Applications of Chalcomoracin and homologues thereof in preparation of drugs for treatment of proliferative vitreoretinopathy
A vitreoretinal and homologous technology, applied in the field of biomedicine, can solve problems such as the influence of PVR that has not been studied, and achieve the effect of good development potential
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
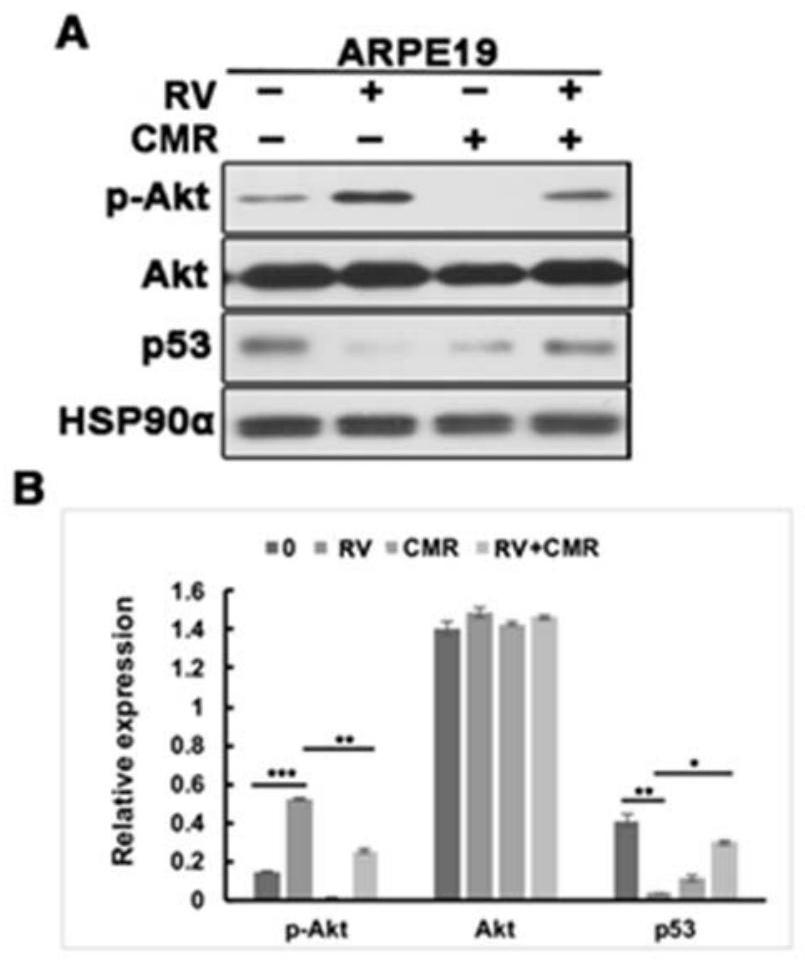
[0024] Example 1 Chalcomoracin inhibits vitreous-induced AKT activation and p53 decline
[0025] After recovery, ARPE-19 cells were placed in a 24-well plate of DMEM / F12 medium containing 10% fetal bovine serum, at 37°C, 5% CO 2 cultured in an incubator. After the cells were fused to 90%, serum-starved culture was performed for 24 hours, and then the drug (PVR rabbit vitreous body (RV, diluted 1:3 with DMEM / F12 medium), medium containing RV and 5 μM Chalcomoracin (CMR) was continuously treated for 8 hours. Wash twice with pre-cooled phosphate-buffered saline (PBS), add the lysate and let stand on ice for 15min, collect the lysate, centrifuge at 12000r / m, 4°C for 15min. After BCA is quantified, add a certain volume of loading buffer and boil Protein samples were obtained in 15min. Separation was carried out by 10% SDS-polyacrylamide gel electrophoresis. Then the proteins in the gel were transferred to polyvinylidene fluoride membranes, and Western blot analysis was carried out...
Embodiment 2
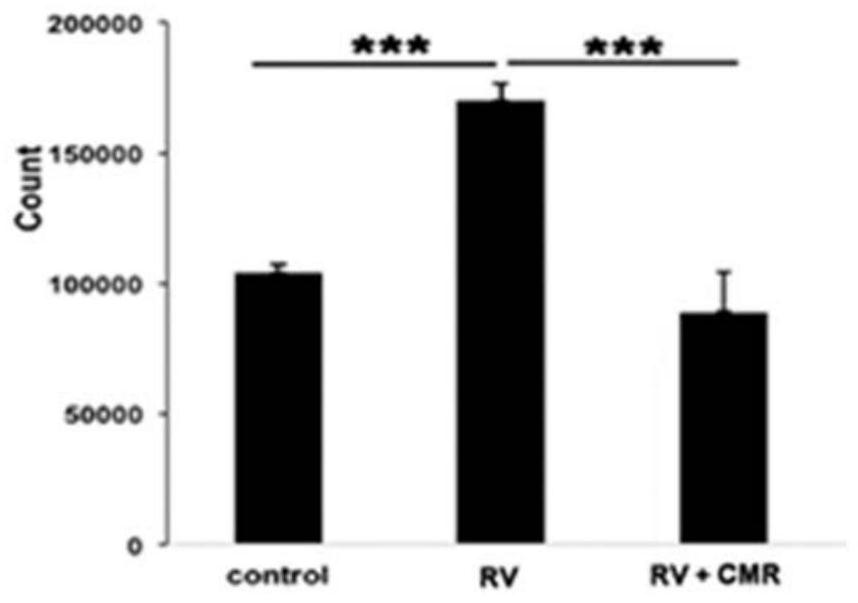
[0027] Example 2 Cell Proliferation Experiment
[0028] After recovery, ARPE-19 cells were placed in a 24-well plate of DMEM / F12 medium containing 10% fetal bovine serum, at 37°C, 5% CO 2 cultured in an incubator. After ARPE-19 reached about 90% confluency, they were digested with 0.5% Tripsin-EDTA and counted at 3 × 10 4 The density of the cells / well was seeded in the wells of a 24-well plate and placed in DMEM / F12 medium containing 10% fetal bovine serum. After the cells were attached to the plate, they were treated with 0.5ml DMEM(-) or RV supplemented with 5μM Chalcomoracin After 48 hours, the cells were digested with trypsin, the blood cell counting method was used to count the cells under an optical microscope, and the results of three experiments were taken for data processing. RV stimulated the proliferation of ARPE-19 (1.6±0.2 fold), while Chalcomoracin completely abolished RV-mediated cell proliferation, see figure 2 .
Embodiment 3
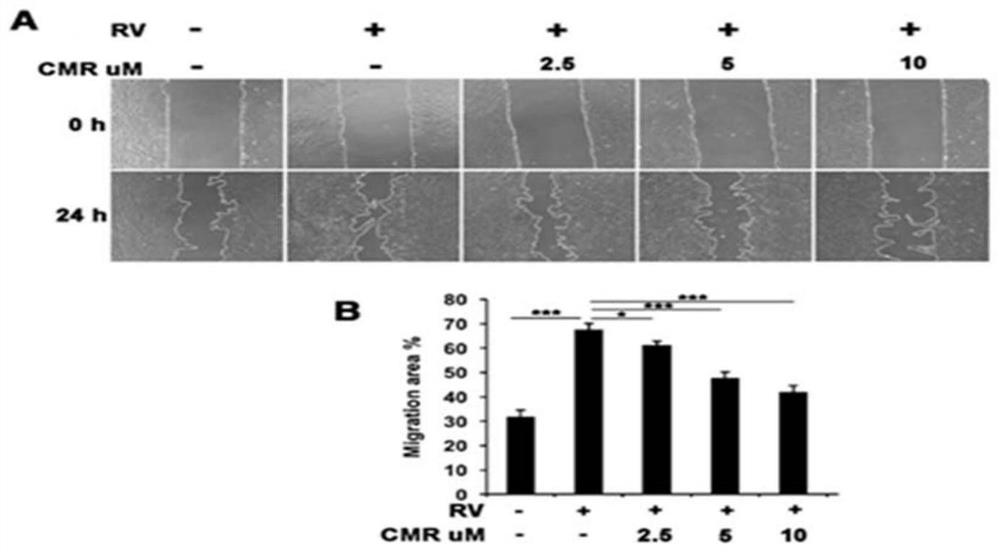
[0029] Example 3 Cell Migration Experiment
[0030] After recovery, ARPE-19 cells were placed in a 24-well plate of DMEM / F12 medium containing 10% fetal bovine serum, and incubated at 37°C and 5% CO 2 cultured in an incubator. When ARPE-19 grows to complete confluence, scrape a "one" scratch with an autoclaved 200 μl pipette tip. Wash 3 times with PBS, take pictures of the initial scratch width, and then treat with RV (diluted 1:3 in DMEM / F12) containing 0, 2.5, 5, 10 μM Chalcomoracin respectively, and take another scratch after 24 hours, and then use Image J and Adobe Photoshop CS4 software were used to analyze the data, and the results of three experiments were taken for data processing. Scratch healing test results see image 3 , indicating that RV stimulated the migration of ARPE-19 ( image 3 A), while Chalcomoracin significantly inhibited RV-induced cell migration in a dose-dependent manner ( image 3 B).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com