Application of circRNA in the preparation of diagnostic reagents for proliferative vitreoretinopathy
A vitreoretinal and diagnostic reagent technology, applied in the field of medical biological detection, can solve the problem of no early diagnostic markers for circRNA proliferative vitreoretinopathy, and achieve the effect of less trauma and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
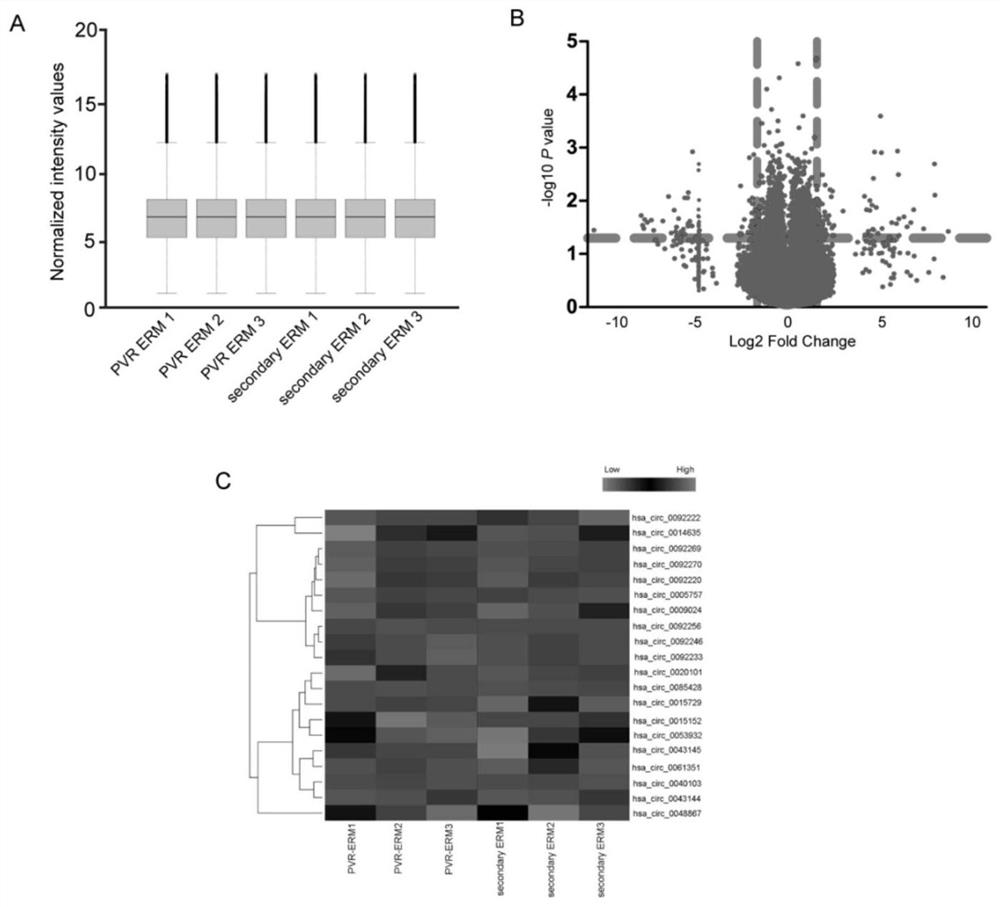
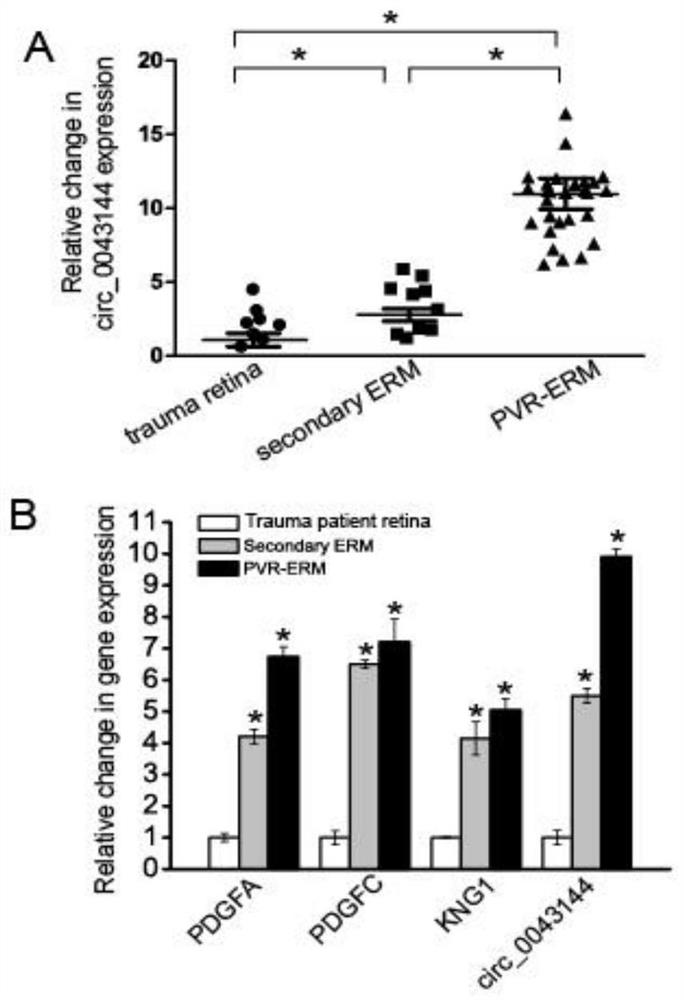
[0053] Example 1 Verification of the correlation between circ_0043144 and proliferative vitreoretinopathy
[0054] Step 1: Sample preparation: Epiretinal membrane specimens (experimental group, n=30) and cataract proliferative membrane specimens (control group, n=30) after ophthalmic vitreous surgery, total RNA was extracted with TRIzol (Invitrogen) reagent, and stored Store at -80°C for later use.
[0055] Step 2: Differential expression screening:
[0056] The expression profiling chip of Agilent Company in the United States was used to analyze the circRNA related to the occurrence of PVR disease; the specific steps of analysis were as follows: the experimental sample RNA was used Agilent expression profiling chip supporting kit, Low Input Quick Amp WTLabeling Kit (Cat.# 5190-2943) and standard The operating procedure is to amplify and label the total RNA of the sample, and use the RNeasy mini kit (Cat.# 74106, QIAGEN) to purify the labeled cRNA; follow the hybridization st...
Embodiment 2
[0060] Embodiment 2: preparation kit of the present invention
[0061] The sequence of circ_0043144 is shown in SEQ ID:NO:1. The upstream and downstream primers for specific quantitative PCR were synthesized by Shanghai Sangon Co., Ltd., with a purity of PAGE grade. The synthesized primers were dissolved in DEPC H2O with a total concentration of 10 μM. The internal reference primer was 18 S rRNA.
[0062] Prepare a kit comprising the following components:
[0063] (a) Extraction system:
[0064] 1) Trizol reagent, 1 tube, 5000 μL / tube;
[0065] 2) Chloroform, 1 tube, 1000 μL / tube;
[0066] 3) Absolute ethanol, 1 tube, 10000 μL / tube;
[0067] 4) DEPC ddH 2 O, 1 tube, 10000 μL / tube;
[0068] 5)ddH 2 O, 1 tube, 10000 μL / tube;
[0069] 6) Isopropanol, 10000 μL / tube;
[0070] (b) Reverse transcription system:
[0071] 1) Total RNA reverse transcription primer (Random 6 mers), 1 tube, concentration: 50 μM, 100 μL / tube;
[0072] 2) Reverse transcriptase (200 U / μL) 100 μL; ...
Embodiment 3
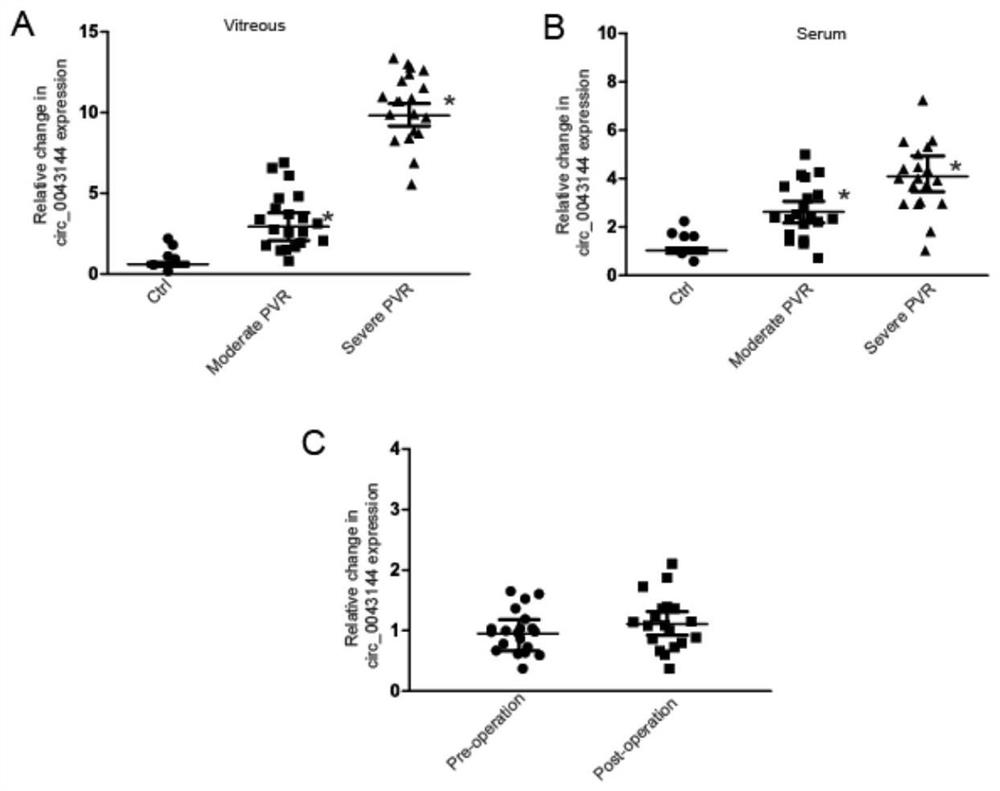
[0083] Embodiment 3: kit detection of the present invention
[0084] 1. Separation of serum
[0085] The blood samples of the examined individual and the healthy individual used as a reference are collected, and the serum and blood cells are separated from the heparin anticoagulated tube by centrifugation for the detection of the disease. The centrifugation conditions were 4°C, 12,000 rpm, 10 min.
[0086] 2. RNA Extraction of Serum and Vitreous Samples
[0087]Using the total RNA extraction system described in the present invention, add TRIzol and place it at room temperature for 10 minutes to fully lyse the sample (Note: If the next step is not performed, the sample can be stored at -80°C for a long time). Add 200 μl of chloroform to every 1 ml of TRIzol, vibrate vigorously and mix well, then place at room temperature for 3-5 min to allow natural phase separation. Centrifuge at 12,000 rpm for 15 min at 4°C. The sample will separate into three layers: a yellow organic pha...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com