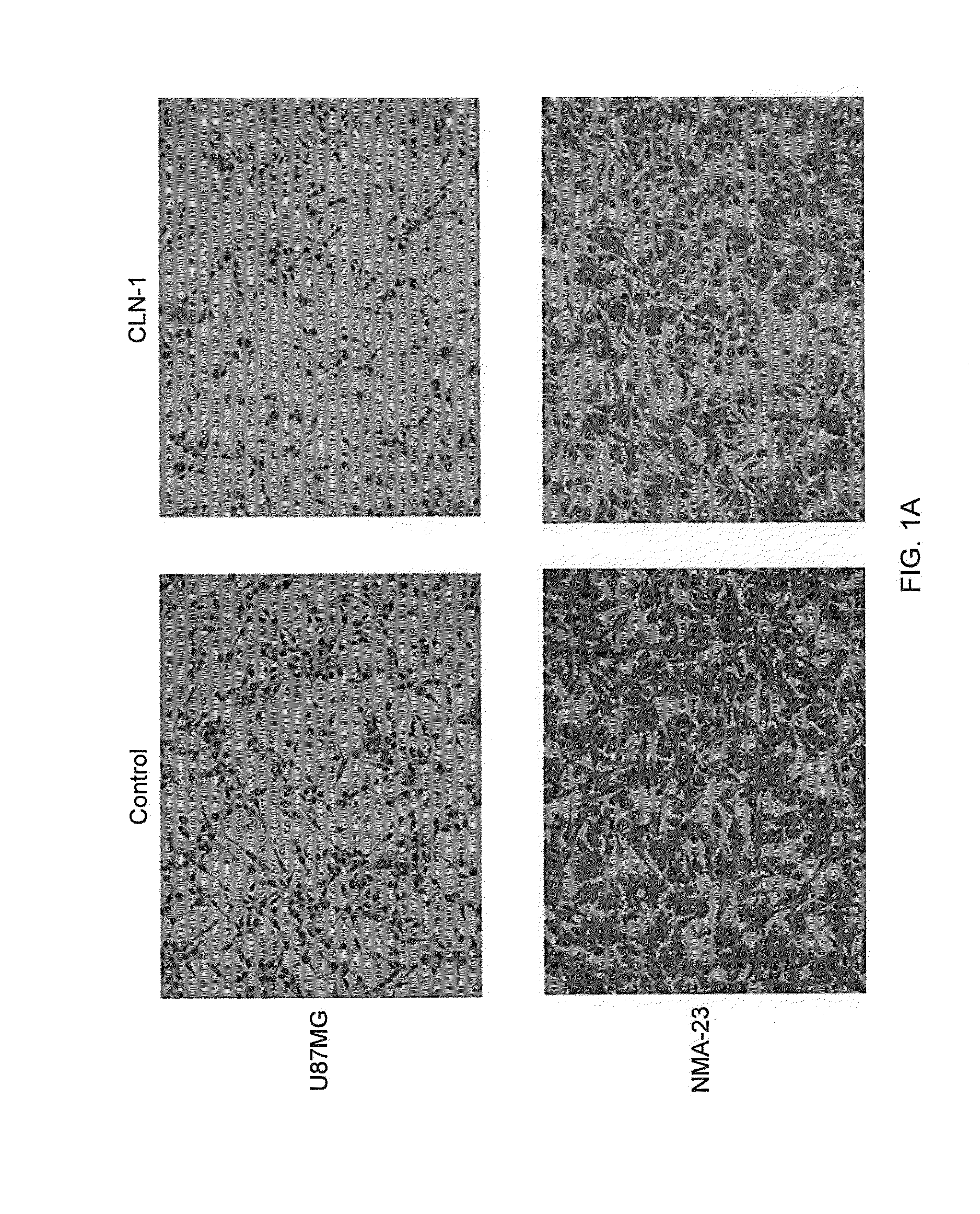
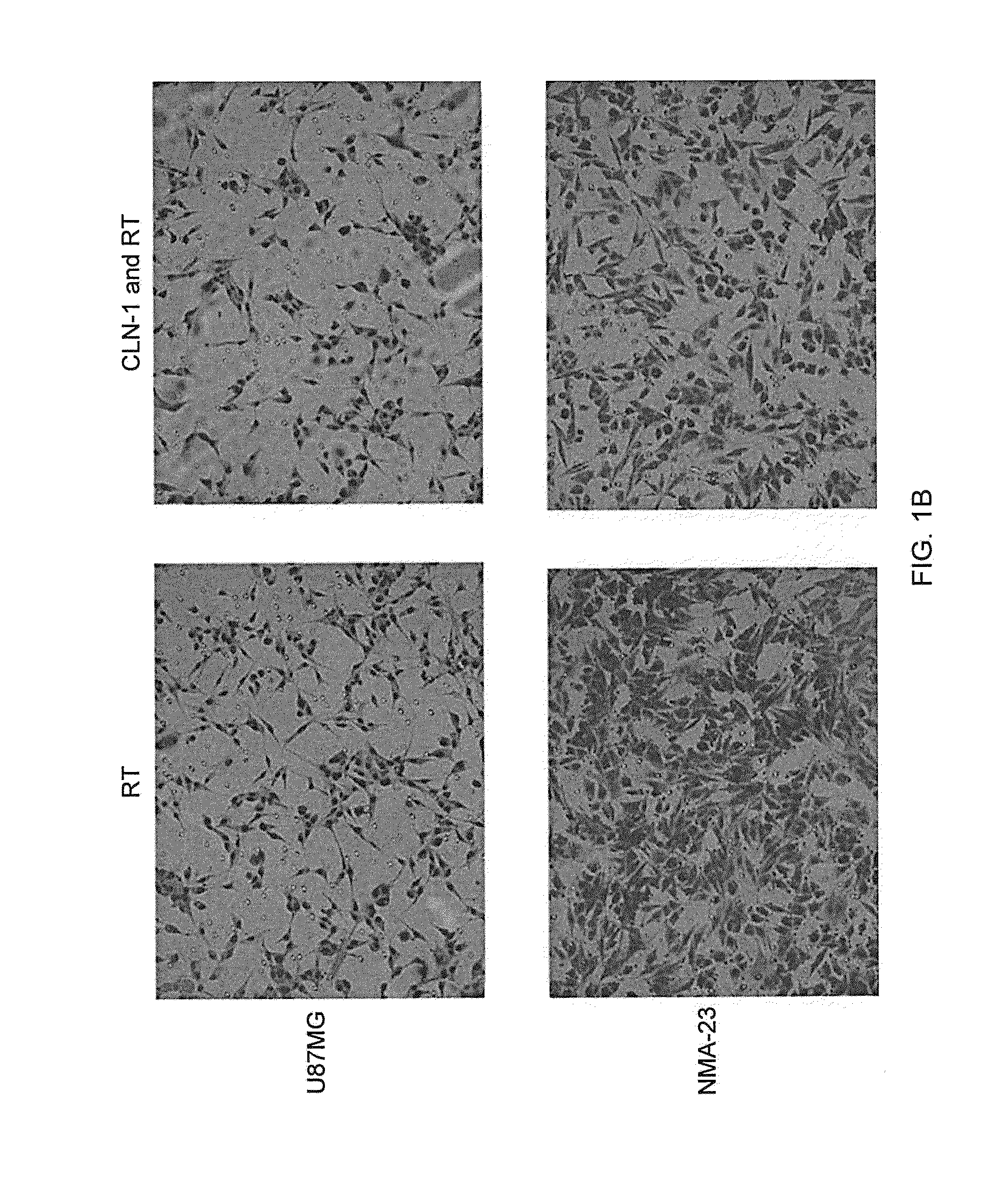
Compositions and Methods for Treating Brain Tumors
InactiveUS20140065162A1Reduce cell proliferationVascularity reducedOrganic active ingredientsHeavy metal active ingredientsCTGFBrain tumor
The present invention relates to methods and medicaments useful for the treatment of brain tumors by administering anti-CTGF agents, particularly anti-CTGF antibodies. Methods and medicaments are provided for reducing tumor cell proliferation and tumor growth, reducing tumor vascularity, inhibiting tumor cell invasion, improving tumor surgical margins and prolonging survival of patients with brain tumors.
Owner:FIBROGEN INC
Monoclonal antibody against connective tissue growth factor and medicinal uses thereof
InactiveUS20060223133A1Easy to operateIncrease valueAnimal cellsEnzymologyConnective tissue fiberCTGF
A human monoclonal antibody useful for the treatment of various diseases caused by human connective tissue growth factor (CTGF) and preventing the onset of the above diseases; medicinal uses thereof; and various monoclonal antibodies having various characteristics against various mammalian CTGFs useful for detecting and assaying CTGFs present in body fluids of mammals suffering from various diseases.
Owner:AMGEN INC
Treatment of disorders associated with elevated blood glucose or blood pressure
InactiveUS20050136502A1Avoid complicationsReducing overproductionBiocidePeptide/protein ingredientsDiseaseCTGF
The present invention relates to methods for treating or delaying the onset of pathologies associated with elevated blood glucose and / or elevated blood pressure. The methods are directed to modulating, regulating, or inhibiting the expression or activity of Connective Tissue Growth Factor (CTGF) or fragments thereof.
Owner:RISER BRUCE +1
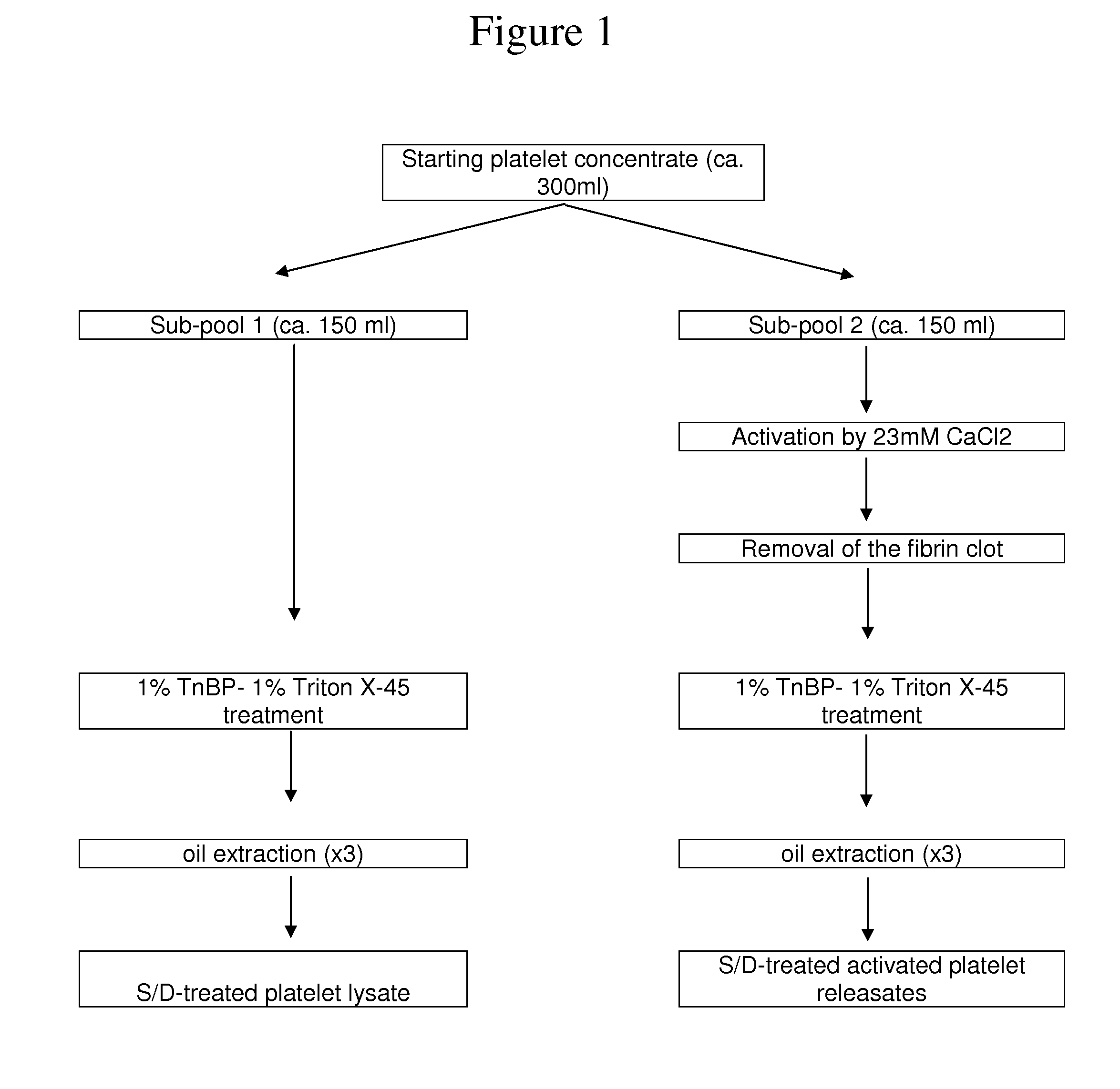
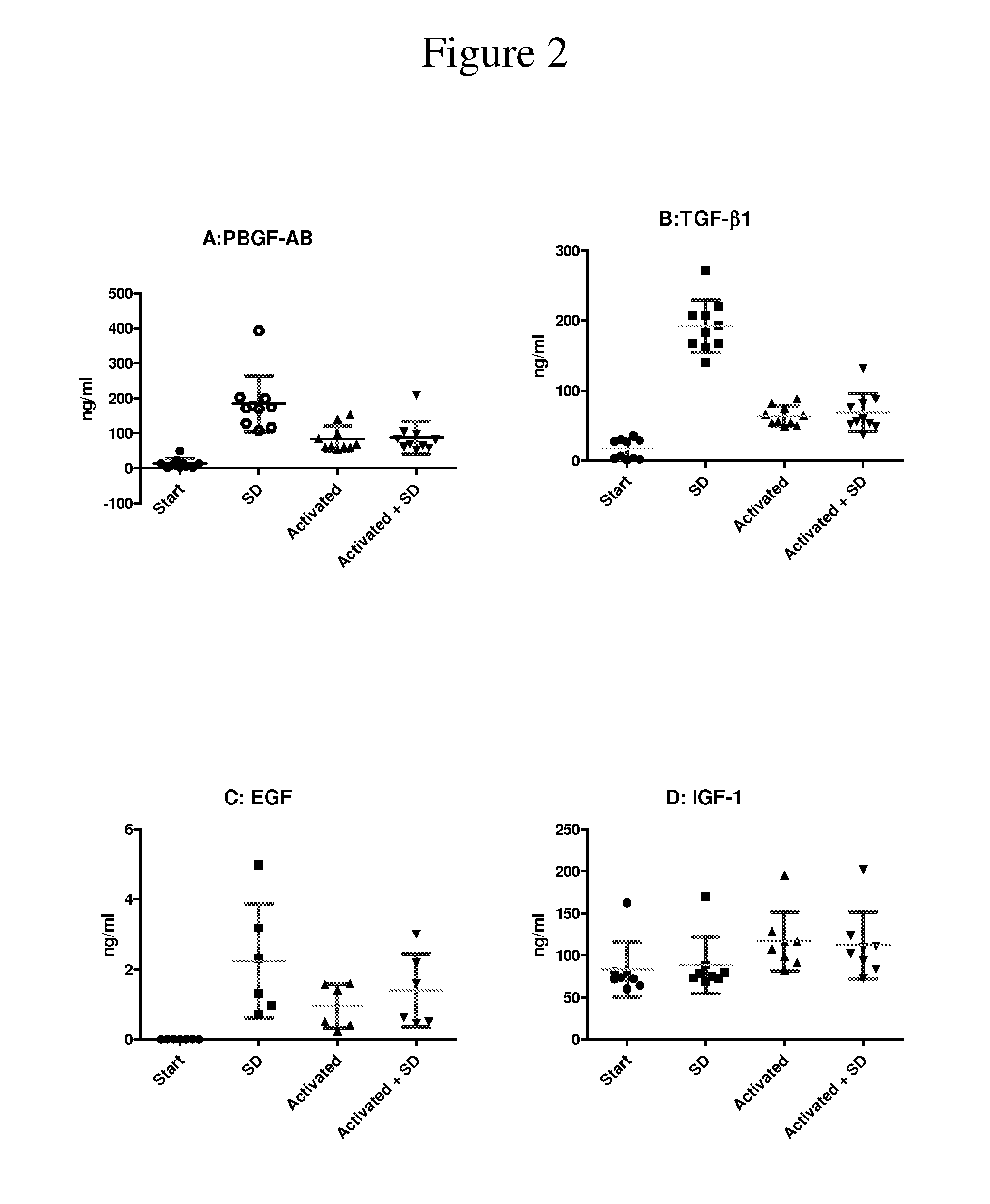
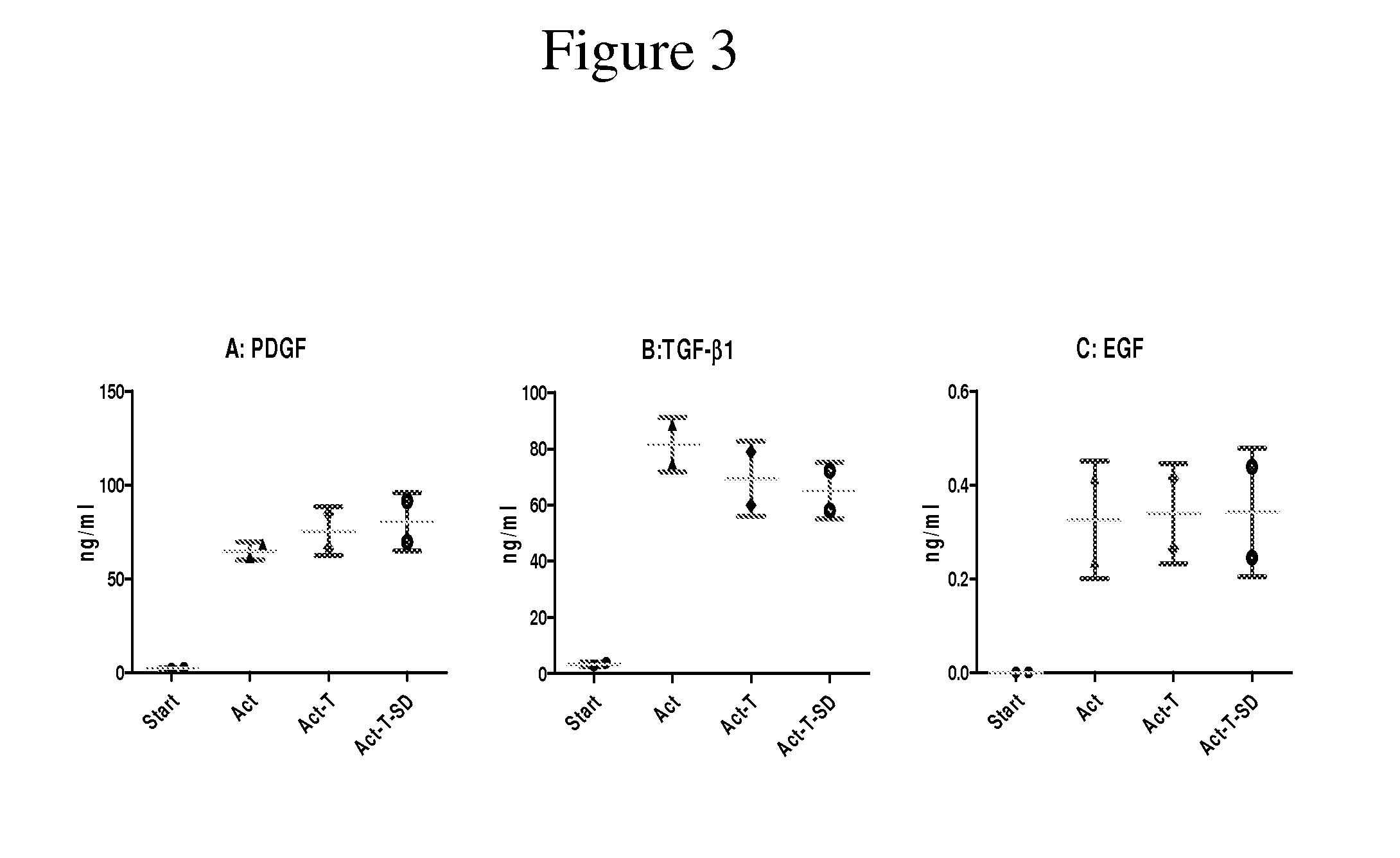
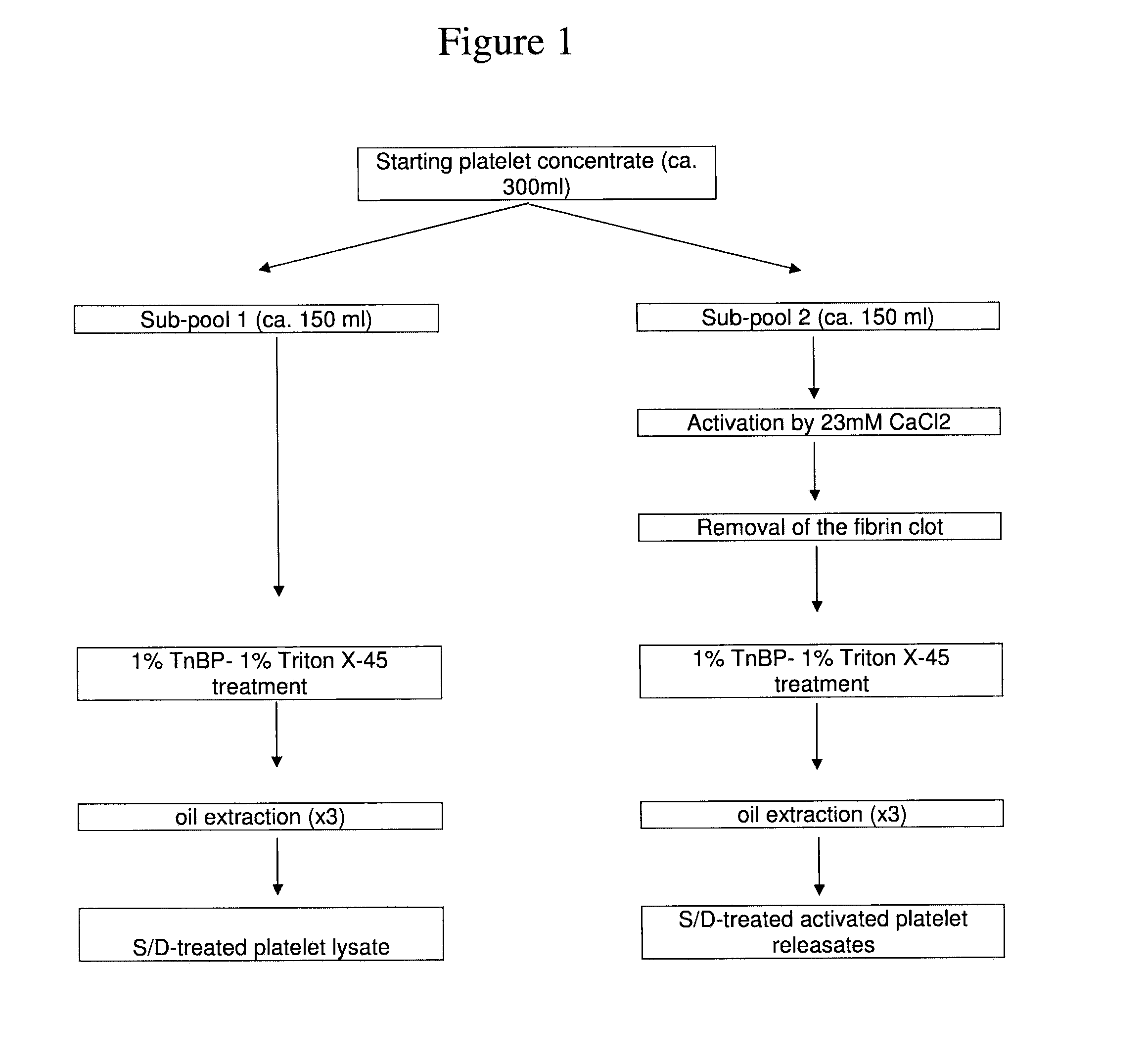
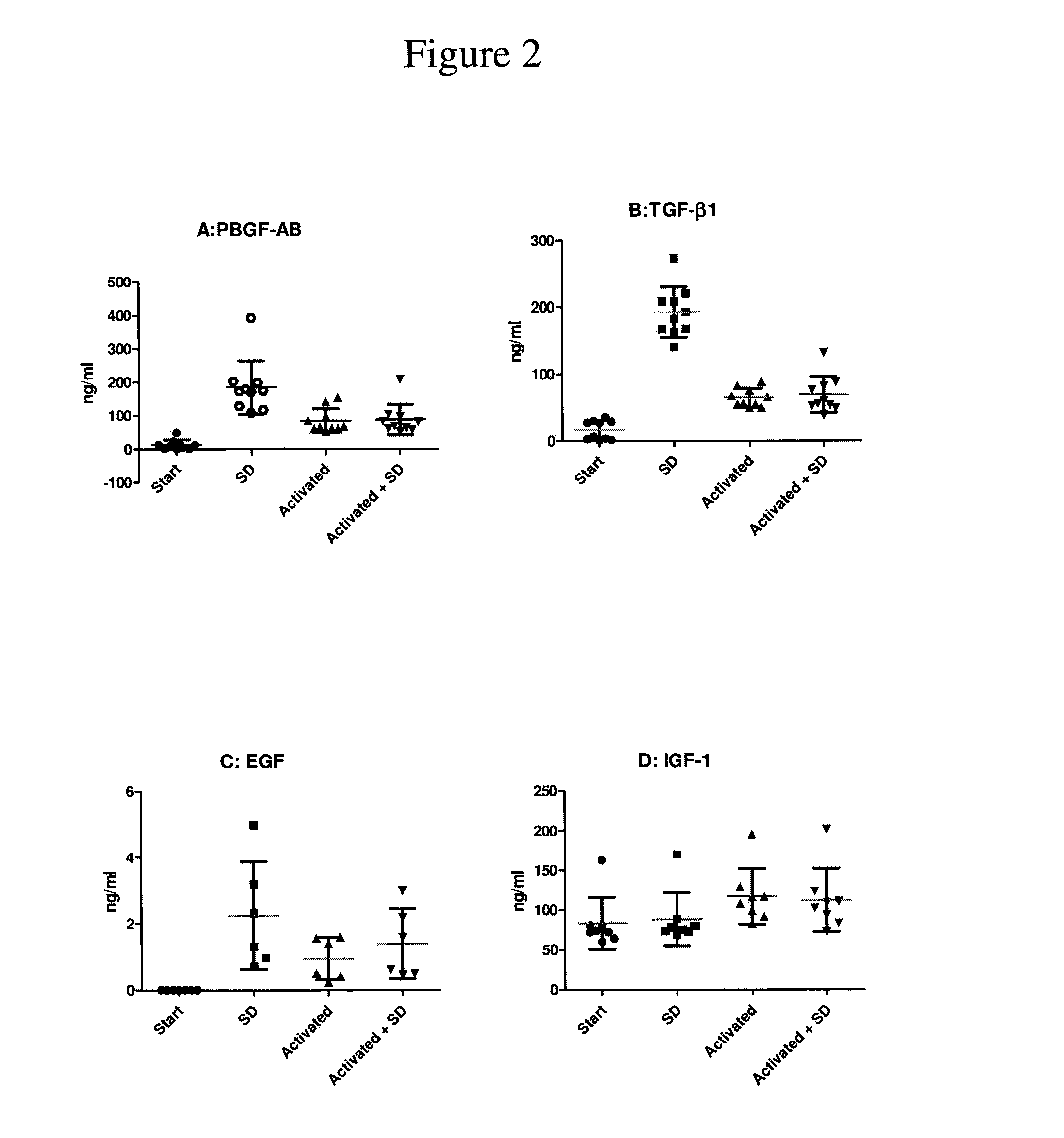
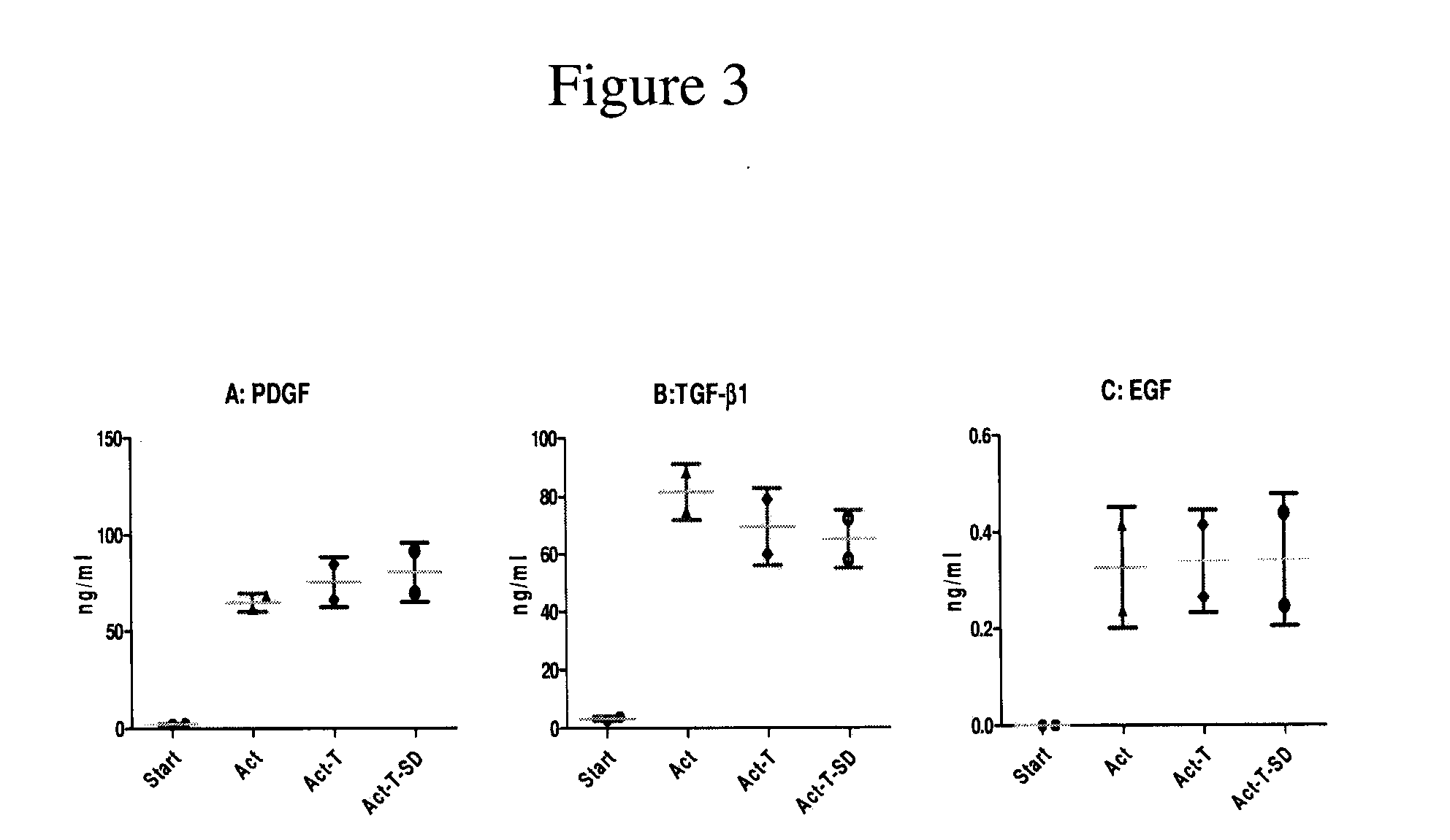
Clottable concentrate of platelet growth factors and preparation method thereof
InactiveUS20110027257A1Improve the level ofCosmetic preparationsPeptide/protein ingredientsCTGFSolvent
The present disclosure relates to a clottable concentrate of platelet growth factors for therapeutic and / or cosmetic use, preferably comprising the growth factors PDGF, TGT-β, IGF, EGF, CTGF, bFGF and VEGF. In a preferred embodiment, the clottable concentrate of platelet growth factors does not induce blood cell-related transfusion reactions. The present disclosure also relates to a method for preparing a clottable concentrate of platelet growth factors including the steps of contacting a platelet concentrate with a solvent and / or a detergent, incubating the platelet concentrate with the solvent and / or detergent for a period of at least 5 minutes to 6 hours, at a pH maintained in a range from about 6.0 to about 9.0, and at a temperature within the range of from 2° C. to 50° C., preferably within the range of from 25° C. to 45° C., and removing the solvent and / or the detergent by oil extraction and / or chromatographic means.
Owner:ZHENG YANG BIOMEDICAL TECH
Connective tissue growth factor fragments and methods and uses thereof
Owner:GROTENDORST GARY R +1
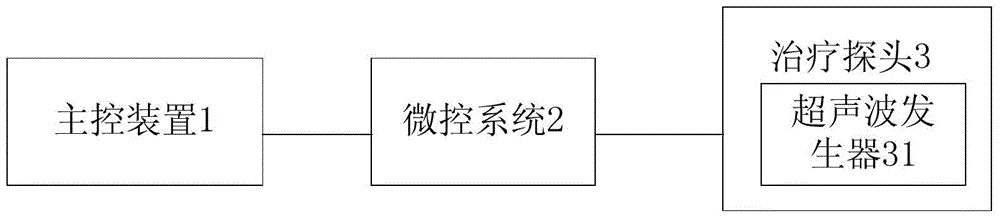
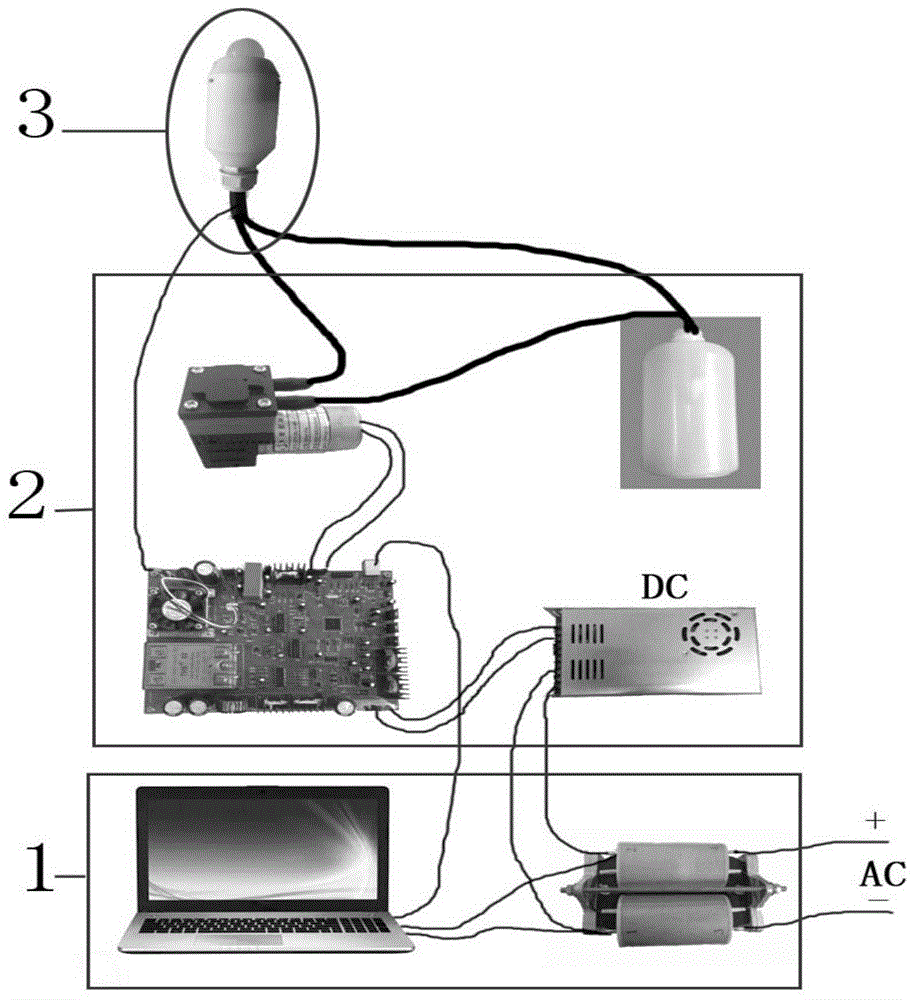
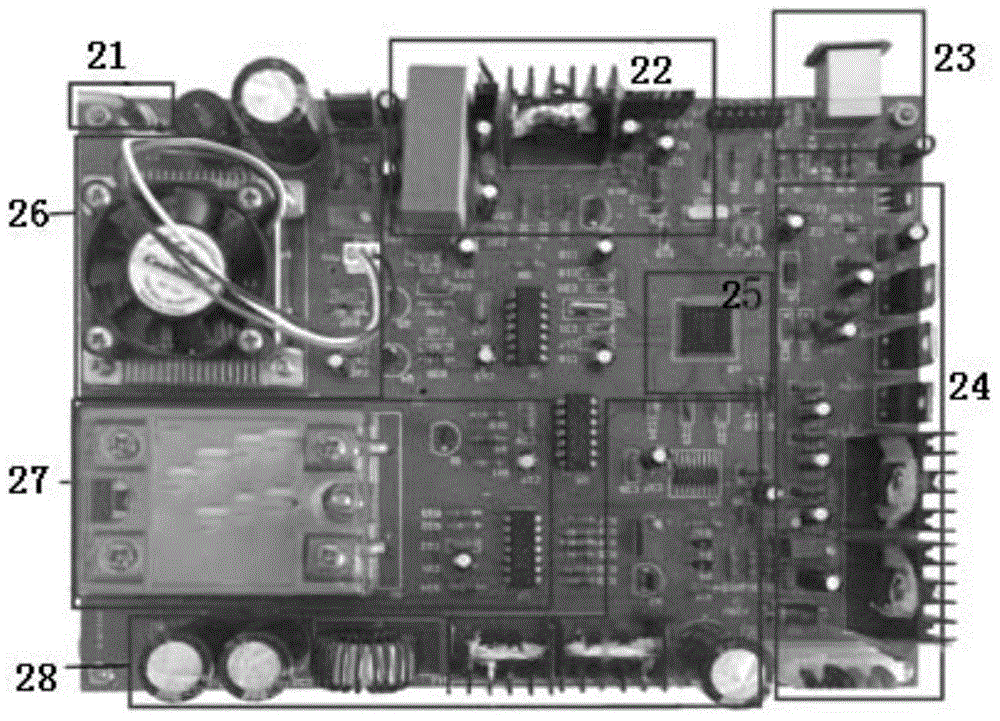
Pulse ultrasound treatment equipment for male erectile dysfunction
ActiveCN105726190AAvoid painAvoid harmUltrasound therapyNon-surgical orthopedic devicesHuman bodyControl signal
The invention discloses pulse ultrasound treatment equipment for male erectile dysfunction.The pulse ultrasound treatment equipment comprises a master control device, a micro-control system and a treatment probe.The treatment probe comprises an ultrasound generator.The master control device sends control signals to the micro-control system, the micro-control system converts the control signals into control instructions and sends the control instructions to the ultrasound generator, and the ultrasound generator generates corresponding ultrasound according to the control instructions.The pulse ultrasound treatment equipment can generate the ultrasound for therapeutic use, treatment is conducted in vitro in a non-intrusive mode, pain brought to a patient by an operation is avoided, and harm of medicine to the human body of dependence of the human body on the medicine are avoided.The low-intensity pulse ultrasound can promote neovascularization, proliferation of cavernous body smooth muscle cells and eNOS and nNOS expression, improve blood flow of the penis, lower a TGF-beta1 / Smad / CTGF signal channel and relieve fibrosis of the penis, the pathological change of the erection organ is improved accordingly, and finally the erection function of the penis is improved.
Owner:北京东方百奥医药开发有限公司 +1
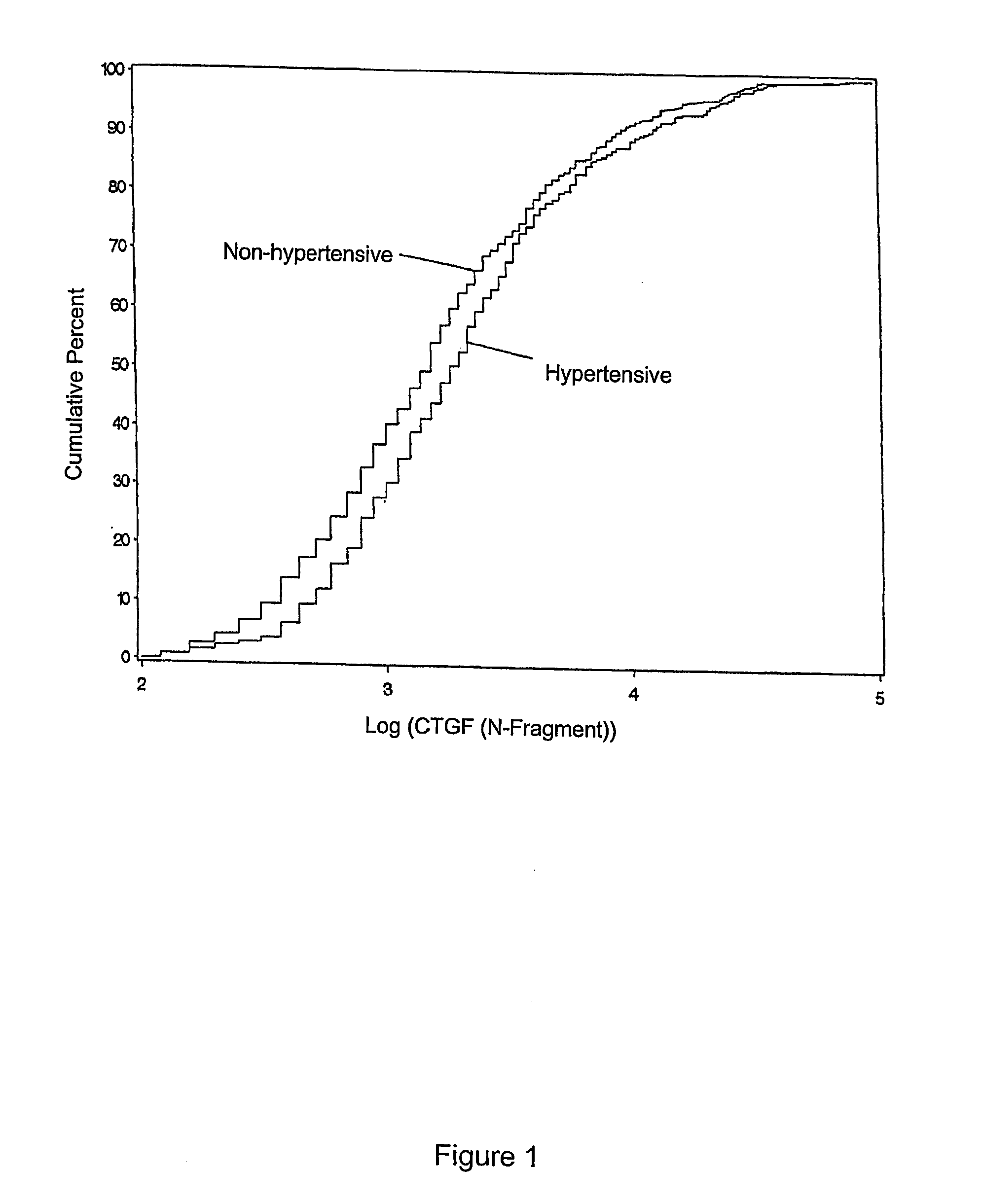
Diagnostic marker for diabetic vascular complications
The present invention relates to the discovery that CTGF is a diagnostic marker indicative of increased risk for development and progression of vascular disease.
Owner:FIBROGEN INC
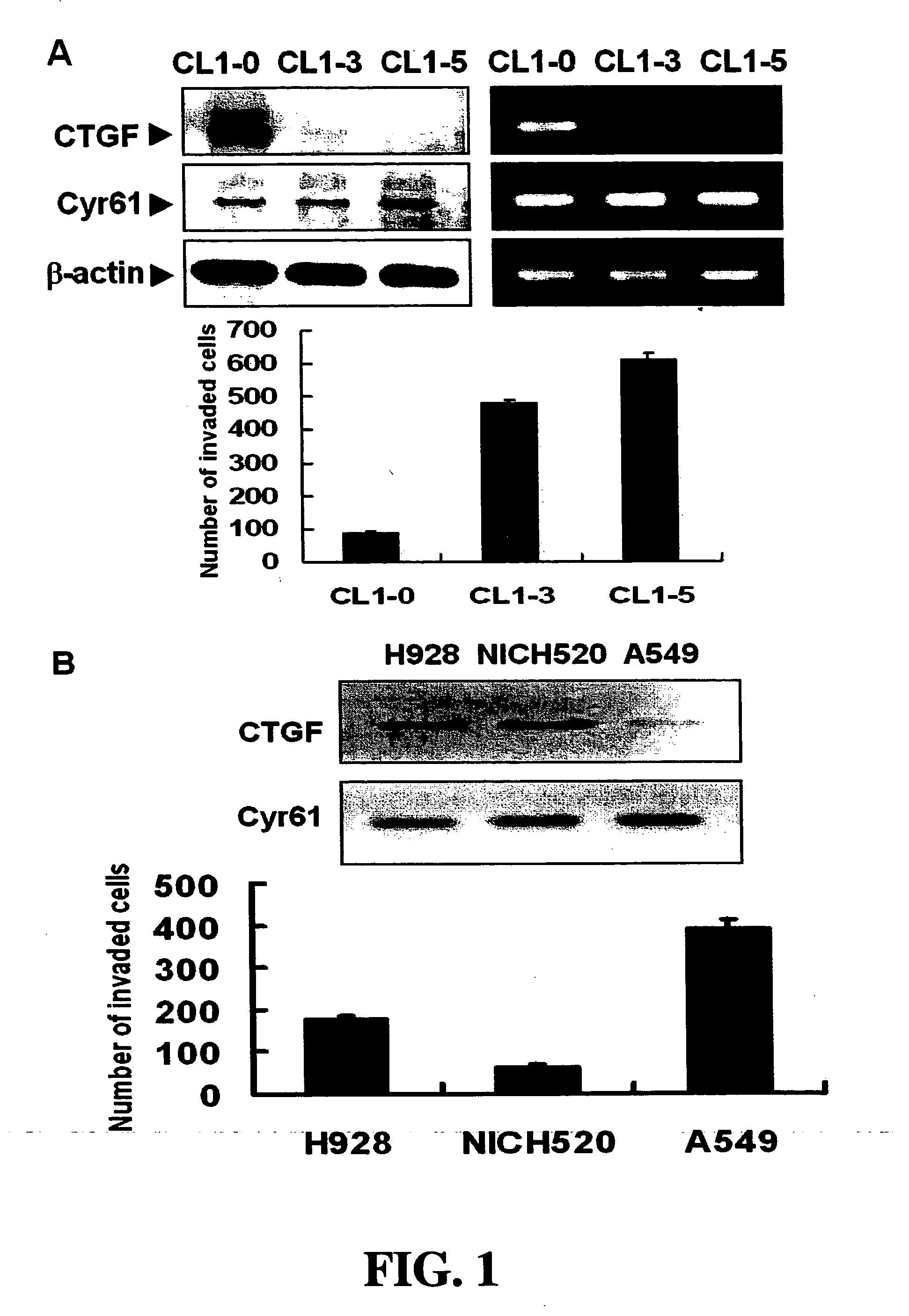
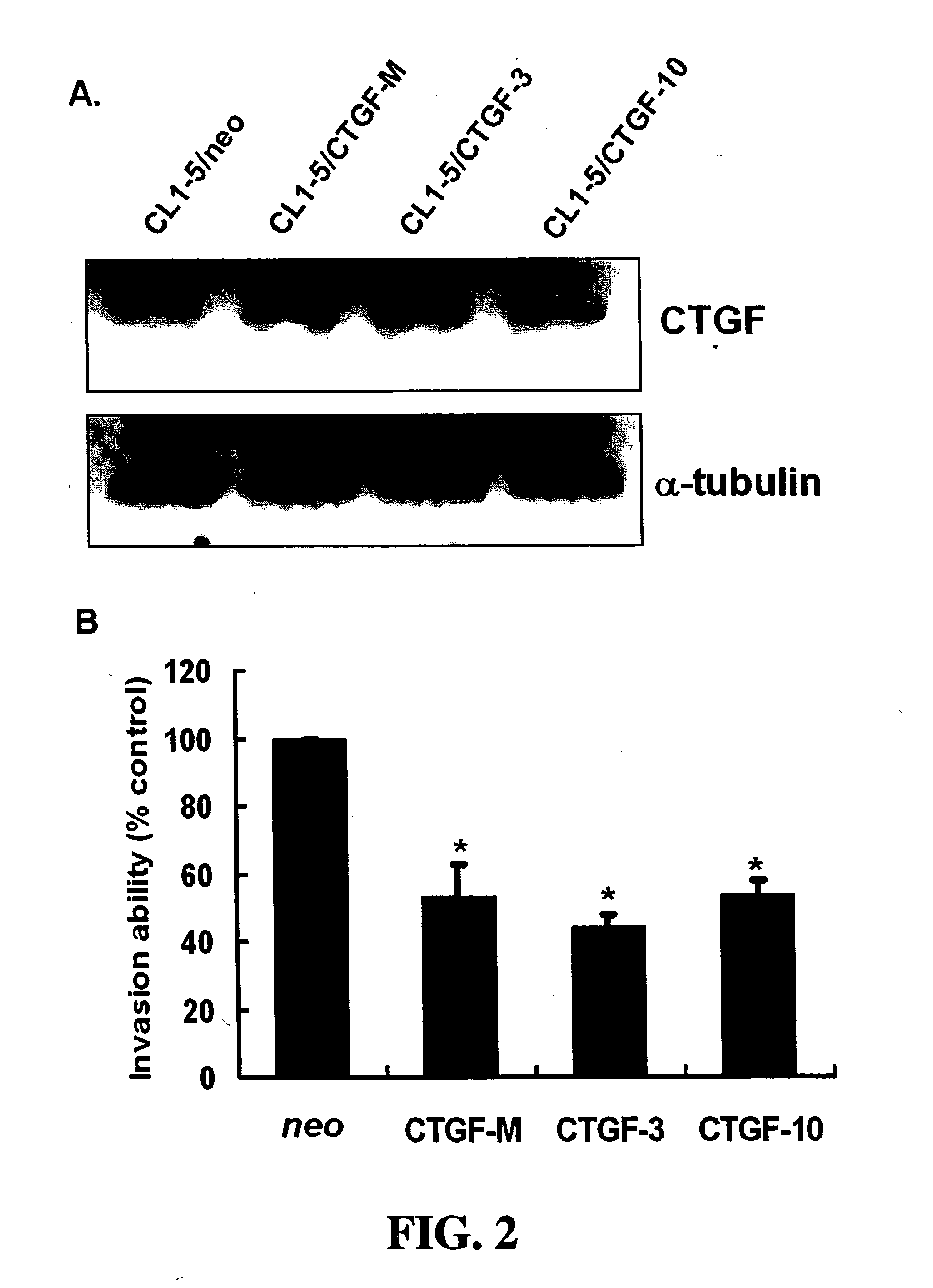
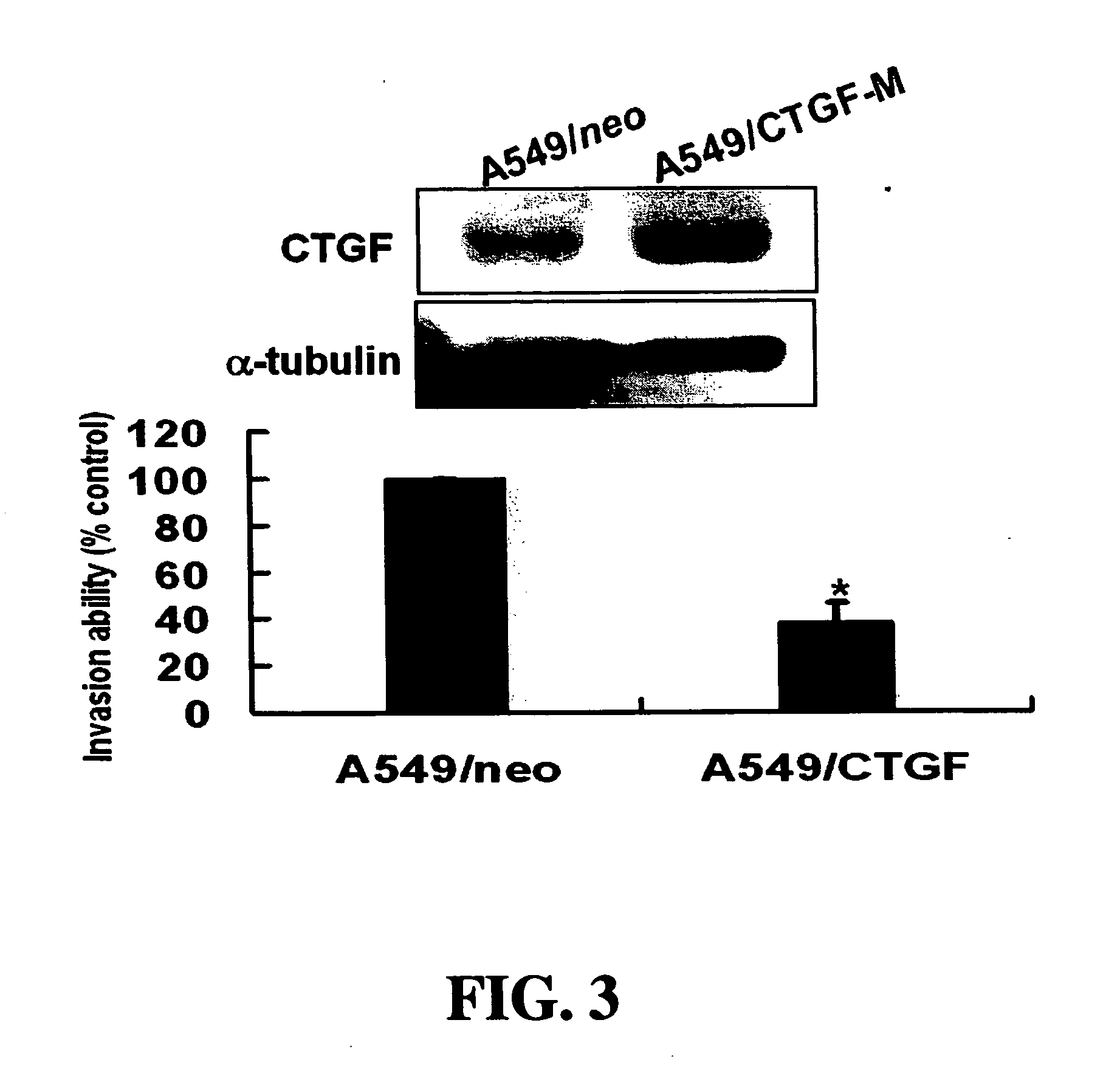
Methods and compositions for diagnosing and suppressing metastasis thereof
ActiveUS20050147986A1Peptide/protein ingredientsMicrobiological testing/measurementAbnormal tissue growthLymphatic Spread
Disclosed is a method for determining metastatic potential of tumors, a method and a composition for treating / suppressing metastasis of cancers, as well as a method for obtaining a metastasis suppressor. A particular aspect of the invention relates to a method and a composition of evaluating expression levels of connective tissue growth factor (CTGF, also known as CCN2) to determine the status of tumor invasion. Another aspect of the invention relates to a method and a composition comprising a tumor suppressor, which maintains or increases an expression level of CTGF to suppress the invasion ability of tumor cells effectively. In another aspect of the invention relates to methods for obtaining a metastasis suppressor by evaluating the expression levels of CTGF after contacting suppressor candidates with a tumor cell line system.
Owner:NAT TAIWAN UNIV
Clottable Concentrate Of Platelet Growth Factors And Preparation Method Thereof
Owner:ZHENG YANG BIOMEDICAL TECH
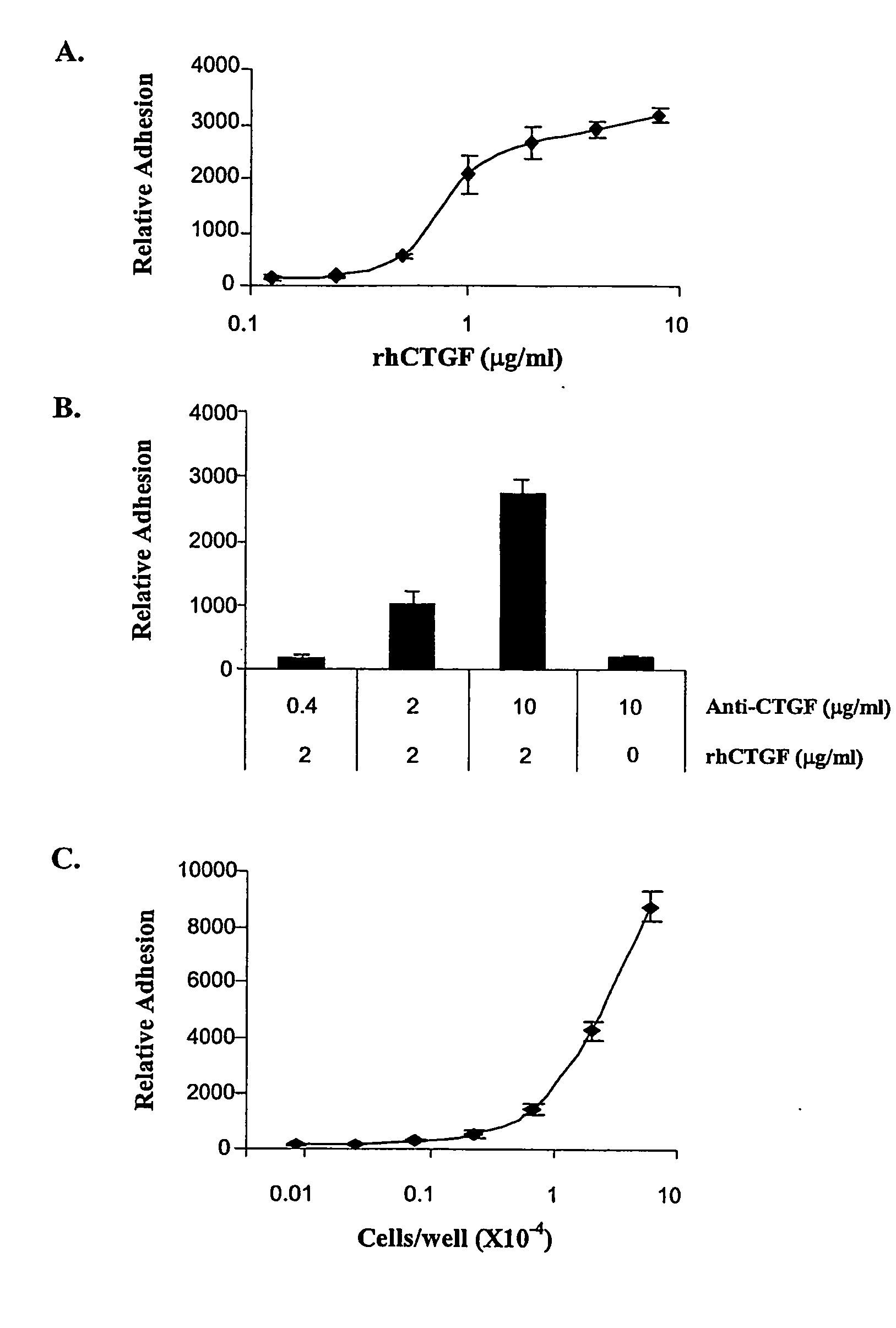
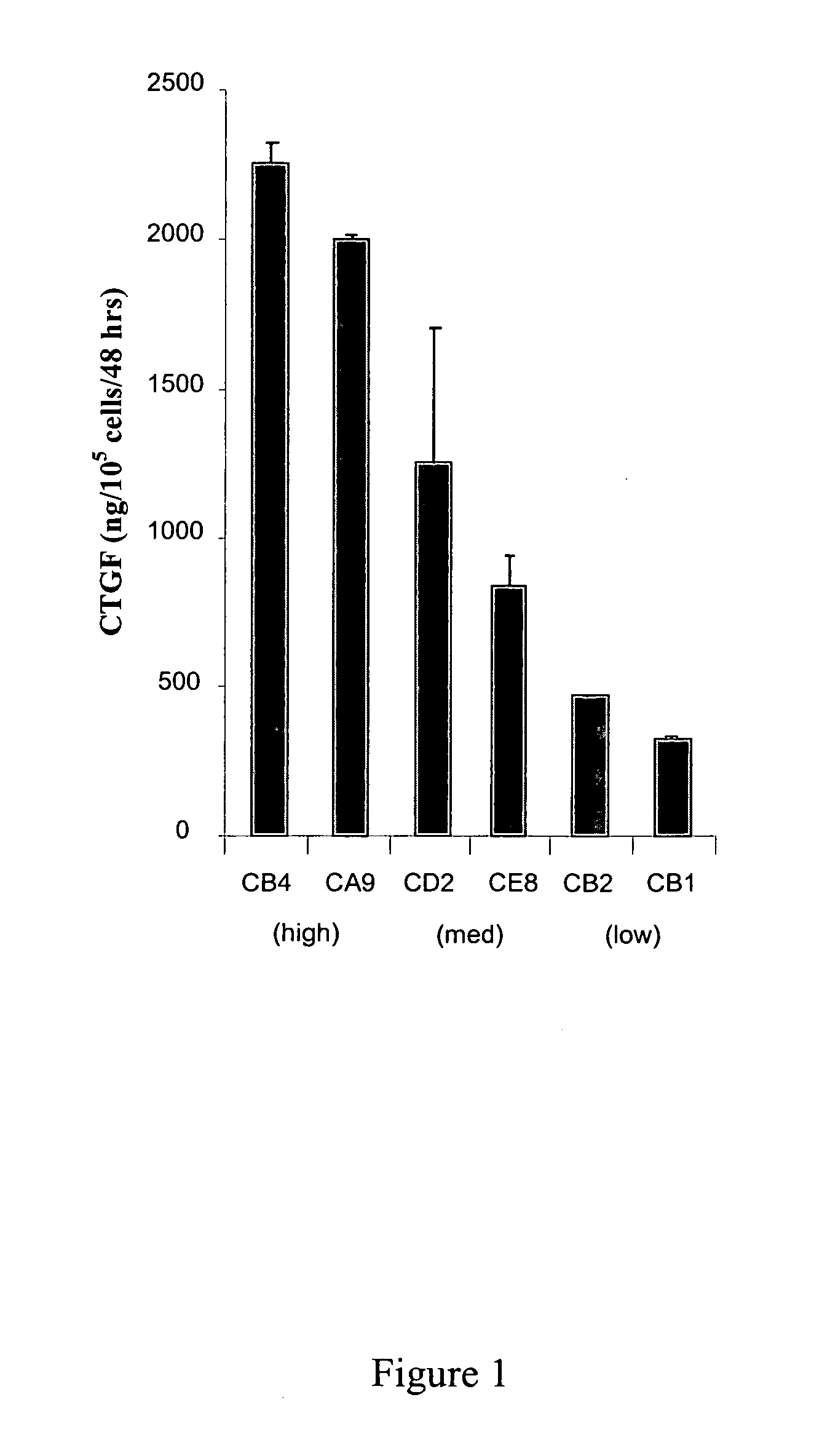
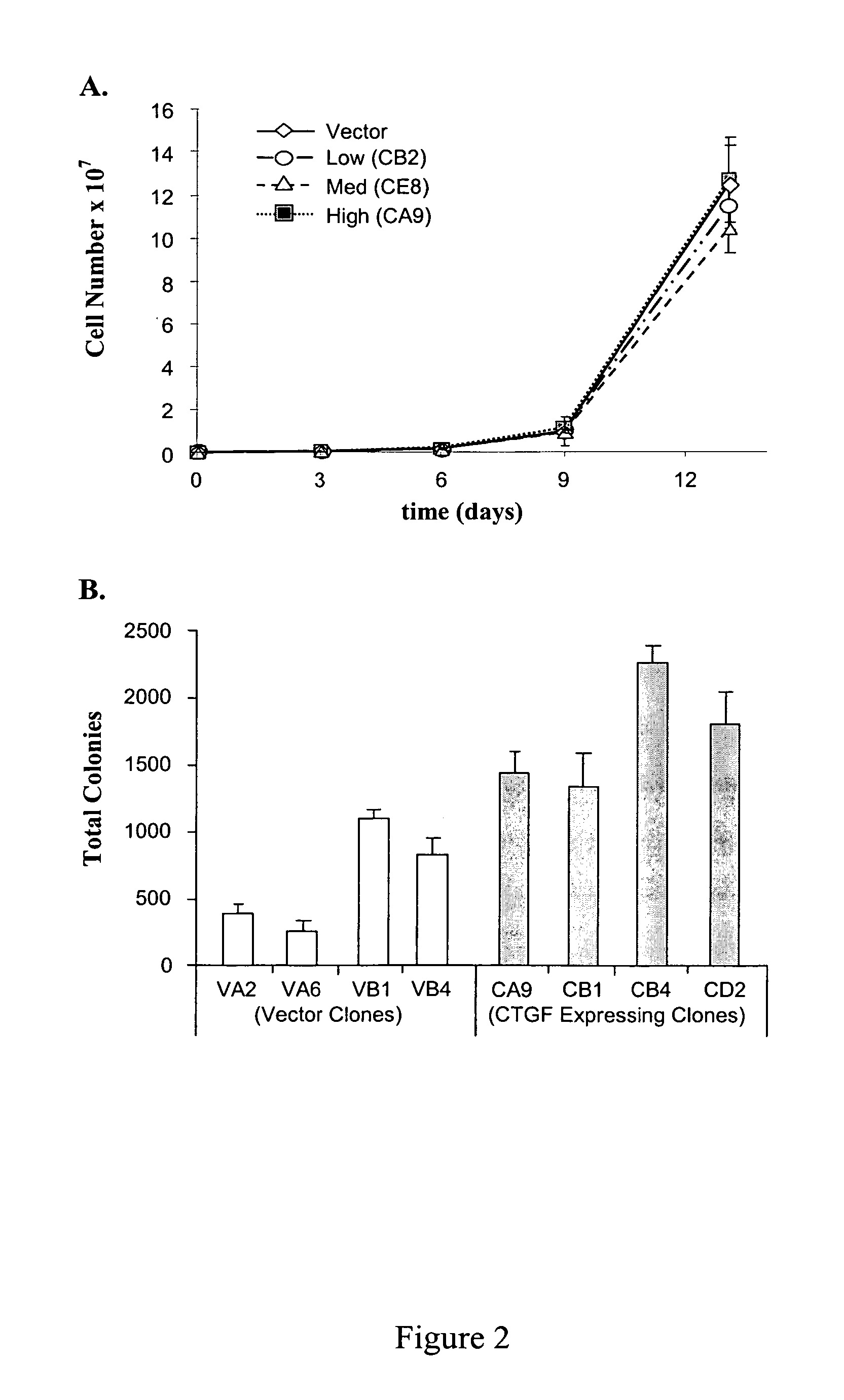
Connective Tissue Growth Factor Signaling
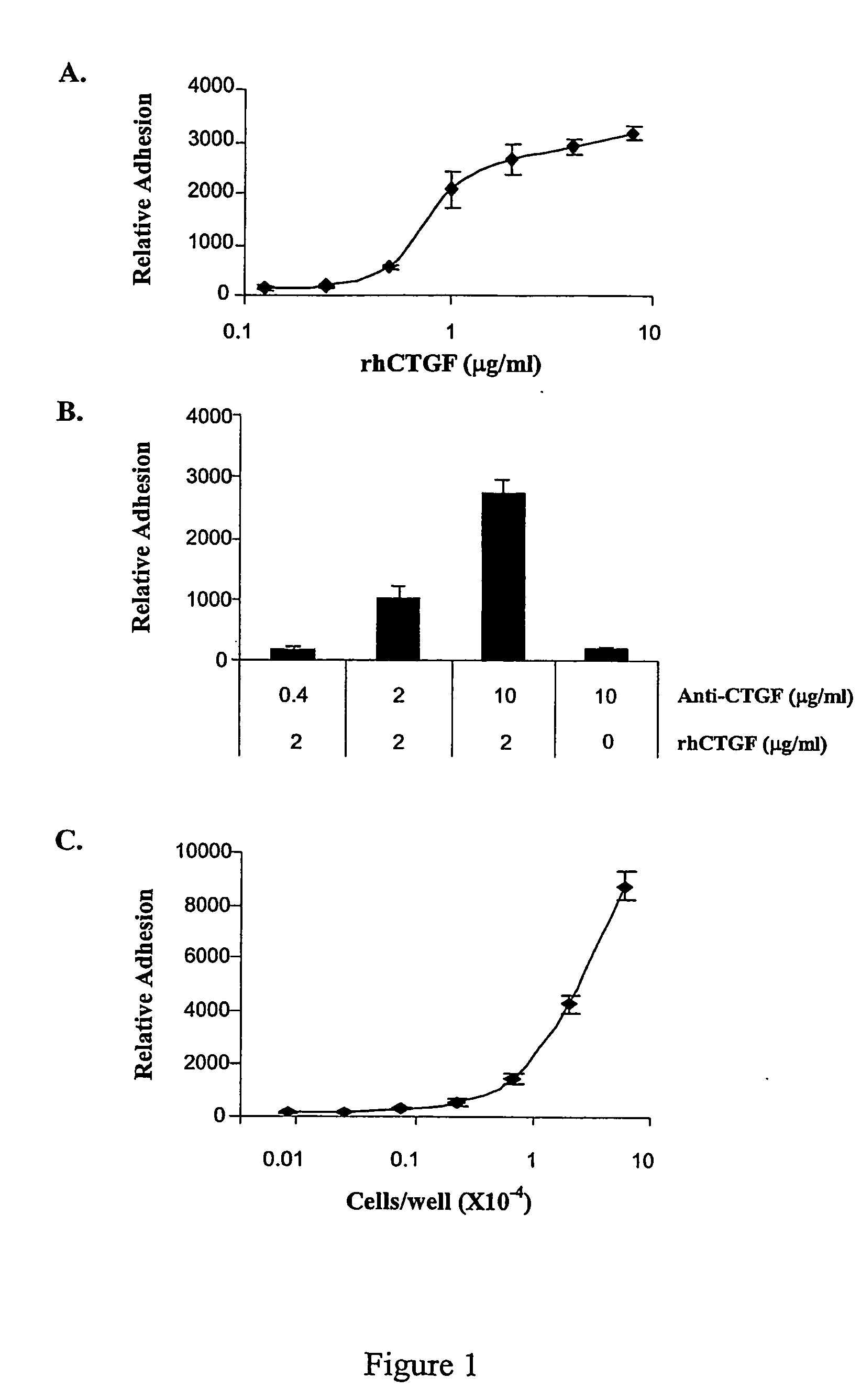
InactiveUS20080254487A1Reduce the possibilityOrganic active ingredientsCompound screeningCell adhesionAdhesion process
The present invention provides compounds and agents that modulate CTGF-mediated cell adhesion and / or binding of CTGF to cells. The invention further provides assays that may be used to identify additional modulators of CTGF-mediated cell adhesion and CTGF binding to cells, and assays that may be used to identify compounds or agents that modulate interaction of CTGF with HSPGs.
Owner:FIBROGEN INC
Treatments for cancer
InactiveUS20050271670A1Reducing pancreatic tumor expansionDecrease in cell survivalMicrobiological testing/measurementGenetic material ingredientsPancreatic tumorCTGF
The present invention provides methods for reducing tumor survival, expansion, and metastasis. In particular, the invention provides methods for reducing pancreatic tumor survival, expansion, and metastasis. The invention also provides agents for use in the methods, particularly agents that reduce the level or activity of connective tissue growth factor (CTGF), and methods for identifying such agents.
Owner:FIBROGEN INC
Ctgf Expression Inhibitor
A CTGF expression inhibitor comprising a compound of the formula I:a pharmaceutically acceptable salt or solvate thereof as an active ingredient, (wherein Y is hydroxy or a group of the formula: —NH—SO2—Y′ (wherein Y′ is optionally substituted aryl or optionally substituted alkyl), andR1 to R9 are each independently hydrogen, halogen, optionally substituted alkyl, optionally substituted alkoxy or the like).
Owner:SHIONOGI & CO LTD
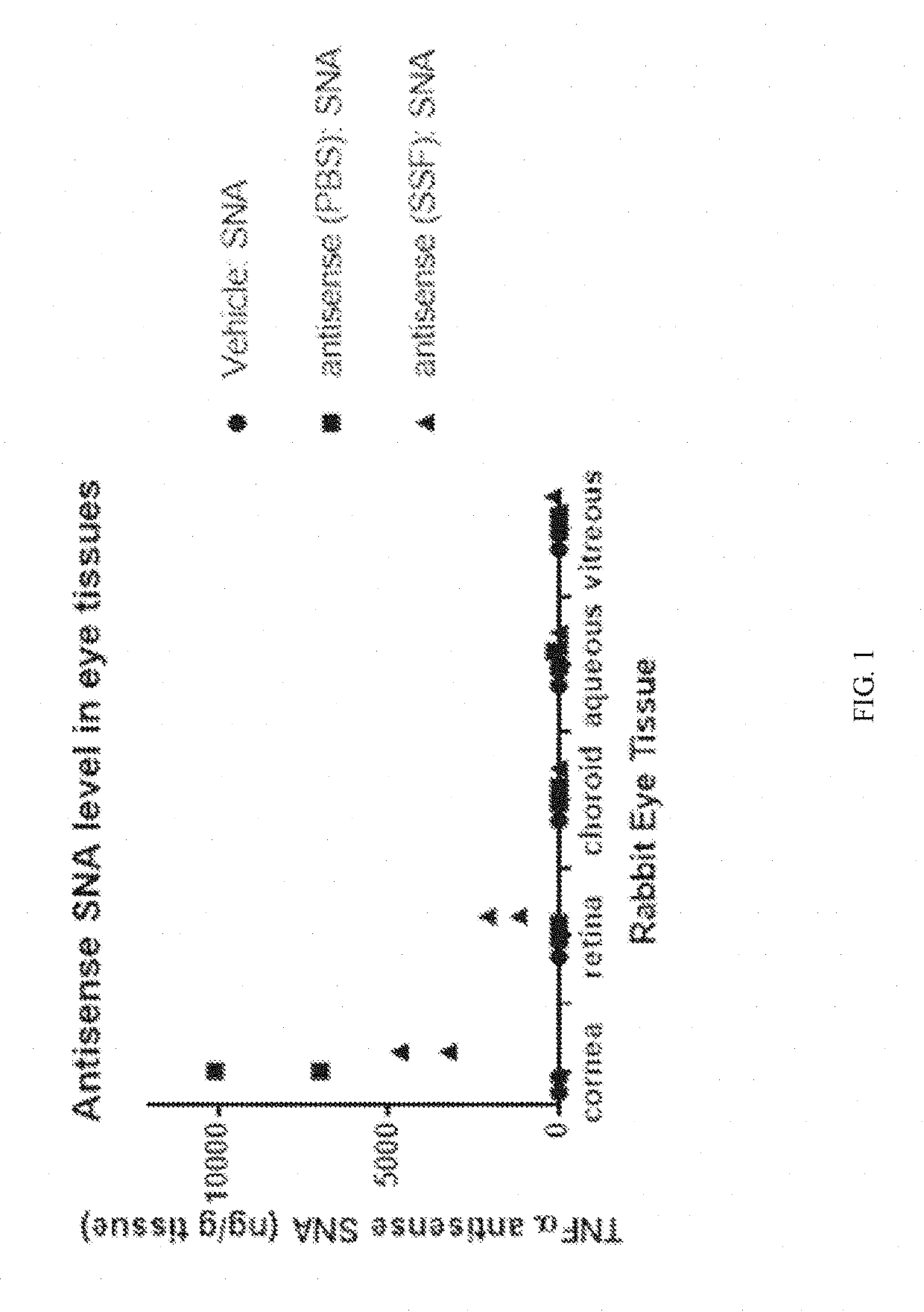
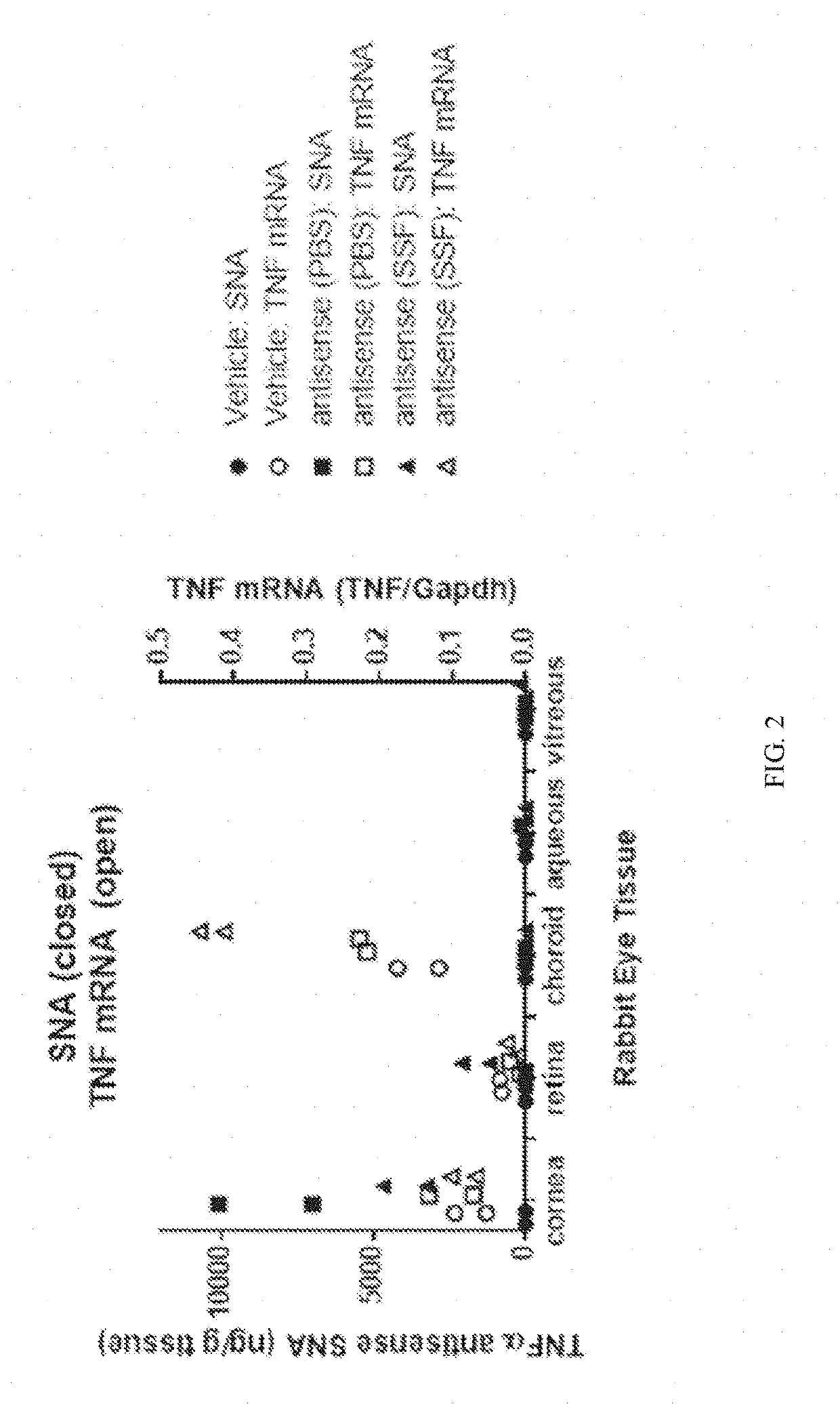
Topical administration of therapeutic agents and oligonucleotide formulations
Aspects of the invention relate to topical and ocular formulations of spherical nucleic acids (SNA), as well as methods of use thereof and compositions thereof. The formulations may include an inhibitor such as an inhibitor of tumour necrosis factor alpha (TNFa), platelet-derived growth factor subunit A (PDGFA), platelet-derived growth factor subunit B (PDGFB), platelet-derived growth factor subunit C (PDGFC), platelet-derived growth factor subunit D (PDGFD), platelet-derived growth factor receptor alpha (PDGFRA), platelet-derived growth factor receptor beta (PDGFRB), platelet-derived growth factor receptor like (PDGFRL), vascular endothelial growth factor A (VEGFA), vascular endothelial growth factor B (VEGFB), vascular endothelial growth factor C (VEGFC) vascular endothelial growth factor D (VEGFD), vascular endothelial growth factor receptor-1 (VEGFR1), vascular endothelial growth factor receptor-2 (VEGFR2), vascular endothelial growth factor receptor-3 (VEGFR3), beta-2 adrenergic receptor (ADRB2), connective tissue growth factor (CTGF), interleukin 1 beta (IL1 β), interleukin 1 receptor-1 (IL1 R1), interleukin 1 receptor-2 (IL1R2), and interleukin 1 receptor-3 (IL1R3). Aspects of the invention further relate to nanostructures comprising self-assembling therapeutic oligonucleotides, such as antisense oligonucleotides, that are linked to a molecular species, wherein the molecular species is positioned in a core of the nanostructure and the oligonucleotides extend radially from the core.
Owner:EXICURE INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com