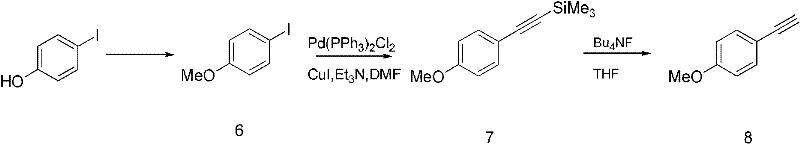Resveratrol dimer derivative and preparation and application methods thereof
A technology of resveratrol and dimer, applied in the field of medical engineering
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
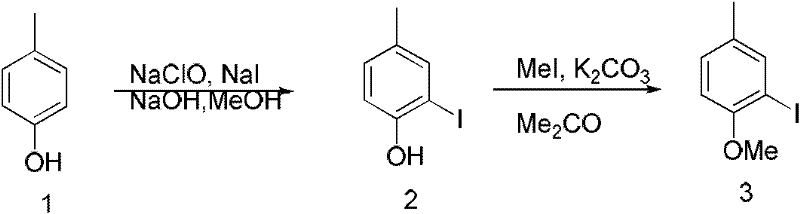
[0079] Preparation of 4-methyl o-iodoanisole:
[0080] Such as figure 1 As indicated, p-cresol (2 g, 18.5 mmol) was dissolved in methanol, and sodium iodide (2.77 g, 18.5 mmol) and sodium hydroxide (0.74 g, 18.5 mmol) were added. The suspension was cooled to 0° C., 30 ml of 5% sodium hypochlorite solution was added dropwise to the suspension, and reacted for 5 hours. After the reaction was completed, the reaction was quenched with sodium thiosulfate, extracted with ether, the organic phase was washed with saturated brine, dried over anhydrous sodium sulfate, filtered and evaporated to dryness to obtain a light yellow solid, which was separated on a silica gel column to obtain a light yellow oily liquid (740 mg, 17%), the oily liquid (740mg, 3.16mmol) was further selected to be dissolved in acetone, potassium carbonate (874mg, 6.32mmol) was added, and stirred. Under the protection of nitrogen, iodomethane (672mg, 4.74mmol) was added, after the dropwise completion, the tempera...
Embodiment 2
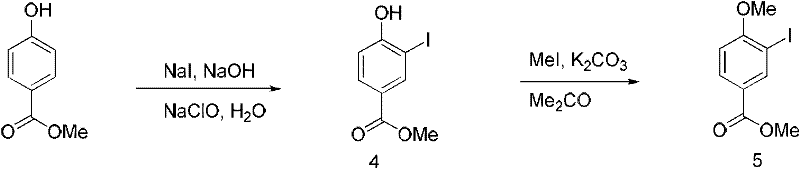
[0082] Preparation of methyl 3-iodo-5-methoxybenzoate:
[0083] Such as figure 2 As shown, with reference to Example 1, methyl p-hydroxybenzoate (5g, 32.8mmol) was iodized and methyl etherified to obtain methyl 3-iodo-5-methoxybenzoate (4.32g, 4.32g, 45%).
Embodiment 3
[0085] Preparation of 4-methoxyphenylacetylene:
[0086] Such as image 3 As indicated, 4-hydroxyiodobenzene (1 g, 4.5 mmol) was dissolved in acetone, potassium carbonate (1.245 g, 9 mmol) was added, and stirred. Under the protection of nitrogen, iodomethane (765mg, 5.4mmol) was added, after the dropwise completion, the temperature was raised to 68°C and refluxed. After reacting for 3 hours, evaporate acetone to dryness, extract with ethyl acetate, wash the organic phase with saturated brine, dry over anhydrous sodium sulfate, filter and evaporate to dryness to obtain a white solid, 4-methoxyiodobenzene (1.05g, 100%)
[0087] Dissolve 4-methoxyiodobenzene (1.05g, 4.5mmol) in triethylamine, add palladium chloride triphenylphosphine (63mg, 0.09mmol) and cuprous iodide (34mg, 0.18mmol), add under nitrogen Trimethylsilylacetylene (530 mg, 5.4 mmol), stirred overnight. After the reaction, triethylamine and excess trimethylsilylacetylene were distilled off to obtain a brown solid. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com