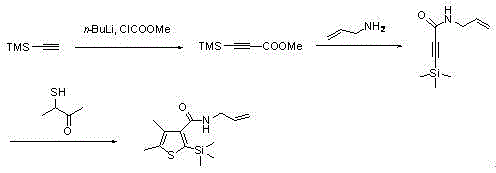Patents
Literature
63 results about "Trimethylsilylacetylene" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
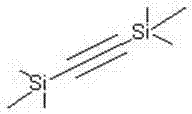
Trimethylsilylacetylene is the acetylene HC₂Si(CH₃)₃. It is a colorless liquid. Called "tms acetylene", it is used as a source of "HC₂⁻". The trimethylsilyl group protected can be desilyated with TBAF. Using this protected alkyne, as opposed to acetylene itself, prevents further coupling reactions and also has the benefit of being a liquid. A less expensive alternative reagent is 2-methylbut-3-yn-2-ol, which after alkynylation is deprotected with base.
Bifunctional 4-TMS-5-I-1,2,3-triazole compound as well as preparation method and application thereof
InactiveCN104592281AWide variety of sourcesEasy to makeGroup 4/14 element organic compoundsPtru catalystIodide
The invention discloses a bifunctional 4-TMS-5-I-1,2,3-triazole compound as well as a preparation method and application thereof. According to the technical scheme, the bifunctional 4-TMS-5-I-1,2,3-triazole compound is characterized by being prepared at normal temperature, wherein trimethyl silyl acetylene and nitrine serve as raw materials, acetonitrile serves as a solvent, cuprous iodide serves as a catalyst, N-chlorosuccinimide serve as an oxidant, and N-di(isopropyl)ethylamine serves as an alkali. The bifunctional 4-TMS-5-I-1,2,3-triazole compound has a structural formula as shown in the specification, wherein R is as shown in the specification. The invention further discloses a preparation method of the bifunctional 4-TMS-5-I-1,2,3-triazole compound and a method of further synthesizing a 1,5-di-substituted-1,2,3-triazole compound from the bifunctional 4-TMS-5-I-1,2,3-triazole compound which serves as an intermediate. The raw materials are wide in source, are easy to prepare and are low in cost, and the cuprous iodide adopted by reaction serves as the catalyst and the reactant, is very low in cost, is simple and is easy to obtain.
Owner:HENAN NORMAL UNIV
Luminescent probe with visual recognition effect for benzene steam as well as preparation method and application of luminescent probe
InactiveCN104194769AEasy to detectHigh sensitivityFluorescence/phosphorescenceTenebresent compositionsBenzene vaporChlorobenzene
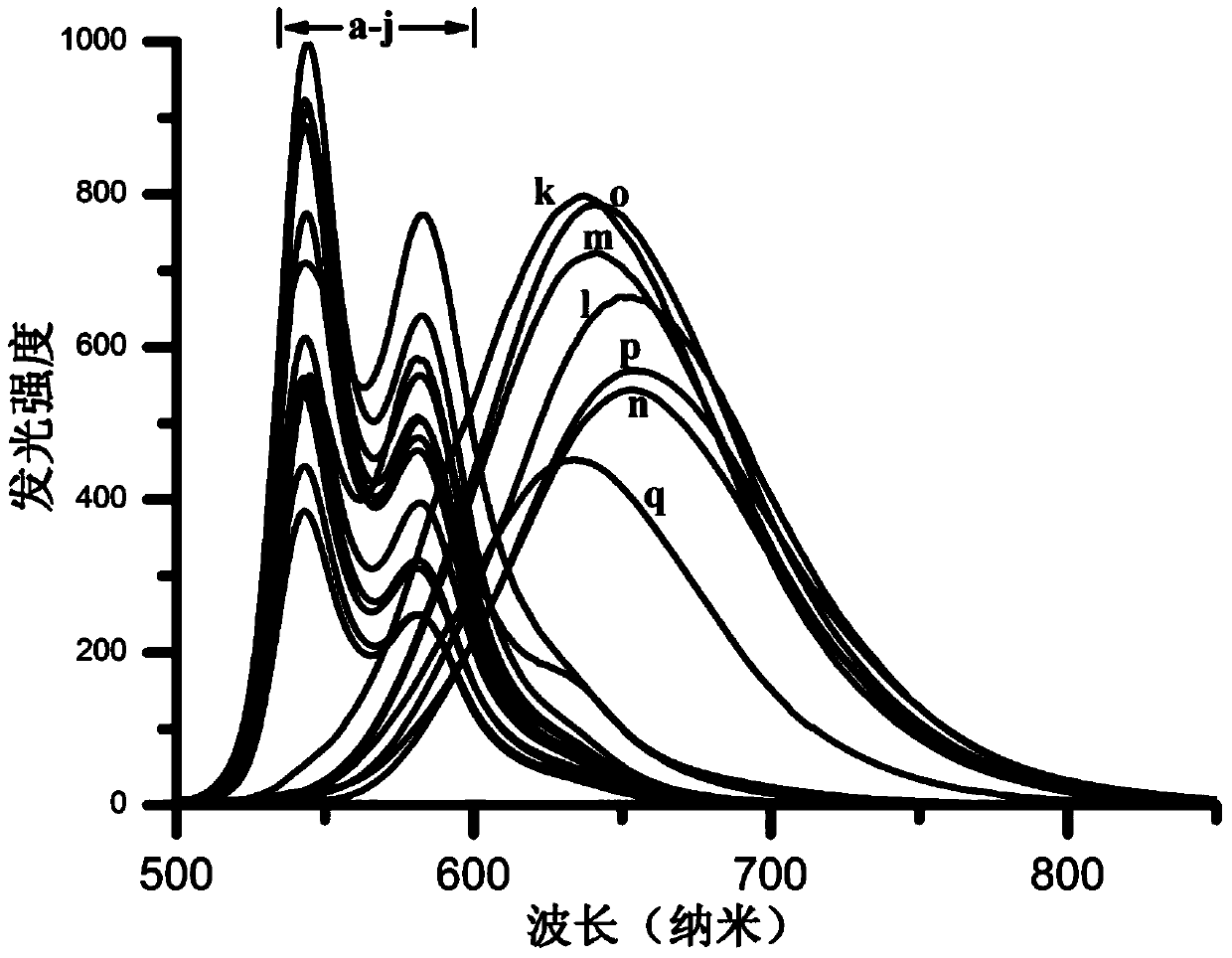
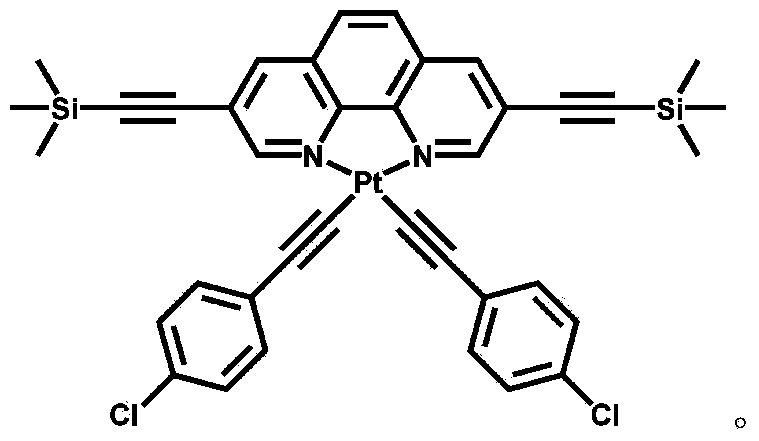
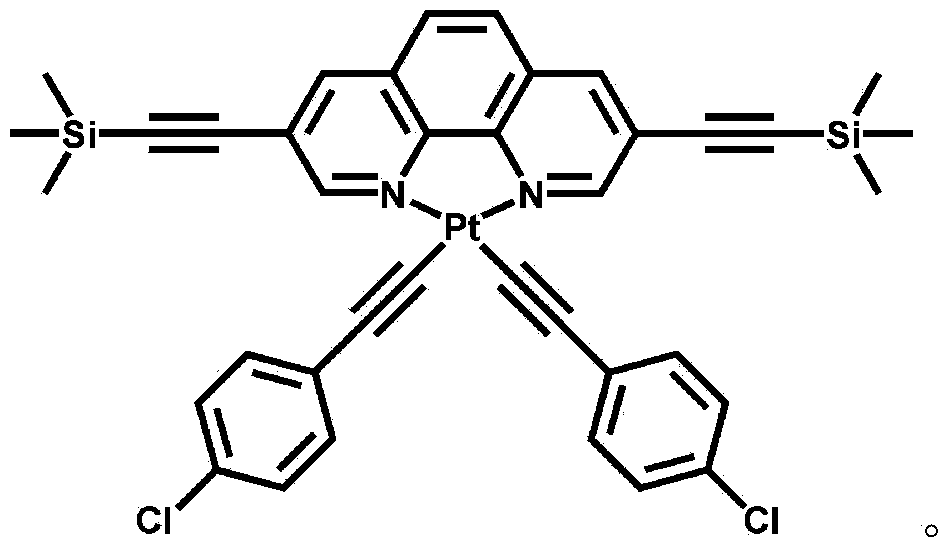
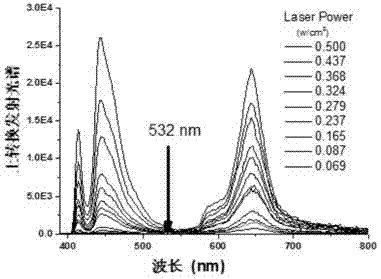
The invention discloses a luminescent probe with a visual recognition effect for benzene steam as well as a preparation method and an application of the luminescent probe. The probe is a complex prepared from platinum (II), a 3, 8-bi(trimethylsilylacetylene)-1, 10-phenanthroline ligand and an auxiliary 4-chlorophenylacetylene ligand. When the probe is not in contact with benzene vapor, emission peaks of the probe are located at the wavelength positions of 543nm and 581nm under the excitation of light with the wavelength of 250-500nm, and the light is yellowish green. When the probe is in contact with the benzene vapor, an emission spectrum is subjected to bathochromic shift to a position near the wavelength of 630-660nm, and the color of the emitted light is remarkably changed from yellowish green to bright red. The probe disclosed by the invention not only has high sensitivity and high selectivity for the benzene vapor, but also has the advantages of high analysis speed, good repeatability, visualization, low detecting cost and simple detecting method so as to have a high application value in the field of environment detection. The probe has good reversibility, so that the performance of the probe is not influenced after the probe is repeatedly used.
Owner:DALIAN UNIV OF TECH
3,4-ethylenedioxythiophene polymer capable of being super-assembled with carbon-based material and preparation method thereof
The invention belongs to the technical field of organic supermolecules and specifically relates to a 3,4-ethylenedioxythiophene polymer which can be super-assembled with a carbon-based material and a preparation method thereof. The polymer of the invention is a high molecular polymer with 3,4-ethylenedioxythiophene-2-acetylene as a main chain and an alkoxy group as a side chain. The preparation method comprises the following steps: 3,4-ethylenedioxythiophene, which is used as a starting material, is subjected to a bromination reaction to obtain 2,5-dibromo-3,4-vinyldioxythiophene; then, 2,5-dibromo-3,4-vinyldioxythiophene reacts with trimethylsilylacetylene to obtain a bistrimethylsilyl3,4-vinyldioxythiophene intermediate; after removal of theTMS protecting group, a Sonogashira reaction between the above reaction product and 2,5-dibromo-3,4-vinyldioxythiophene is carried out to obtain the 3,4-ethylenedioxythiophene polymer. The polymer and carbon nanotubes can form a super-assembly system with main chain Pi-Pi adsorption and side chain winding. The 3,4-vinyldioxythiophene polymer / carbon nanotube composite formed by the super-assembly system has good stability and has a broad application prospect in composite materials.
Owner:FUDAN UNIV
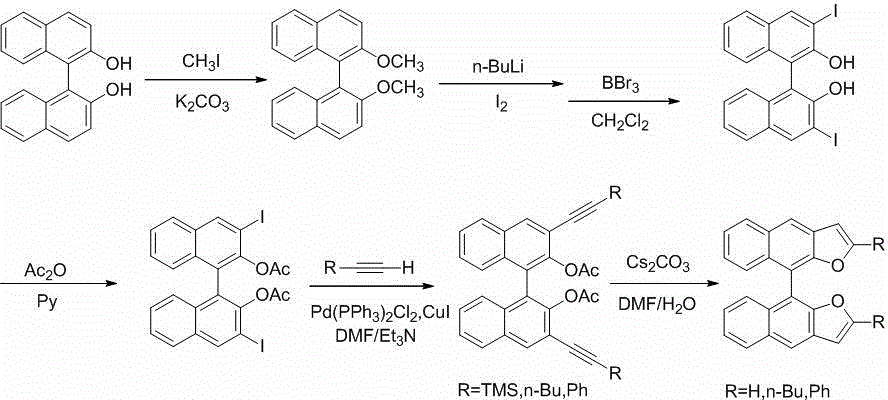
(S)-/(R)-difurodinaphthalene as well as derivatives thereof and preparation method
The invention discloses (S)- / (R)-difurodinaphthalene as well as derivatives thereof and a preparation method. The compounds have two spatial configurations which are (S)- / (R)-, and in the structure, the site 2 of furan has substituent groups which are hydrogen, and linear or branched alkyl groups or phenyl groups of C4 or below. The preparation method comprises the following steps of: reacting (S)- / (R)-binaphthol as a raw material and potassium carbonate as alkali with iodomethane in acetone so as to obtain (S)- / (R)-2, 2'-dimethoxy-1, 1'-dinaphthalene; then carrying out diiodo-reaction of a product obtained in the previous step, removing protecting groups of phenolic hydroxyl groups, and esterifying with acetic anhydride, thus obtaining (S)- / (R)-3, 3'-diiodo-2, 2'-diacetoxy-1, 1'-dinaphthalene; carrying out Sonogashira coupled reaction of (S)- / (R)-3, 3'-diiodo-2, 2'-diacetoxy-1, 1'-dinaphthalene with alkyne under the catalysis action of palladium to obtain a series of (S)- / (R)-3, 3'-di(trimethylsilylacetylene, hexynyl or phenyl ethynyl)-2, 2'-diacetoxy-1, 1'- dinaphthalene; and finally under the action of cesium carbonate, carrying out ring closing reaction to obtain the (S)- / (R)-double furodinaphthalene and derivatives thereof. A series of compounds provided by the invention broaden the range of organic electroluminescence materials.
Owner:EAST CHINA NORMAL UNIV
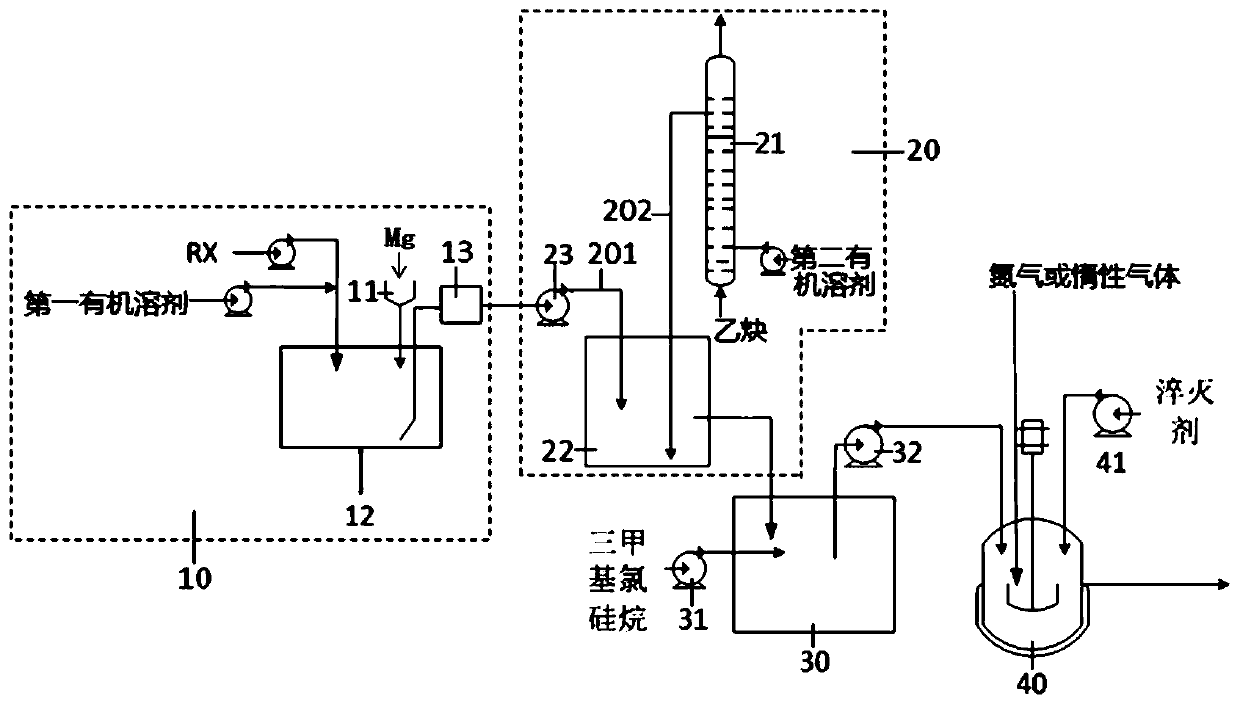
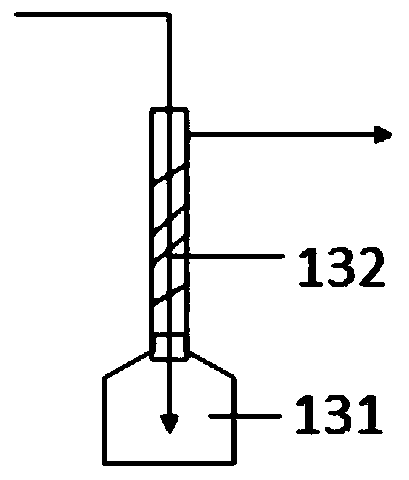
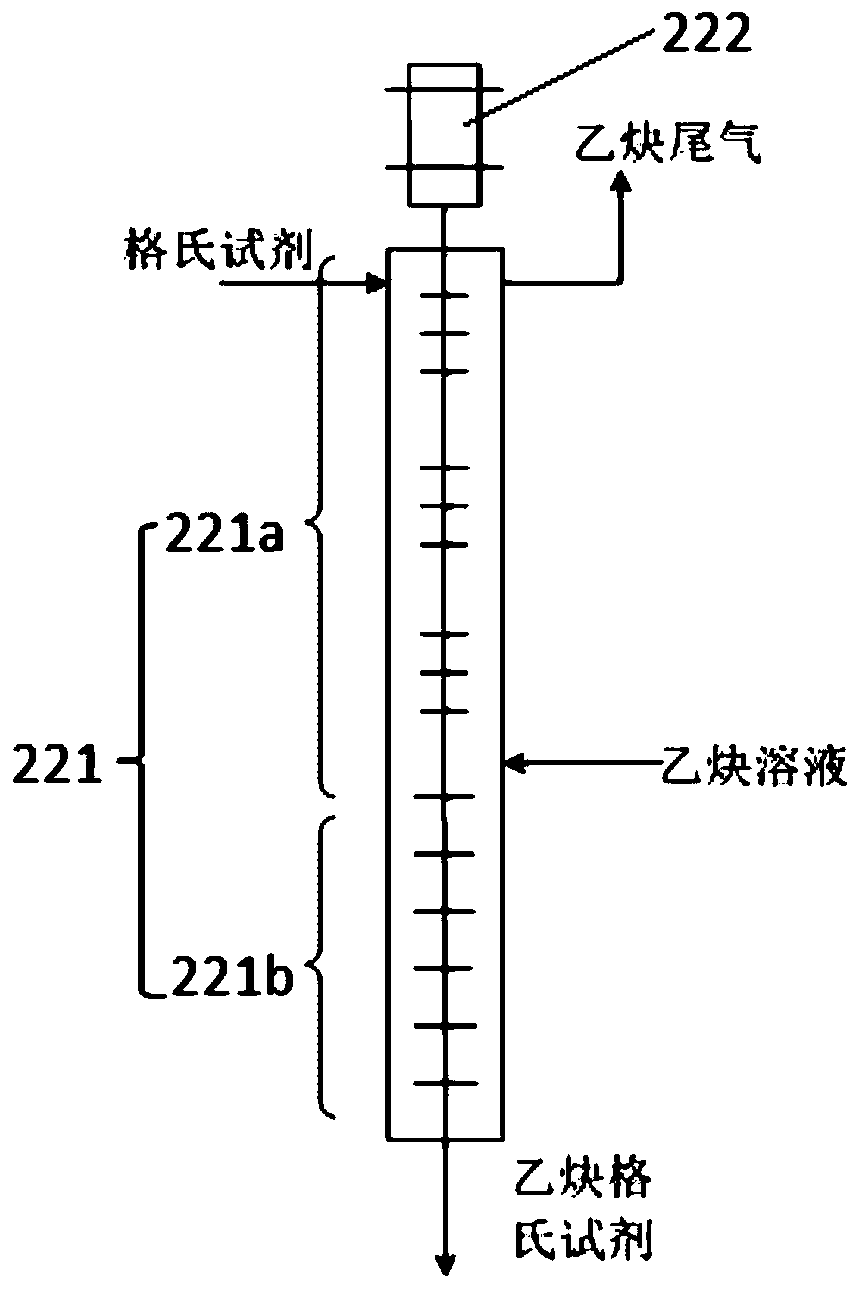
Continuous synthesis system and method for trimethylsilylacetylene
ActiveCN110183480ALarge specific surface areaShorten the production cycleSilicon organic compoundsTrimethylsilylSynthesis methods
The invention provides a continuous synthesis system and a continuous synthesis method of trimethylsilylacetylene. The continuous system comprises a Grignard reagent continuous preparation unit, an acetylene Grignard reagent continuous synthesis unit and a trimethylsilylacetylene continuous preparation device, wherein the Grignard reagent continuous preparation unit is provided with a first feeding port and a Grignard reagent outlet; the acetylene Grignard reagent continuous synthesis unit is provided with a Grignard reagent inlet, an acetylene solution inlet and an acetylene Grignard reagentoutlet, and the Grignard reagent inlet is communicated with the Grignard reagent outlet through a Grignard reagent transfer pipe; the trimethylsilylacetylene continuous preparation device is providedwith a trimethylhalosilane inlet, an acetylene Grignard reagent inlet and a trimethylsilylacetylene outlet, the acetylene Grignard reagent inlet is communicated with the acetylene Grignard reagent outlet through an acetylene Grignard reagent transfer pipe. By adopting the continuous synthesis system for preparing trimethylsilylacetylene, the production cost can be reduced, the period can be shortened, and safety of the whole process can also be improved.
Owner:LIAONING ASYMCHEM LAB CO LTD
Organic microporous material with metal coordination containing heteroatoms and preparation and application thereof
ActiveCN109134872AImprove oxidation capacityHigh Nitrogen Adsorption CapacityHeteroatomStructural formula
The invention relates to an organic microporous material with metal coordination containing heteroatoms and preparation and application thereof. An organic conjugated precursor of the organic microporous material has a structural formula of I or II. The preparation comprises the steps of preparation of 4-trimethylsilylacetylene-2-bromopyridine or 5-trimethylsilylacetylene-3-bromopyridine, preparation of 4-ethynyl-2-bromopyridine or 5-ethynyl-3-bromopyridine, preparation of bis(2-bromopyridine) acetylene or bis(3-bromopyridine) acetylene, preparation of bis(2-thiophene pyridine) acetylene or bis(3-thiophene pyridine) acetylene, preparation of the organic conjugated precursor, and preparation of the organic microporous material with the metal coordination containing the heteroatoms. The organic microporous material has a controllable form and structure, thereby having different properties and functions, and can be applied to the development of novel functional materials such as gas adsorption and storage, molecular separation, catalysis, sustained release of drugs and the like.
Owner:DONGHUA UNIV
Preparation method for metalloporphyrin metal complexes with different structure frameworks and application
ActiveCN105440044AVisible light intensityStrong absorption capacityOrganic chemistryColor/spectral properties measurementsPhotodynamic therapyTrimethylsilyl
The invention belongs to the technical field of organic photoelectric materials, and concretely relates to synthesis and application of porphyrin metal complexes with different frameworks. The complexes are obtained through coordination of porphyrin rings with different structures and a metal, and the structure general formula is shown in the specification. A porphyrin compound is synthesized from pyrrole, pentafluorobenzaldehyde and trimethylsilylacetylene, and then porphyrin complexes are formed through coordination of the porphyrin compound and different metals. TTA (triplet-triplet annihilation) transition phenomenon is generated when the prepared target metal complex is combined with an acceptor, and the metal complexes have good application prospects on solar cells and cell imaging. Additionally, the porphyrin metal complexes can generate single oxygen quantum dot with extremely high efficiency, and possesses potential application prospect on anoxia detection and photodynamic therapy. The prepared target complexes are shown in the specification.
Owner:NANJING UNIV OF POSTS & TELECOMM
Synthetic method for silthiopham
ActiveCN105111229AAvoid pollutionRaw materials are cheap and easy to getGroup 4/14 element organic compoundsOrganic baseOrganic synthesis
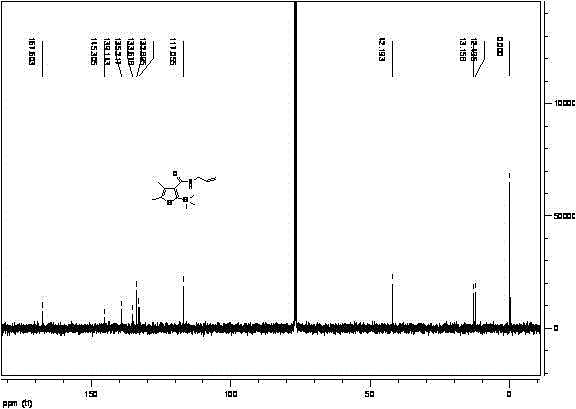
The invention provides a synthetic method for silthiopham, which belongs to the technical field of organic synthesis. The objective of the invention is to overcome the problems of complicated synthesis process, a great number of byproducts and severe environmental pollution of conventional synthetic methods for silthiopham. The synthetic method provided by the invention comprises the following steps: (1) with trimethylsilylacetylene as a raw material, under the protection of inert gas, reacting trimethylsilylacetylene with methyl chloroformate under the action of organic base so as to obtain methyl (trimethylsilyl)propiolate; (2) reacting methyl (trimethylsilyl)propiolate with allyl amine in a solvent under the action of a catalyst so as to obtain N-allyl-3-(trimethylsilyl)propiolamide; and (3) subjecting N-allyl-3-(trimethylsilyl)propiolamide with 3-mercapto-2-butanone to a heating reflux reaction under the action of an alkali catalyst and carrying out dehydration so as to obtain the final product silthiopham. The method has the advantages of usage of cheap and easily available raw materials, simple route, high yield and no usage of reagents severely polluting the environment, e.g., t-butyl nitrous acid and thionyl chloride.
Owner:HANGZHOU NORMAL UNIVERSITY
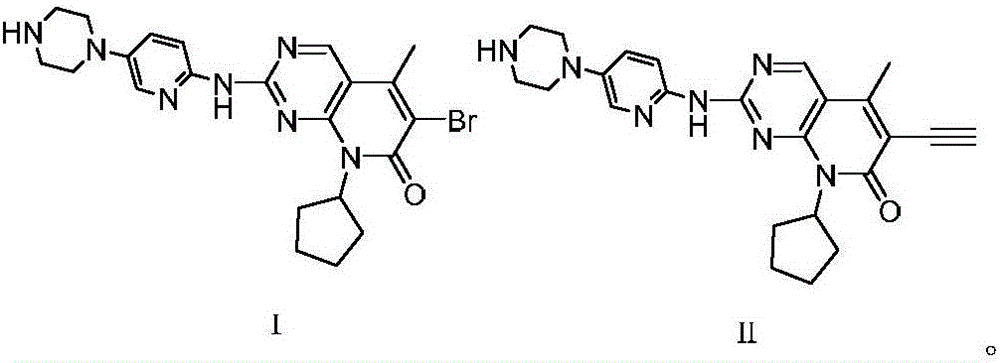
Method for preparing CDK46 kinase inhibitor Palbociclib
ActiveCN106008499AReduce usageMild reaction conditionsOrganic chemistryAcid waterPotassium tert-butoxide
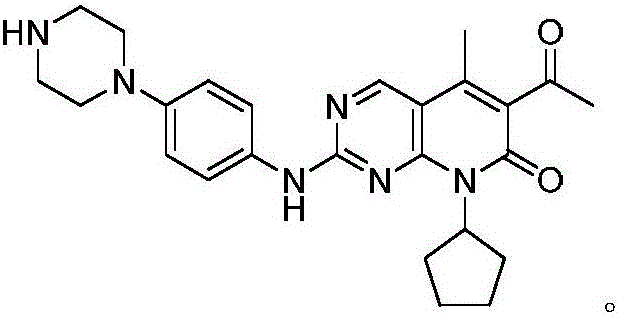
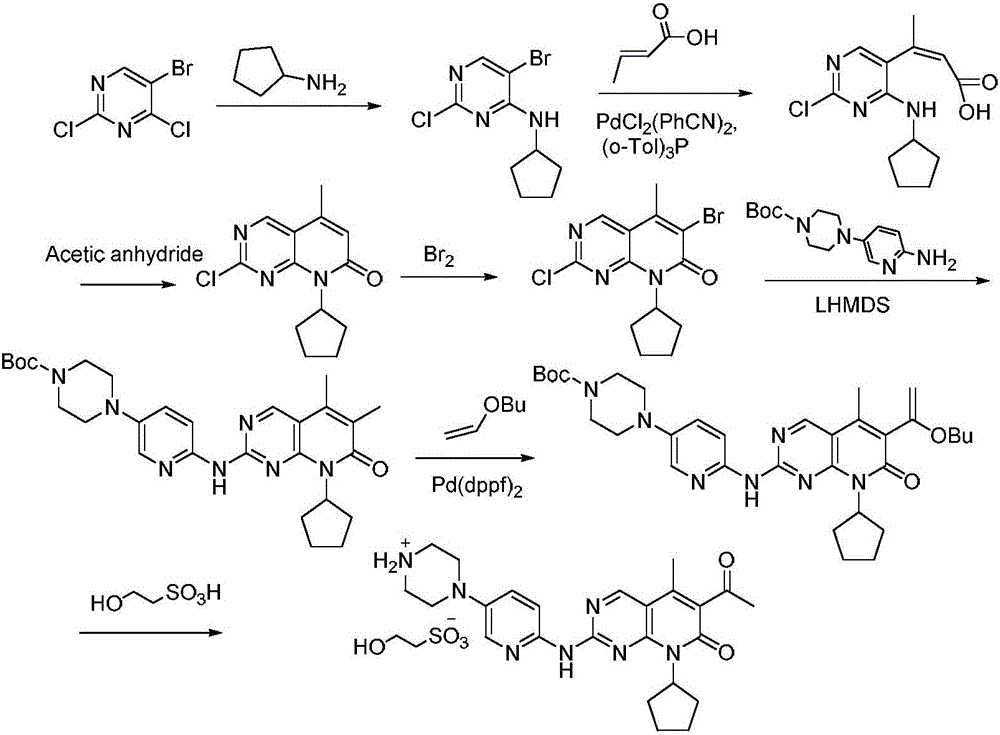
The invention discloses a method for preparing the CDK46 kinase inhibitor Palbociclib. The method includes the following steps that 1, the compound N-cyclopentyl-5-methyl-2-[[5-(1-piperazinyl)-2-pyridyl]amino]-6- bromopyridine -[2,3-d]-pyrimidine-7(8H)-ketone shown in the formula (I) reacts with trimethylsilylacetylene in the presence of cuprous bromide and potassium tert-butoxide to generate the compound N-cyclopentyl-5-methyl-6-acetenyl-2-[[5-(1-piperazinyl)-2-pyridyl]amino]pyridino-[2,3-d] pyrimidine-7(8H)-ketone shown in the formula (II); 2, the compound N-cyclopentyl-5-methyl-6-acetenyl-2-[[5-(1-piperazinyl)-2-pyridyl]amino]pyridino-[2,3-d] pyrimidine-7(8H)-ketone shown in the formula (II) and obtained in the step 1 is hydrolyzed in an acid water solution to obtain Palbociclib. By means of the method, a new way for preparing Palbociclib is developed, conditions are mild, and the yield is high.
Owner:刘淑兰 +1
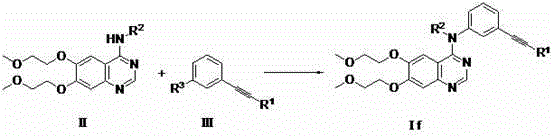
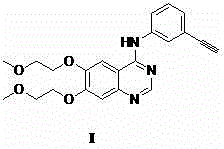
Preparation method of erlotinib and derivatives of erlotinib
The invention discloses a preparation method of erlotinib and derivatives of erlotinib. The preparation method is characterized in that a compound II (when R2=H, the compound II is 6,7-(2-methoxyl-oxethyl)-quinoline-4-amine and a compound III (when R1=SiMe and R3=I, the compound III is 3- trimethylsilylacetylene-iodobenzene have a coupling reaction in an organic solvent under the actions of alkali, elementary substance copper and / or copper salt to obtain erlotinib and derivatives of erlotinib. The elementary substance copper and / or copper salt or palladium reagent is used as the base of the catalytic reaction, the N-(3-phenylacetylene)-6,7-di(2-methoxyl oxethyl)-4-quinoline amino acid salt (erlotinib, I) and the derivatives of erlotinib are prepared from midbody compoundS II and III. The preparation method has the advantages of use of low-price and easily available raw materials, , moderate reaction conditions, few side reaction, high yield and small environmental pollution and is applicable to the industrial production.
Owner:云南现代民族药工程技术研究中心 +1
Preparation method of intermediate of medicine for treating chronic dry eye
The invention relates to a preparation method of an intermediate of a medicine for treating chronic dry eye. The method comprises the following steps: (a) adding methyl alcohol, m-hydroxybenzoic acid,NaOH and NaI to a reaction vessel; and dropwise adding NaClO water solution with stirring in order to react under the temperature of -10 to -5 DEG C; (b) adding tetrahydrofuran, a compound II, a catalyst and trimethylamine to another reaction vessel; dropwise adding trimethylsilylacetylene under inert gas; and stirring to react; and (c) adding methyl alcohol, a compound III, cuprous iodide and trimethylamine to a third reaction vessel; reacting under the temperature of 50-80 DEG C; filtering; collecting filtrate; dropwise adding NaOH solution; stirring to react; removing methyl alcohol and trimethylamine in a pressure reduction manner; regulating pH through an acid solution; filtering; and drying to obtain the product. According to the method, the finished product can be prepared by threesteps; the yield and the purity are high; the cost is low; and industrial production can be carried out.
Owner:刘可
Method for preparing 3-ethyl-4-methylol acetophenone
InactiveCN109456156AHigh yieldLow costGroup 4/14 element organic compoundsOrganic compound preparationHydrogenCoupling reaction
Owner:荆门医药工业技术研究院 +1
Fluorescent film sensor for fluoride ion detection and preparation method of fluorescent film sensor
ActiveCN106770077AEnsure transparencyEnsuring Fluorescent EfficiencyOrganic chemistryElectrolysis componentsMonomerTrimethylsilylacetylene
The invention discloses a fluorescent film sensor for fluoride ion detection and a preparation method of the fluorescent film sensor and relates to the field of fluorescent molecular sensors. According to the fluoride ion detecting fluorescent film sensor and the preparation method thereof, firstly, an electropolymerizable fluorescent sensing material monomer is prepared from 2-bromo-9,9-(N-carbazole-hexyl) fluorene and trimethylsilylacetylene through a reaction; then the fluorescent film sensor poly1,4-bis(4-(9,9-bis(N-carbazole-hexyl) fluorenyl)-1,2,3-triazolyl) benzene is obtained from the fluorescent sensing material monomer through electrochemical polymerization, and Fe<3+> can be added to the obtained fluorescent film sensor to enhance the detection performance of the sensor. The fluorescent film sensor can effectively guarantee fast diffusion of metal ions in a film and improves the film detection sensitivity, the used fluorescent sensing raw materials are cheap and available, the synthetic method is relatively simple, and a constructed device is good in reversibility and high in practicability.
Owner:JIANGXI PURUIFENG ECOLOGICAL TECH CO LTD
Preparing method of ponatinib
The invention discloses a preparing method of ponatinib. The preparing method of ponatinib comprises the steps of building a reaction system in copper salt and alkaline organic solvent with 3-halogen-parazole [1,5-a]pyrimidine being the starting point, with trimethylsilylacetylene, tetrabutylammonium fluoride and the like being raw materials in sequence and with acridine salt being a photocatalyst, and thus indirectly building a synthesizing path of ponatinib. The synthesis conditions are mild, the cost is low, and the yield of ponatinib is high.
Owner:深圳蓝新科技有限公司
Preparation method of five-membered ring polymer used for OLED
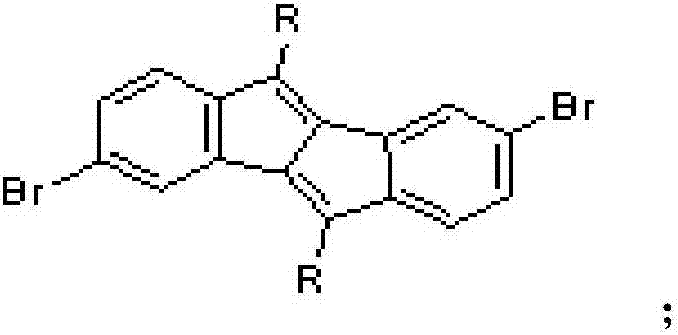
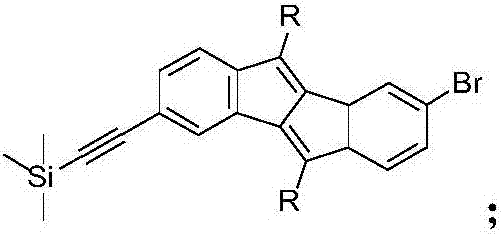
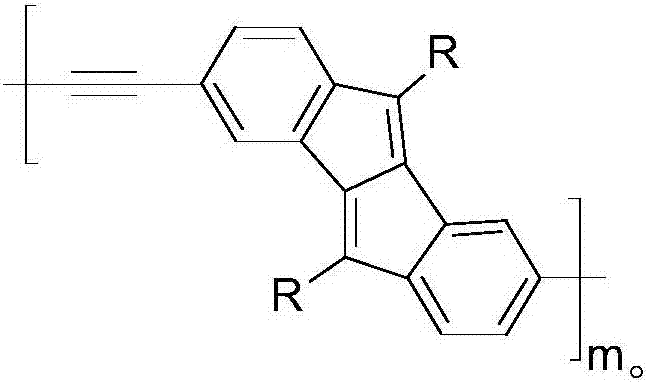
The invention discloses a preparation method of a five-membered ring polymer used for OLED. The preparation method comprises following steps: 2,5-dibromoaniline is dissolved in acetate acid gracial, a NaNO2 aqueous solution is added, a urea aqueous solution is added dropwise, a KI aqueous solution is added drop by drop, extraction is carried out, and an obtained product 1 is mixed with a derivative of R molecular containing terminal alkynyl; the product 1 is dissolved in a mixed solution of tetrahydrofuran and triethylamine, argon is introduced, ultrasonic treatment is carried out, dichlorobispalladium and cuprous iodide are added, purification is carried out, and an obtained product 2 is dissolved in 1,4-dioxane, argon is introduced, ultrasonic treatment is carried out, an obtained product 3 is added into a mixture of hydroquinone, Cs2CO3, CsF, Pd2(dba)3, and P(tBu)3; purification is carried out, an obtained product 4 is dissolved in a mixed solution of tetrahydrofuran and triethylamine, argon is introduced, ultrasonic treatment is carried out, trimethylsilylacetylene is added, dichlorobispalladium and cuprous iodide are added, separation purification is carried out, an obtained product 5 is dissolved in a mixture of absolute methanol and tetrahydrofuran; separation purification is carried out, an obtained product 6 is dissolved in a mixed solution of methylbenzene and tetramethyl ethylenediamine; cuprous chloride is added, and separation purification is carried out so as to obtain a finished product. The five-membered ring polymer is capable of improving the stability and prolonging the service life of OLED.
Owner:XIJING UNIV
Carbon nanotube dispersing phenylacetylene-based polyfunctional group polymer and preparation method thereof
The invention discloses a carbon nanotube dispersing phenylacetylene-based polyfunctional group polymer and a preparation method thereof. The backbone chain of the carbon nanotube dispersing phenylacetylene-based polyfunctional group polymer is phenylacetylene, and the side chain of the carbon nanotube dispersing phenylacetylene-based polyfunctional group polymer is alkoxy. The preparation methodof the carbon nanotube dispersing phenylacetylene-based polyfunctional group polymer comprises taking 1, 4-dialkoxy substituted bisphenol ether 1 as the raw material for bromination to obtain dibromo-bisphenol ether 2, subjecting the dibromo-bisphenol ether 2 to reaction with trimethylsilylacetylene to obtain an intermediate 3, removing TMS (trimethylsilyl) of the intermediate 3 to obtain terminalalkyne 4, subjecting the terminal alkyne 4 to Sonogashira reaction with the dibromo-bisphenol ether 2 to produce a high-molecular polyfunctional group polymer containing functional backbone and sidechains. The prepared carbon nanotube dispersing phenylacetylene-based polyfunctional group polymer can produce backbone chain pi-pi adsorption and side chain entanglement with carbon nanotubes to achieve well dispersion of the carbon nanotubes under the premise of not damaging the carbon nanotubes; according to difference in the structure of the carbon nanotube dispersing phenylacetylene-based polyfunctional group polymer and the sizes of the carbon nanotubes, the dispersion degrees ranges between 2% and 7%.
Owner:NEW MATERIAL INST OF SHANDONG ACADEMY OF SCI +1
Method for polymerizing 8-hydroxyquinoline metal complex
The invention provides a method for polymerizing an 8-hydroxyquinoline metal complex, which comprises the following steps: synthesizing 5-bromo-8-tert-butoxycarbonylquinoline;synthesizing 5-trimethylsilylacetylene-8-tert-butoxycarbonylquinoline by the Sonagashira coupling reaction between 5-bromo-8-tert-butoxycarbonylquinoline and trimethylsilylacetylene; synthesizing 5-acetenyl-8-tert-butoxycarbonylquinoline by an elimination reaction; synthesizing polystyrene of which terminal group is 8-tert-butoxycarbonylquinoline by the click reaction between 5-bromo-8-tert-butoxycarbonylquinoline and azido-polystyrene; synthesizing polystyrene macromolecular ligand of which the terminal group is 8-hydroxyquinolinyl; and synthesizing a polystyrene metal complex by the coordination reaction between polystyrene macromolecular ligand of which the terminal group is 8-hydroxyquinolinyl, 8-hydroxyquinoline and metal ions. The polymer metal complex synthesized by the method of the invention shows a wide application prospect in the fields of electroluminescence, magnetic functional polymer, electrochemical catalysis and polymer photovoltaic devices.
Owner:HARBIN ENG UNIV
Production process of trimethylsilylacetylene
InactiveCN103896976ALow priceReduce manufacturing costSilicon organic compoundsTetrahydrofuranTrimethylsilylacetylene
The invention discloses a production process of trimethylsilylacetylene, comprises the following steps: (1) adding the measured tetrahydrofuran and magnesium metal into a reaction still, adding iodine and 1,2-dibromoethane, warming to 50-60 DEG.C, introducing the measured chloroethylene, heat-insulating for 3 hours, cooling to 20-30 DEG.C, adding a catalyst, stirring, and then adding the measured mixed solution of tetrahydrofuran and trimethylchlorosilane, warming to 50-60 DEG.C, heat-insulating for 5 hours; distilling the produced vinyl trimethylsilyl to be dissolved in methanol, controlling temperature at 15-20 DEG.C, dropwise adding equal bromine, reacting until floor fading, slowly dropwise adding equal alkaline methanol solution, controlling temperature at 30-40 DEG.C, reacting HPLC monitoring until the reaction is finished, adding dichloromethane, filtering to remove salt, and concentrating to obtain a finished product. The raw materials adopted by the invention are available and low in price, the production cost is low, the product quality is high, the reaction condition is mild, the yield is high, and the production process is capable of producing the trimethylsilylacetylene on large scale.
Owner:ANQING FENGYUAN CHEM
Synthetic method of 7-azaindole-5-chloro-6-carboxylic acid
The invention relates to a synthetic method of 7-azaindole-5-chloro-6-carboxylic acid, which mainly solves the technical problem that no effective synthetic method suitable for the industrialization is provided. The synthetic method comprises the following steps: dissolving 3,6-dichloropyridine-2-carboxylic acid in a solvent of tertiary butanol and pyridine, adding paratoluensulfonyl chloride, obtaining a compound 1, wherein the product is unnecessary to purify; coupling the compound 1 and diphenyl amine under the catalysis of Pd2(dba)3 and xant-Phos, adding hydroxylamine hydrochloride, and purifying a crude product by virtue of column chromatography, thus obtaining a compound 2; dissolving the compound 2 in acetic acid, adding NIS, heating to facilitate the reaction, and purifying a crudeproduct by virtue of column chromatography to obtain a compound 3; dissolving the compound 3 in tetrahydrofuran, adding trimethylsilylacetylene, cuprous iodide, Pd(PPh3)2Cl2 and triethylamine, and reacting under the protection of nitrogen, thus obtaining a product 4 which does not require to be purified; and dissolving the compound 4 in dry N-methyl pyrrolidone, adding potassium tert-butoxide, heating, reacting, standing overnight, washing a crude product by using acetate, and filtering to obtain a target product.
Owner:KANGHUA SHANGHAI DRUG RES DEV CO LTD
Method for synthesizing polystyrene high molecular ligand taking 8-hydroxyquinoline as ligand group
The invention provides a method for synthesizing a polystyrene high molecular ligand taking 8-hydroxyquinoline as a ligand group. The method comprises the following steps of: (1) synthesizing 5-bromo-8-tert-butoxycarbonyloxyquinoline; (2) synthesizing 5-trimethylsilylacetylene-8-tert-butoxycarbonyloxyquinoline; (3) removing a trimethyl silicane group of 5-trimethylsilylacetylene-8-tert-butoxycarbonyloxyquinoline, and synthesizing 5-acetylenyl-8-tert-butoxycarbonyloxyquinoline; (4) reacting the 5-acetylenyl-8-tert-butoxycarbonyloxyquinoline with click of azido-polystyrene to synthesize polystyrene of which a terminal group is 8-tert-butoxycarbonyloxyquinoline; and (5) synthesizing a polystyrene high molecular ligand taking 8-hydroxyquinoline as a ligand group. The ligand prepared with the method takes 8-hydroxyquinoline as a ligand group, has the potential of forming a metal complex together with metal ions such as zinc, magnesium, aluminum and the like, and has wide application prospects in the fields of biology, electrofluorescence, magnetic functional high molecules, electrochemical catalysis, polymer photovoltaic devices and the like.
Owner:HARBIN ENG UNIV
Method for synthesizing second-level trifluoromethyl propargyl alcohol
InactiveCN106892936ANothing producedGood chemical stabilitySilicon organic compoundsOxygen-containing compound preparationBenzoic acidKetone
The invention discloses a method for synthesizing second-level trifluoromethyl propargyl alcohol. The method comprises the following steps: enabling 2-iodo benzoic acid and sodium periodate to react so as to generate 1-(hydroxyl)-1,2-iodobenzoyl-3(1H)-ketone (BIOH), generating trimethylsilylacetylene and triisopropylchlorosilane to generate trimethylsilyl (triisopropyl silicon) acetylene, synthesizing 1-[(triisopropyl silicon) acetylene]-1,2-iodobenzoyl-3(1H)-ketone from BIOH and trimethylsilyl (triisopropyl silicon) acetylene, enabling 1-[(triisopropyl silicon) acetylene]-1,2-iodobenzoyl-3(1H)-ketone and trifluoroethylamine hydrochloride and sodium nitrite to synthesize 1-[(triisopropyl silicon) acetylene]-1,2-iodobenzoyl-3(1H)-ketone, and finally performing hydrolysis, thereby synthesizing a target product. The trifluoromethyl propargyl alcohol prepared by using the method is an important fluorine-containing building block molecule, and has very great significances for synthesis of fluorine-containing functional organic molecules and medicinal intermediates.
Owner:HEFEI UNIV OF TECH
Marine natural product (+)-(4E, 15Z)-4, 15-docosadienoic-1- alkyne-3-alcohol and synthetic method of enantiomer thereof
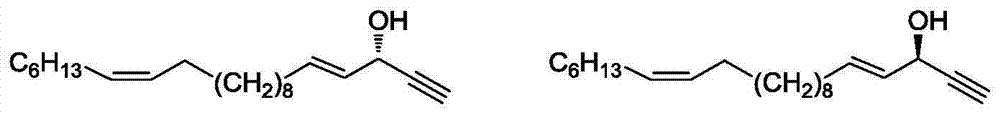
InactiveCN104725193AHigh optical purityOvercoming productivityGroup 4/14 element organic compoundsOxygen-containing compound preparationChemical synthesisEnantiomer
The invention discloses a marine natural product (+)-(4E, 15Z)-4, 15- docosadienoic-1- alkyne-3-alcohol and a synthetic method of an enantiomer thereof, and belongs to the field of chemical synthesis. With propargyl alcohol as an initial raw material, the method provided by the invention comprises multiple reactive synthesis steps, such as coupling, triple bond transposition, oxidation, selective reduction, asymmetric alkynylation, esterification and hydrolysis and the like, and the key step is to carry out an asymmetric addition reaction on trimethylsilylacetylene and aldehyde to directly obtain a chiral propargyl alcohol structure. The synthetic method disclosed by the invention has the advantages of simple steps, high total yield and high optical purity of the product.
Owner:CHINA AGRI UNIV
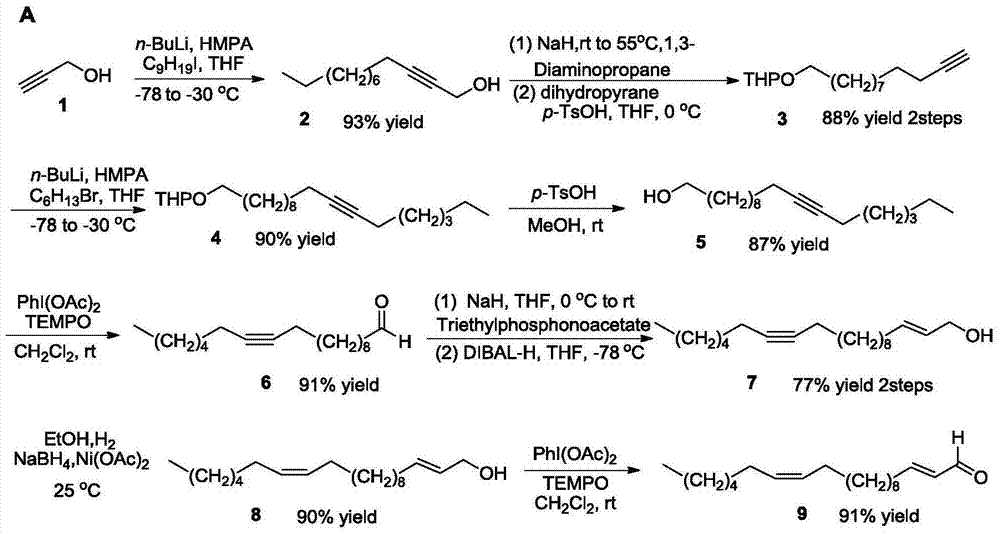
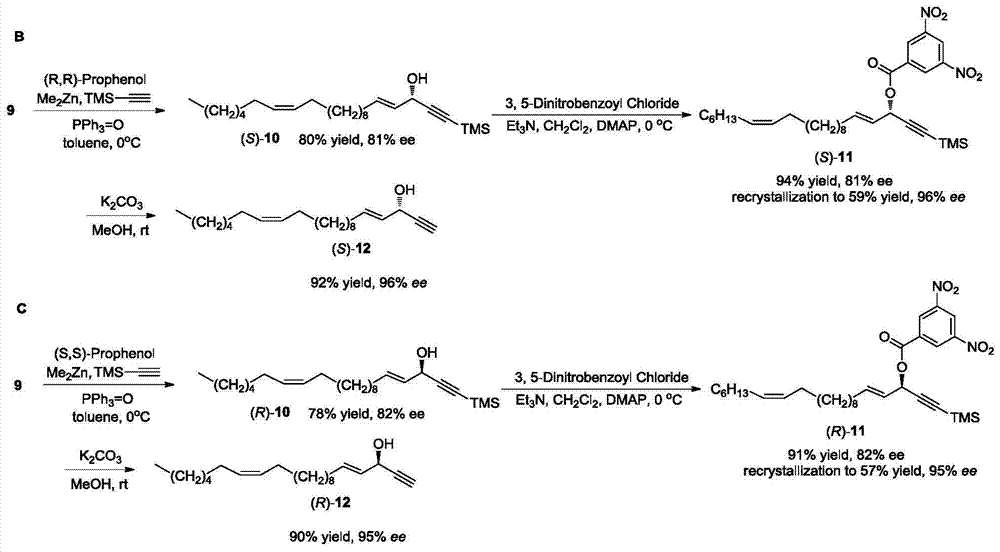
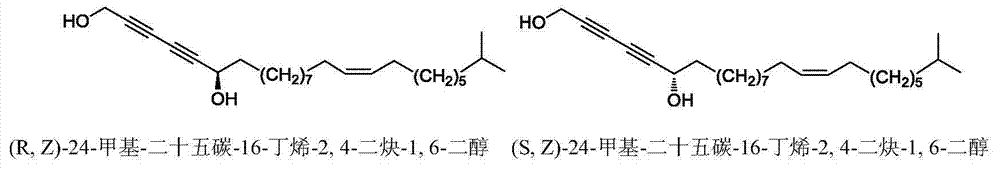
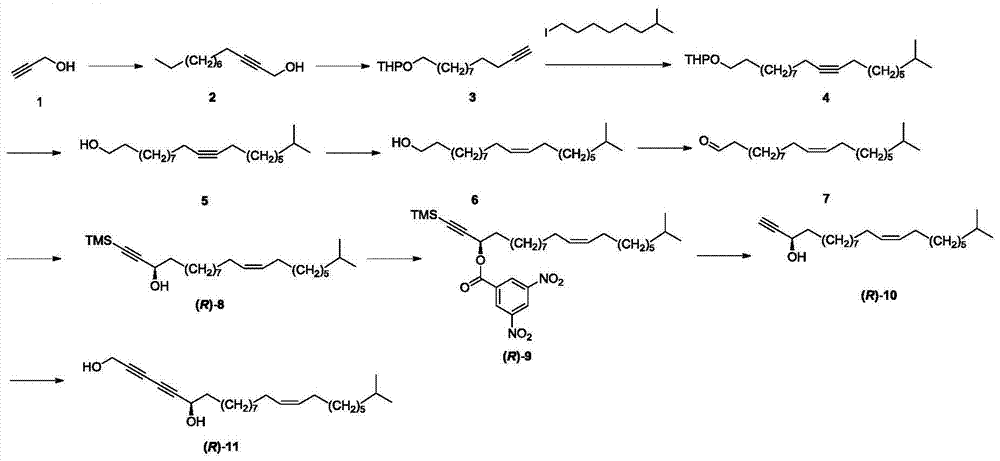
Synthetic method of marine natural product namely (R, Z)-24-methyl-25-carbon-16-butylene-2,4-diyne-1,6-diol and enantiomer thereof
ActiveCN104774138AHigh stereoselectivitySimple stepsOrganic compound preparationHydroxy compound preparationChemical synthesisIodide
The invention relates to a synthetic method of a marine natural product namely (R, Z)-24-methyl-25-carbon-16-butylene-2,4-diyne-1,6-diol and an enantiomer thereof, and belongs to the field of chemical synthesis. The synthetic method comprises the following steps: firstly, preparing long chain alkyl iodide by using a series of simple reactions including bromination, oxidation, esterification, reduction and the like; and then, performing multiple steps of reactions including coupling, dislocation, oxidation, selective reduction, asymmetric alkynylation addition, esterification, hydrolysis and the like by taking propargyl alcohol and the long chain alkyl iodide as starting materials to synthesize the marine natural product namely (R, Z)-24-methyl-25-carbon-16-butylene-2,4-diyne-1,6-diol and the enantiomer thereof, wherein the key step is that trimethylsilylacetylene and alkynal are subjected to asymmetric addition reaction to generate alkynol segments with high optical purity by one step. The synthetic method provided by the invention reports the synthesis of the natural product of the type for the first time, and has the characteristics of simple and convenient steps, relatively high total yield, good product stereoselectivity and the like, and the optical purity of each of the two types of synthesized products is more than 99%ee.
Owner:河南源博新材料有限公司
Synthesis method of 3-hydroxy-4-((trimethylsilyl) ethynyl) methyl benzoate
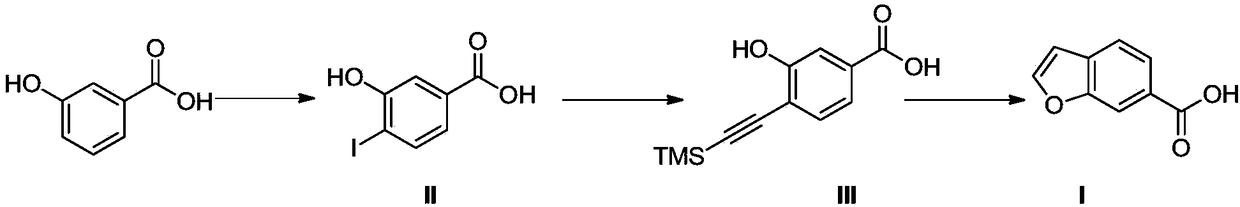
ActiveCN112625057AThe synthesis method is simpleGood catalyticSilicon organic compoundsChemical recyclingBenzoic acidPtru catalyst
The invention relates to a synthetic method of 3-hydroxy-4-((trimethylsilyl) ethynyl) methyl benzoate, and belongs to the technical field of synthesis of medical intermediates. According to the method, 4-iodo-3-hydroxybenzoic acid and trimethylsilylacetylene are taken as raw materials, and under an alkaline system, the 3-hydroxy-4-((trimethylsilyl) ethynyl) methyl benzoate is synthesized under the catalysis of a palladium-loaded ordered mesoporous carbon catalyst and a cuprous iodide-triphenylphosphine complex. The palladium-loaded ordered mesoporous carbon catalyst used in the synthesis method is simple to synthesize and beneficial to recovery. The catalytic synthesis rate of the 3-hydroxy-4-((trimethylsilyl) ethynyl) methyl benzoate is increased, the reaction period is shortened, the synthesis yield is greatly increased and reaches 94%, and the obtained product is stable in quality and relatively high in purity.
Owner:SHANDONG JINCHENG KERUI CHEMICAL CO LTD
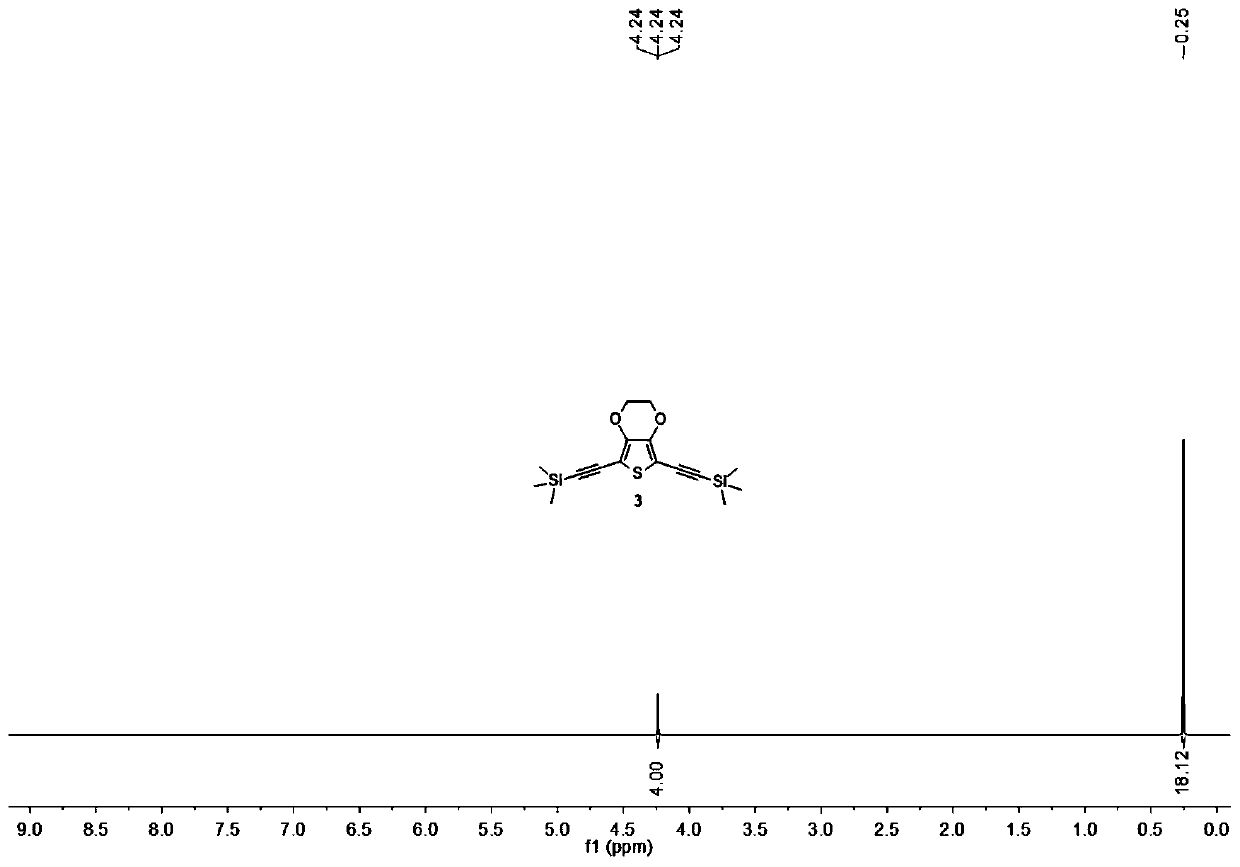
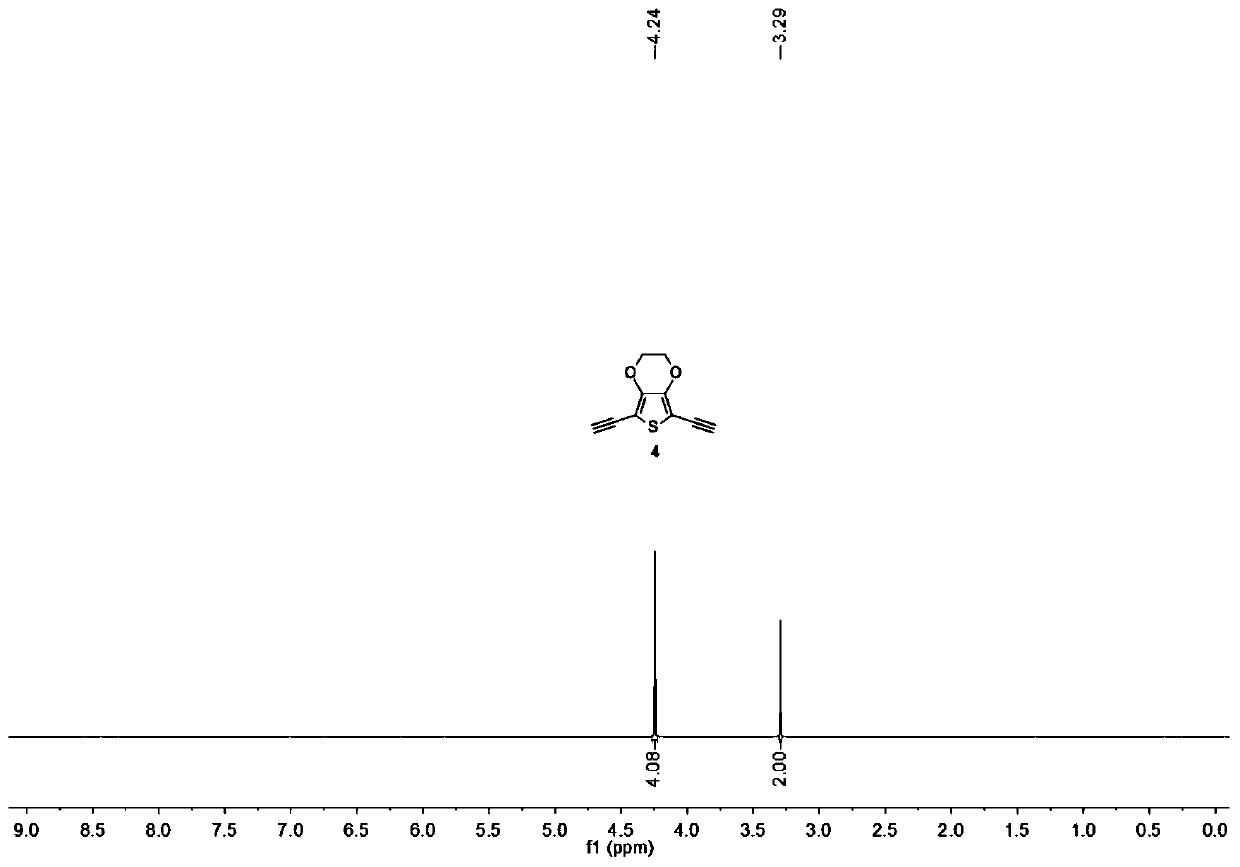
Synthetic method of di(trimethyl silicon-based)acetylene
InactiveCN102391298ACheap manufacturingHigh recovery rateSilicon organic compoundsGrignard reagentPhysical chemistry
The invention relates to a synthetic method of di(trimethyl silicon-based)acetylene. The method comprises the following steps: 1. taking diacetylenes metal salt and trimethyl halogenosilane as raw materials, wherein the mole mass ratio of diacetylenes metal salt to trimethyl halogenosilane is 1:2.0-1:2.2, suspending diacetylenes metal salt in toluene, adding trimethyl halogenosilane drop by drop, heating to the temperature of 50-60 DEG C after dripping is finished and reacting for 2 hours, wherein the diacetylenes metal salt is acetylene disodium and / or acetylene dibromo; 2, terminating the reaction by water, extracting, drying, reducing the pressure, concentrating, vacuum rectifying to obtain the di(trimethyl silicon-based)acetylene with the purity of more than 99%, wherein the yield is more than 90%. The synthetic method omits the preparation of a Grignard reagent, so that toluene which has advantages of cheap price and convenient recovery is capable of replacing tetrahydrofuran with expensive price, so that the synthesis cost is substantially reduced, because the toluene solvent used in the method of the invention has high recovery rate, so that the generation and discharge of toxic waste can be substantially minimized, and the toluene solvent is in favor of environmental protection.
Owner:SHANGHAI RECORD PHARM CO LTD +1
Synthesizing method of (E)-5-methyl-1H-pyrrolo-[2,3-b] pyridine-3-formaldoxime
InactiveCN107056781AEasy to purifySave raw materialsOrganic chemistrySodium acetatePharmaceutical industry
The invention relates to a synthesizing method of (E)-5-methyl-1H-pyrrolo-[2,3-b] pyridine-3-formaldoxime, which solves the technical problem that an effective synthesizing method is not discovered at present. The synthesizing method provided by the invention comprises the following steps: brominating 2-amino-5-picoline, so as to obtain a compound 1; performing a reaction on the compound 1 and trimethylsilylacetylene under a condition of a Sonogashira coupling reaction, so as to generate a compound 2; performing a reaction on the compound 2 and sodium hydride to form a pyrrole ring, so as to generate a compound 3; performing a reaction on the compound 3 and urotropine, so as to generate a compound 4; performing the compound 4, hydroxylamine hydrochloride and sodium acetate, so as to obtain the target product (E)-5-methyl-1H-pyrrolo-[2,3-b] pyridine-3-formaldoxime. As a sodium acetate, the (E)-5-methyl-1H-pyrrolo-[2,3-b] pyridine-3-formaldoxime is widely applied in the pharmaceutical industry.
Owner:KANGHUA SHANGHAI DRUG RES DEV CO LTD
Method for synthesizing 6-chloro furan [3, 2-B] pyridine
The invention relates to a method for synthesizing 6-chlorofuro[3,2-B]pyridine. Mainly solve the technical problem of its synthetic method. The synthetic method of the present invention comprises the following steps: 5-chloro-3-hydroxypyridine reacts with iodine and sodium carbonate at room temperature to generate compound 1; compound 1 reacts with trimethylethynyl silicon to generate compound under the conditions of the scorpion coupling reaction 2; Compound 2 reacted with tetrabutylammonium fluoride at room temperature to generate target compound 3. The reaction formula is as follows: , as an expensive pharmaceutical intermediate, 6-chlorofuro[3,2-B]pyridine is widely used in the pharmaceutical industry.
Owner:上海吉尔多肽有限公司
Triazole-functionalized 4,4'-difluorodiphenylsulfone compounds and synthesis method thereof
The invention relates to a triazole-functionalized 4,4'-difluorodiphenyl sulfone compound and a preparation method thereof. The structural formula of the compound is: . This kind of compound is synthesized from 4,4'-difluorodiphenyl sulfone through bromination, coupling, click and other reactions. It is divided into four steps, including 3,3'-dibromo-4,4'-difluoro Synthesis of Diphenylsulfone, Synthesis of 3,3'-Ditrimethylsilylethynyl-4,4'-Difluorodiphenylsulfone, 3,3'-Diethynyl-4,4'-Difluorodiphenyl Synthesis of sulfones, synthesis of triazole-functionalized 4,4'-difluorodiphenylsulfone. The compound synthesized in the present invention can be polycondensed with bisphenol compounds to obtain polymer polysulfone material. The skeleton structure of polysulfone makes it have excellent alcohol resistance effect, anti-oxidation, anti-acid catalytic hydrolysis performance, and excellent mechanical properties and thermal stability, and the triazole basic functional group provides proton defects, which can be used to prepare acid-base amphoteric high-temperature proton conductive materials.
Owner:SHANGHAI UNIV
A metal-coordinated heteroatom-containing organic microporous material and its preparation and application
ActiveCN109134872BImprove oxidation capacityHigh Nitrogen Adsorption CapacityPolymer scienceHeteroatom
Owner:DONGHUA UNIV
Preparation method and application of different structural framework metalloporphyrin metal complexes
ActiveCN105440044BStrong absorption capacityLong triplet lifetimeOrganic chemistryColor/spectral properties measurementsPhotodynamic therapyTrimethylsilyl
The invention belongs to the technical field of organic photoelectric materials, and concretely relates to synthesis and application of porphyrin metal complexes with different frameworks. The complexes are obtained through coordination of porphyrin rings with different structures and a metal, and the structure general formula is shown in the specification. A porphyrin compound is synthesized from pyrrole, pentafluorobenzaldehyde and trimethylsilylacetylene, and then porphyrin complexes are formed through coordination of the porphyrin compound and different metals. TTA (triplet-triplet annihilation) transition phenomenon is generated when the prepared target metal complex is combined with an acceptor, and the metal complexes have good application prospects on solar cells and cell imaging. Additionally, the porphyrin metal complexes can generate single oxygen quantum dot with extremely high efficiency, and possesses potential application prospect on anoxia detection and photodynamic therapy. The prepared target complexes are shown in the specification.
Owner:NANJING UNIV OF POSTS & TELECOMM
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com