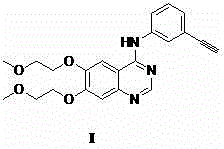
Preparation method of erlotinib and derivatives of erlotinib
A technology of derivatives and compounds, applied in the field of pharmaceutical compound preparation, can solve the problems of difficulty in post-processing, unsuitable for industrialized production, etc., and achieve the effects of low cost, few side reactions and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] Unless otherwise specified, the raw materials or reagents used in the examples are commercially available.
[0071] The synthesis of compound IV can be found in Bioorganic & Medicinal Chemistry Letters, 2006, 16, 4102.
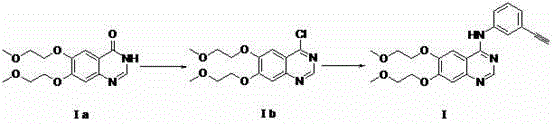
[0072] step one:
[0073] Synthesis of Compound II (R2 = H): Dissolve 5.0 g and 18.8 mmol of Compound IV in 50 ml of formamide, stir the resulting reaction solution at 175°C for three hours under nitrogen protection, and distill the resulting tan solution under vacuum The organic solvent was removed, and the remaining solid was purified by silica gel column (dichloromethane:methanol=10:1) to obtain the desired product compound II, a total of 3.8g of white solid powder, with a yield of 69%.
[0074] The structural identification data of its compound II (R2 = H) are as follows:
[0075] 1 H NMR (d 6 -DMSO): 8.25 (s, 1H), 7.60 (s, 1H), 7.40 (br, 2H), 7.09 (s, 1H), 4.22 (m, 4H), 3.75 (m, 4H), 3.35 (s, 6H).
[0076] Step two:
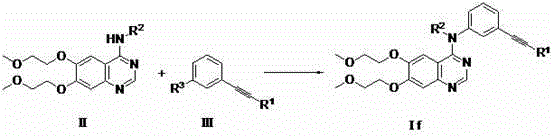
[0077] Its compound If(...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com