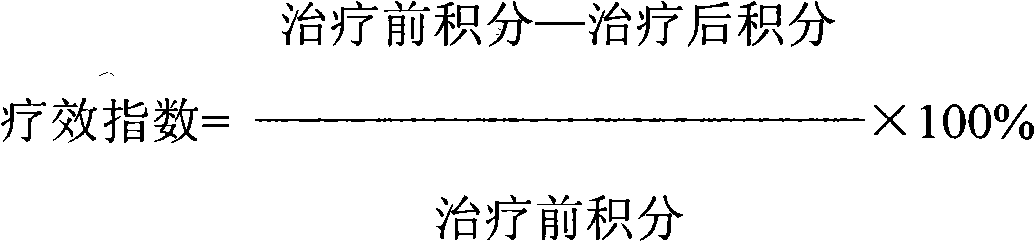
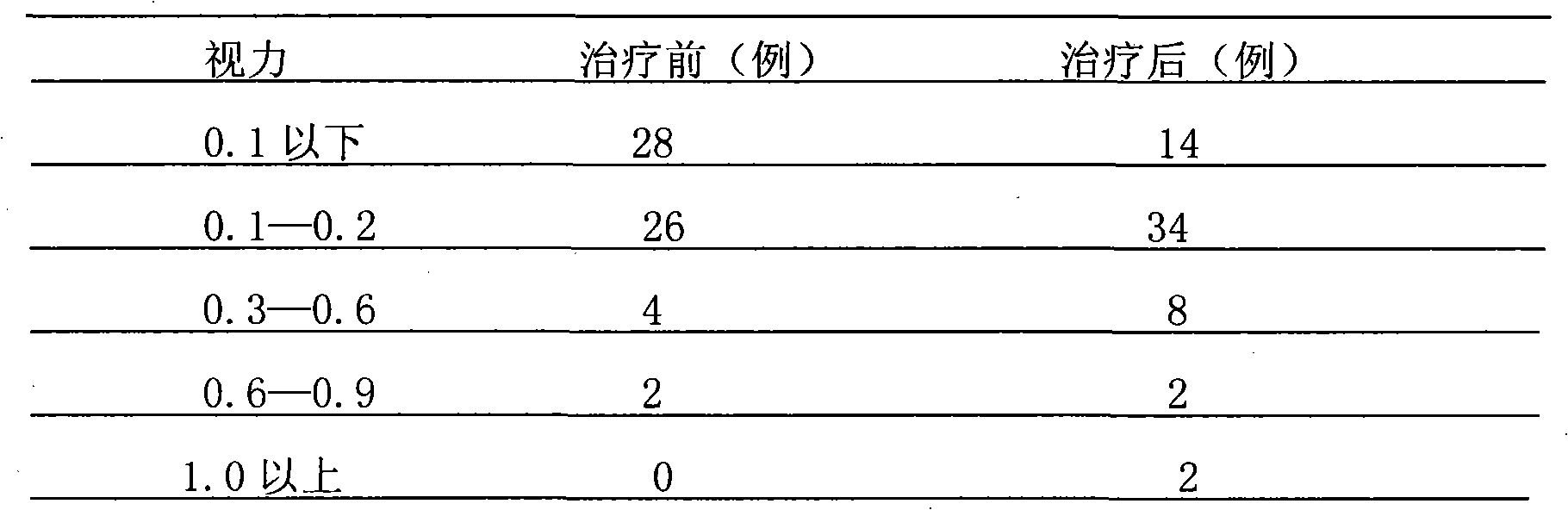
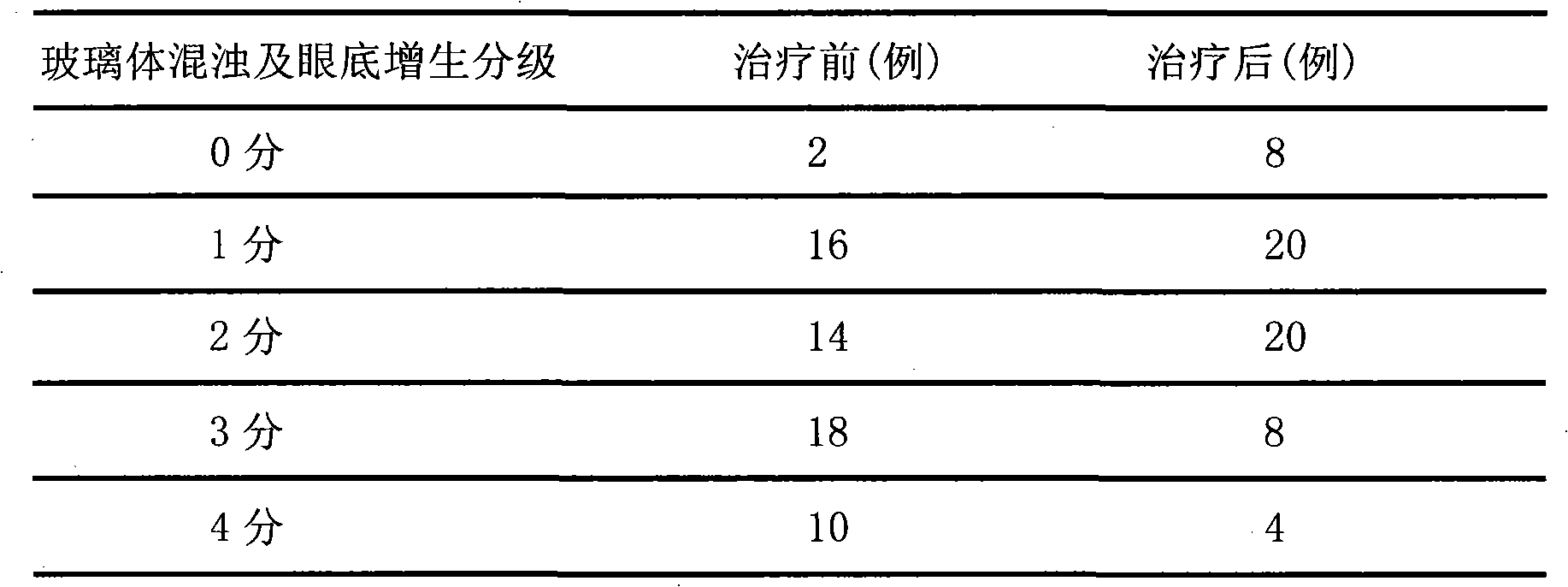
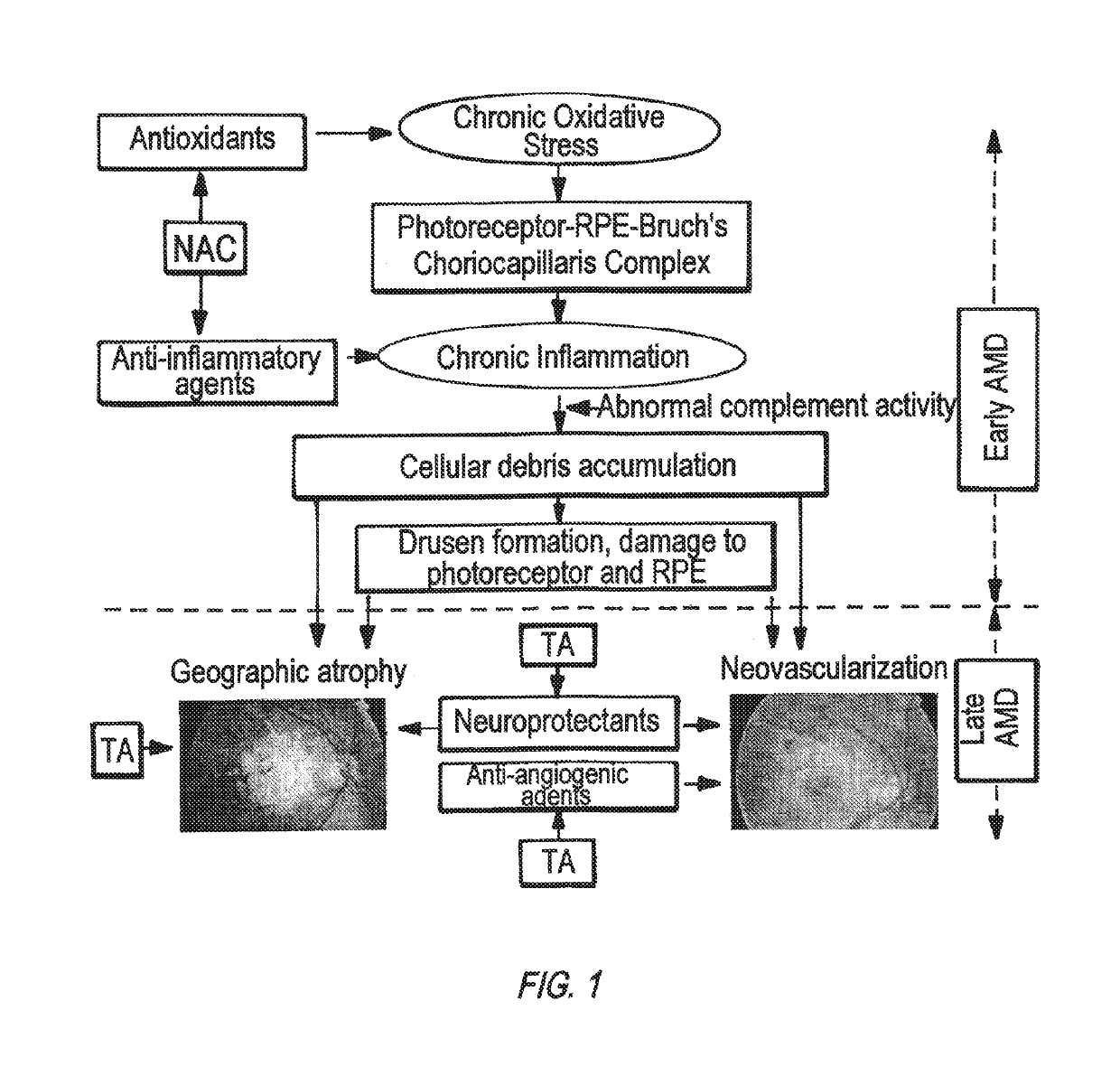
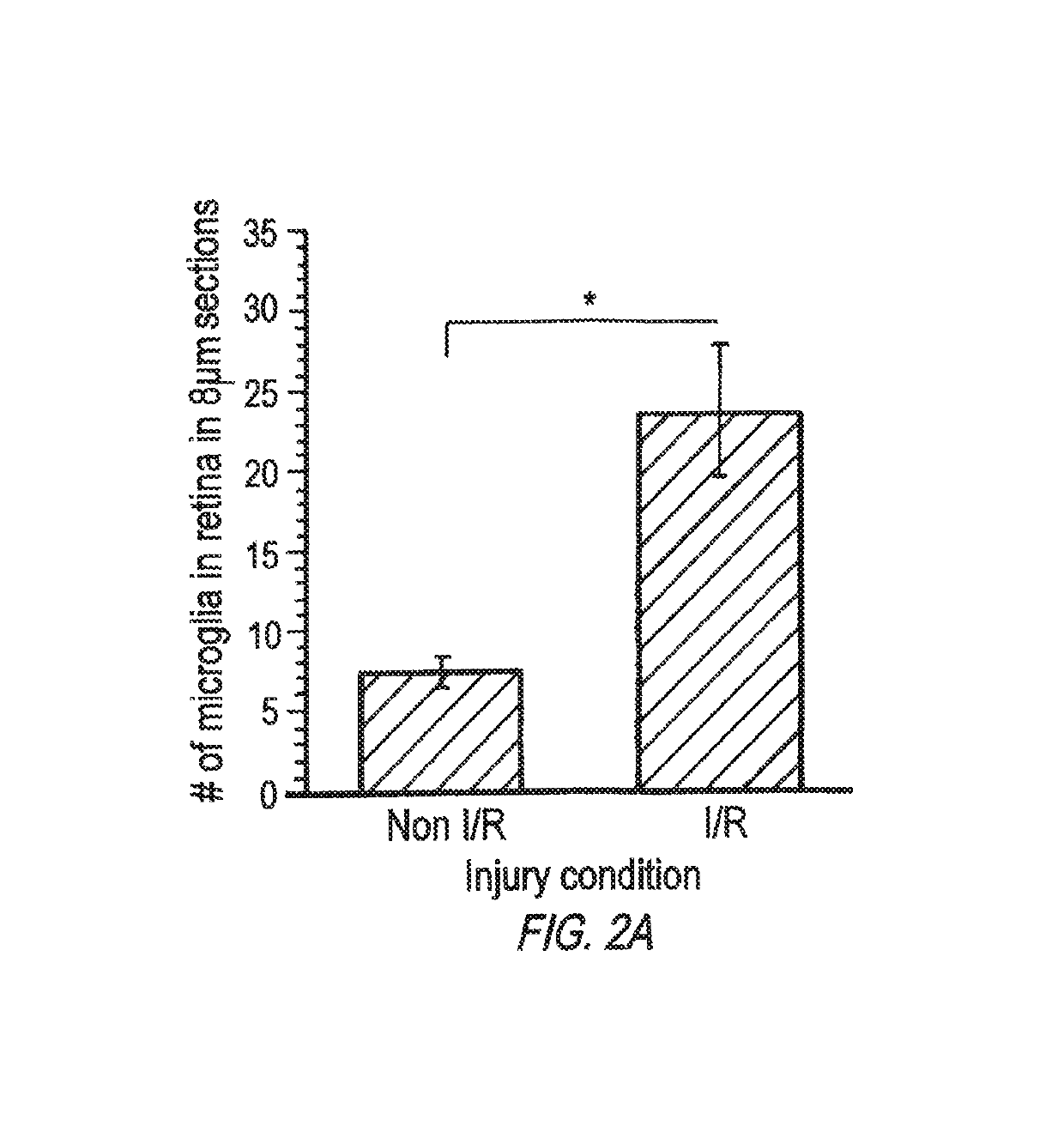
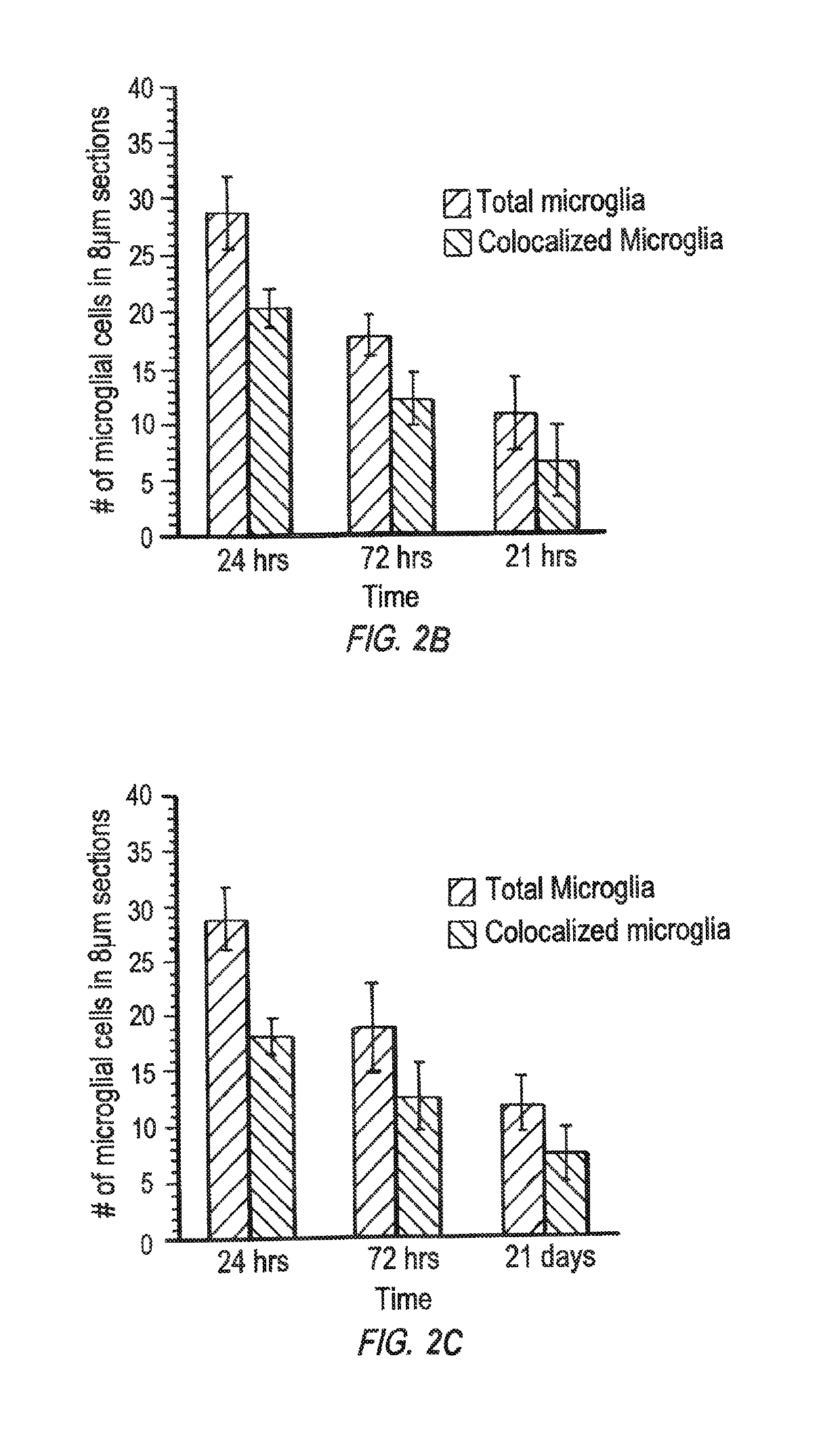
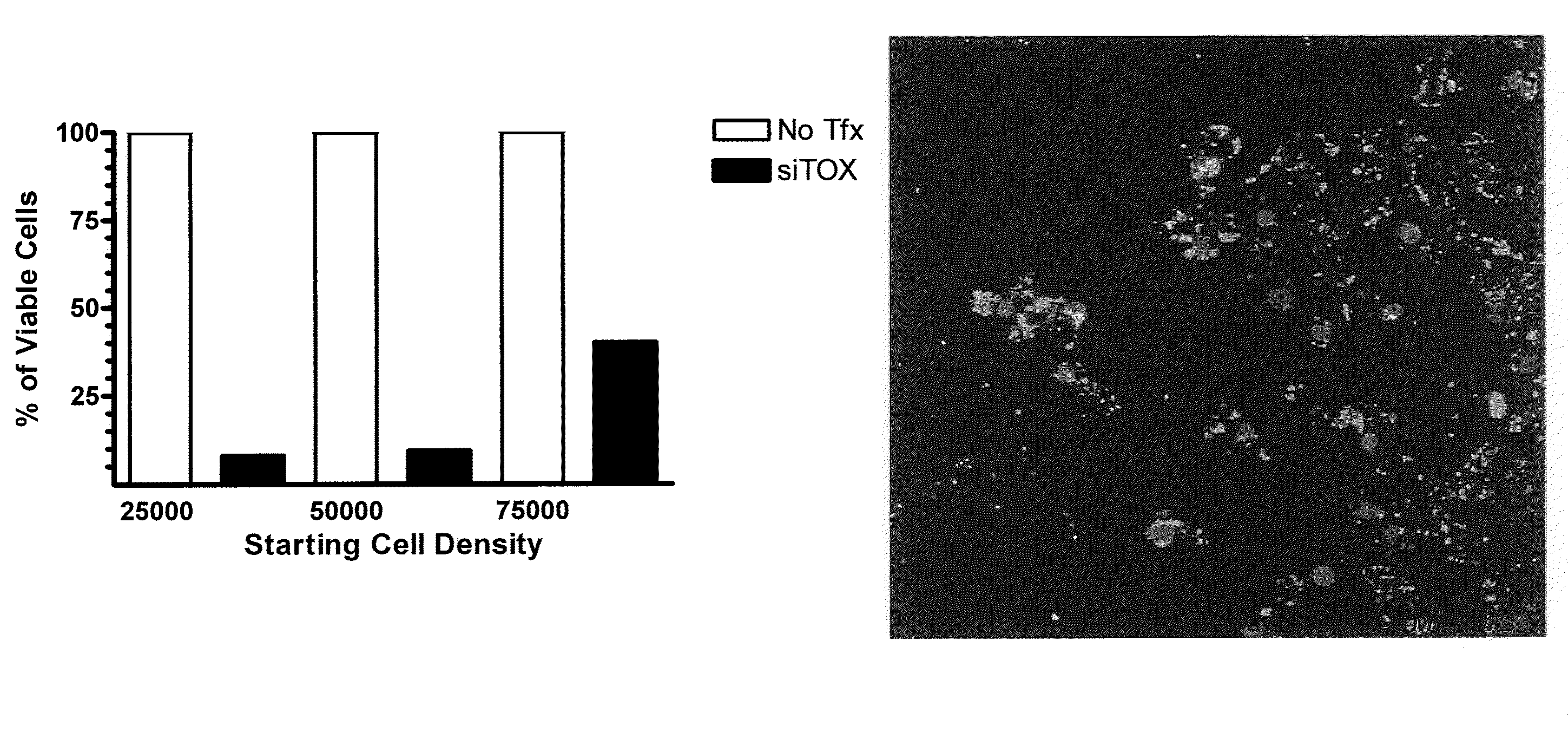
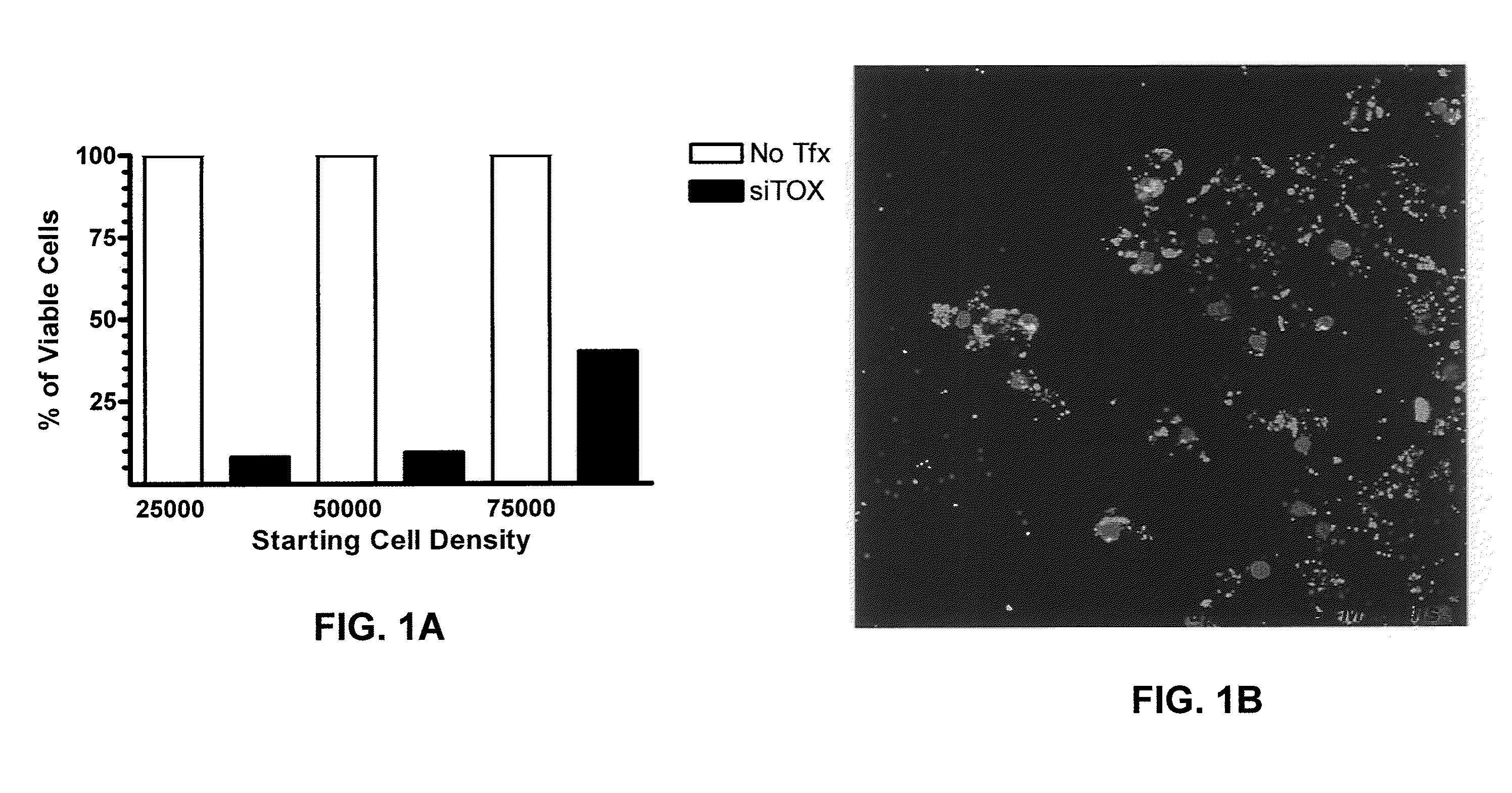
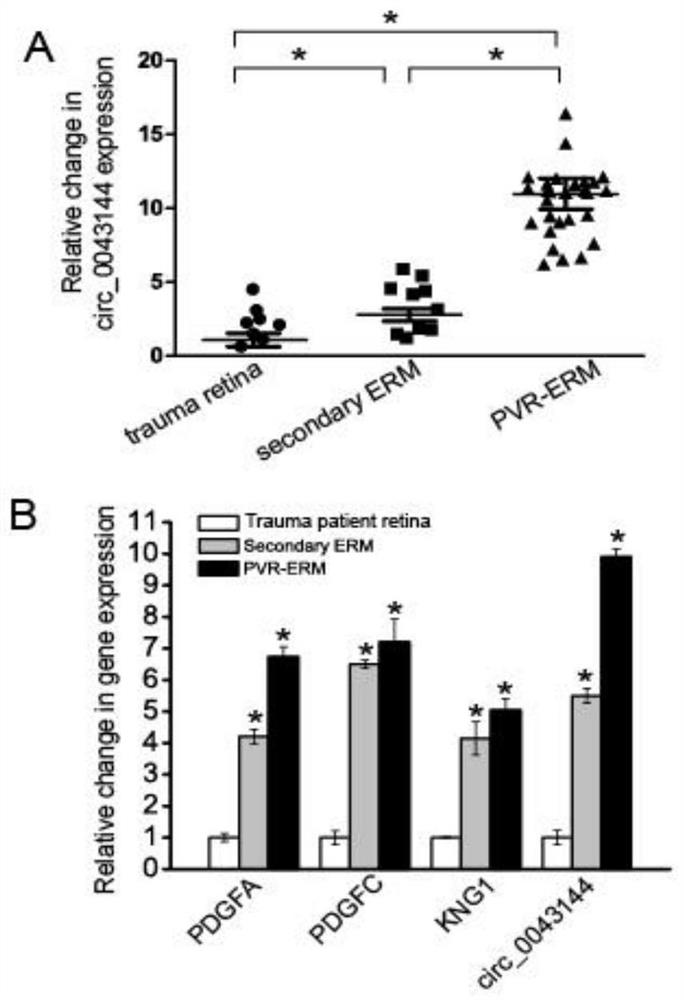
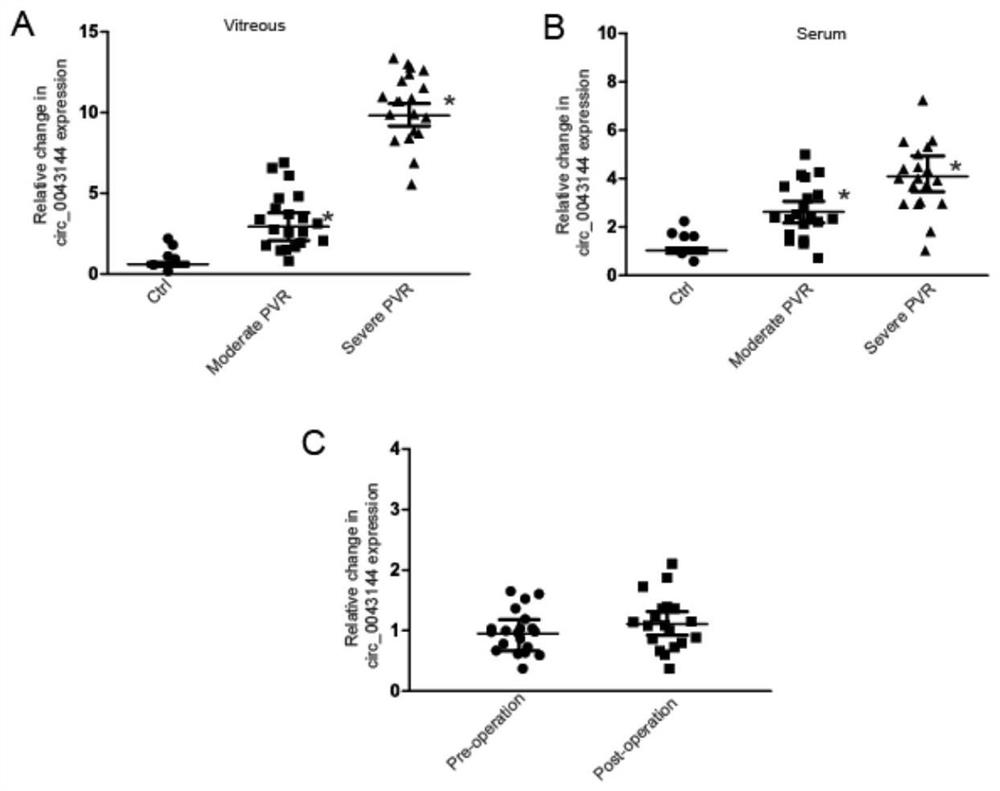
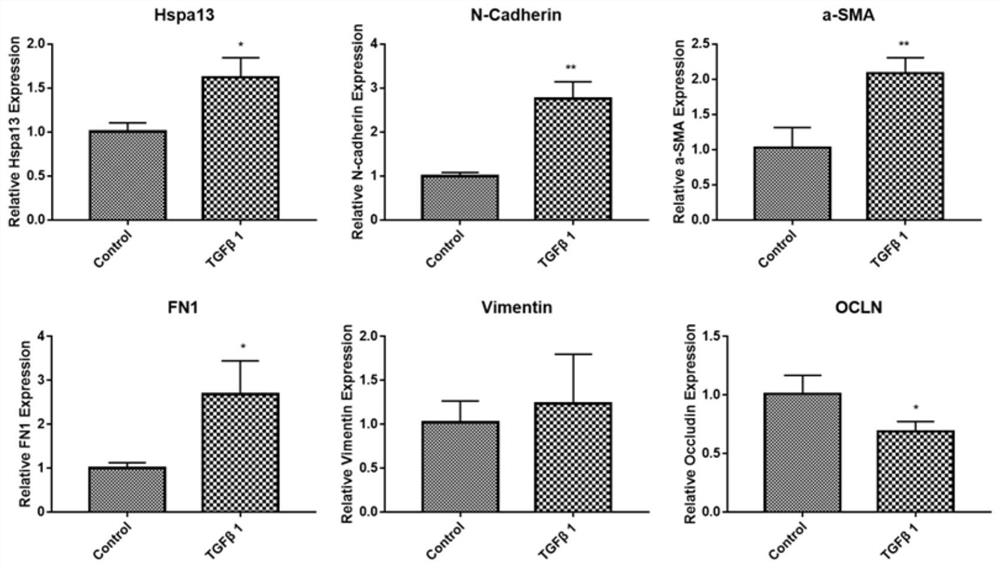
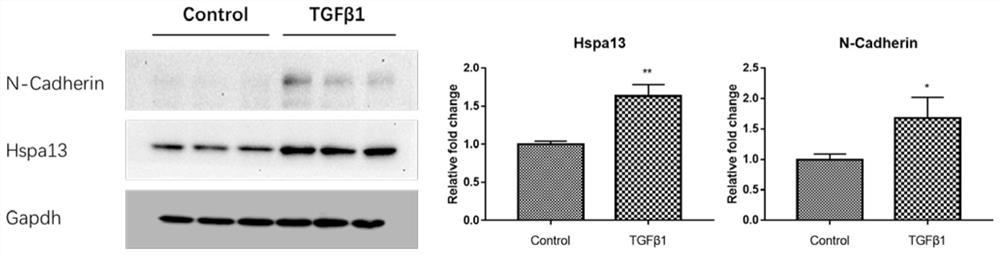
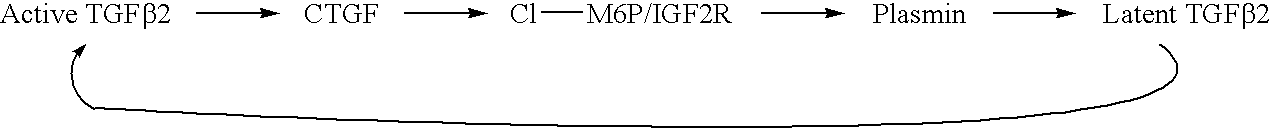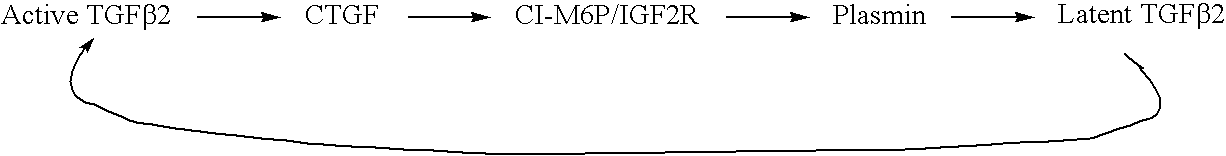Patents
Literature
42 results about "Proliferative vitreoretinopathy" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Proliferative vitreoretinopathy (PVR) is a disease that develops as a complication of rhegmatogenous retinal detachment. PVR occurs in about 8–10% of patients undergoing primary retinal detachment surgery and prevents the successful surgical repair of rhegmatogenous retinal detachment. PVR can be treated with surgery to reattach the detached retina but the visual outcome of the surgery is very poor.
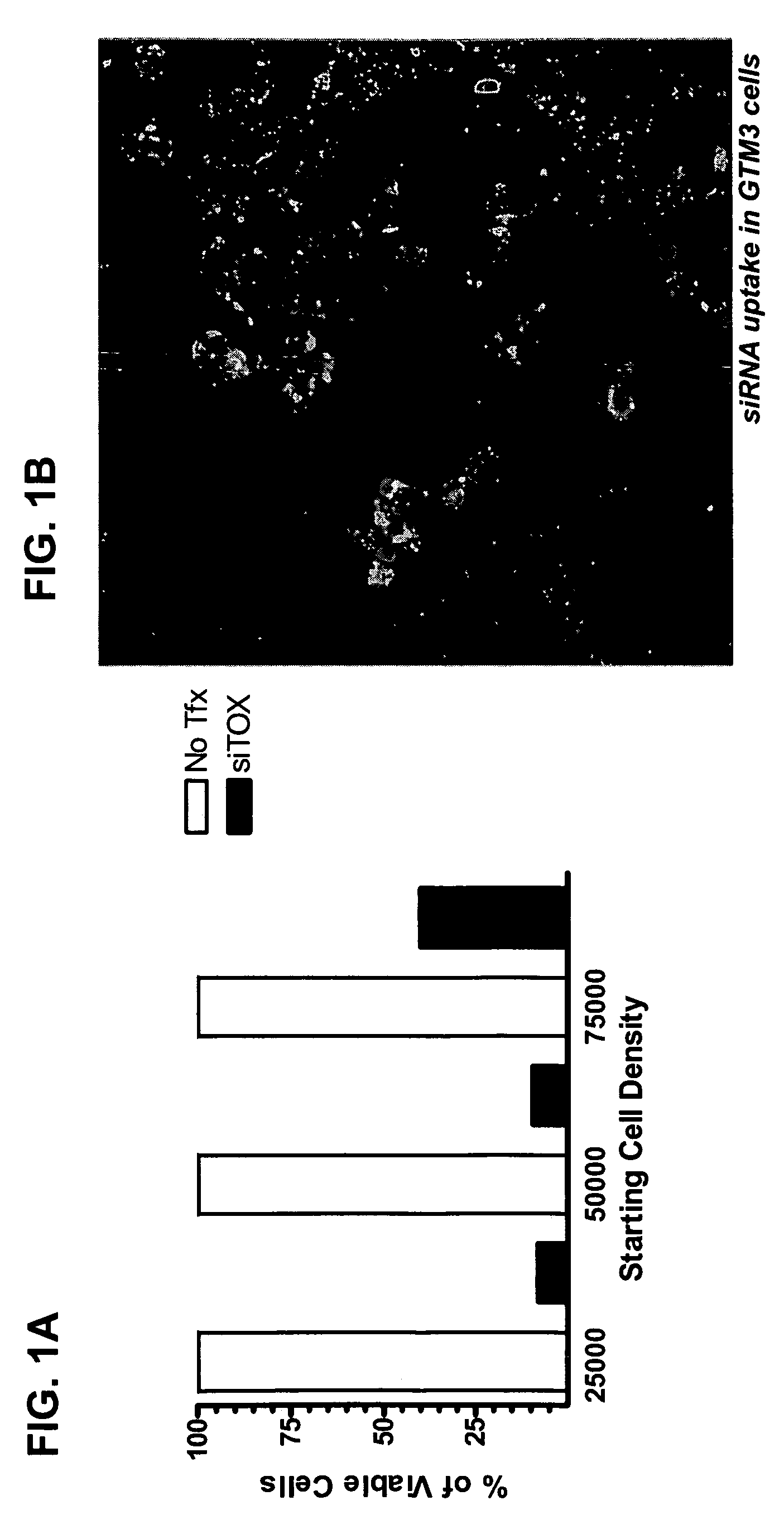
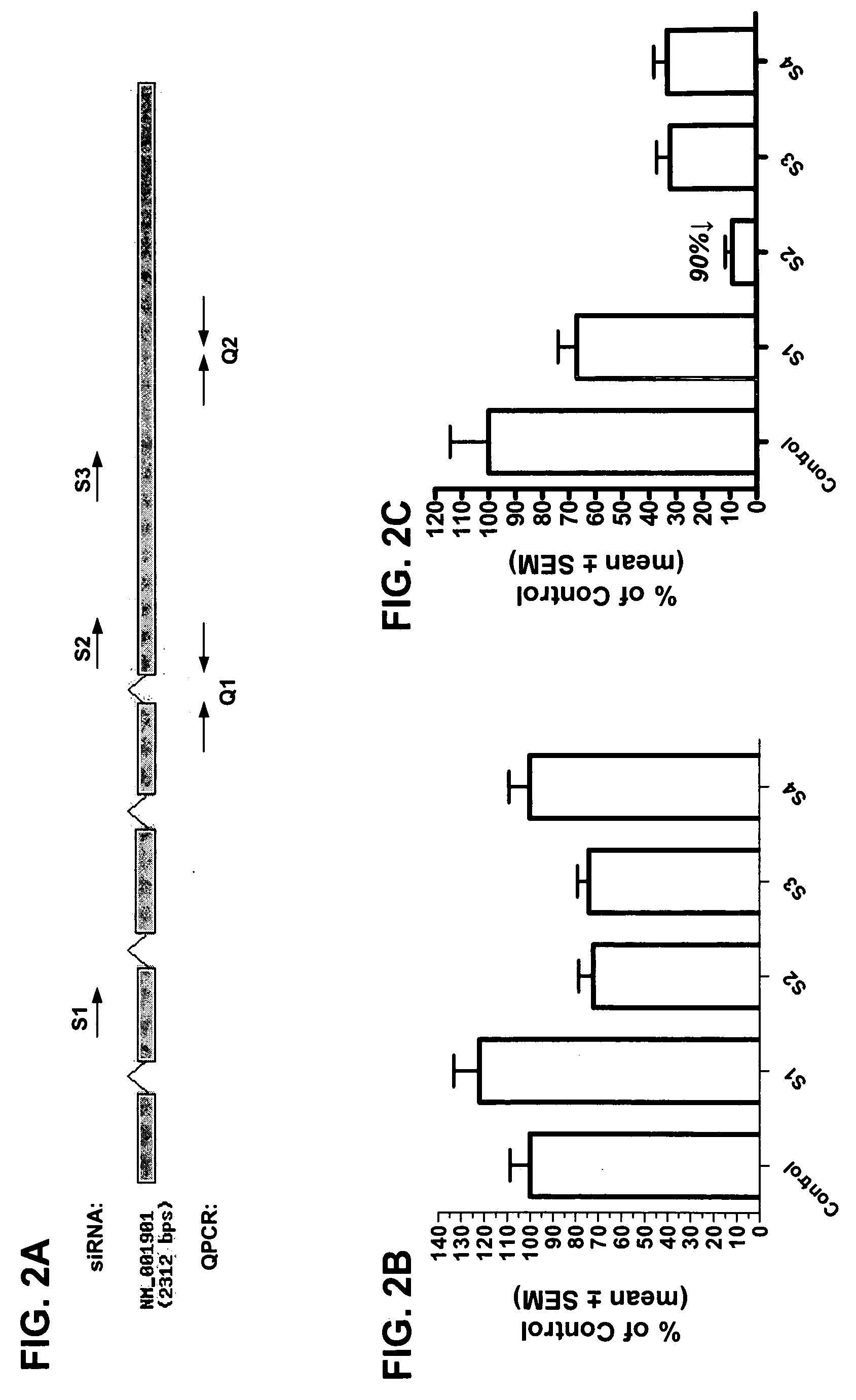
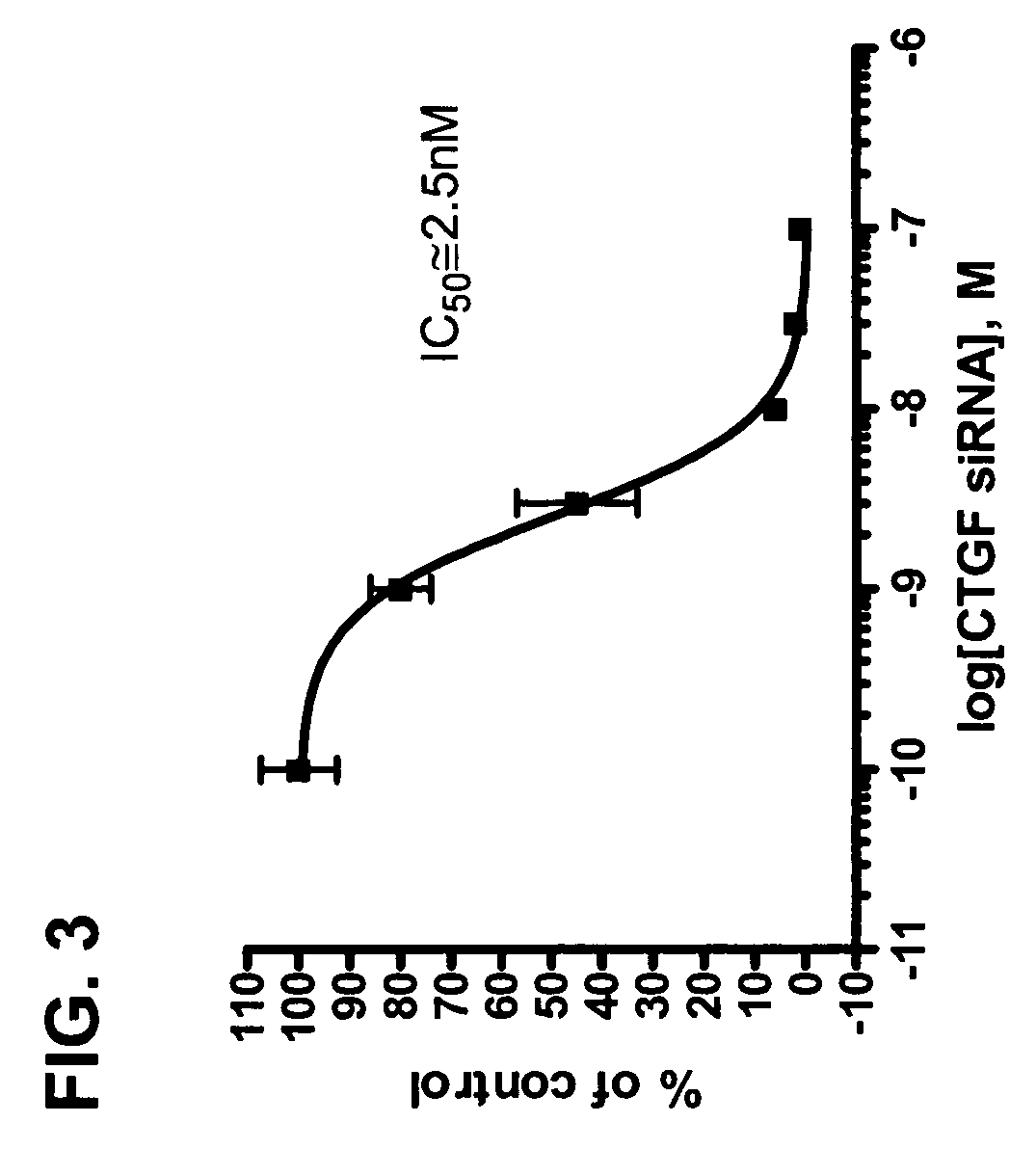
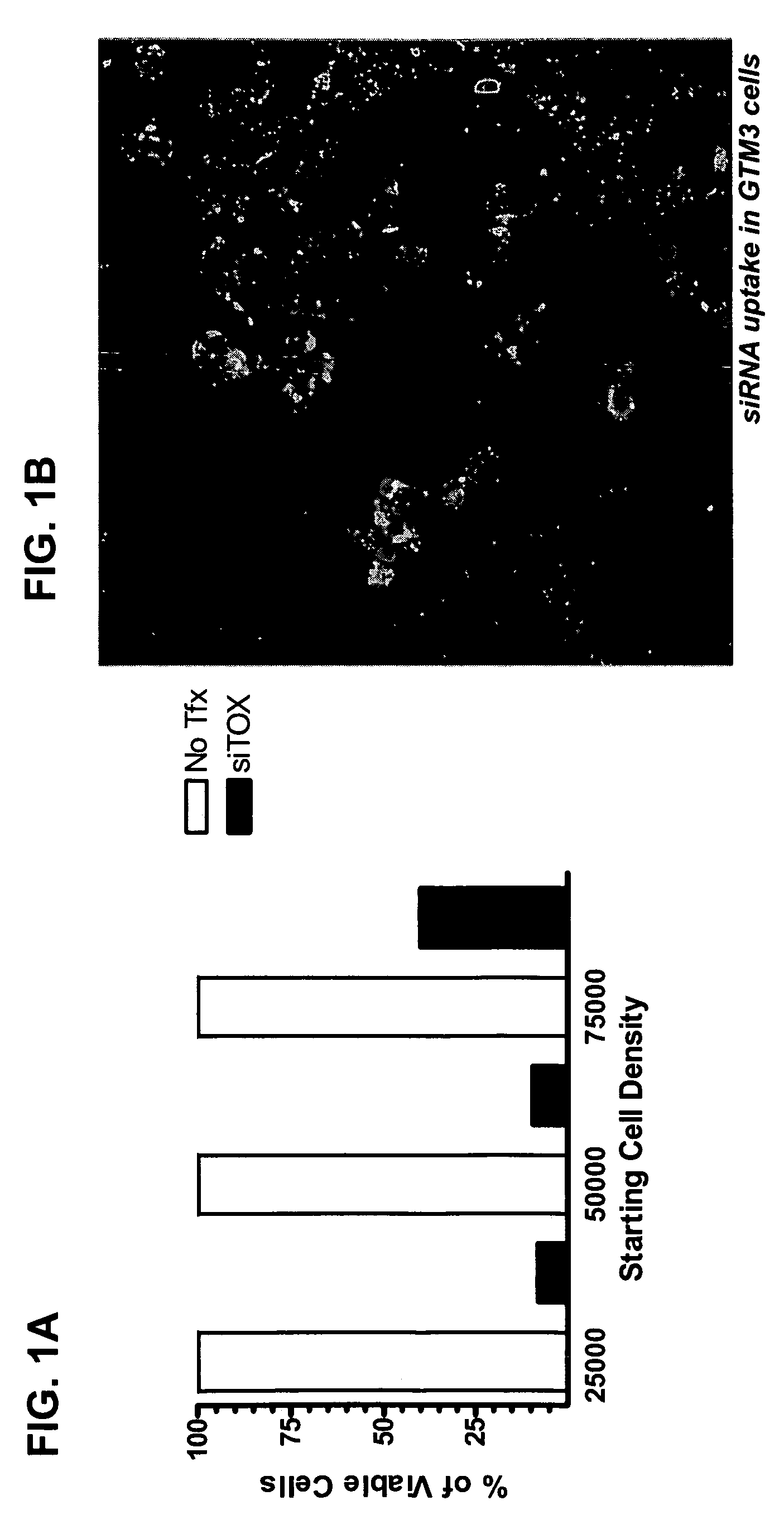
RNAi inhibition of CTGF for treatment of ocular disorders
RNA interference is provided for inhibition of connective tissue growth factor mRNA expression in ocular disorders involving CTGF expression. Ocular disorders involving aberrant CTGF expression include glaucoma, macular degeneration, diabetic retinopathy, choroidal neovascularization, proliferative vitreoretinopathy and wound healing. Such disorders are treated by administering interfering RNAs of the present invention.
Owner:NOVARTIS AG
RNAi inhibition of CTGF for treatment of ocular disorders
Owner:NOVARTIS AG
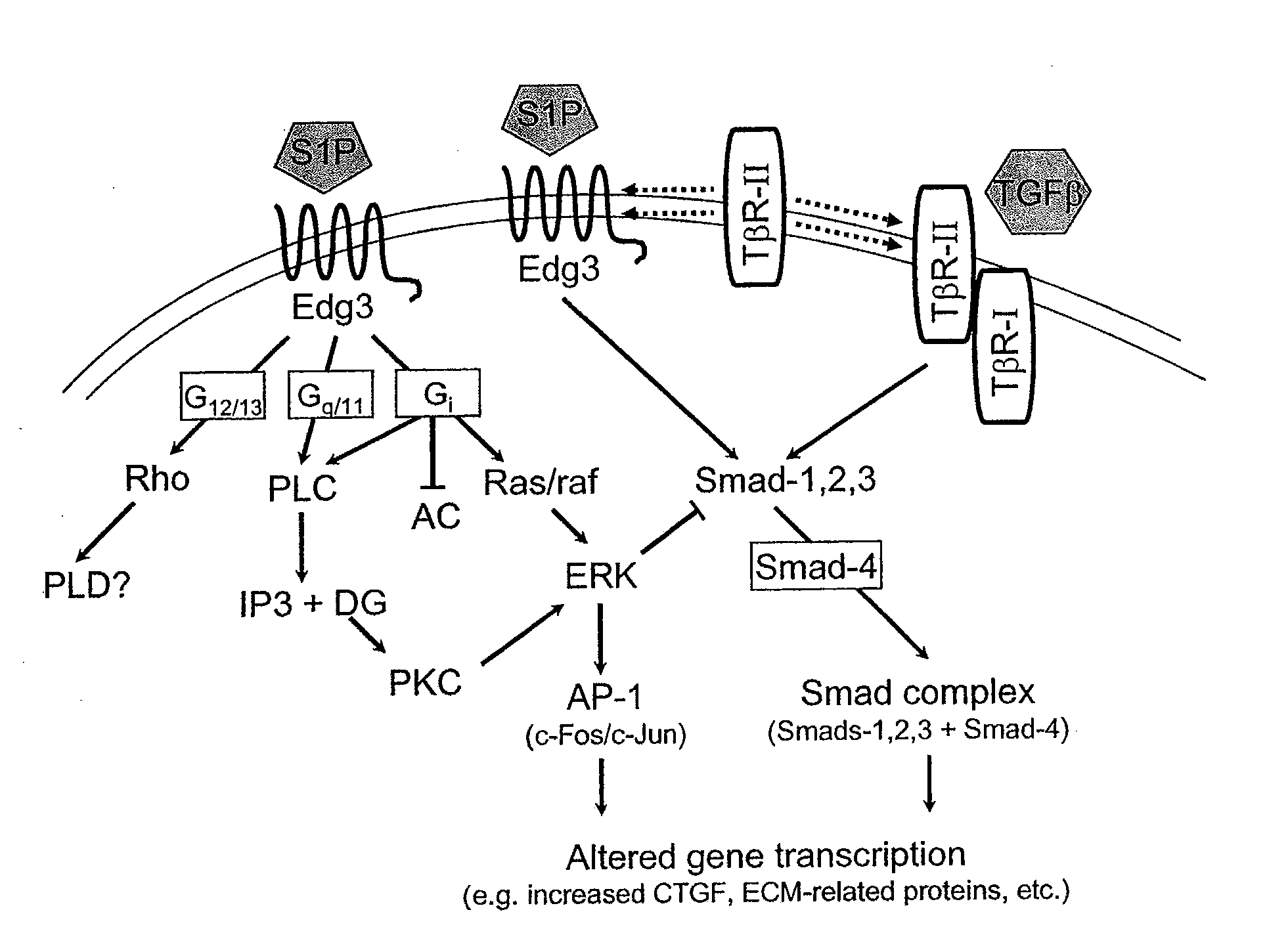
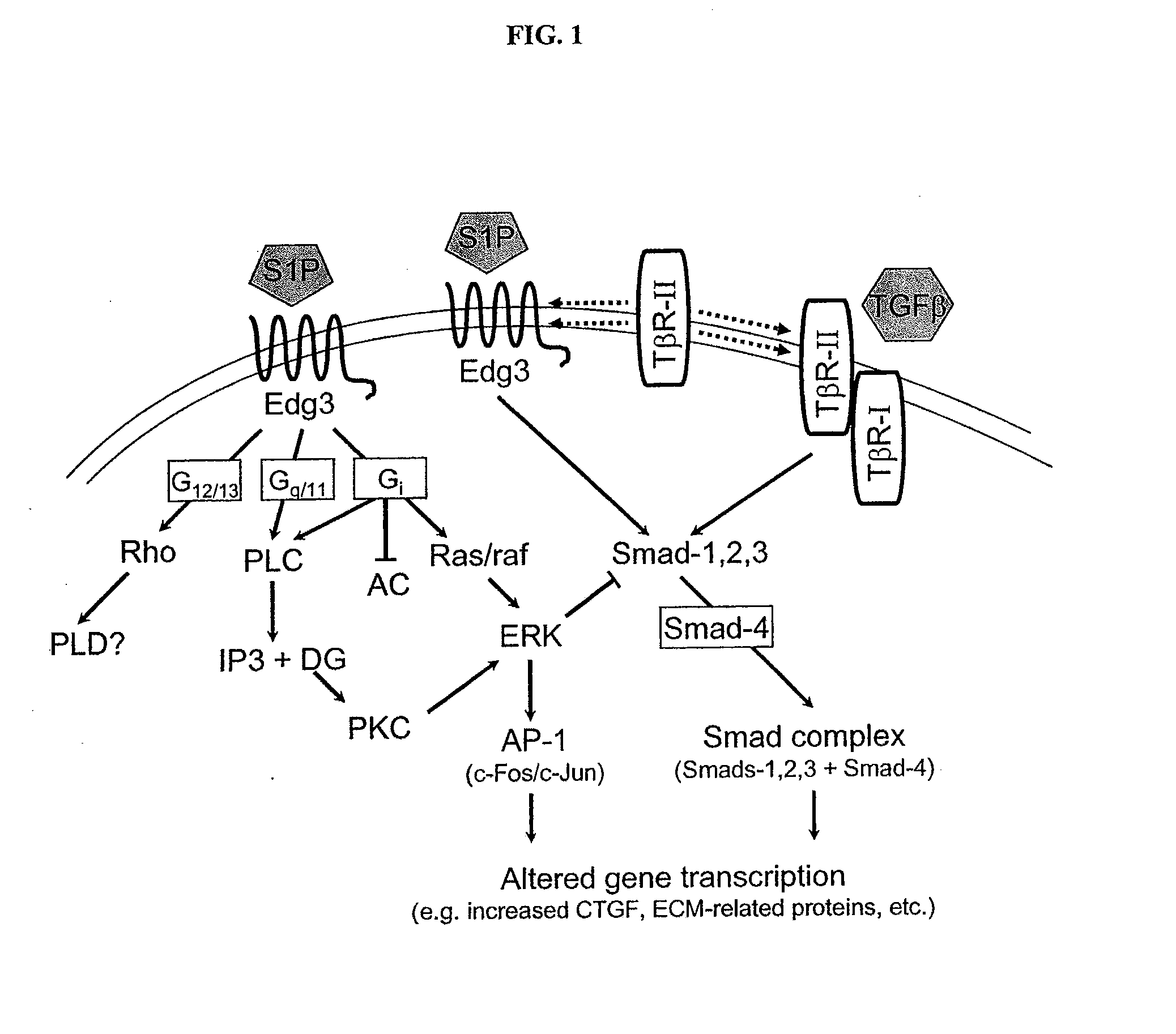
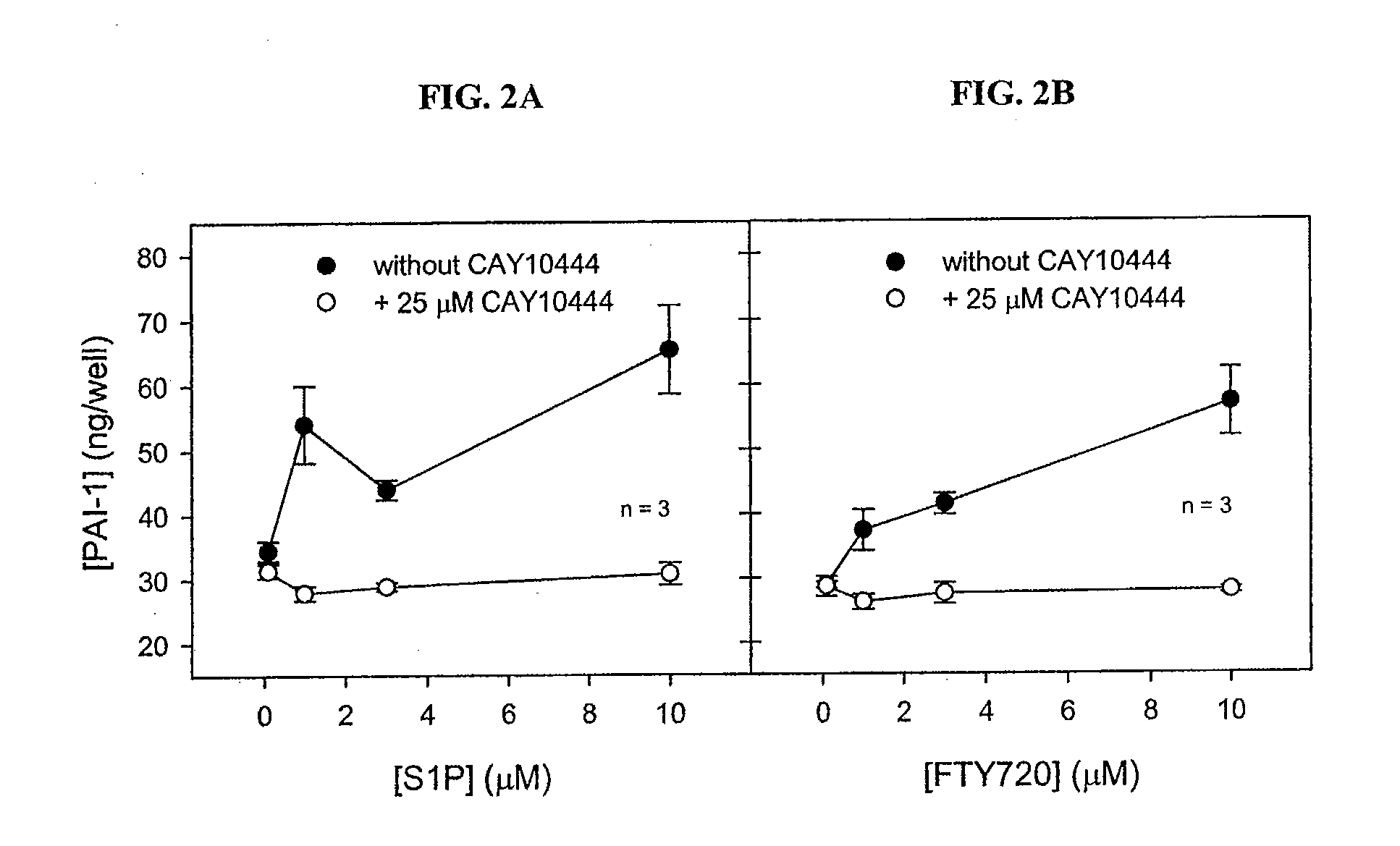
Antagonists of endothelial differentiation gene subfamily 3 (edg-3, s1p3) receptors for prevention and treatment of ocular disorders
InactiveUS20080025973A1Attenuating Smad signalingReduce signalingBiocideSenses disorderDiabetic retinopathyDisease
Antagonists of S1P3 (Edg-3) receptors are provided for attenuation of Smad signaling in a method of down-regulation of receptor signaling and downstream decreased production of connective tissue growth factor in ocular disorders involving CTGF accumulation. Ocular disorders involving inappropriate CTGF accumulation include ocular hypertension, glaucoma, glaucomatous retinopathy, optic neuropathy, macular degeneration, diabetic retinopathy, choroidal neovascularization, proliferative vitreoretinopathy and ocular wound healing, for example. Such disorders are treated by administering antagonists of the present invention.
Owner:ALCON RES LTD
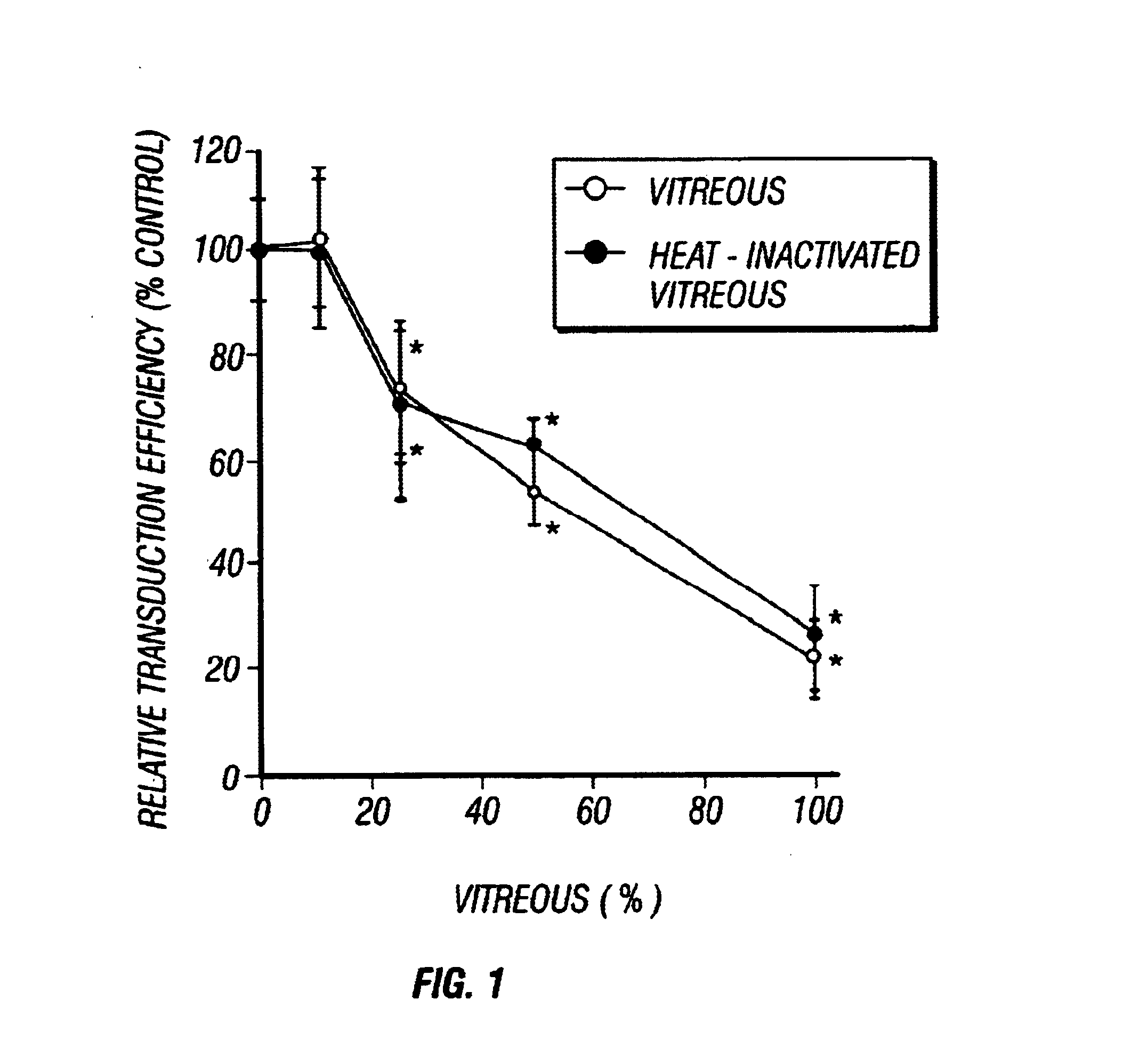
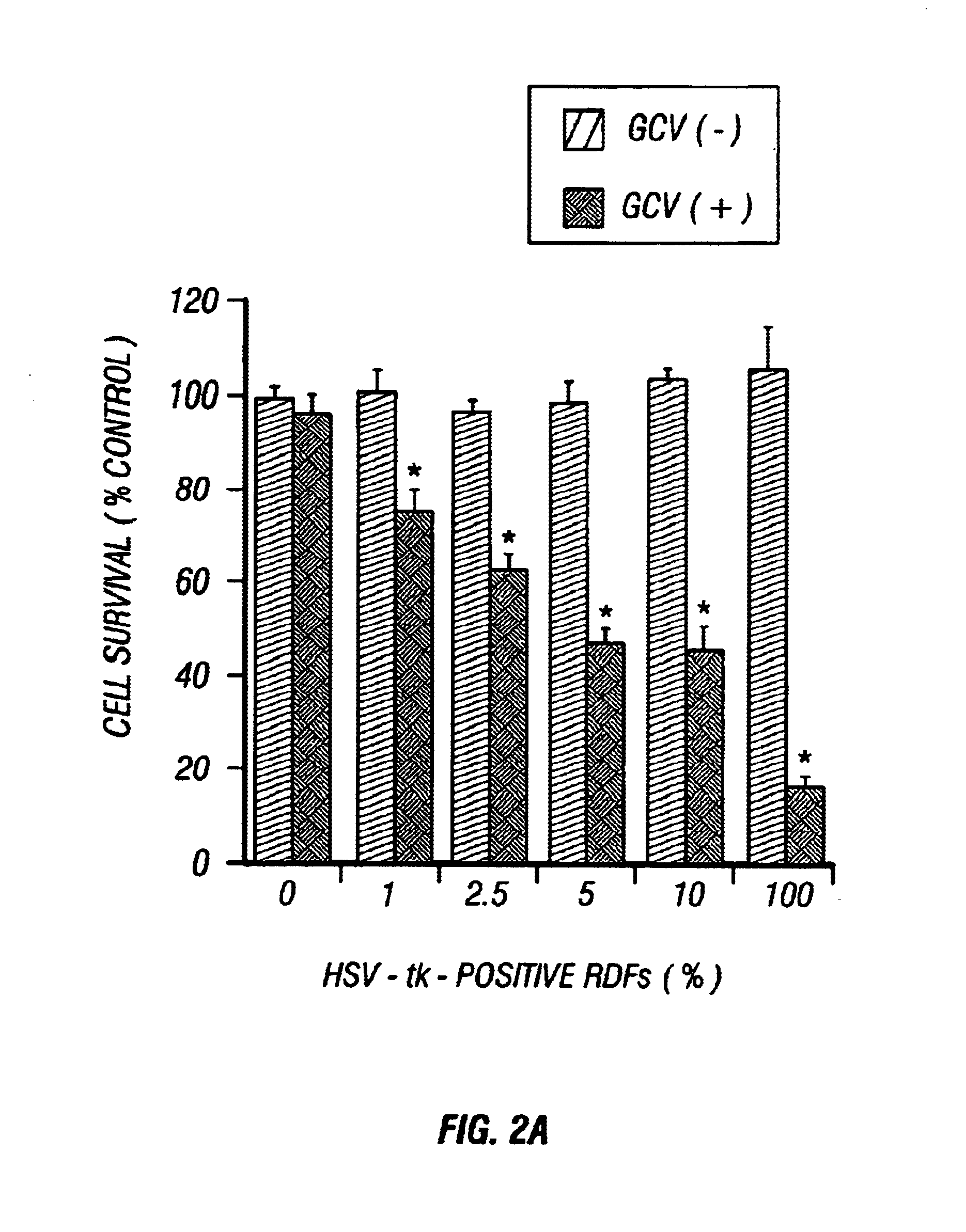
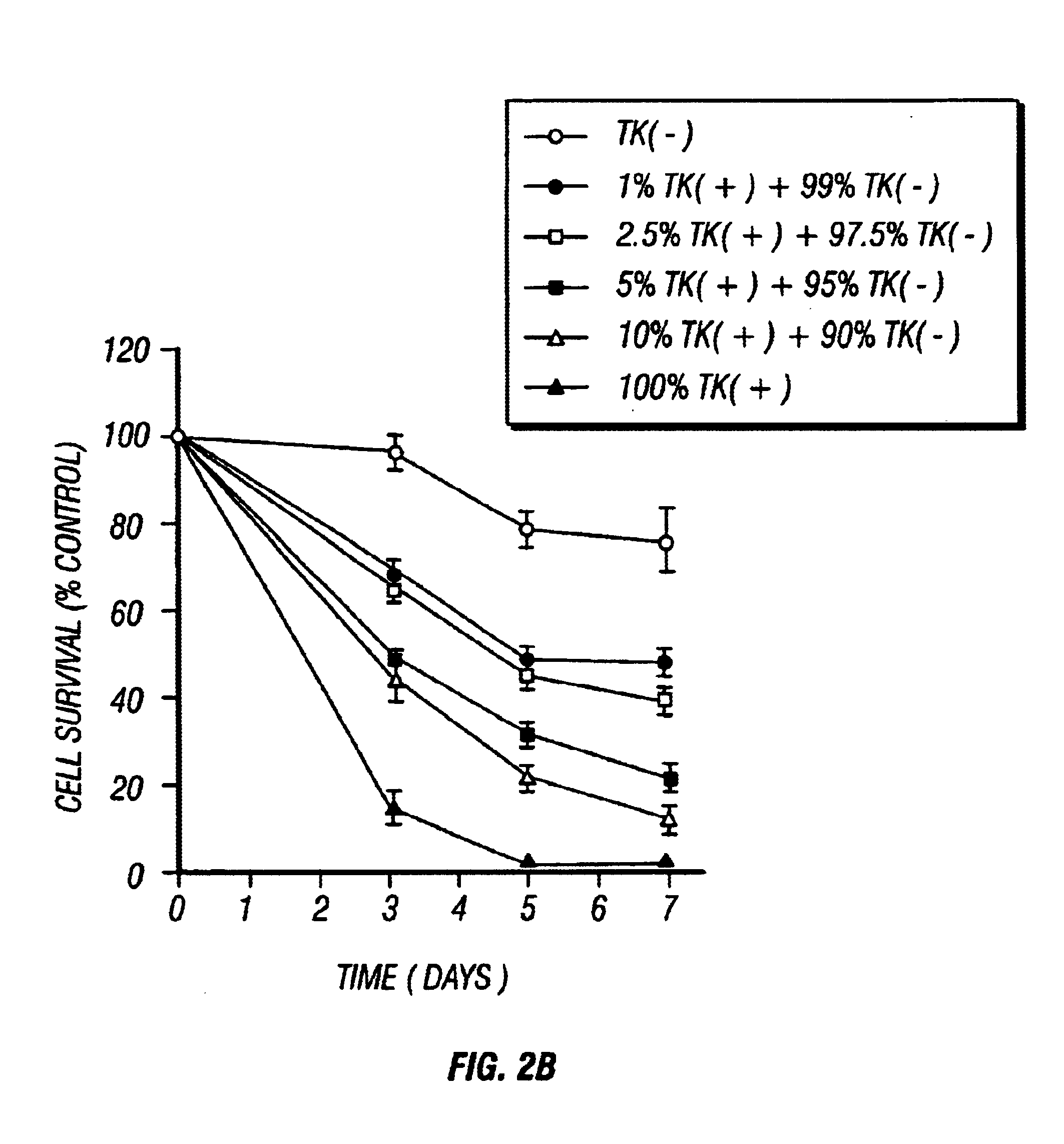
Gene Therapy for proliferative vitreoretinopathy
A method of treating ocular disorders (such as, for example, proliferative vitreoretinopathy or PVR) associated with replicating ocular cells by transfecting replicating ocular cells in vivo with a polynucleotide encoding an agent which is capable of providing for the inhibition, prevention, or destruction of the growth of the replicating ocular cells upon expression of the agent. The agent may be a viral thymidine kinase, and the polynucleotide encoding the agent may be contained in a retroviral vector. Once the replicating ocular cells are transduced with the retroviral vector, the patient is given a chemotherapeutic or interaction agent, such as ganciclovir, which kills the transfected replicating ocular cells.
Owner:UNIV OF SOUTHERN CALIFORNIA
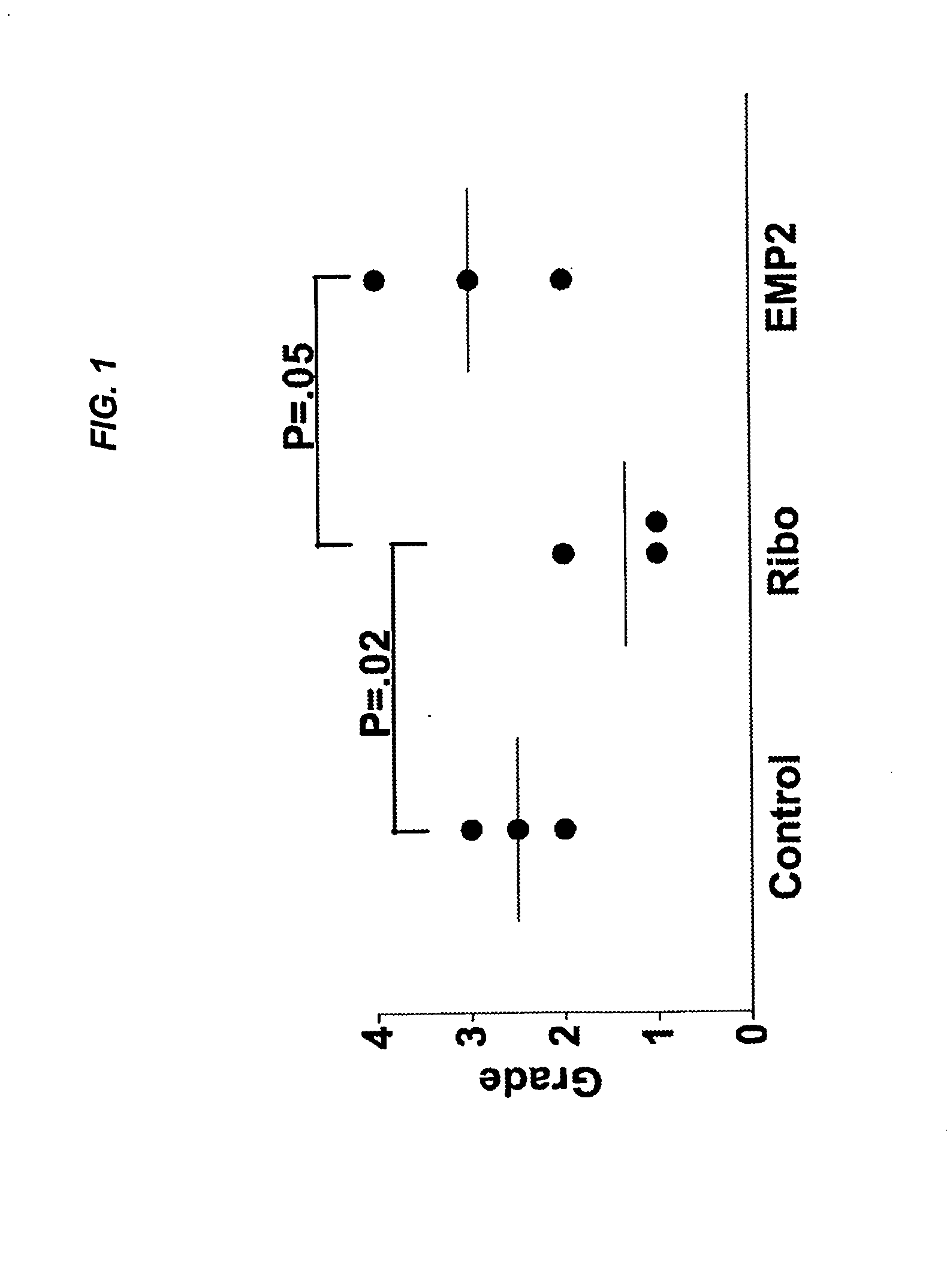
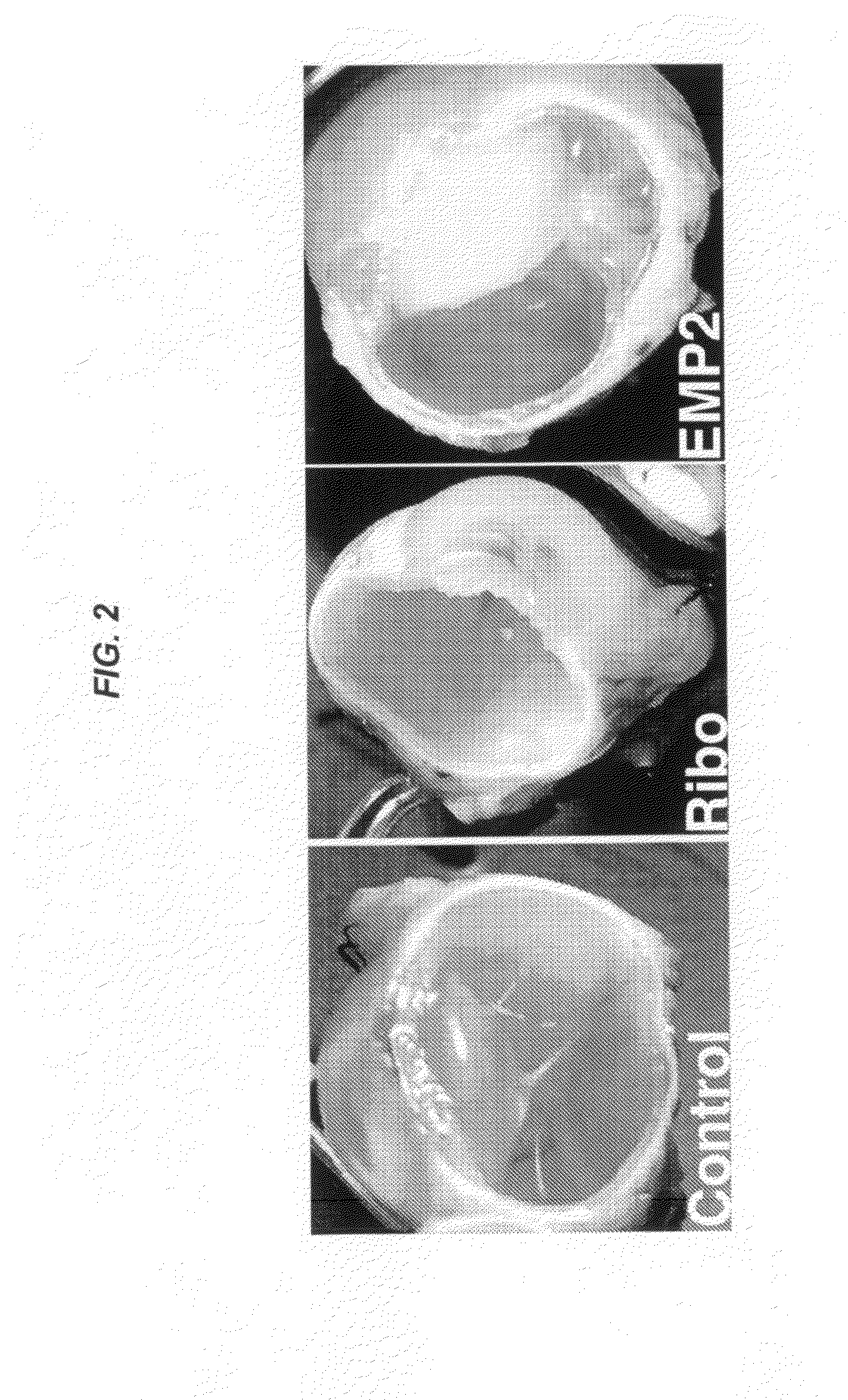
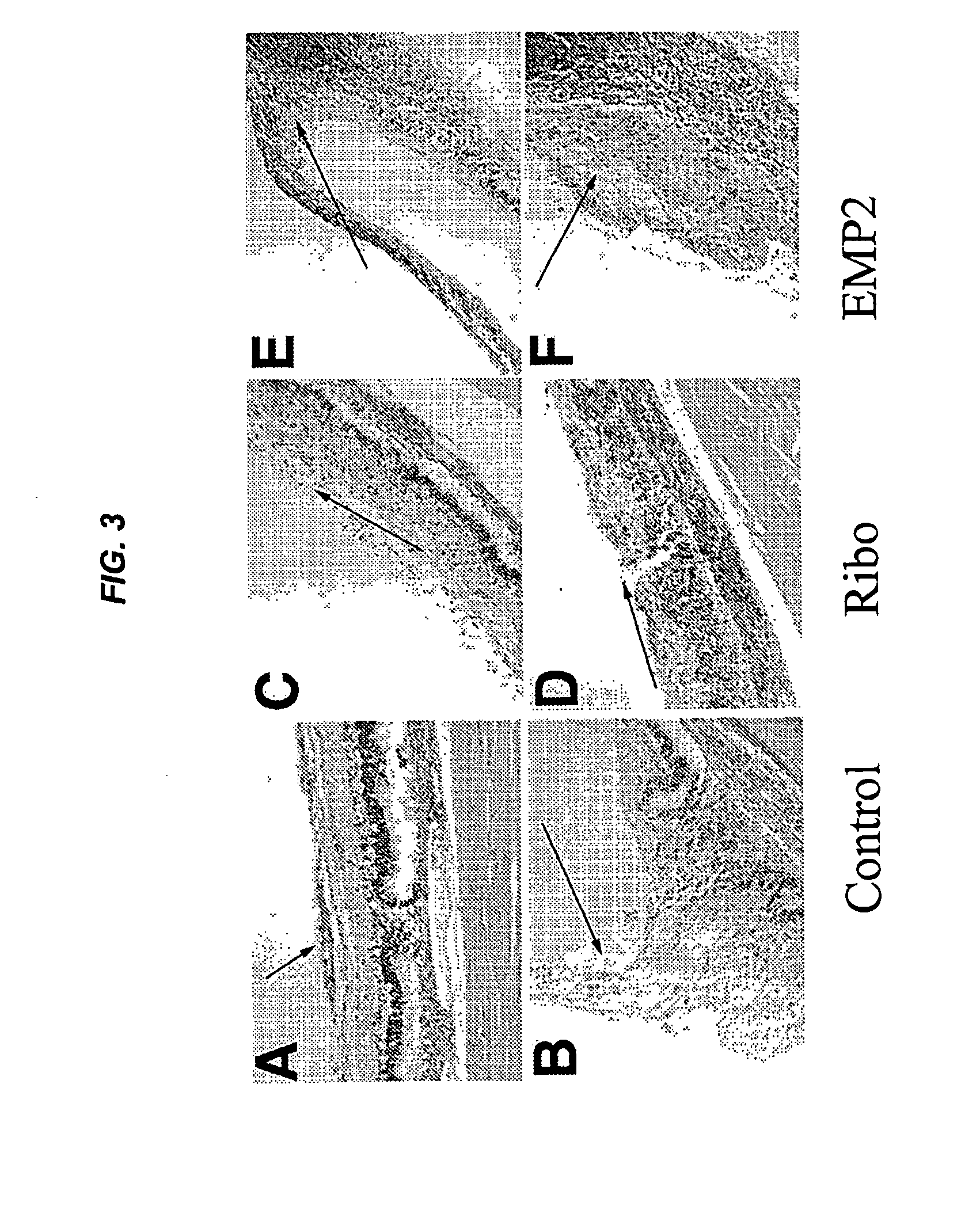
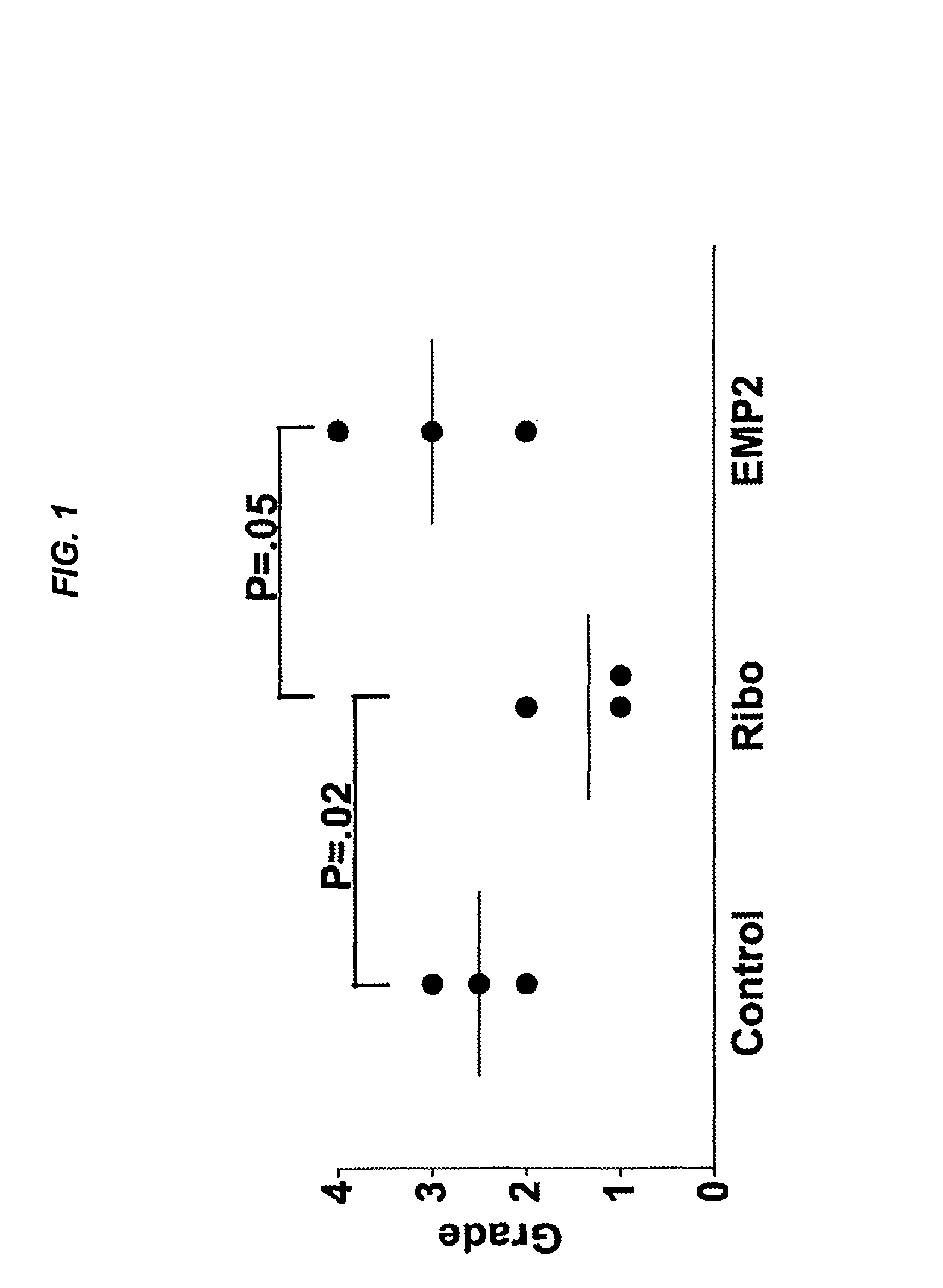
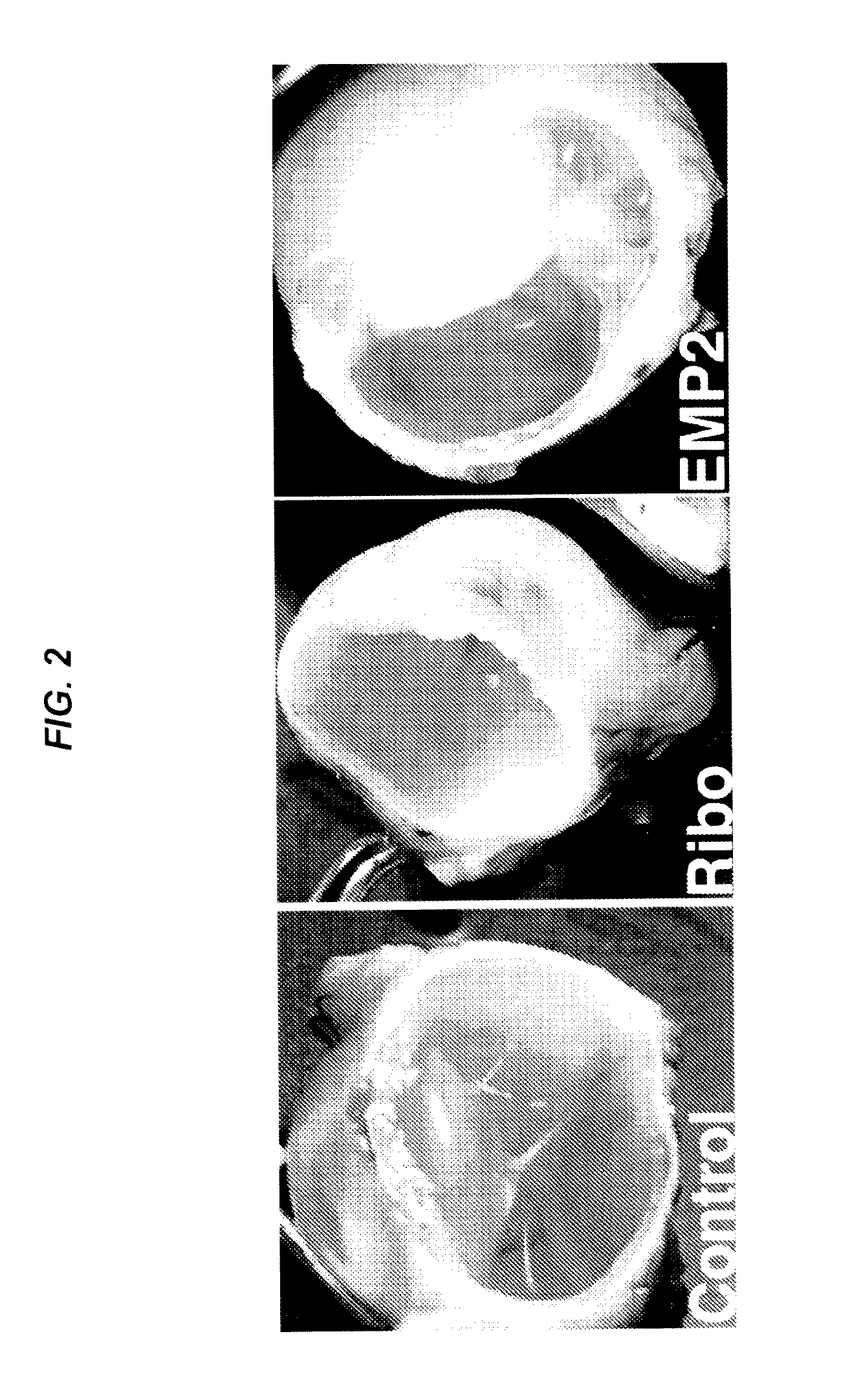
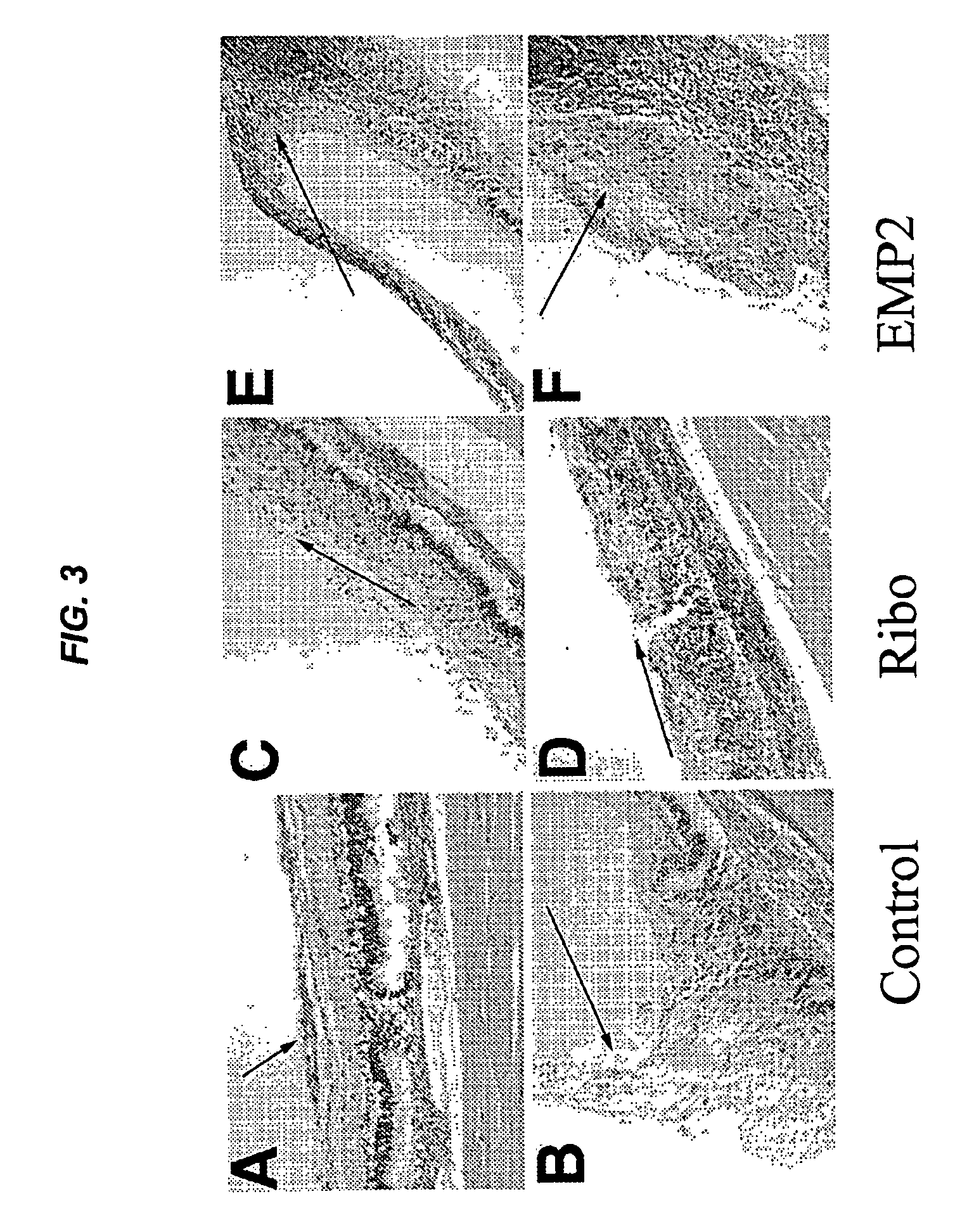
Epithelial membrane protein-2 (EMP2) and proliferative vitroretinopathy (PVR)
ActiveUS20130004493A1Reduces EMP activityReduce riskSenses disorderPharmaceutical delivery mechanismPVR - Proliferative vitreoretinopathyCvd risk
Methods of preventing retinal detachment associated with proliferative vitreoretinopathy are provided by administering agents which antagonize the activity or function of EMP2 to subjects at risk of the detachment.
Owner:RGT UNIV OF CALIFORNIA +1
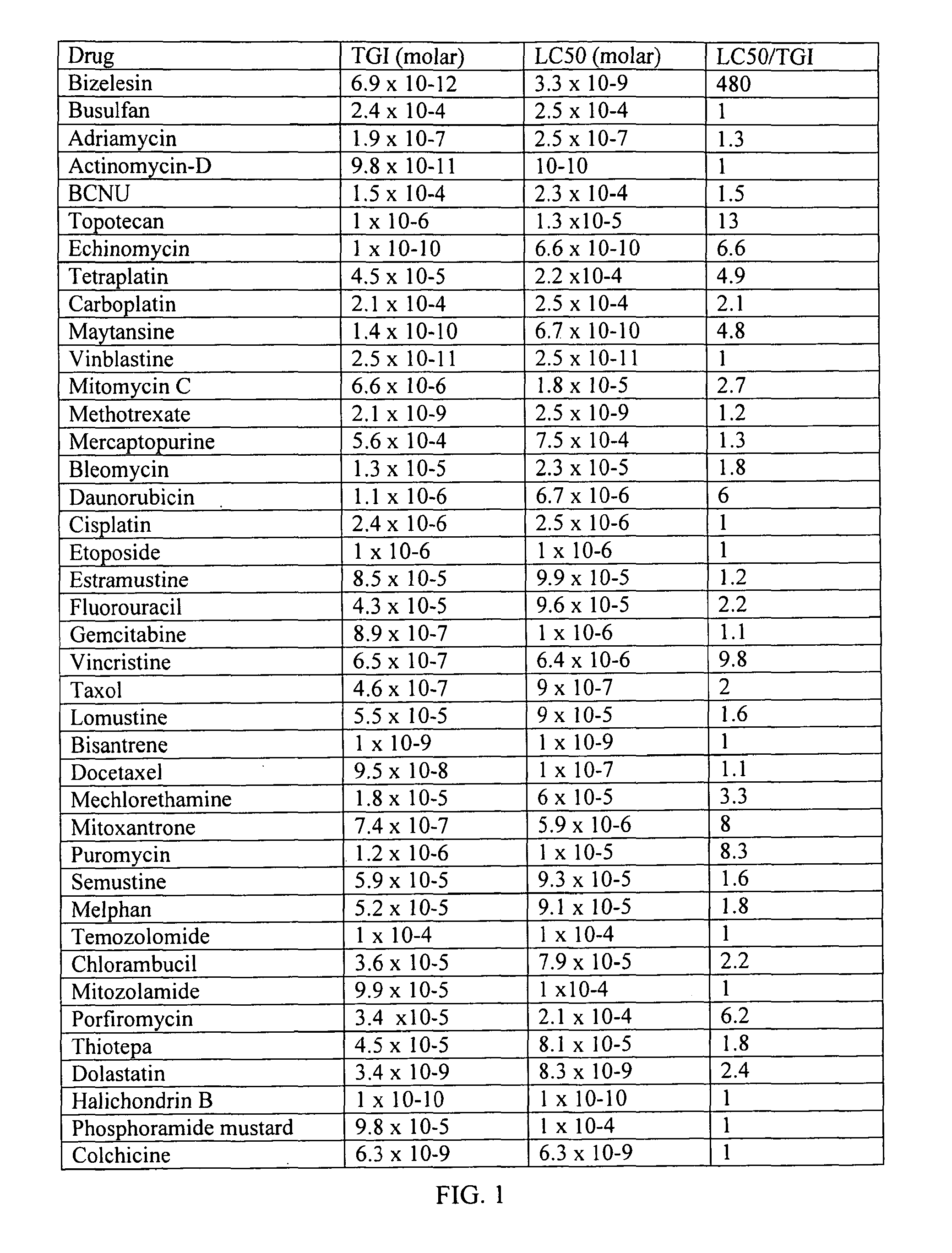
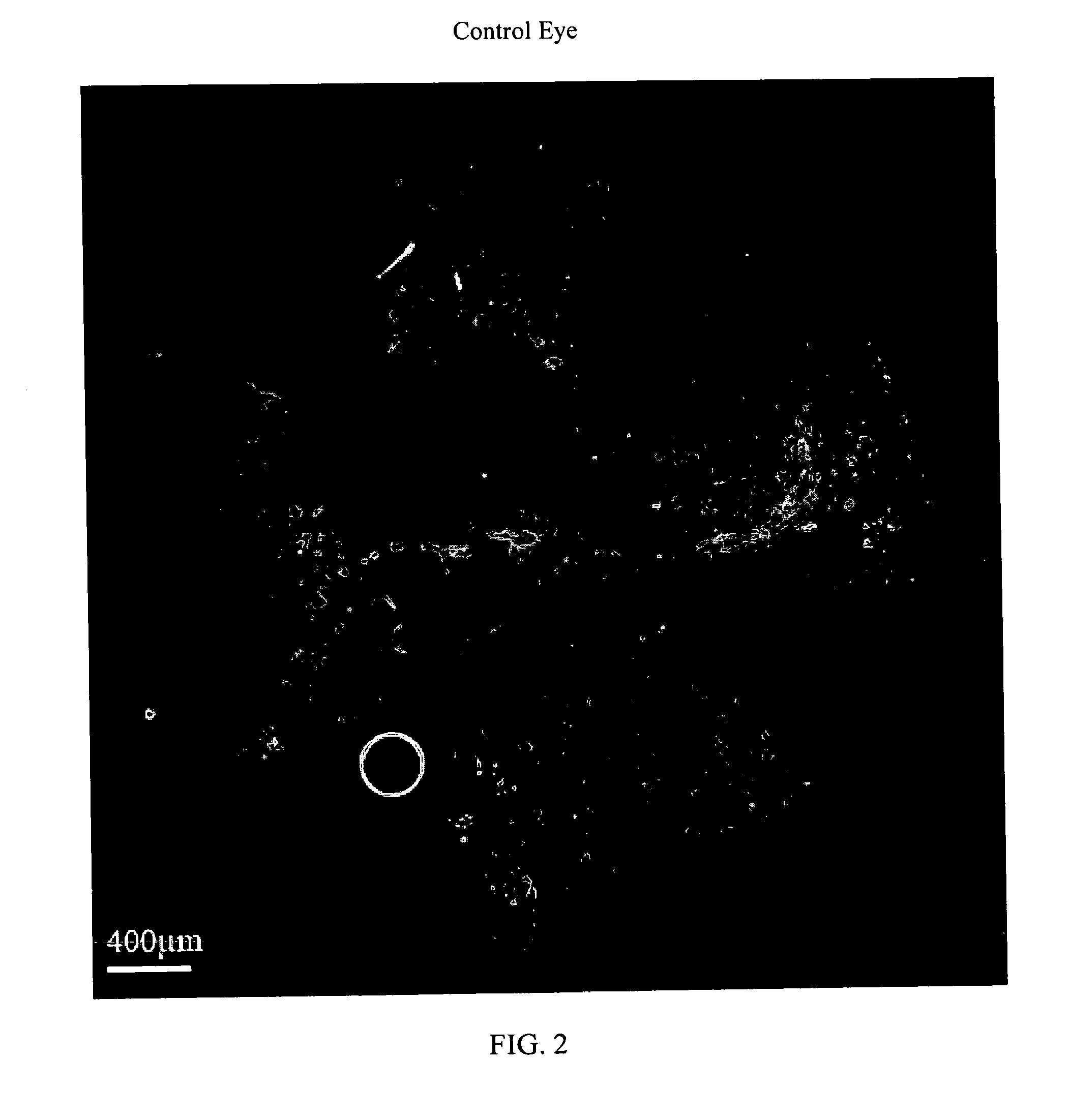
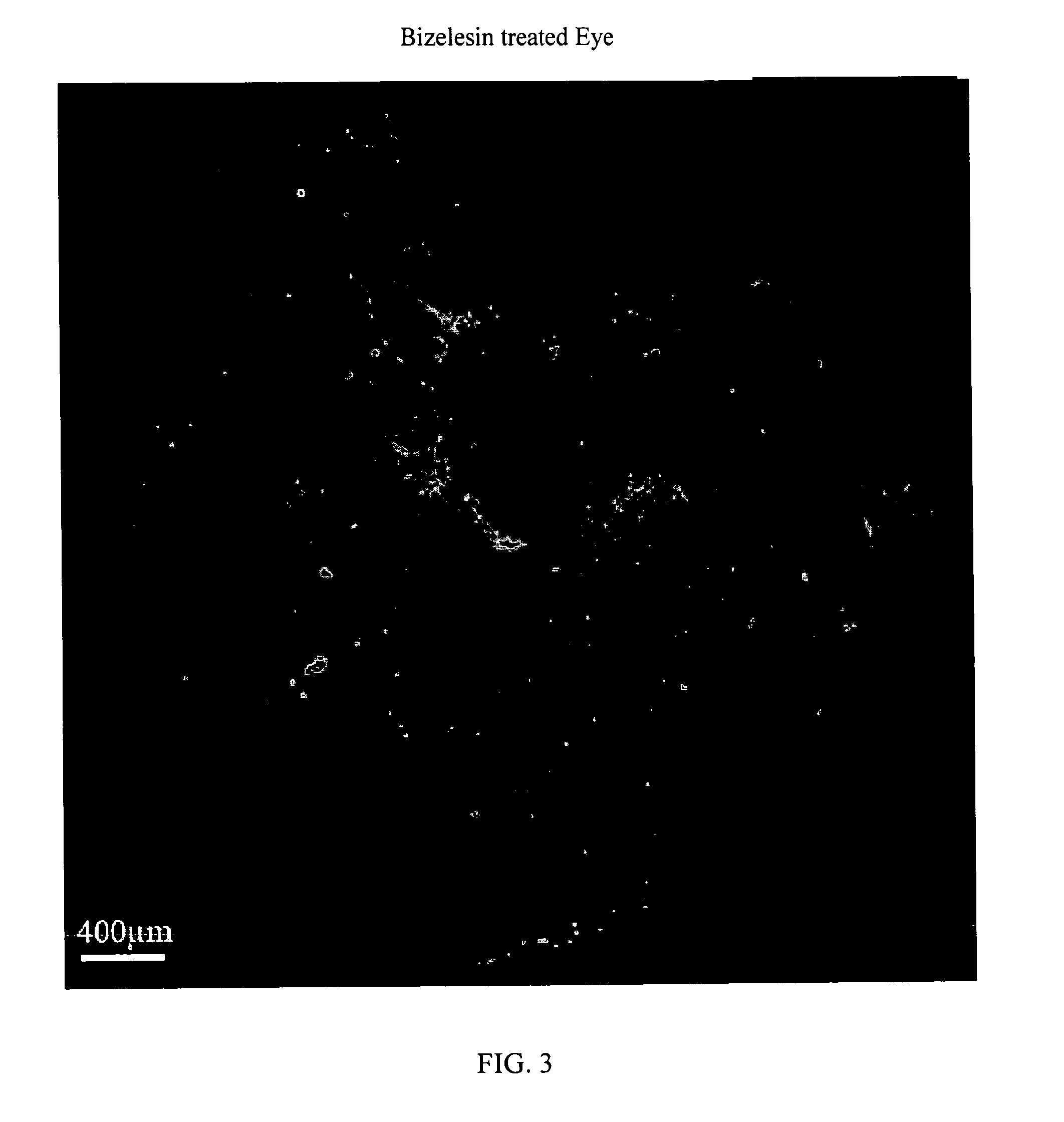
Method For The Treatment Of Proliferative Disorders Of The Eye
InactiveUS20110200662A1Inhibit cell proliferationLarge therapeutic indexBiocideSenses disorderUveitisMelanoma
The present invention relates to method for the treatment or prevention and of proliferative eye diseases including but not limited to: age related macular degeneration associated proliferative retinopathy, proliferative diabetic retinopathy, proliferative vitreoretinopathy, posterior capsular opacification, scaring and fibrosis after glaucoma filtration surgery, uveal melanoma, and retinoblastoma. The method comprises contacting cells in the eye by means of intra-ocular injection or infusion, with a drug that irreversibly inhibits cellular proliferation without causing extensive tissue necrosis or cytotoxicity. In a preferred embodiment the drug is bizelesin.
Owner:ONCOTX
Insoluble biological activity protein as vitreous body oral medicine for treating ophthalmic diseases
InactiveCN101422603AEfficient degradationEffective blockingPeptide/protein ingredientsFibrin depositionProliferative vitreoretinopathy
The invention relates to a method for curing a plurality of ophthalmonogy diseases by degrading, blocking, combining and inhabiting vitreous bodies and / or pathogenic restricted substances in the retinal and using an insoluble biological activity protein as a intravitreal medicament according to the unique structure feature of the vitreous body cavity in eyeballs and the pathogenic functions of a plurality of vitreous bodies and / or the pathogenic restricted substances in the retinal of ophthalmonogy patients in a plurality of the ophthalmonogy diseases. For example, diabetic retinopathy, senile macular degeneration, ophthalmonogy diseases caused by retinal artery or vein occlusion, macular edema, proliferative vitreoretinopathy, intravitreal living cells, vitreous macular traction, macular pucker, macular exudate, fibrin deposition, retinopathy of prematurity, floaters, retinal tearing, retinal detachment, macula hole, epiretinal membrane, retinal internal limiting membrane causing surface tension, collagen fibers causing the traction function to the retinal and the like.
Owner:马万超
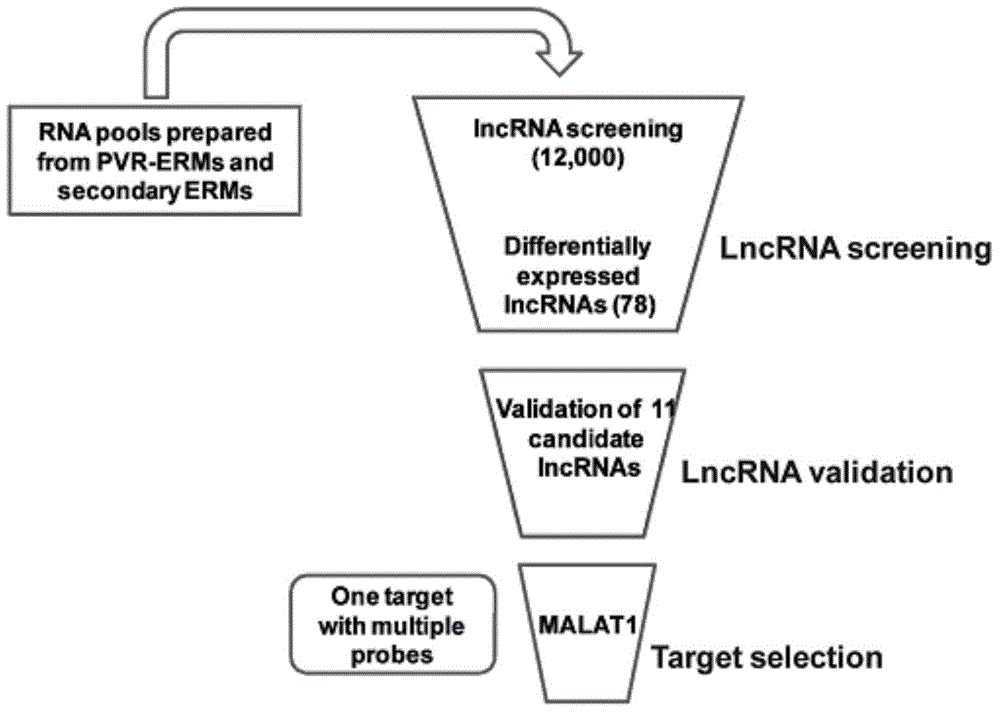
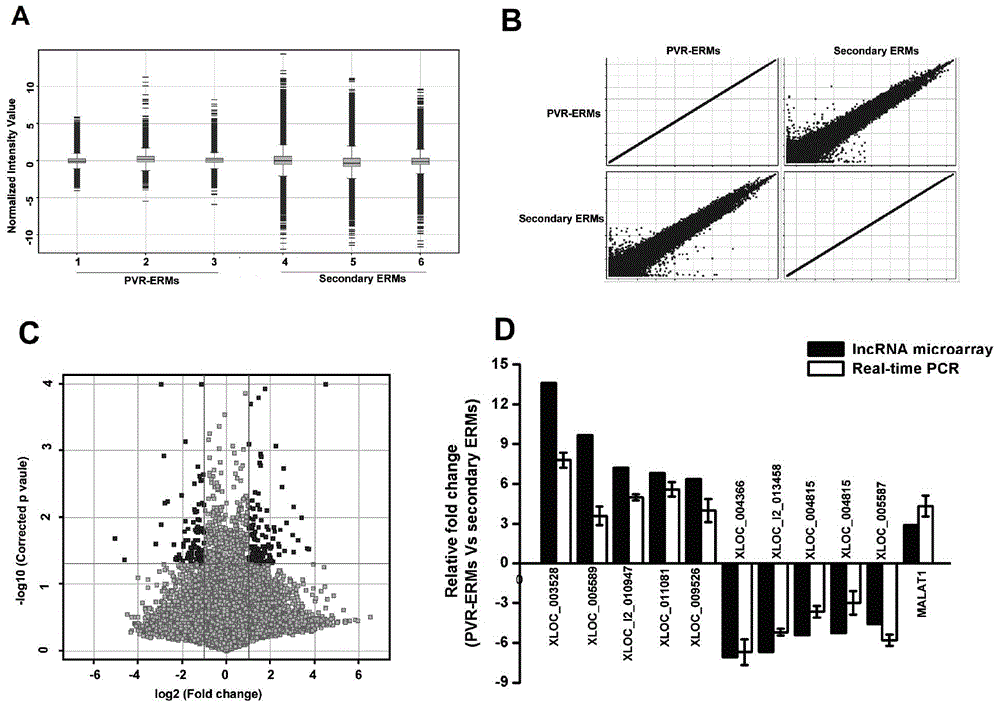
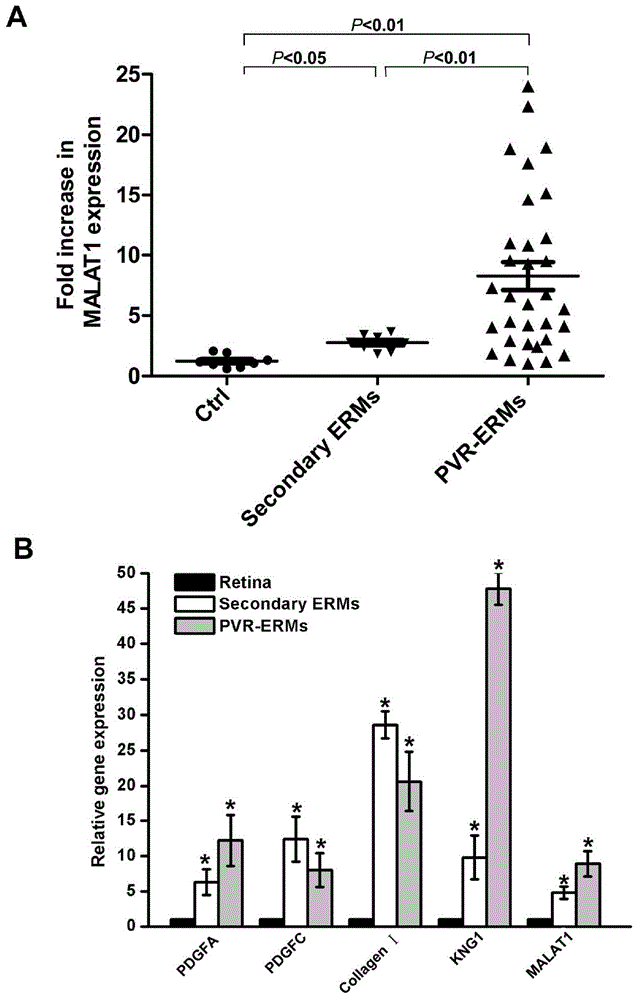
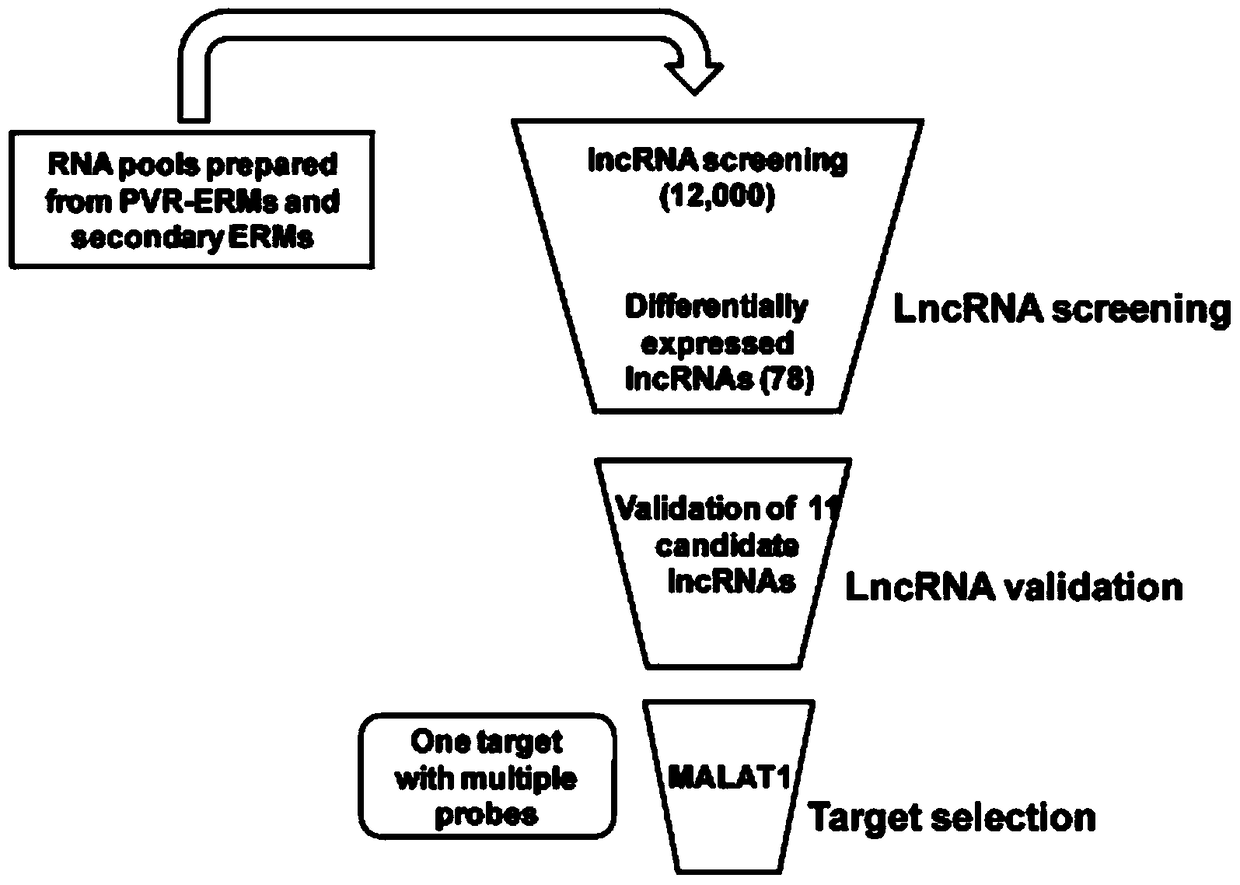
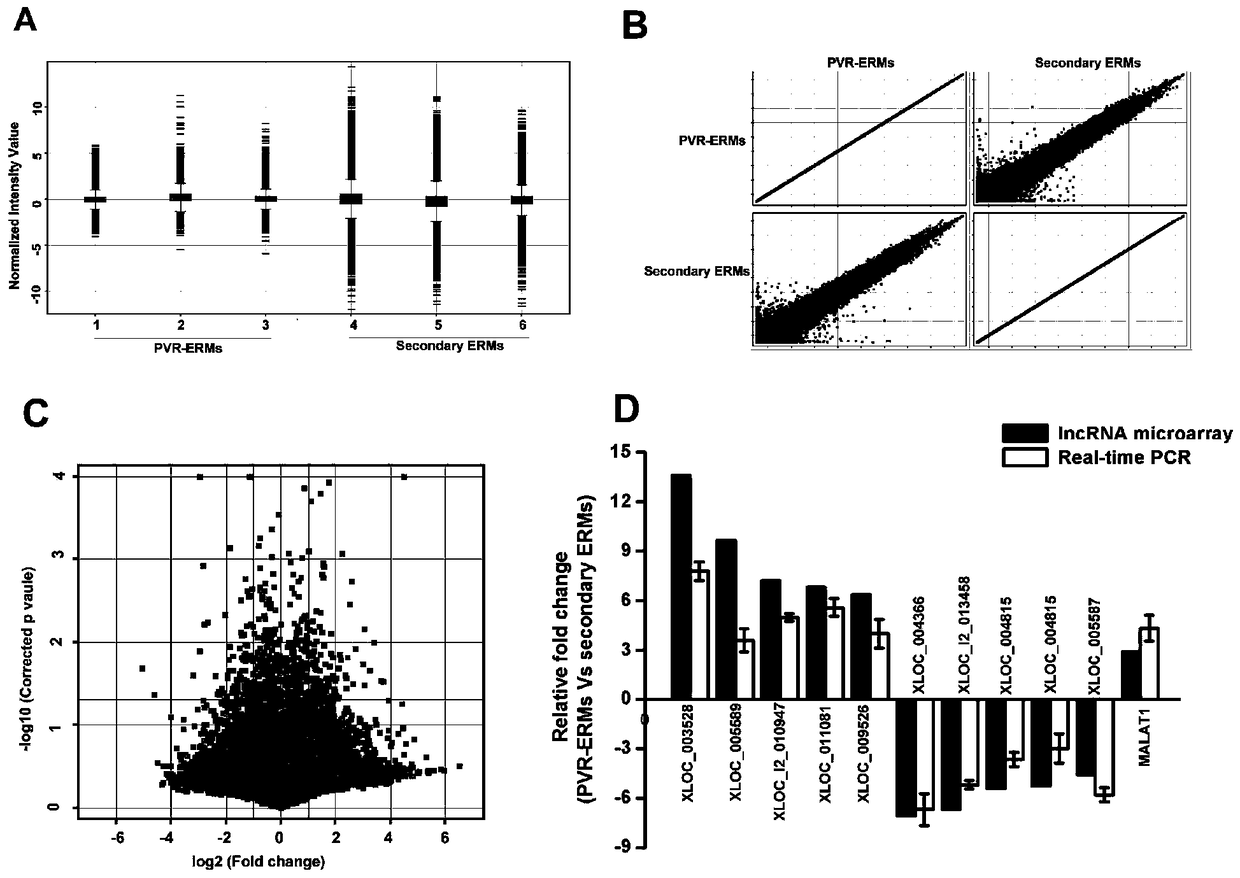
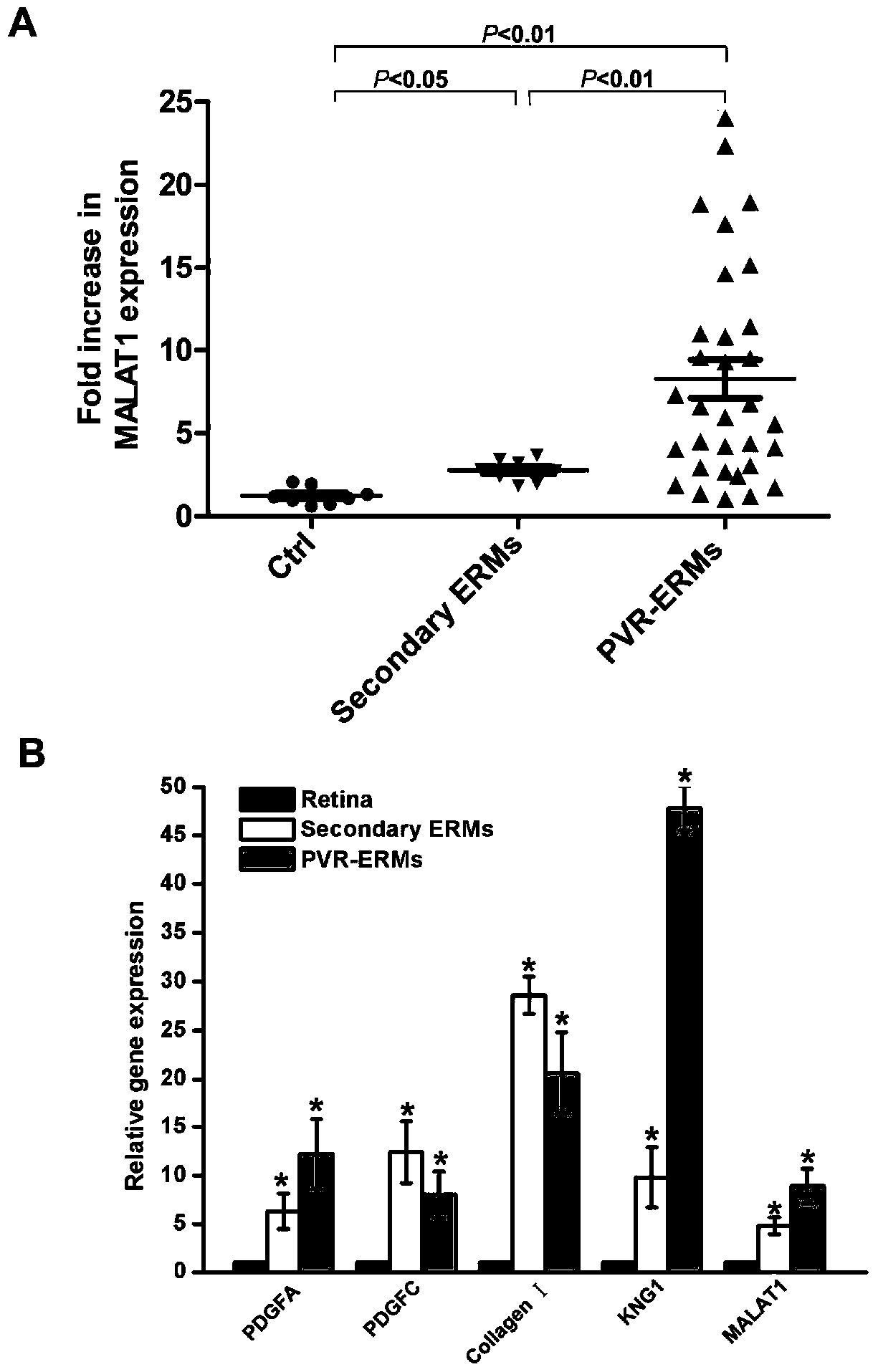
Application of lncRNA-MALAT1 in preparing proliferative vitroretinopathy (PVR) diagnosis reagent
The invention provides application of lncRNA-MALAT1 in preparing a proliferative vitroretinopathy (PVR) diagnosis reagent. The invention also provides an MALAT1 detection kit for PVR diagnosis. The kit comprises an RNA (ribonucleic acid) extraction system, a reverse transcription reaction system and a PCR (polymerase chain reaction) reaction system. On the basis of real-time fluorescent quantitative PCR, the kit can be used for carrying out PVR early diagnosis by detecting the relative content of MALAT1 in the serum or plasma. The method has the characteristics of small trauma and the like, and is convenient to operate.
Owner:南京医科大学眼科医院
Antagonists of ci-m6p/igf2r for prevention and treatment of ctgf-mediated ocular disorders
Antagonists of cation-independent mannose 6-phosphate / insulin-like growth factor-II receptor are provided for attenuation of CTGF signaling in a method of down-regulation of receptor signaling and downstream decreased signaling of connective tissue growth factor in ocular disorders involving inappropriate CTGF signaling. Ocular disorders involving inappropriate CTGF signaling include ocular hypertension, glaucoma, glaucomatous retinopathy, optic neuropathy, macular degeneration, diabetic retinopathy, choroidal neovascularization, and proliferative vitreoretinopathy, for example. Such disorders are treated by administering antagonists of the present invention.
Owner:ALCON RES LTD
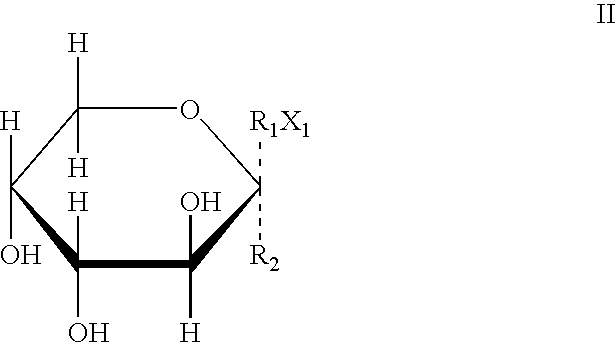
Applications of Chalcomoracin and homologues thereof in preparation of drugs for treatment of proliferative vitreoretinopathy
ActiveCN111617066ABlock proliferationInhibit migrationOrganic active ingredientsSenses disorderPVR - Proliferative vitreoretinopathyPharmaceutical drug
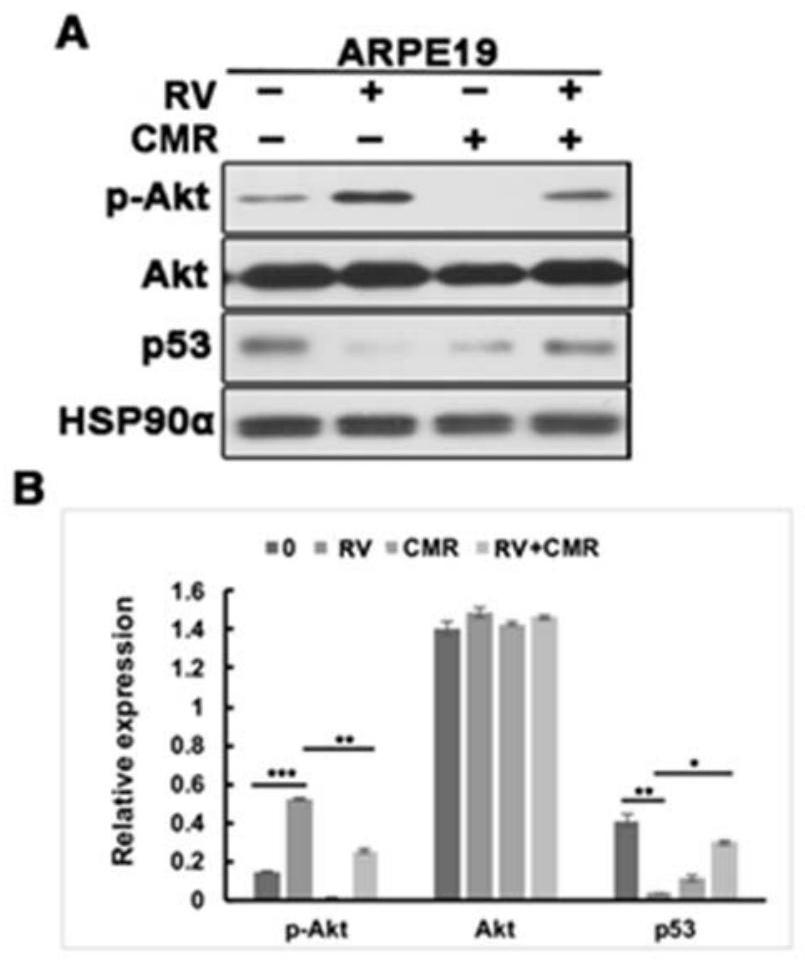
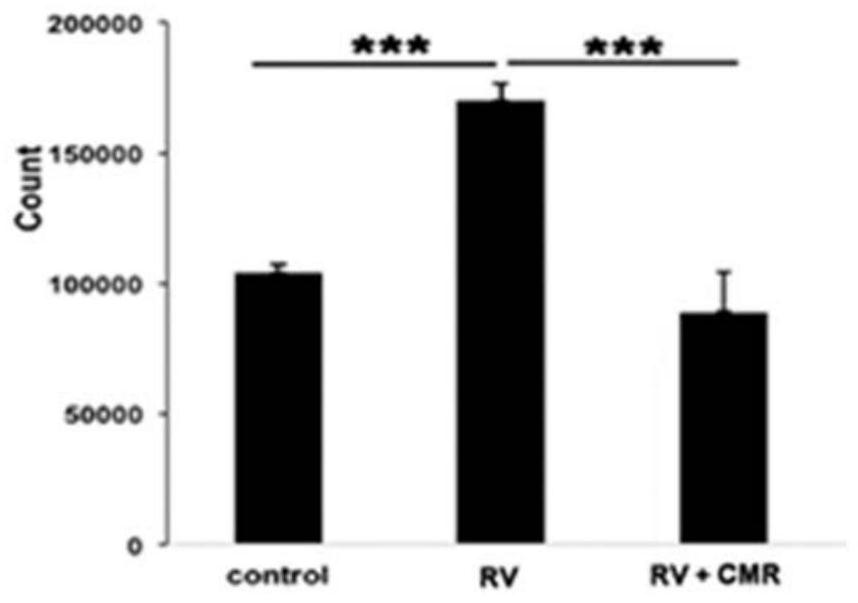
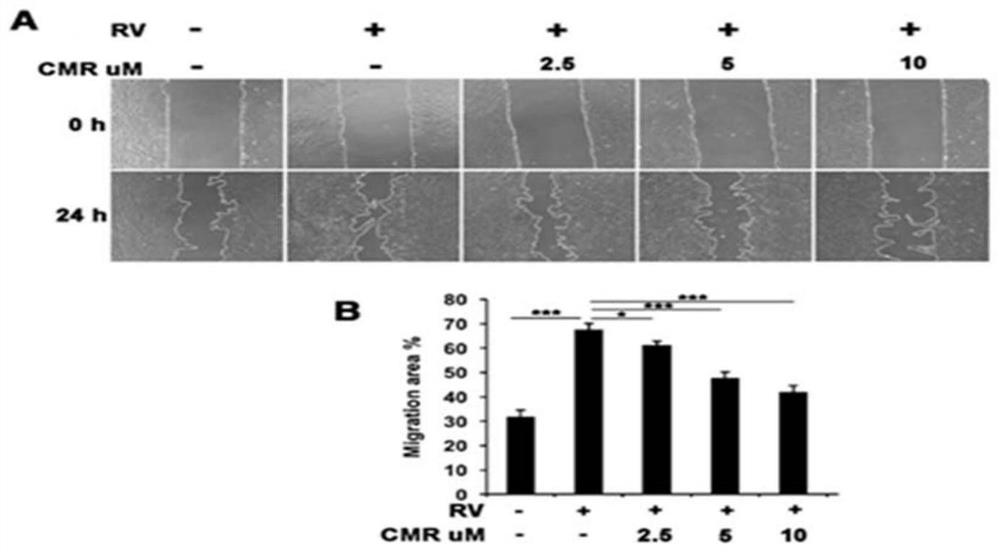
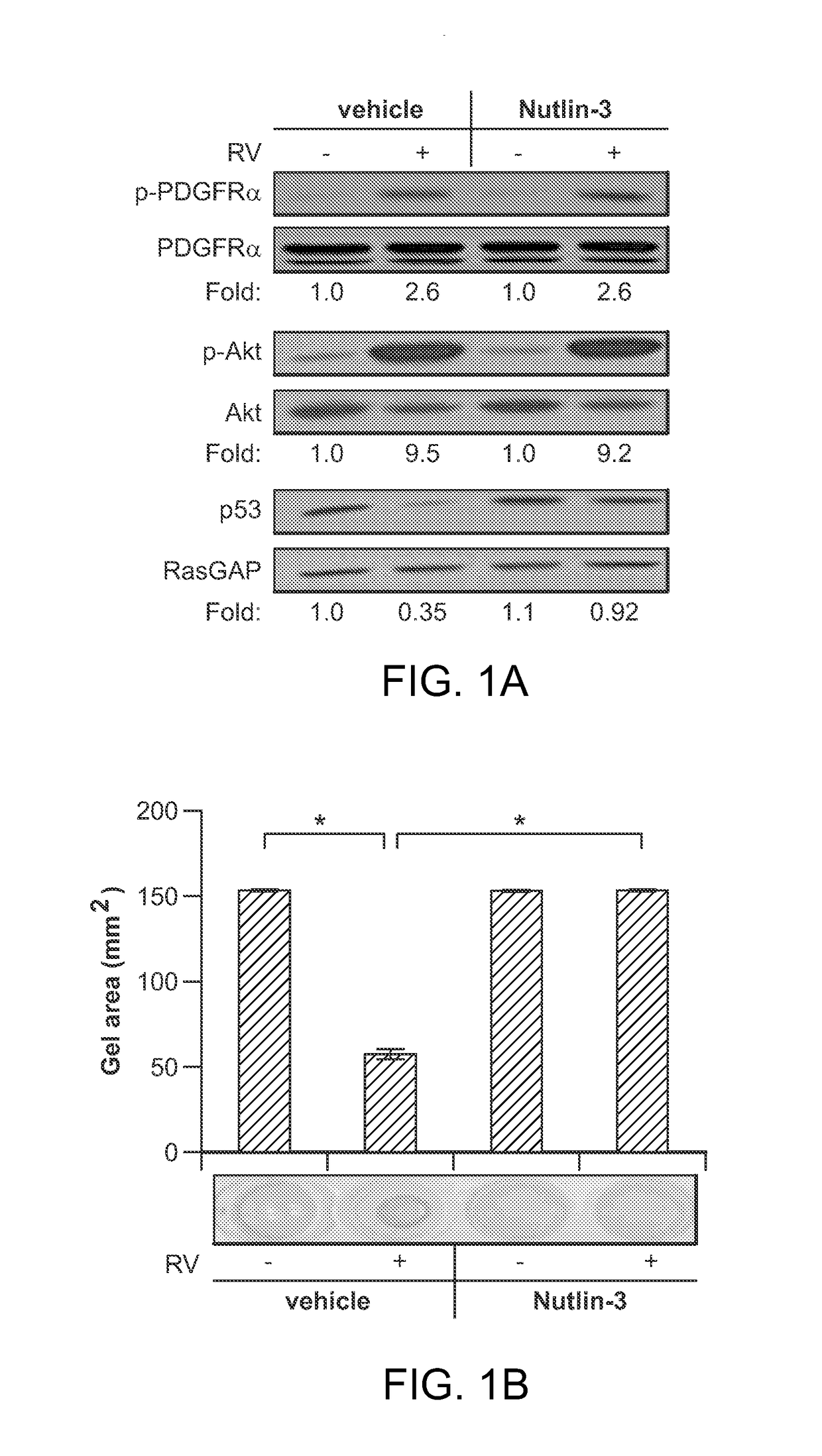
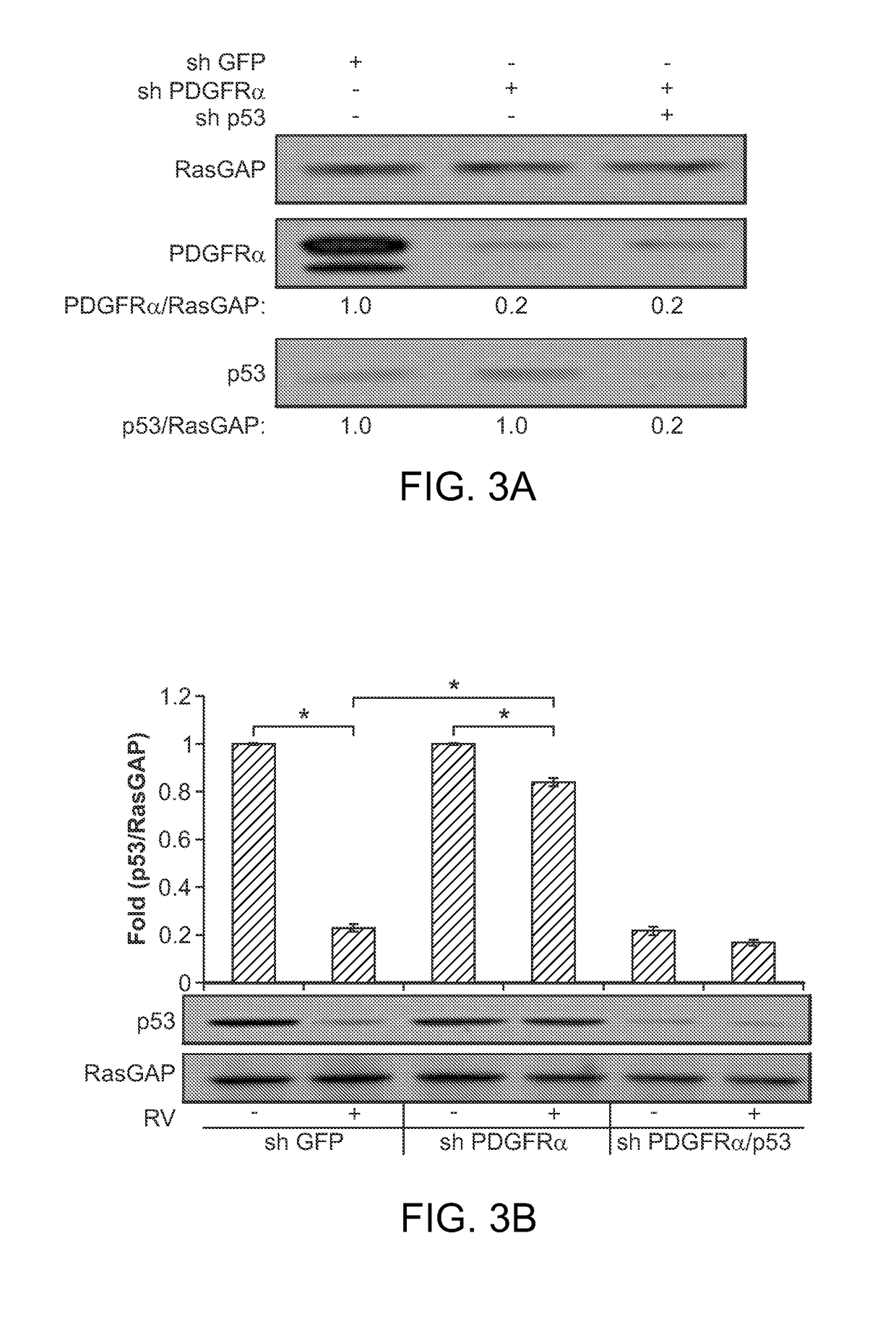
The present invention discloses applications of Chalcomoracin and homologues thereof in preparation of drugs for treatment and prevention of proliferative vitreoretinopathy. The Chalcomoracin and homologues thereof can inhibit vitreous-induced AKT activation and p53 decline, block proliferation, migration and contraction of ARPE-19 cells stimulated by vitreous, and have effects of treating and preventing the proliferative vitreoretinopathy.
Owner:ZHEJIANG UNIV
Calcium blockers to treat proliferative vitreoretinopathy
InactiveUS20050192322A1Preventing and reducing proliferative vitreoretinopathyAlleviate neuronal damageBiocideSenses disorderProliferative vitreoretinopathyNeuroglia
Glutamate causes migration and proliferation of retinal pigment epithelium and / or glial cells, and glutamate antagonists can prevent, treat or reduce retinal pigment epithelium and / or glial migration and the subsequent development of proliferative vitreoretinopathy. Avoidance or management of proliferative vitreoretinopathy can be achieved by administering to the patient a compound capable of reducing glutamate-induced retinal cell migration in a concentration effective to reduce such migration.
Owner:ALLERGAN INC
Antagonists of ci-m6p/igf2r for prevention and treatment of ctgf-mediated ocular disorders
Antagonists of cation-independent mannose 6-phosphate / insulin-like growth factor-II receptor are provided for attenuation of CTGF signaling in a method of down-regulation of receptor signaling and downstream decreased signaling of connective tissue growth factor in ocular disorders involving inappropriate CTGF signaling. Ocular disorders involving inappropriate CTGF signaling include ocular hypertension, glaucoma, glaucomatous retinopathy, optic neuropathy, macular degeneration, diabetic retinopathy, choroidal neovascularization, and proliferative vitreoretinopathy, for example. Such disorders are treated by administering antagonists of the present invention.
Owner:ALCON RES LTD
Treatments for proliferative vitreoretinopathy
Disclosed herein are methods for treatment for proliferative vitreoretinopathy (PVR) by inhibiting the activity of activated transforming growth factor Beta activated kinase 1 (TAK-1), or activation thereof. Pharmaceutical compositions for use in the described treatments are also provided.
Owner:MOR RES APPL LTD
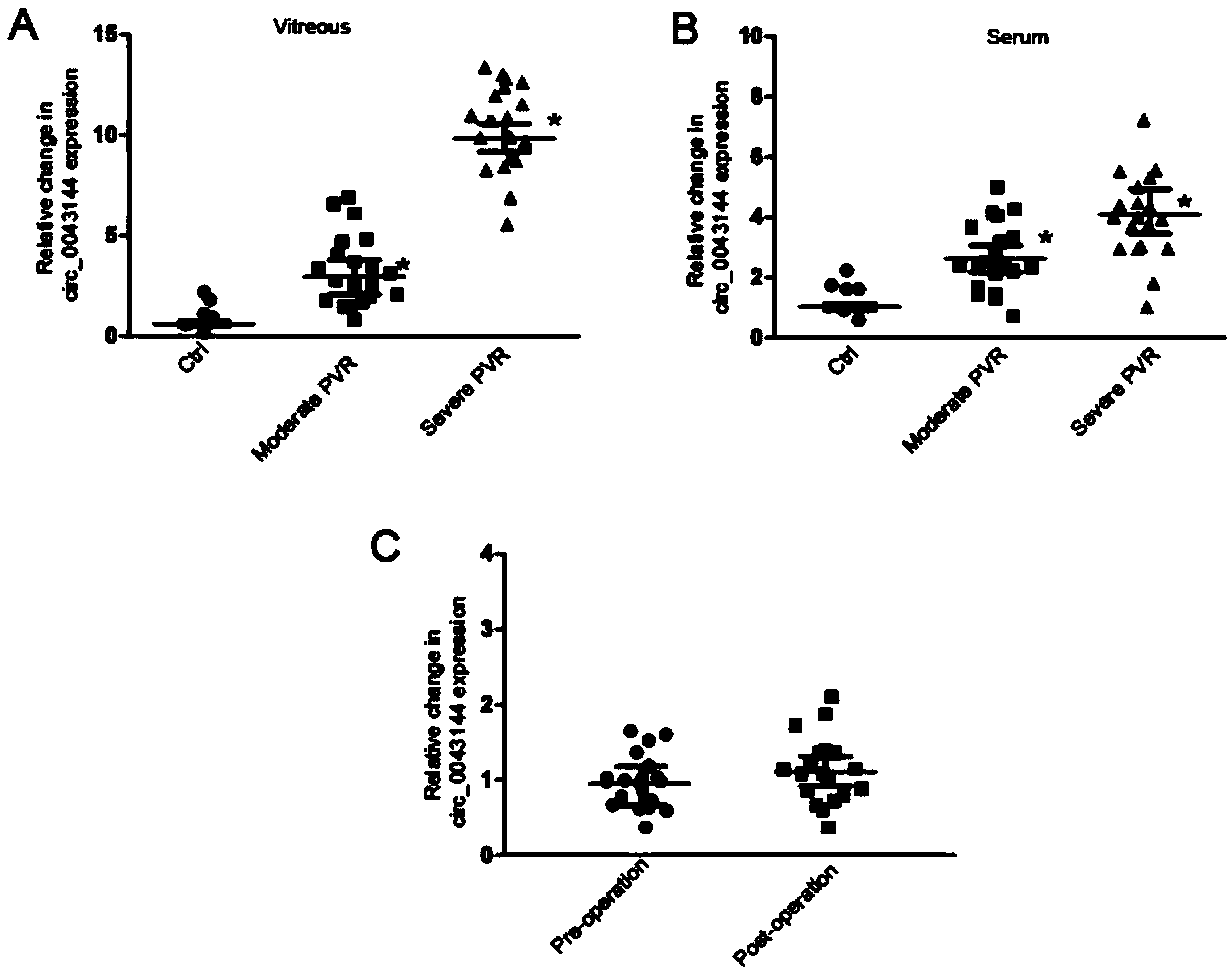
Application of circRNA in preparation of diagnostic reagent for proliferative vitreoretinopathy
ActiveCN109355373ALess traumaticEasy to operateOrganic active ingredientsSenses disorderFluorescenceProliferative vitreoretinopathy
The invention discloses an application of circRNA in preparing a diagnostic reagent for proliferative vitreoretinopathy (PVR). The invention also discloses a circular RNA detection kit for PVR diagnosis. The kit mainly comprises an RNA extraction system, a reverse transcription reaction system, and a PCR reaction system. The relative contents of circRNA in vitreous and serum are detected by real-time fluorescence quantitative PCR technique for early diagnosis of PVR. The application of circRNA in preparing the diagnostic reagent for PVR has the advantages of small wound, high sensitivity and simple operation.
Owner:EYE & ENT HOSPITAL SHANGHAI MEDICAL SCHOOL FUDAN UNIV
Application of lncrna-malat1 in the preparation of diagnostic reagents for proliferative vitreoretinopathy
ActiveCN104962654BLess traumaticEasy to operateOrganic active ingredientsSenses disorderDiagnosis earlySerum ige
Owner:南京医科大学眼科医院
Slow-released 5-fluorouracil system in vitreous chamber
InactiveCN1846700AAvoid dangerPrevent diseaseOrganic active ingredientsSenses disorderSurgical operationPolyvinyl alcohol
The present invention provides one kind of slow released 5-fluorouracil system in vitreous chamber. The slow released 5-fluorouracil system has medicine carrying amount of 10.44%, medicine carrier of synthesized biodegradable polymer polylactic acid, and emulsifier of water soluble polymer PVA. The slow released 5-fluorouracil system is suitable for use after the surgical operation on vitreoretinopathy, and after being injected into vitreous chamber, it may maintain smooth medicine release for 28 days to inhibit the proliferation of active cell inducing proliferative vitreoretinopathy, block the acting path of the cell factors and prevent proliferative vitreoretinopathy effectively.
Owner:毕宏生 +2
Traditional Chinese medicine for treating proliferative vitreoretinopathy
ActiveCN101797348ASenses disorderPharmaceutical delivery mechanismTraditional medicineActive ingredient
The invention relates to a traditional Chinese medicine for treating proliferative vitreoretinopathy, which is prepared from leech, trigone, hawthorn, kelp, seaweed and other traditional Chinese medicines as raw materials.
Owner:XIAN BEILIN PHARMA
Dendrimer compositions and their use in treatment of diseases of the eye
ActiveUS10369124B2Inhibition is effectiveAntibacterial agentsOrganic active ingredientsDiseaseDendrimer
The treatment of many ocular disorders is hampered because of poor penetration of systemically administered drugs into the eye. The tight junctional complexes (zonulae occludens) of the retinal pigment epithelium and retinal capillaries are the site of the blood-ocular barrier. This barrier inhibits penetration of substances, including antibiotics, into the vitreous. Over the last 18 years we have evaluated the nontoxic doses of various drugs. These include antibiotics and antifungals for treatment of bacterial and fungal endophthalmitis, antivirals for treatment of viral retinitis (specifically, when medication with these drugs poses the threat of toxicity to other organs). Intravitreal antineoplastic drugs have been studied to prevent cell proliferation in the vitreous cavity after retinal attachment surgery, which can lead to proliferative vitreoretinopathy (PVR). Furthermore, we evaluated the anti-inflammatory action of dexamethasone and cyclosporine A to reduce intraocular inflammation after intraocular surgery or in uveitis. Because these studies had been performed in the presence of the vitreous, which can slow down the diffusion of the drugs toward the retina, it was necessary to reevaluate the concentration of drugs which could be administered intravitreally in the vitrectomized eye. The nontoxic dose of numerous drugs when added to vitrectomy infusion fluid has also been evaluated. Furthermore, the role of vitrectomy in the treatment of bacterial fungal endophthalmitis has been studied and the role of vitrectomy in this ocular disorder is defined.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Traditional Chinese medicine for treating proliferative diabetic retinophathy
InactiveCN104940754ALower eye pressureReduce thicknessSenses disorderMetabolism disorderDiabetes retinopathyLife quality
The invention belongs to the technical field of traditional Chinese medicine, in particular to traditional Chinese medicine for treating proliferative diabetic retinopathy. The traditional Chinese medicine comprises, by weight, 5-13 parts of American ginseng, 8-16 parts of radix pseudostellariae, 8-16 parts of atractylodes macrocephala koidz, 11-15 parts of Chinese yam, 8-16 parts of prepared rehmannia roots, 8-16 parts of fruits of Chinese wolfberry, 8-16 parts of glossy privet fruits, 8-16 parts of seeds of Chinese dodder, 5-13 parts of ligusticum wallichii, 5-13 parts of corydalis tuber, 8-16 parts of salviae miltiorrhizae, 5-13 parts of Rhizoma Sparganii, 1-3 parts of leeches, 5-13 parts of rhizoma arisaematis, 2-4 parts of rhizoma typhonii, 8-16 parts of semen cassiae and 8-16 parts of seeds of feather cockscomb. The traditional Chinese medicine can effectively reduce the intraocular pressure and the thickness of yellow spots of an affected eye, reduce the leakage area of retinal neovascularization, promote retinal neovascularization to gradually vanish, improve the vision of a patient and improve the life quality of the patient.
Owner:JINAN BANGWEN MEDICAL TECH
RNAi INHIBITION OF CTGF FOR TREATMENT OF OCULAR DISORDERS
RNA interference is provided for inhibition of connective tissue growth factor mRNA expression in ocular disorders involving CTGF expression. Ocular disorders involving aberrant CTGF expression include glaucoma, macular degeneration, diabetic retinopathy, choroidal neovascularization, proliferative vitreoretinopathy and wound healing. Such disorders are treated by administering interfering RNAs of the present invention.
Owner:NOVARTIS AG
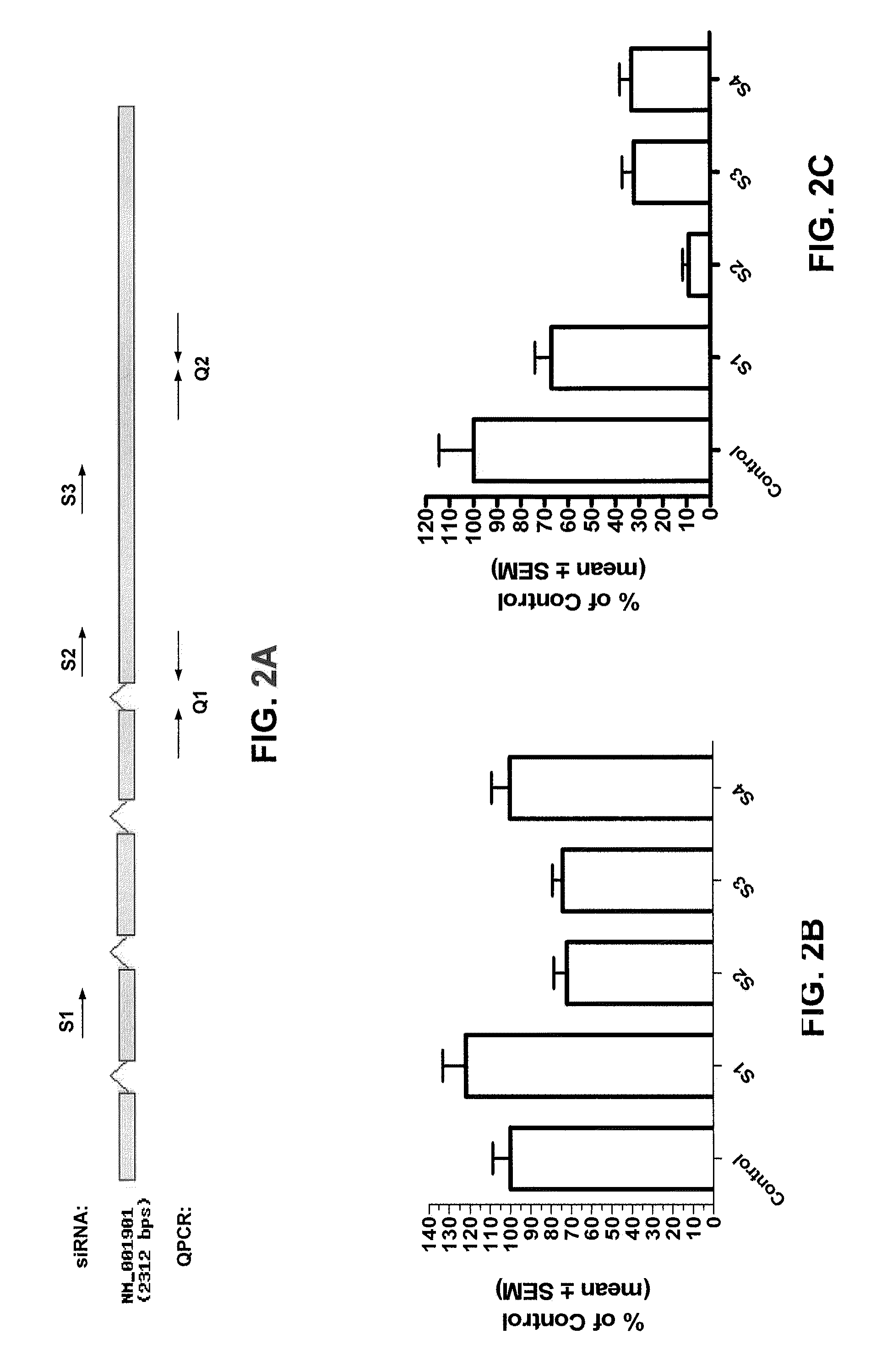
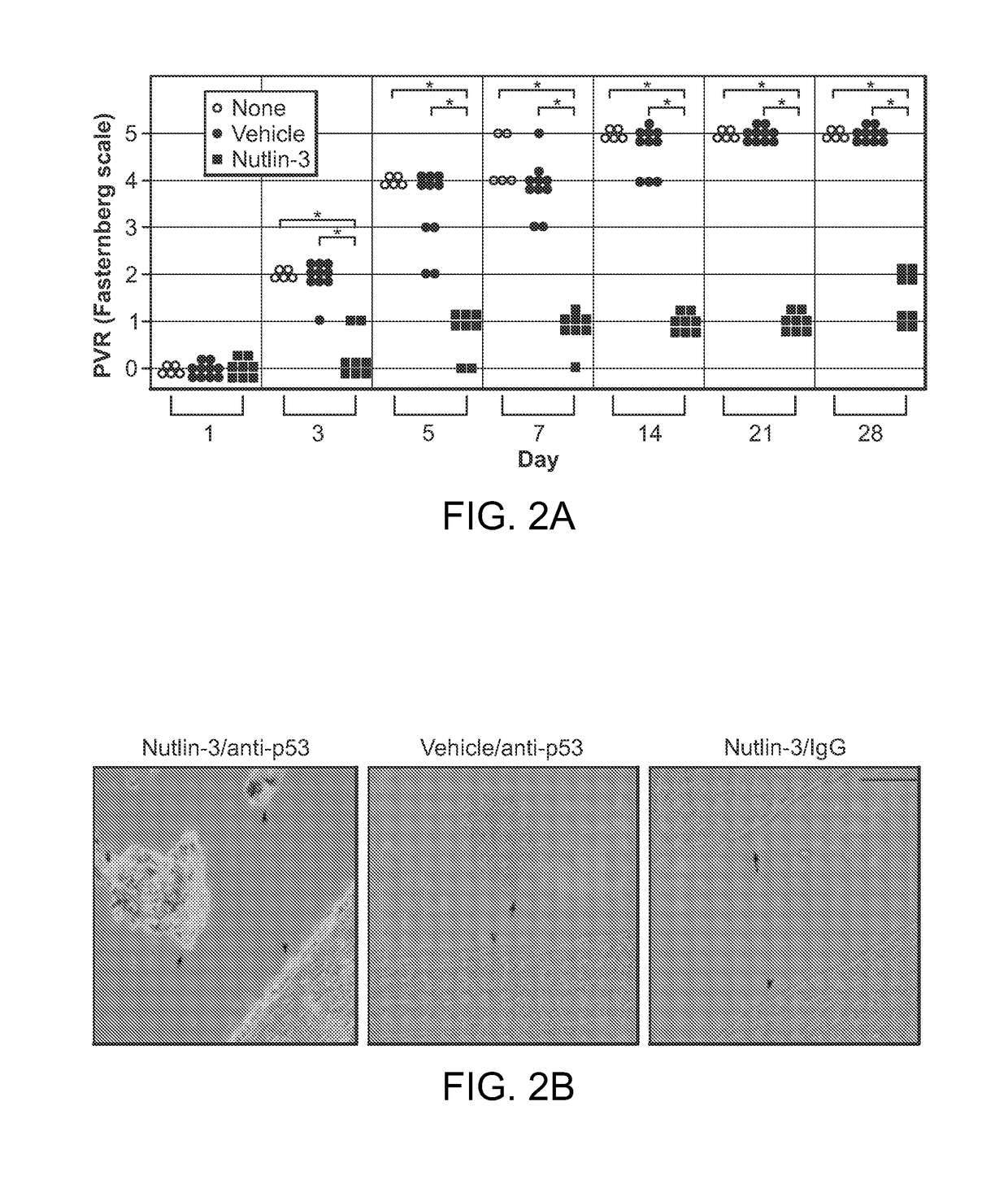
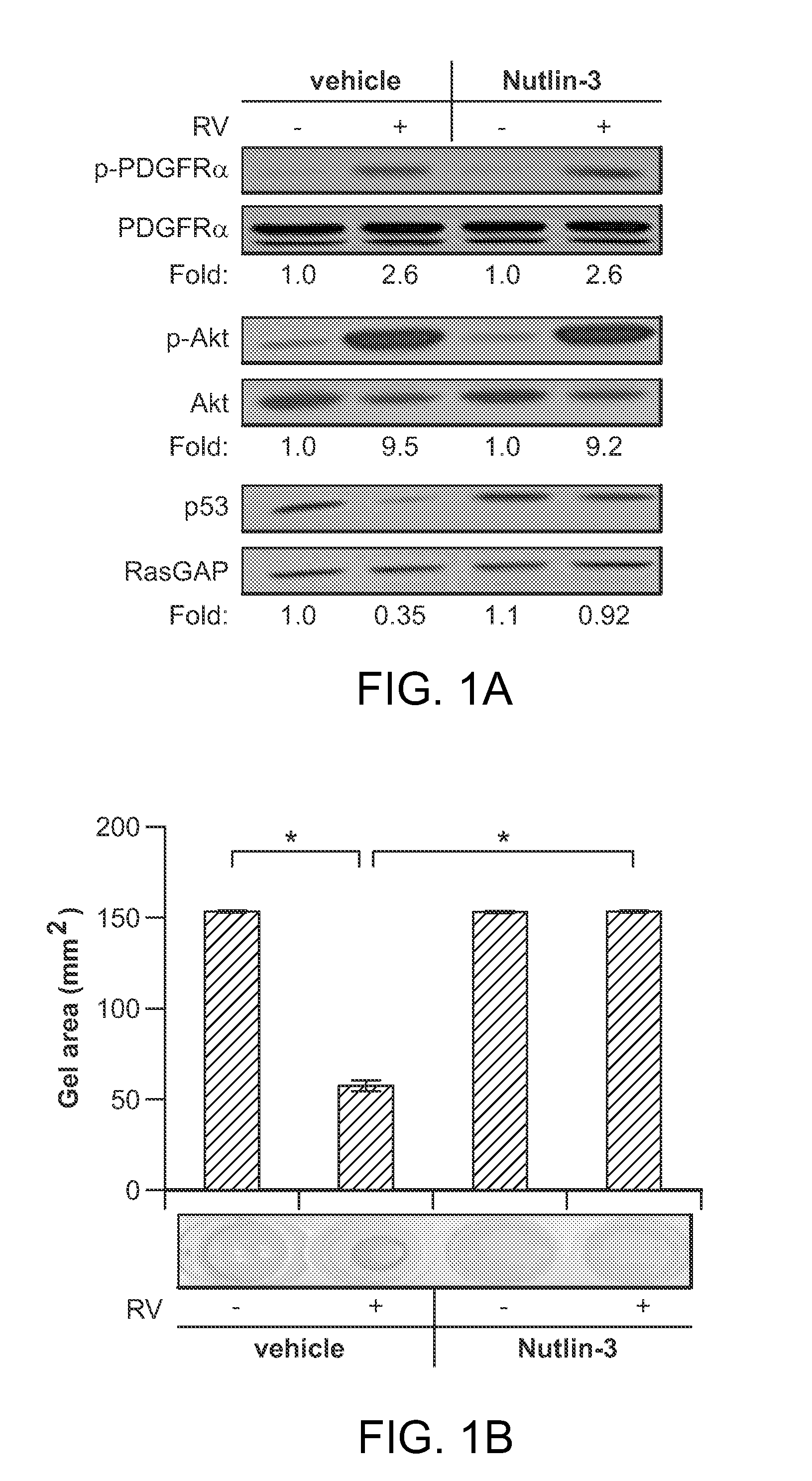
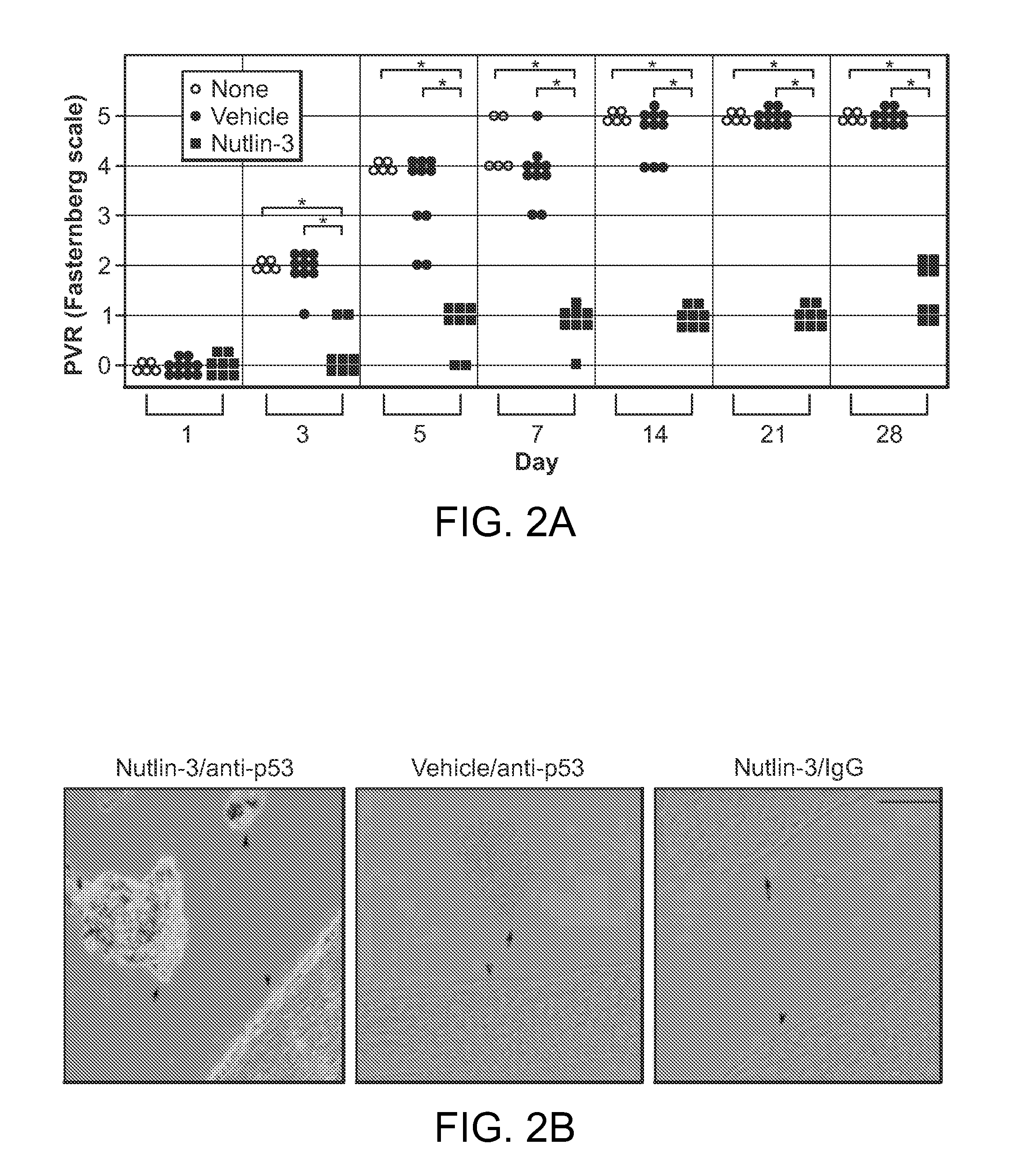
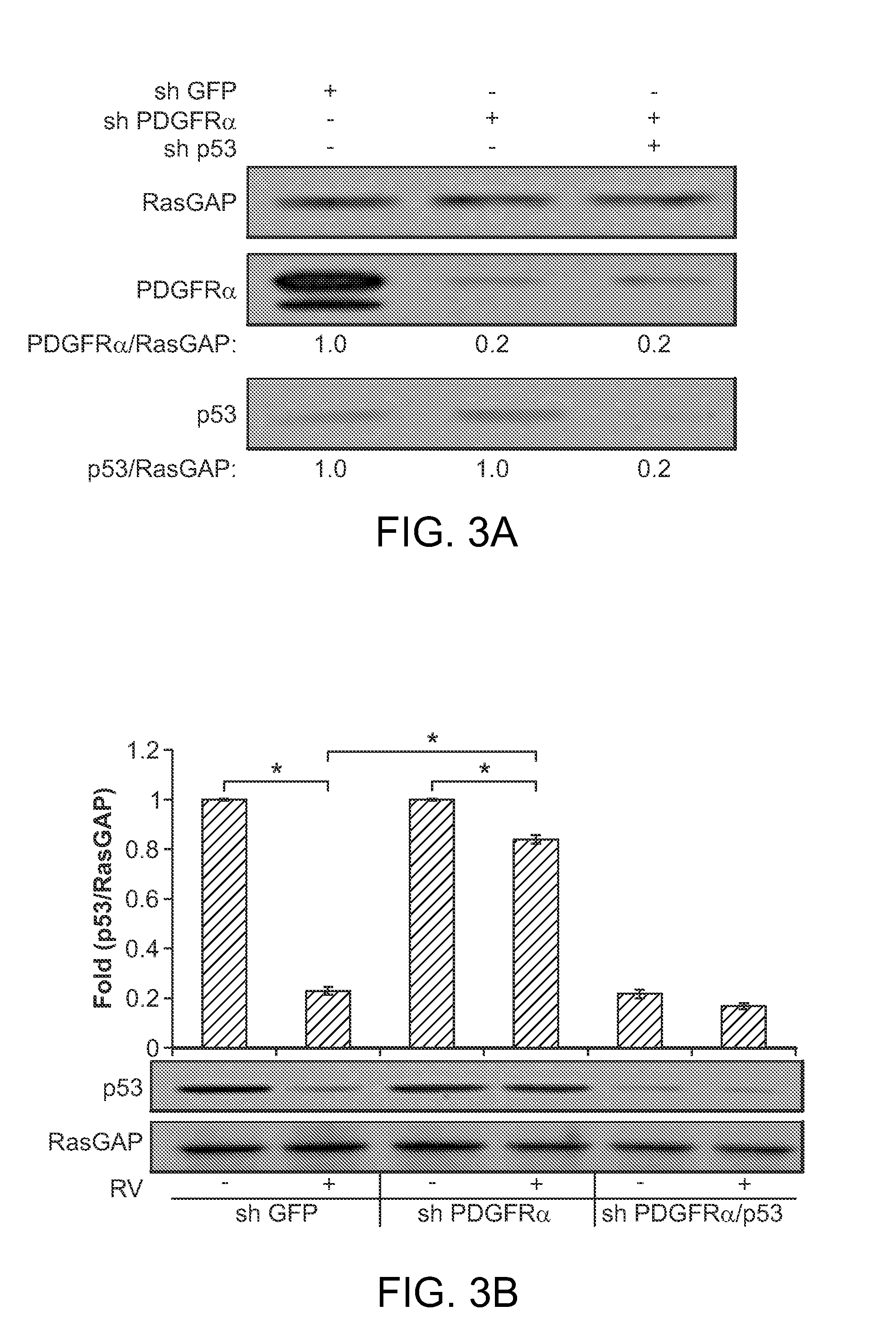
Nutlin-3A for treatment of proliferative vitreoretinopathy
ActiveUS9623024B2Organic active ingredientsSenses disorderVitreous retinaProliferative vitreoretinopathy
Owner:THE SCHEPENS EYE RES INST
Nutlin-3A For Treatment of Proliferative Vitreoretinopathy
ActiveUS20150313893A1Reduce severityReduce frequencyOrganic active ingredientsSenses disorderProliferative vitreoretinopathyCancer research
Owner:THE SCHEPENS EYE RES INST
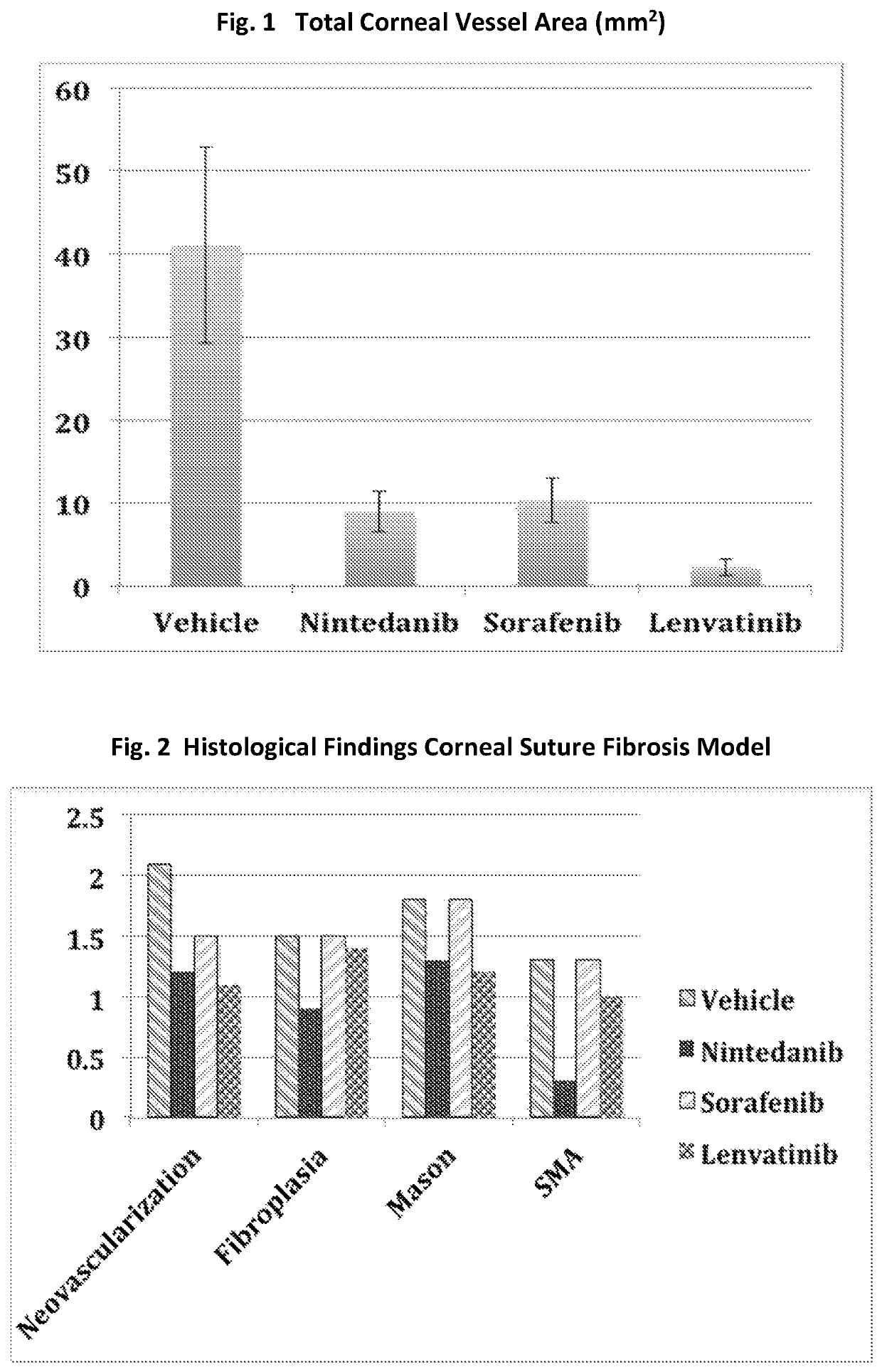
Multikinase inhibitors and uses in ocular fibrosis
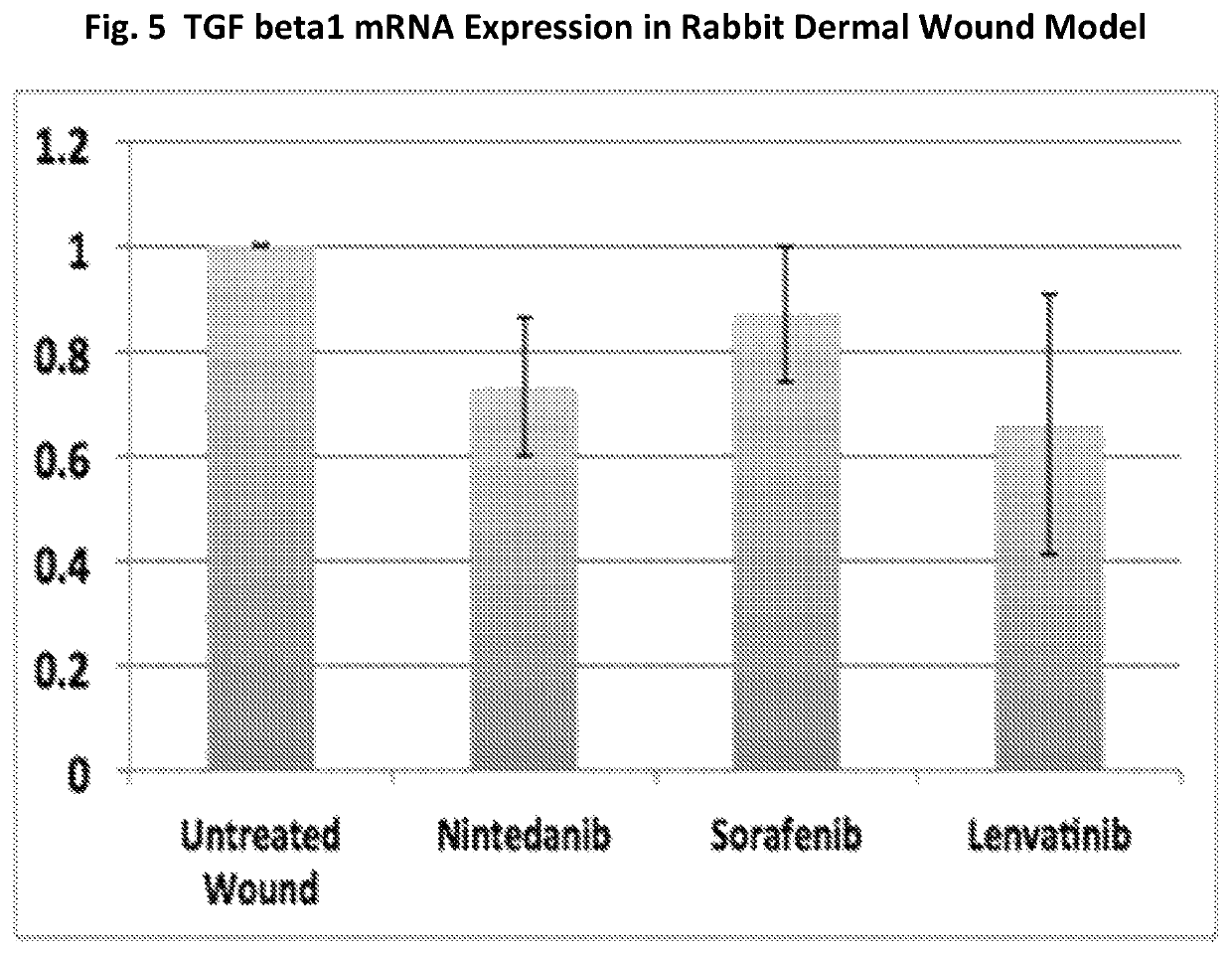
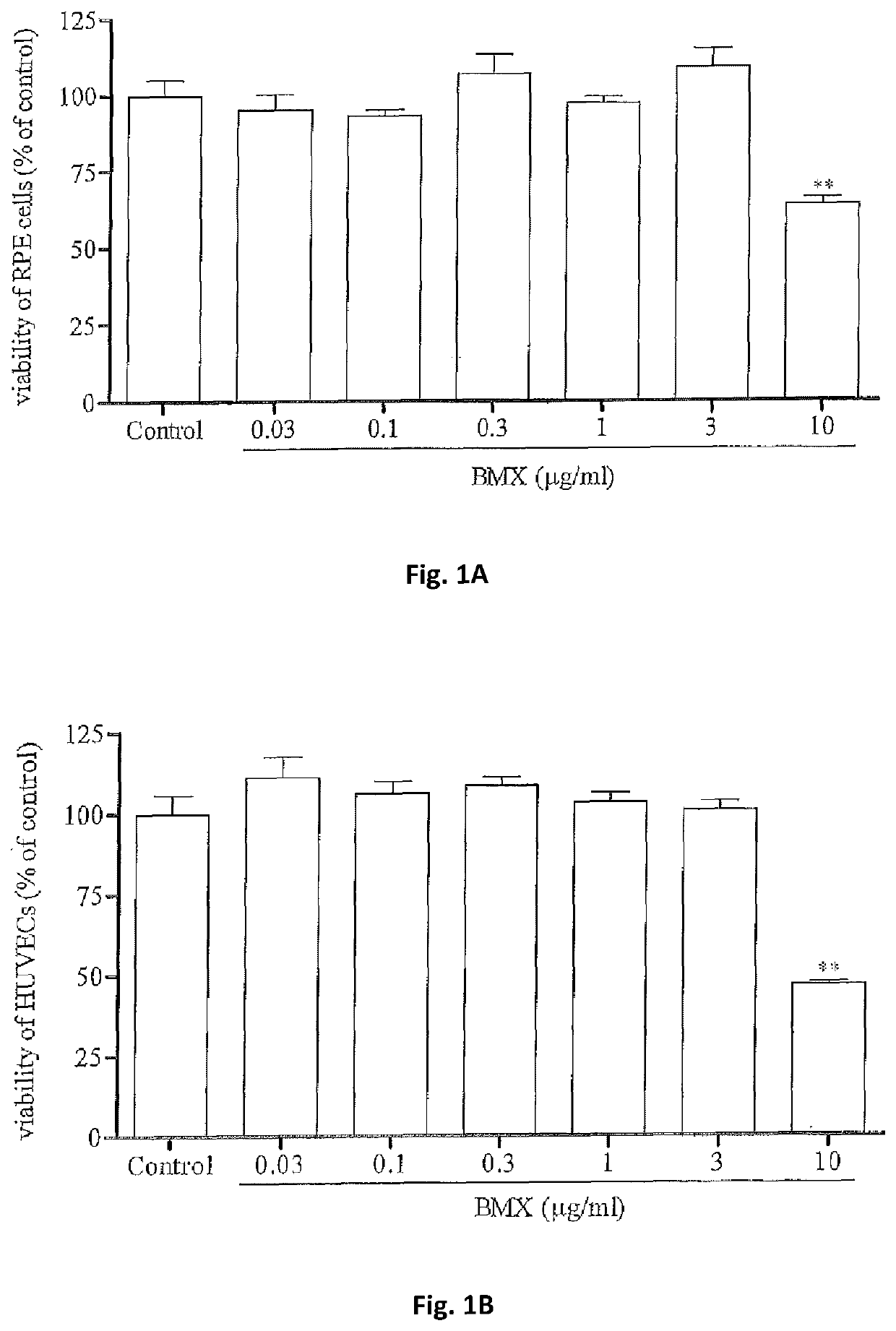
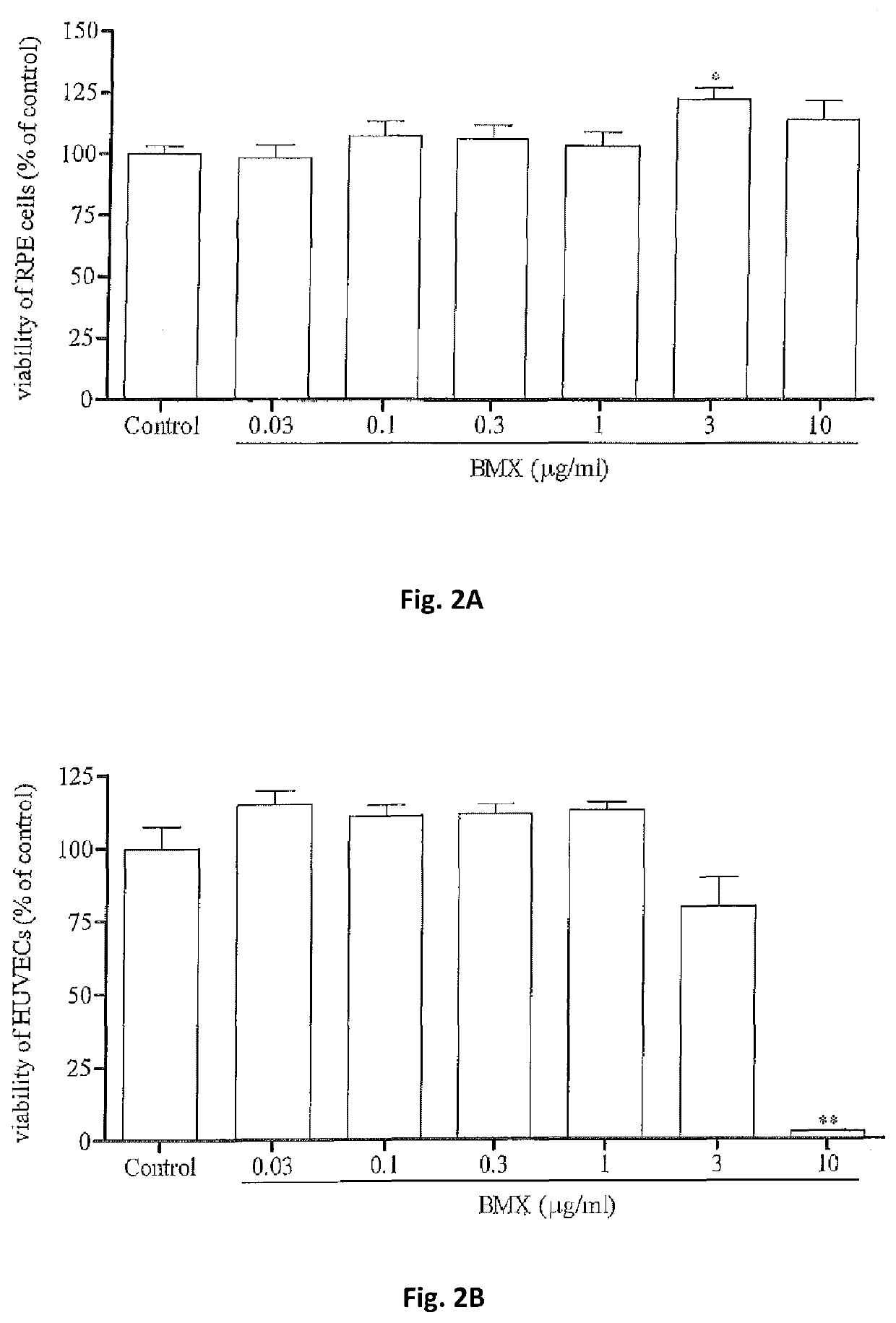
A method for preventing and / or treating fibrosis associated with an eye-related disease or disorder includes administering an effective amount of a multikinase inhibitor to a subject in need thereof. The multikinase inhibitor is nintedanib, lenvatinib, a combination thereof, or a salt thereof. The eye-related disease or disorder is corneal transparency, corneal scar formation, secondary cataract formation, glaucoma filtration surgery, ocular surgical procedures and implants, photorefractive keratectomy, laser in situ keratomileusis, formation and contraction of pre- and epiretinal membranes, proliferative vitreoretinopathy, subretinal fibrosis / scarring, retinal gliosis, or formation of choroidal membranes.
Owner:AIVIVA BIOPHARMA INC
Methods for treating ocular diseases
ActiveUS11045435B2Prevent the breakdown of blood eye barrierEffective preventionSenses disorderPharmaceutical delivery mechanismUveitisDisease
The present invention is related to a method for treating an ocular disease, particularly a diabetes related ocular disease, comprising administering to a subject in need thereof an effective amount of a group of compounds having a structure of Formula A1, wherein the ocular disease is selected from the group consisting of proliferative vitreoretinopathy (PVR), uveitis, glaucoma and age related macular degeneration (AMD), and the diabetes related ocular disease is selected from the group consisting of diabetic retinopathy (DR) and diabetic macular edema (DME).
Owner:NATURE WISE BIOTECH & MEDICALS CORP
Long-acting low molecule heparin intraocular sustained-release system
InactiveCN101259098BOvercome the disadvantage of easy bleedingPrevent proliferationOrganic active ingredientsSenses disorderEpitheliumProliferative vitreoretinopathy
Owner:SHANDONG EYE INST
Application of circRNA in the preparation of diagnostic reagents for proliferative vitreoretinopathy
ActiveCN109355373BLess traumaticEasy to operateOrganic active ingredientsSenses disorderRNA extractionPVR - Proliferative vitreoretinopathy
The invention discloses the application of circRNA in the preparation of proliferative vitreoretinopathy (PVR) diagnostic reagents. The invention also discloses a circRNA detection kit for PVR diagnosis. The kit mainly includes RNA extraction system, reverse transcription reaction system and PCR reaction system. The relative content of circRNA in vitreous and serum was detected by real-time quantitative PCR technology for early diagnosis of PVR. This method has the characteristics of less trauma, high sensitivity and simple operation.
Owner:EYE & ENT HOSPITAL SHANGHAI MEDICAL SCHOOL FUDAN UNIV
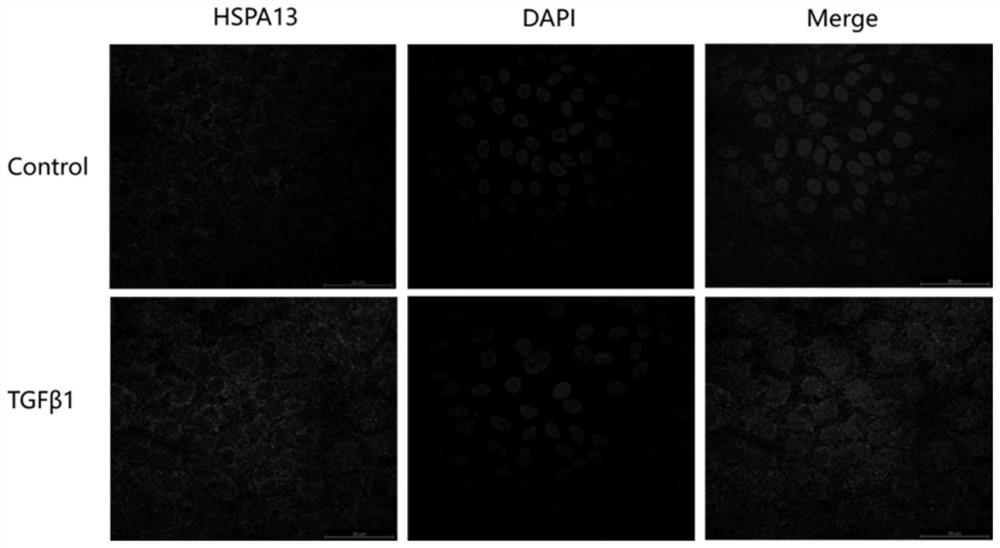
siRNA interfering with hspa13 expression and its application
ActiveCN109207478BReduce recurrenceExpress new ideasOrganic active ingredientsSenses disorderPVR - Proliferative vitreoretinopathyPharmaceutical drug
The invention relates to siRNA interfering with HSPA13 expression and application thereof, in particular to siRNA molecule interfering with HSPA13 expression and application of the siRNA molecule as therapeutic drug for preparing proliferative vitreoretinopathy. The siRNA molecule is adopted to interfere with the expression of HSPA13, realizes the occurrence and development of effective treatmentof PVR from the gene molecule level, and can avoid the risks caused by other treatment methods.
Owner:TONGJI UNIV
Epithelial membrane protein-2 (EMP2) and proliferative vitroretinopathy (PVR)
ActiveUS9464135B2Reduces EMP activityReduce riskSenses disorderImmunoglobulins against cell receptors/antigens/surface-determinantsCell Membrane ProteinsProliferative vitreoretinopathy
Methods of preventing retinal detachment associated with proliferative vitreoretinopathy are provided by administering agents which antagonize the activity or function of EMP2 to subjects at risk of the detachment.
Owner:RGT UNIV OF CALIFORNIA +1
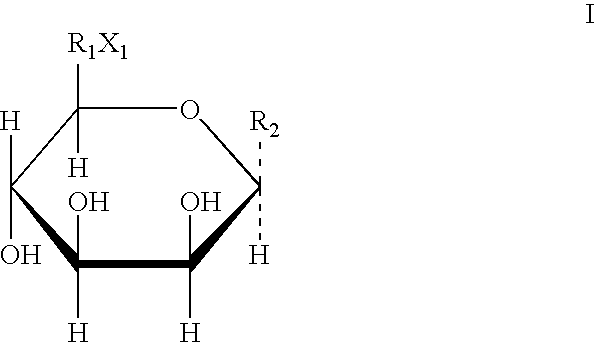
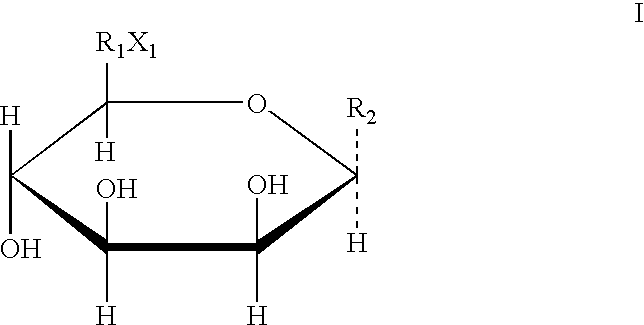
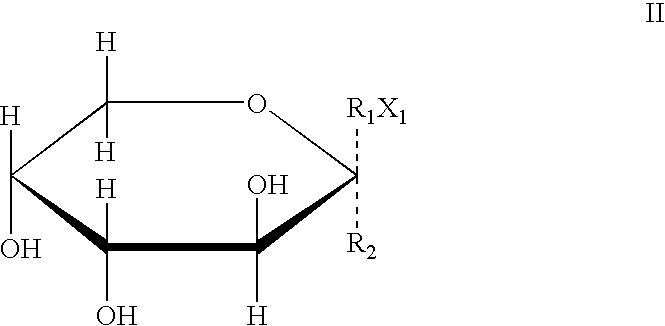
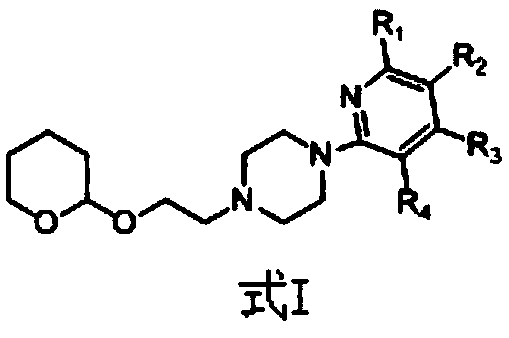
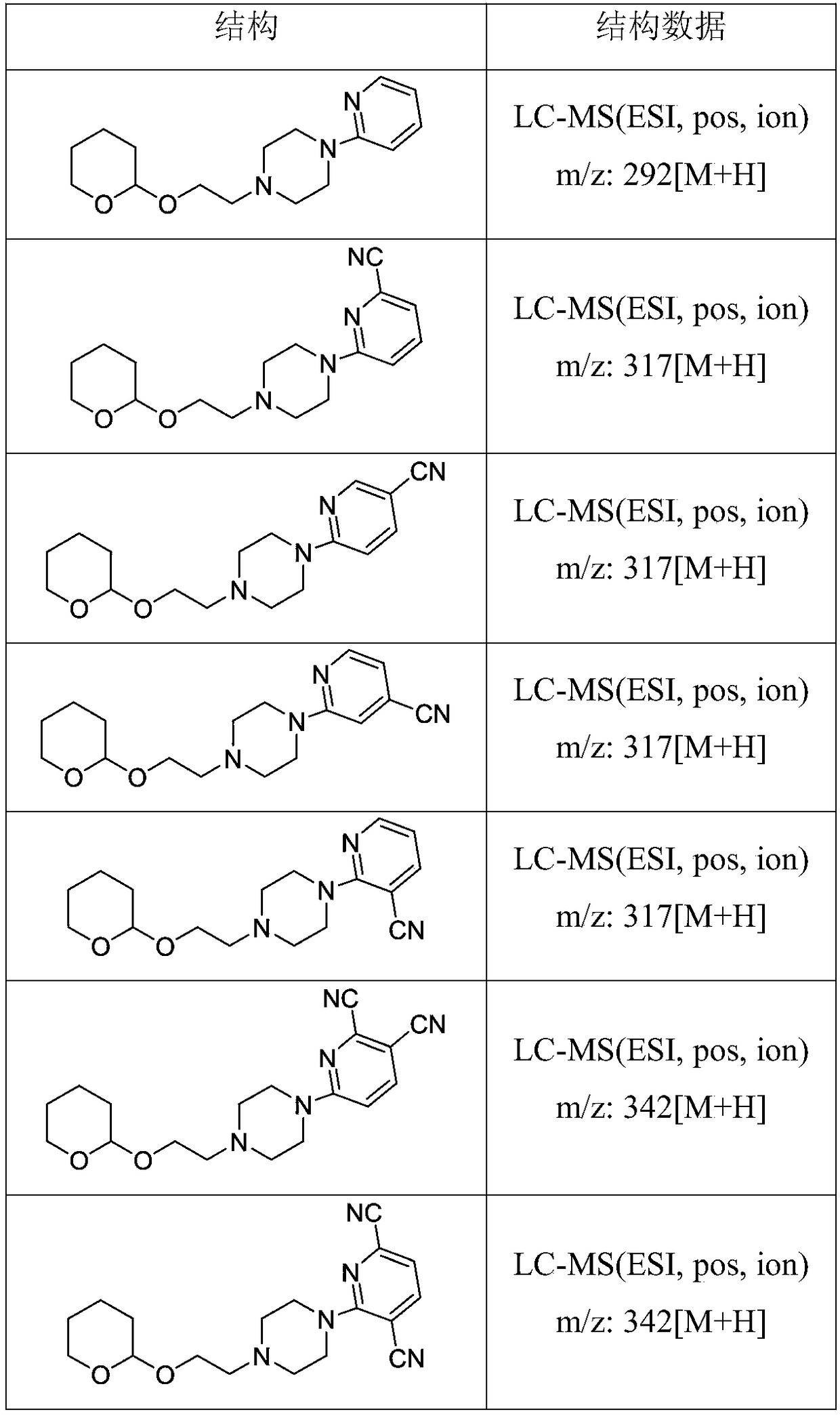
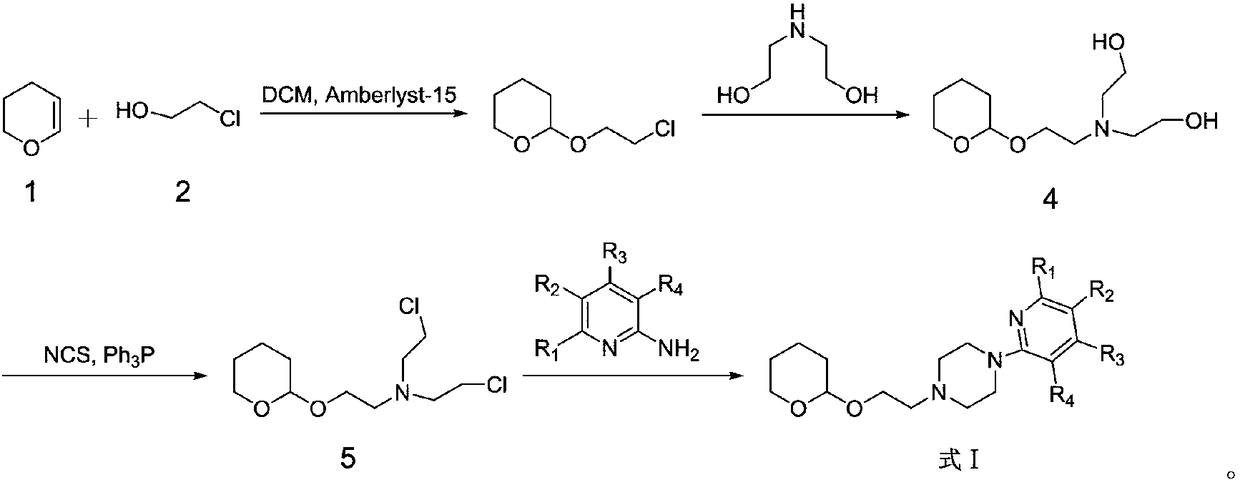
Derivative containing nitrogen heterocyclic rings and application of derivative in retinal neovascularization diseases
The invention discloses a derivative containing nitrogen heterocyclic rings and application of the derivative in retinal neovascularization diseases. The derivative has the biological structural formula shown in the description, wherein R1, R2, R3 and R4 are respectively and independently selected from -H or -CN. Under the same concentration of 5microg / mL, the growth inhibition ratios of the embodiment II, the embodiment III and the embodiment IV to HRCEC are superior to a monoclonal antibody drug Avastin. By injecting the compound into a vitreous body, the formation of pathologic new vesselscan be reduced, so that the derivative of the formula I or the salt of the derivative can be used for deeply researching and developing retinal neovascularization relevant diseases such as retinal vein occlusion, proliferative diabetic retinopathy, subretinal neovascular membrane, neovascular glaucoma, retinopathy of prematurity and proliferative vitreoretinopathy.
Owner:翟学旭
Calcium blockers to treat proliferative vitreoretinopathy
InactiveUS7230032B2Reduce and preventAvoid damageBiocideSenses disorderOphthalmologyProliferative vitreoretinopathy
Glutamate causes migration and proliferation of retinal pigment epithelium and / or glial cells, and glutamate antagonists can prevent, treat or reduce retinal pigment epithelium and / or glial migration and the subsequent development of proliferative vitreoretinopathy. Avoidance or management of proliferative vitreoretinopathy can be achieved by administering to the patient a compound capable of reducing glutamate-induced retinal cell migration in a concentration effective to reduce such migration.
Owner:ALLERGAN INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com