Treatments for proliferative vitreoretinopathy
A vitreoretinal and proliferative technology, applied in sensory diseases, organic active ingredients, medical preparations containing active ingredients, etc., can solve problems such as the unknown role of TAK1
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0091] Example 1: TGF-β1 activates TAK1MAP kinase in RPE cells
[0092] TAK1 is a known mediator of TGF-β1 signaling, but was not detected (inactive or active form) in RPE cells. This example shows that TAK1 is expressed and activated in RPE cells.
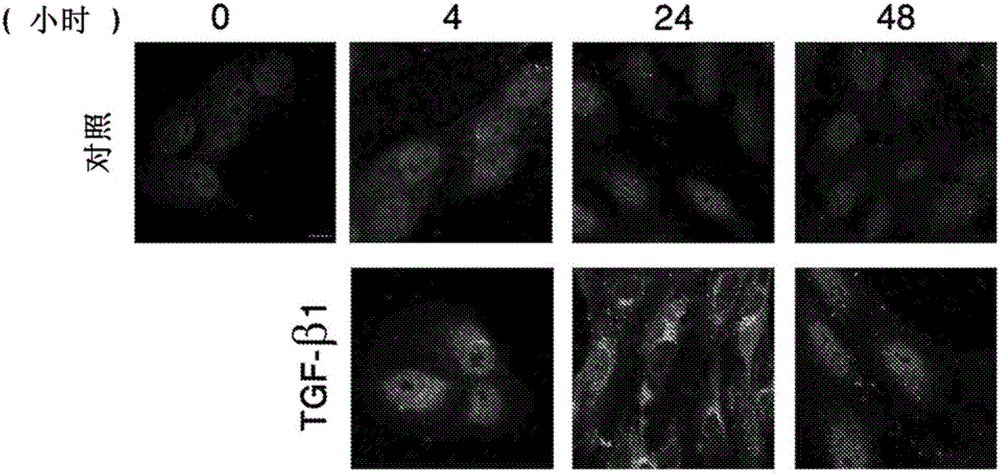
[0093] To study the kinetics and expression pattern of activated TAK1 in RPE cells upon TGF-β1 treatment, RPE cells were immunostained with a phospho-TAK1 antibody that specifically recognizes the activated form of TAK1 phosphorylated on Thr187 . Such as figure 1 As shown, TAK1 activation, which can be observed in non-nuclear staining, gradually increases in treated cells, peaks at 24 hours after treatment and then decreases. In contrast, control cells showed only critical levels of activated TAK1 without significant changes, thus demonstrating specific activation of TAK1 by TGF-β1 in RPE cells.
[0094] Abnormal expression and activity of TAK1 in diseased retina and RPE cells Figure 6A , Figure 6B (retina), Figure 7A an...
Embodiment 2
[0095] Example 2: Inhibition of TAK1 in RPE cells stops the EMT process under TGF-β1 stimulation
[0096] EMT is known to be mediated by various factors. This example shows that TGF-β1-stimulated EMT that occurs in RPE can be mediated by activated TAK1.
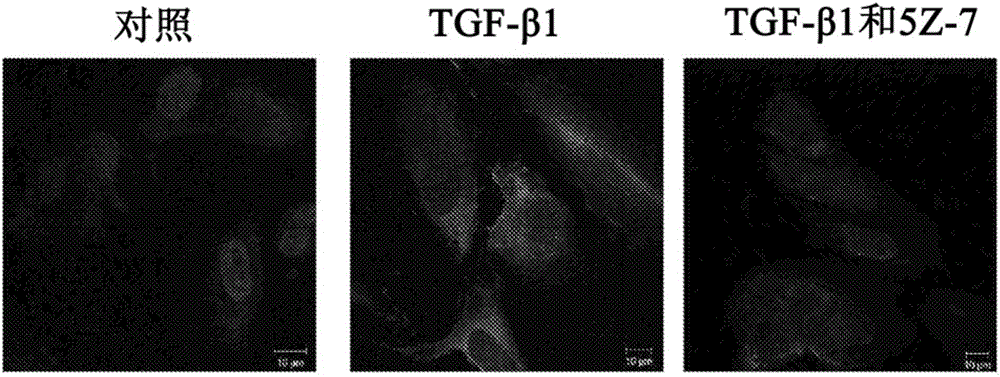
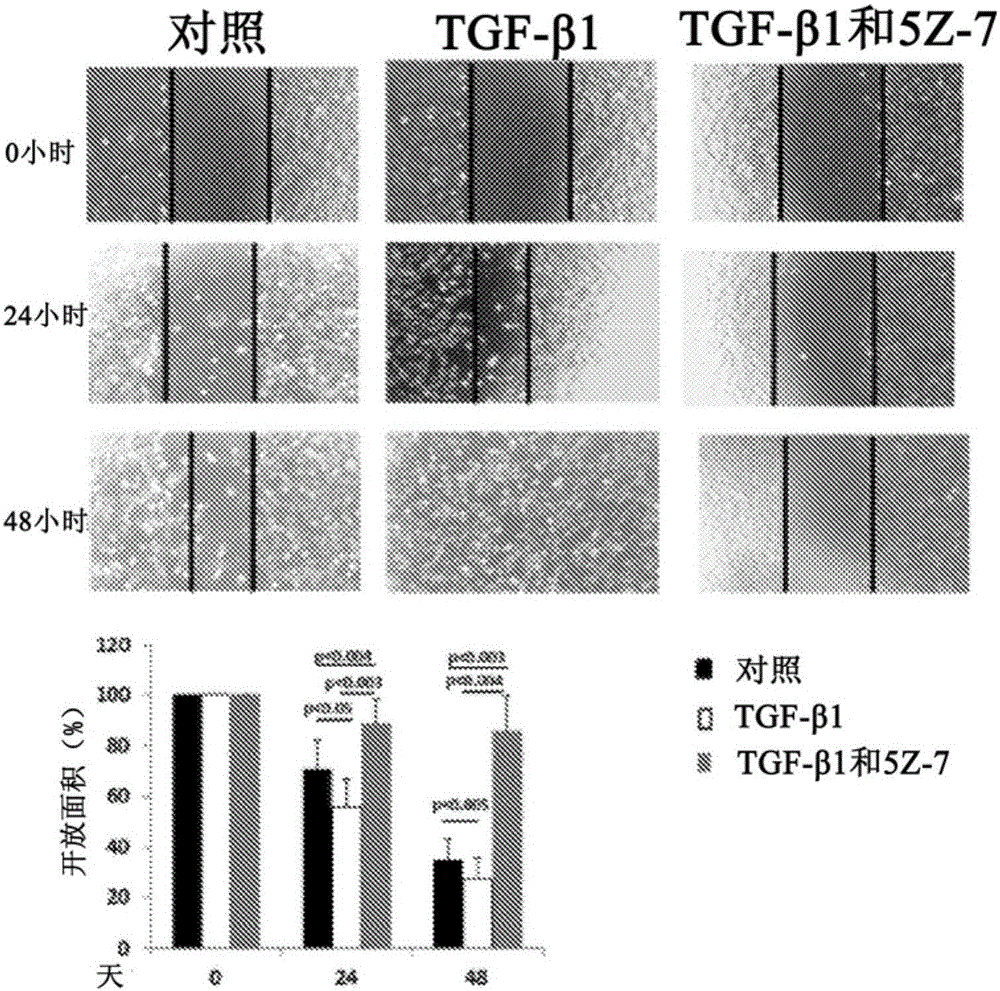
[0097] EMT of RPE cells is known to be mediated through TGF-β1 and is characterized by increased expression of α-SMA, enhanced cell migration and secretion of MMPs. To address the possible involvement of TAK1 in the differentiation and transformation of RPE cells induced by TGF-β1, the effect of specific TAK1 inhibitors on this process was investigated. The key step in the differentiation of RPE cells into myofibroblasts is the de novo synthesis of α-SMA (Hinz et al., AmJ Pathol 2001, 159:1009-1020). In order to observe this process in vitro, RPE cells were stimulated with TGF-β1 for 2 days in the presence or absence of TAK1 inhibitor (5Z-7-oxozeaenol) under serum-free conditions, or with DMSO as a control group ( Figure ...
Embodiment 3
[0103] Example 3: TAK1 regulates TGF-β signal transduction in RPE cells
[0104] The results shown in Example 2 indicate that inhibition of TAK1 reduces α-SMA expression and cell migration. These processes are known to be regulated by TGF-β1 signaling through Smad2 / 3 in RPE cells (MuY et al., Cell Tissue Res 2011, 347:11-20). This example explores whether TAK1 inhibition has an effect on the phosphorylation of Smad2 / 3.
[0105] RPE cells were treated with or without 5Z-7-oxozeaenol, followed by TGF-β1, and the phosphorylation pattern of Smad2 / 3 was examined by immunoblotting. Phospho-Smad2 / 3 was observed to increase immediately and reach maximum activation at 60 minutes in TGF-β1-treated cells ( Figure 4A ). In contrast, Smad2 / 3 activation was barely detectable in cells pretreated with 5Z-7-oxozeaenol. Calculation of the ratio of phosphorylated-Smad2 / 3 to total Smad2 / 3 at each time point clearly demonstrated that inhibition of TAK1 activity abolished Smad2 / 3 activation in...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com