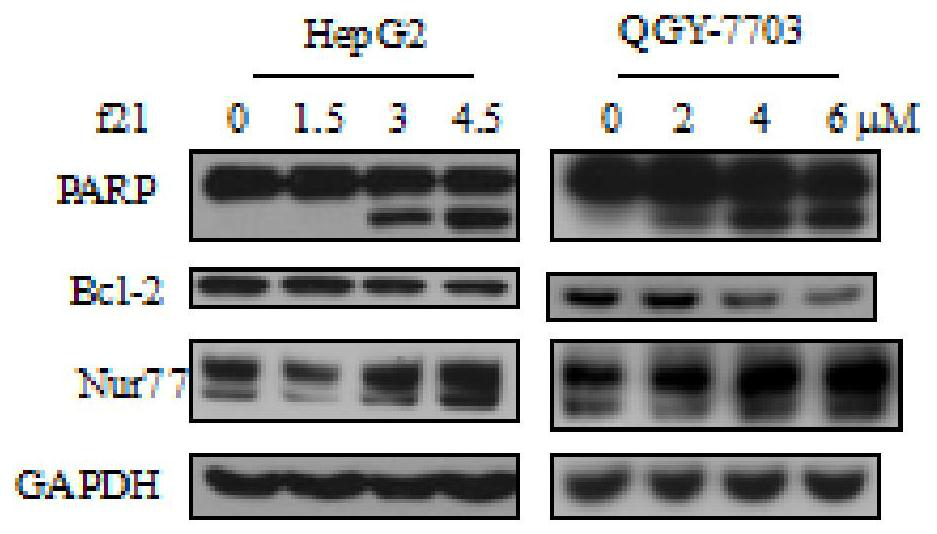
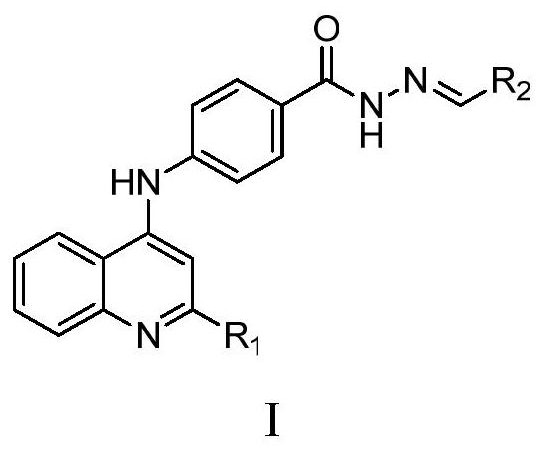
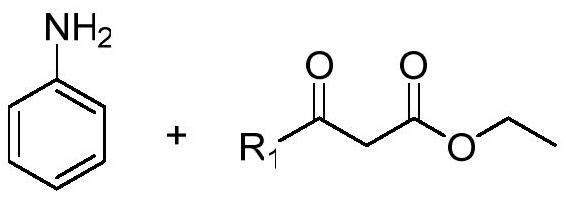
A class of 4-((2-substituted quinolin-4-yl) amino) benzoylhydrazine derivatives and its preparation method and application
A technology for benzoylhydrazide and derivatives, which is applied in the field of 4-amino)benzohydrazide derivatives and their preparation, can solve problems such as toxic and side effects, and achieve the effects of low cost, high yield, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Example 1: Preparation of N'-((naphthalene-2-yl)methylene)-4-((quinolin-4-yl)amino)phenylhydrazine hydrazide:
[0048]
[0049] Preparation of ethyl 4-((quinolin-4-yl)amino)benzoate:
[0050]
[0051] Add 4-hydroxyquinoline (2.90g, 0.020mol) and phosphorus oxychloride (30mL) into a dry and sealed 100mL reaction bottle, stir the reaction, seal and heat to 120°C to continue the reaction for 3h, TLC detects that the reaction is complete, stop reaction. The reaction solution was cooled to room temperature, concentrated under reduced pressure to remove the solvent, added 50 mL of ice water, decolorized with activated carbon, filtered with suction, collected the filtrate, adjusted the pH to 8-9 with 10% NaOH in an ice bath, and distilled to obtain a colorless liquid 4-chloroquinoline 2.80g, yield 87%.
[0052] In a dry 100mL round bottom flask, add 4-chloroquinoline (1.60g, 0.010mol), ethyl p-aminobenzoate (1.70g, 0.010mol), n-butanol (35mL), 2 drops of concentrated h...
Embodiment 2
[0057] Example 2: 4-((quinolin-4-yl)amino)-N'-((2,3,4-trimethoxyphenyl)methylene)benzohydrazide:
[0058]
[0059] The reaction steps are the same as those in Example 1, except that 2-naphthaldehyde is replaced by 2,3,4-trimethoxybenzaldehyde (21.6mg, 0.11mmol), and finally 24.6mg of a light yellow solid product is obtained by isolation, with a yield of 54% . Spectral data: 1 H NMR (600MHz, DMSO-d6): 11.96(s, 1H), 10.96(br.s., 1H), 8.73(d, J=8.4Hz, 1H), 8.68(s, 1H), 8.64(d, J=6.8Hz, 1H), 8.13(d, J=8.6Hz, 2H), 8.04~8.07(m, 2H), 7.85(ddd, J=2.3, 6.0, 8.4Hz, 1H), 7.61~7.69(m ,3H),7.04(d,J=7.0Hz,1H),6.96(d,J=9.0Hz,1H),3.87(d,J=3.95Hz,6H),3.79(s,3H); 13 C NMR (151MHz, DMSO-d6): 162.4, 159.1, 158.8, 158.6, 155.7, 154.8, 153.1, 144.0, 143.9, 142.0, 140.9, 139.0, 134.5, 132.2, 129.8, 127.8, 125.0, 121.10.9, 12 120.8, 118.0, 116.7, 109.2, 101.0, 62.3, 61.0, 56.5; ESI-MS(+): [M+H] + ,457.5.
Embodiment 3
[0060] Example 3: N'-((4-hydroxy-3-methoxyphenyl)methylene)-4-((quinolin-4-yl)amino)benzohydrazide:
[0061]
[0062] The reaction steps were the same as in Example 1, except that 2-naphthaldehyde was replaced by 4-hydroxy-3 methoxybenzaldehyde (16.7mg, 0.11mmol), and finally 23.0mg of the yellow-white solid product was isolated with a yield of 56%. Spectral data: 1 H NMR (600MHz, DMSO-d 6 ):11.88(s,1H),10.99(br.s.,1H),9.70(br.s.,1H),8.74(d,J=8.4Hz,1H),8.64(d,J=7.0Hz, 1H), 8.39(s, 1H), 8.13(d, J=8.4Hz, 2H), 8.05~8.08(m, 2H), 7.86(ddd, J=2.3, 5.9, 8.3Hz, 1H), 7.67(d ,J=8.4Hz,2H),7.36(d,J=1.5Hz,1H),7.11(dd,J=1.7,8.1Hz,1H),7.04(d,J=7.0Hz,1H),6.9(d ,J=8.07Hz,1H),3.85(s,3H);ESI-MS(+):[M+H] + , 413.4.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com