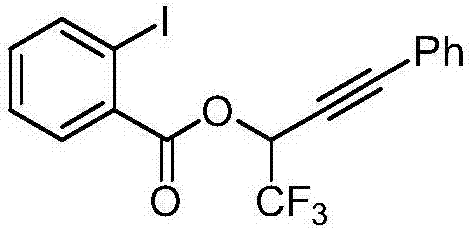
Method for synthesizing second-level trifluoromethyl propargyl alcohol
A technology of trifluoromethyl propargyl alcohol and synthesis method, which is applied in the field of synthesis of fluorine-containing organic compounds, and achieves the effect of good chemical stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] This example synthesizes secondary trifluoromethyl propargyl alcohol 1,1,1-trifluoro-4-triisopropylsilyl-3-butyn-2-ol according to the following steps:
[0042] a. Preparation of 1-(hydroxyl)-1,2-phenyliodide-3(1H)-ketone
[0043]Add 2-iodobenzoic acid (8.00g, 32.2mmol) and sodium periodate (7.24g, 33.8mmol) in a dry 250mL flask equipped with a magnetic stirring bar, and then add 30vt% (volume percent) acetic acid aqueous solution ( 48mL). Fix the flask in an oil bath, connect the condensate return line and turn on the condensate. Heat the oil bath to 120°C and reflux for 4 hours; remove the oil bath and reflux tube after the reaction, add 180 mL of ice-water mixture (0°C) to the flask under dark conditions, cool to room temperature, and After standing for 1 h, it was filtered, washed with water (3*20 mL) and acetone (3*20 mL) successively, and dried to obtain 8.3 g of white crystals with a yield of 98%.
[0044] 1 H NMR (400MHz, (CD3)2SO) δ8.02 (dd, J = 7.7, 1.4H...
Embodiment 2
[0056] This example synthesizes secondary trifluoromethyl propargyl alcohol 1,1,1-trifluoro-4-phenyl-3-butyn-2-ol according to the following steps:
[0057] a. Preparation of 1-(hydroxyl)-1,2-phenyliodide-3(1H)-ketone
[0058] b, preparation of trimethylsilyl (phenyl) acetylene
[0059] in N 2 Under protection, trimethylsilylacetylene (4.2mL, 30mmol) and THF (48mL) were added to a dry 100mL flask equipped with a magnetic stir bar, then n-butyllithium (12mL) was added at -78°C and stirred 15min, then added iodobenzene (6.73g, 33mmol) and stirred for 5min, warmed up to room temperature and stirred for 6h; after the reaction was completed, NH 4 Cl saturated solution (40mL), then CH 2 Cl 2 (2*60mL) extraction, the organic phase was extracted with water, washed with brine, dried, filtered, concentrated, and separated by column chromatography (petroleum ether as the eluent) to obtain 3.65 g of a colorless liquid with a yield of 70%.
[0060] c. Preparation of 1-[(phenyl)eth...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com