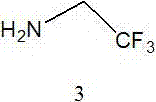
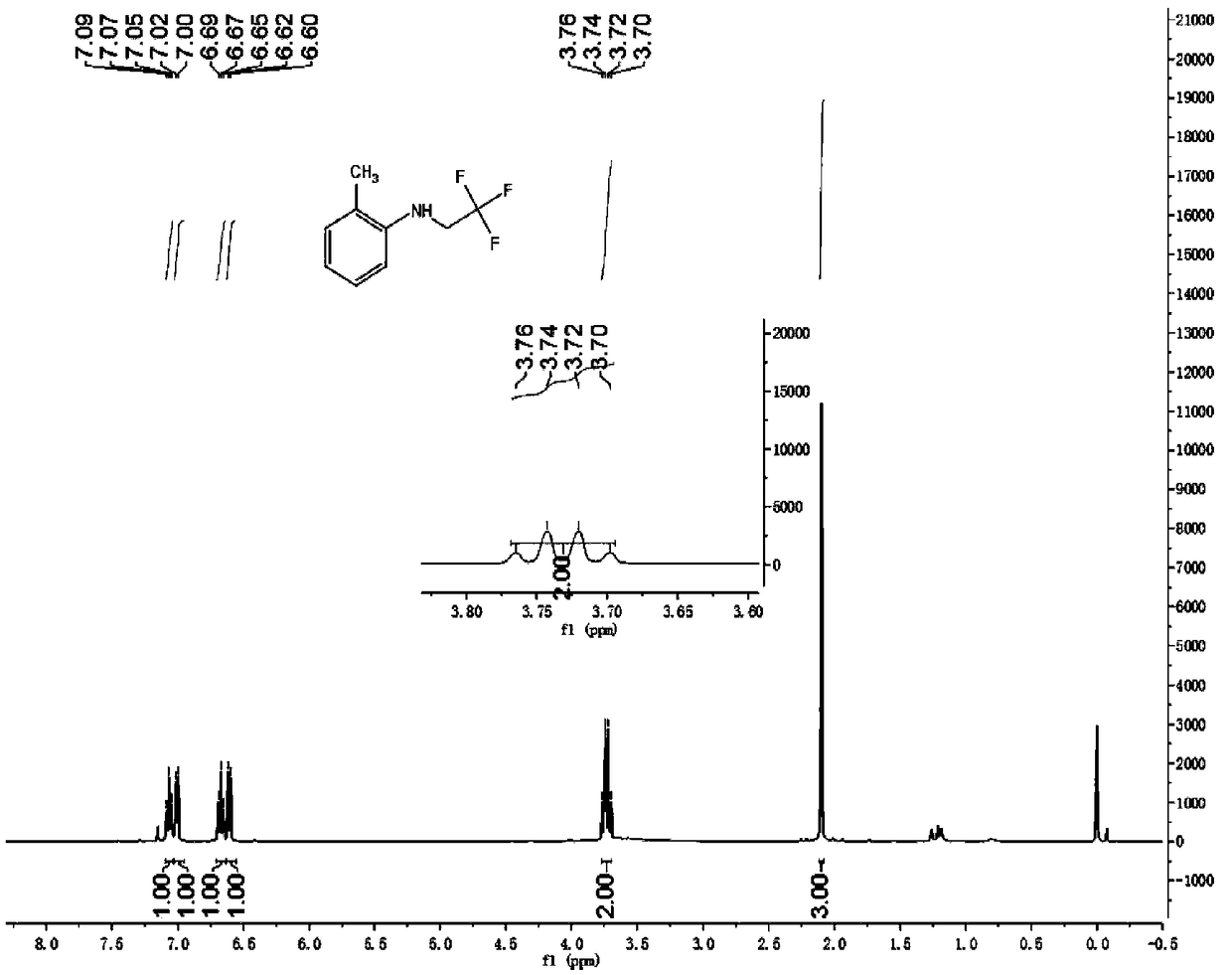
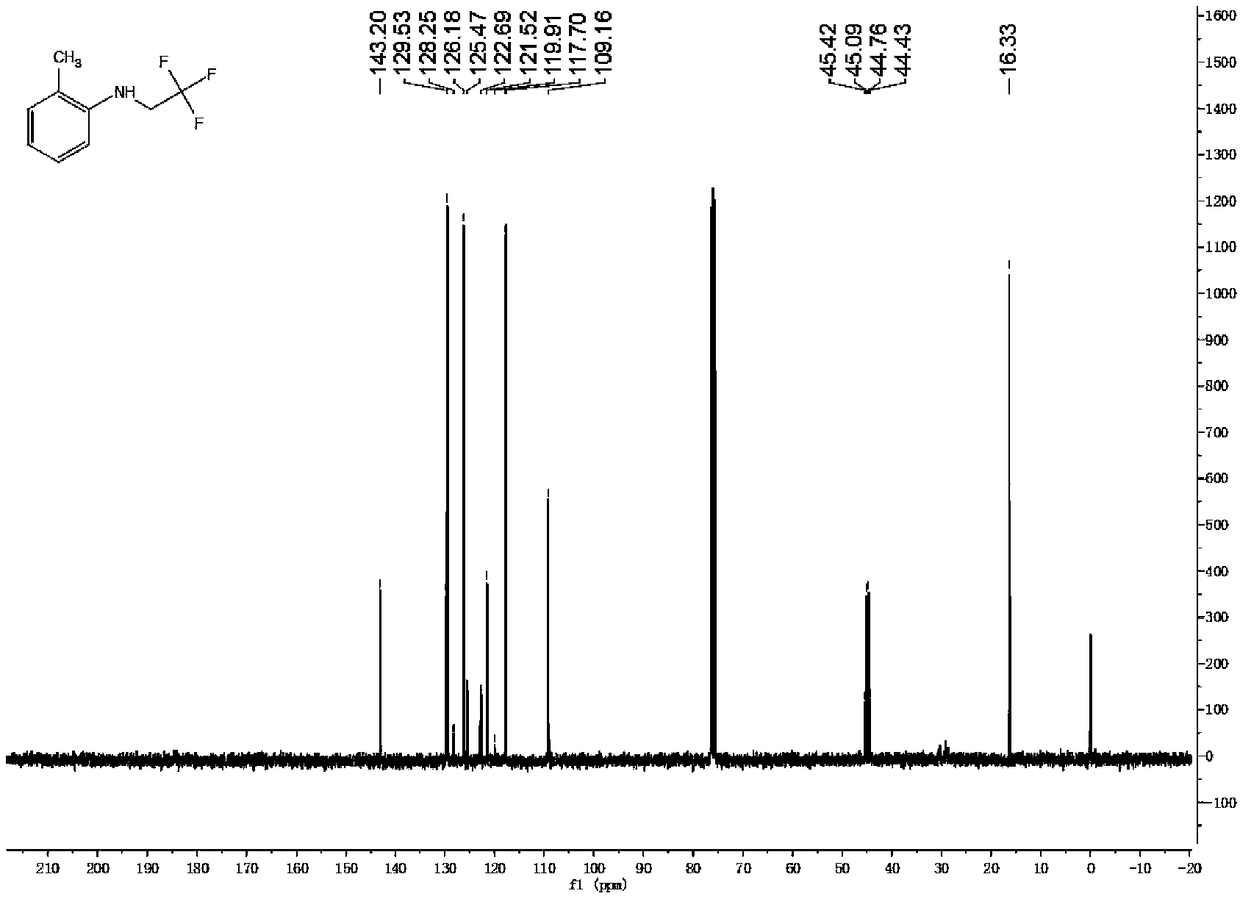
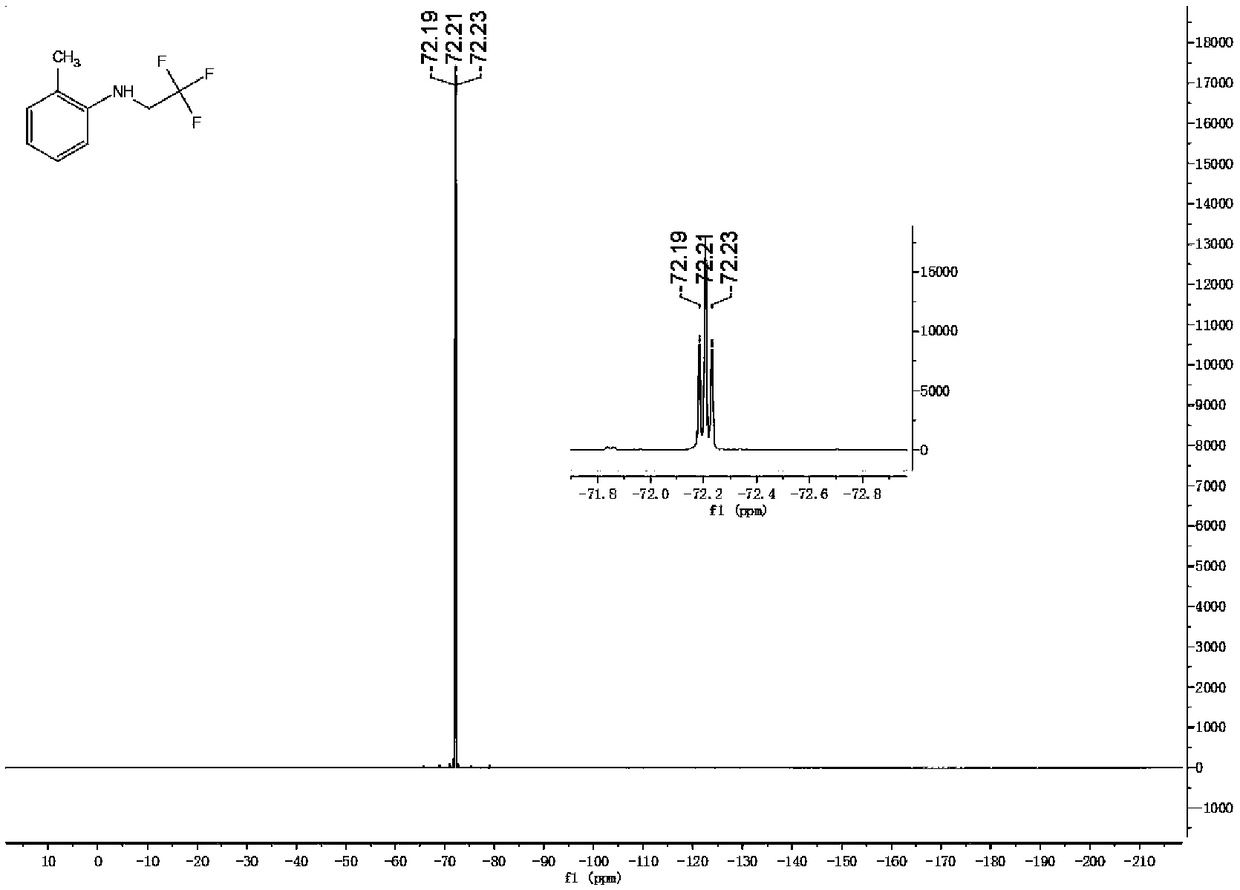
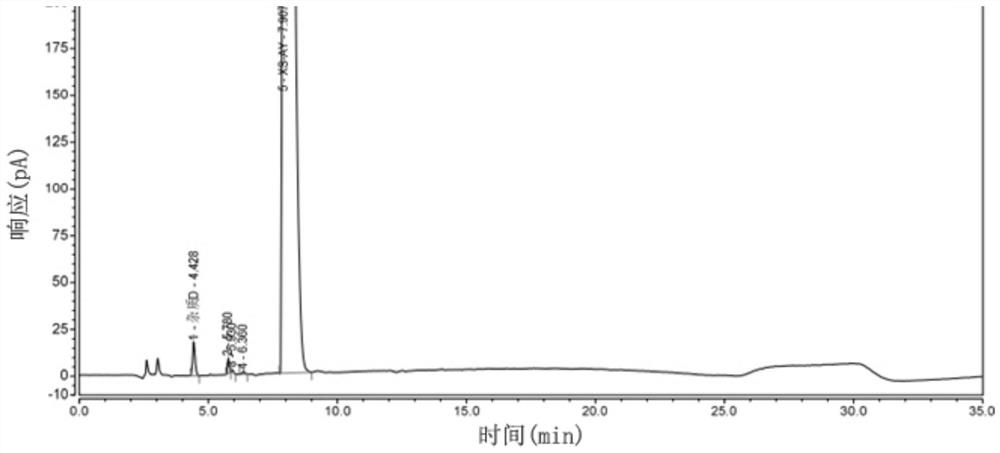
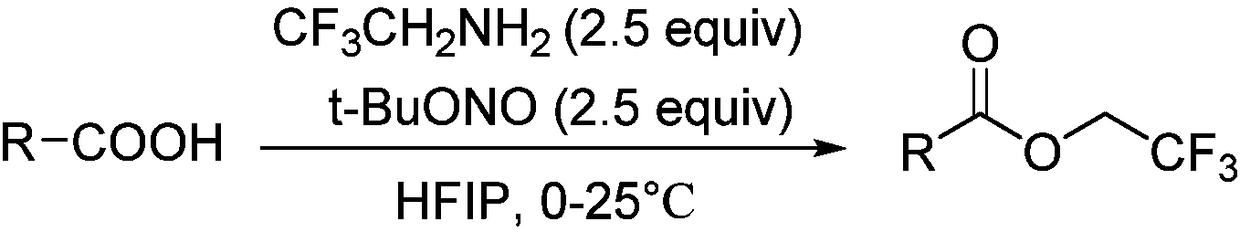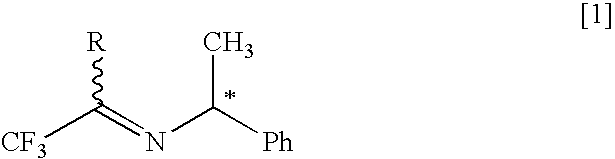Patents
Literature
33 results about "Trifluoroethylamine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Packaging 1, 5 g in ampule Application 2,2,2-Trifluoroethylamine was used in the determination of ibuprofen (2-(p-isobutylphenyl)propionic acid), a non-steroid anti-inflammatory drug (NSAID) residue in surface and wastewater samples.General description
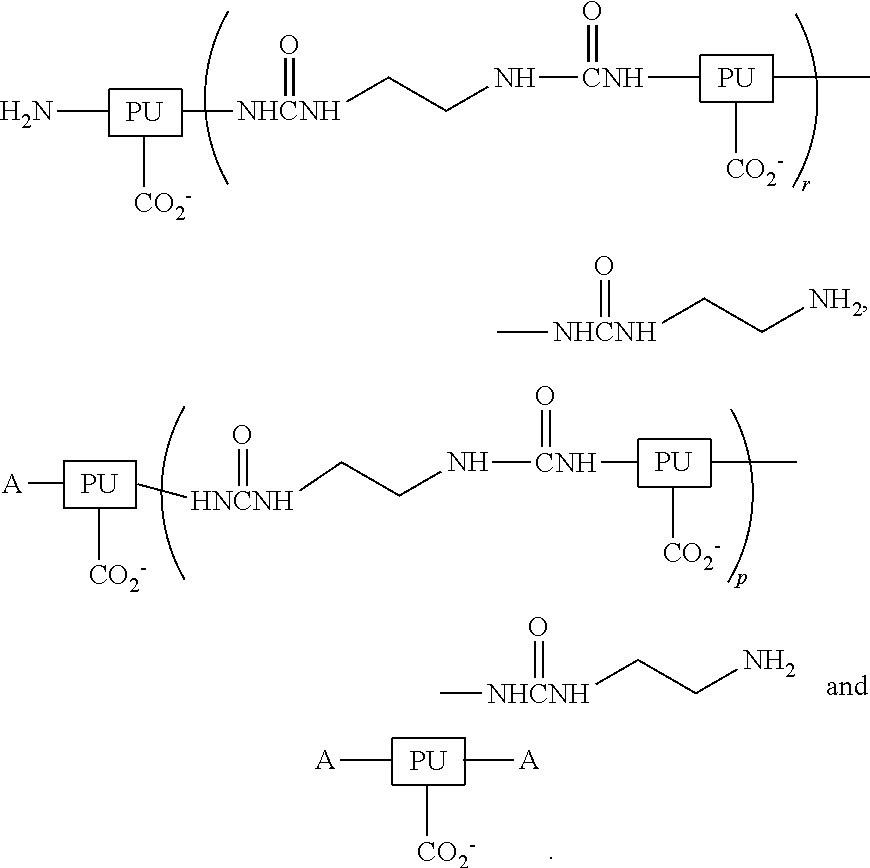
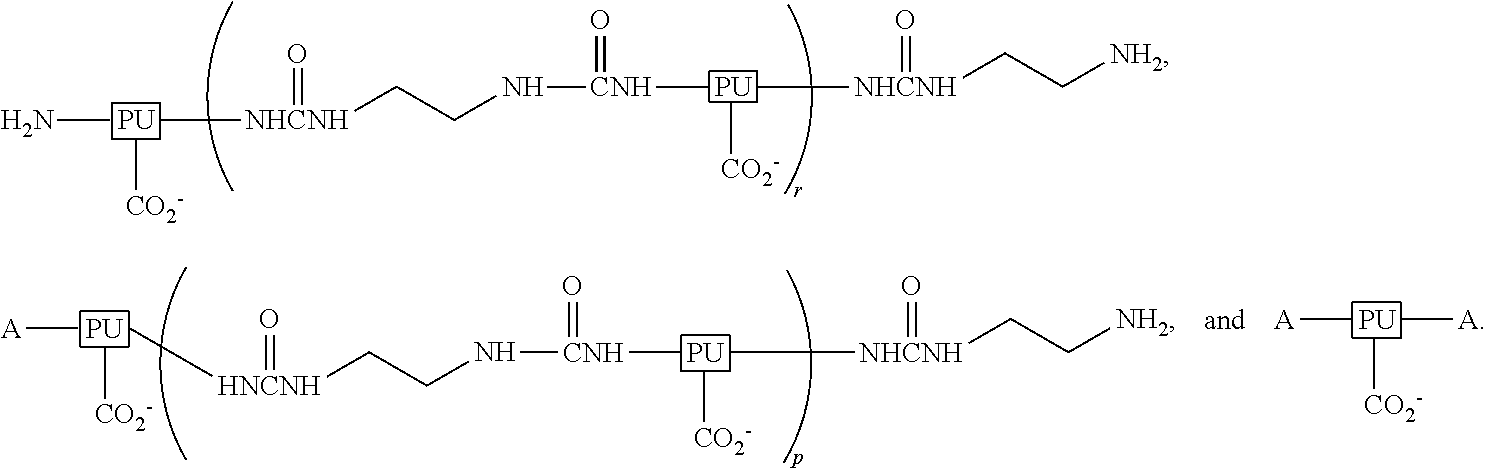
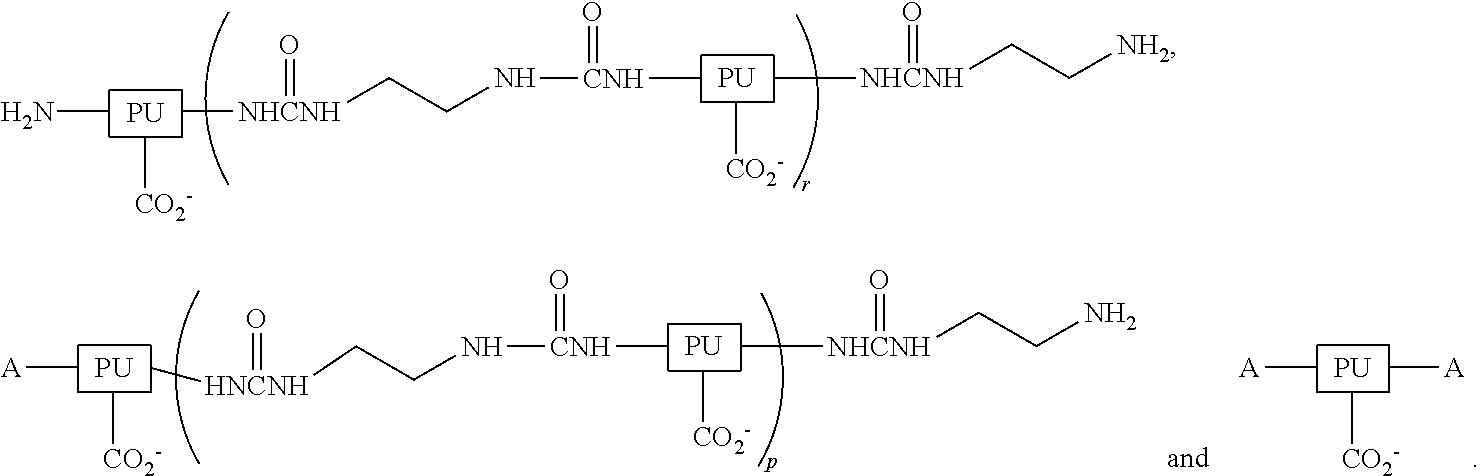
Ambient-temperature self-curable and fluorine containing aqueous-based polyurethane (PU) dispersion and method of manufacturing the same and its modified coated film applications
InactiveUS20120108741A1Improve adhesionImprove permeabilityPolyurea/polyurethane coatingsCross-linkPolyurethane dispersion
An ambient-temperature self-curable fluorine containing aqueous-based polyurethane dispersion, a method of manufacturing the same and its modified coated film applications are provided. The ambient-temperature self-curable fluorine containing aqueous-based PU dispersion includes water, an ambient temperature cross-linking agent and a fluorine containing PU resin dispersed in water phase. The fluorine containing PU resin includesA includes octa-fluoropentanol, hexafluoroisopropanol, trifluoroethanol, tetrafluoropropanol or trifluoroethylamine.Treated fabric with the self-cured fluorine containing aqueous-based PU resin and it becomes a long-lasting water-repellent and stain-proof.
Owner:CHOU TAI CHANG
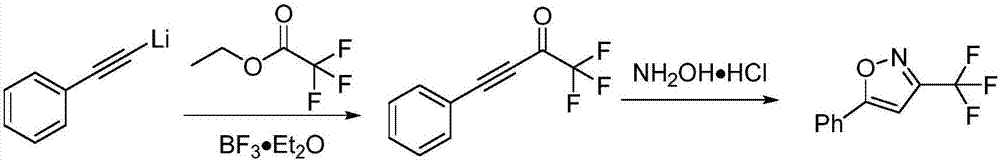
Method for preparing 3-trifluoromethylisooxazole compound by one-pot
ActiveCN107963996AEasy to operateMild conditionsGroup 4/14 element organic compoundsCopperCoupling reaction
The invention discloses a novel method for preparing a 3-trifluoromethylisooxazole compound by one-pot. According to the method, trifluoroethylamine which can be purchased in markets is prepared intofluorinated diazomethane, and then the fluorinated diazomethane and an alkynes compound are in coupled reaction under catalysis of low-price copper. The method is simple to operate, reaction conditions are mild, the cost is low, by-products are fewer, the yield is high, tolerance of a functional group is high, and reaction can be amplified. Meanwhile, much deeper mechanism study is carried out, and a mechanism that a trifluoromethyl ketoximes compound intermediate may be produced in the reaction is proposed.
Owner:JIANGXI NORMAL UNIV
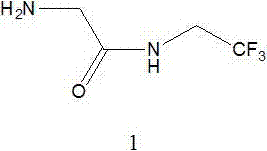
Method for preparing 2-amino-N-(2,2,2-trifluoroethyl)acetamide
InactiveCN107353222ALow priceEasy to operateOrganic compound preparationCarboxylic acid amides preparationHydrazine compoundReaction temperature
The invention relates to a method for preparing 2-amino-N-(2,2,2-trifluoroethyl)acetamide and its salt. The method for preparing 2-amino-N-(2,2,2-trifluoroethyl)acetamide and its salt comprises the following steps: reacting N-phthaloyl-protected glycine with trifluoroethylamine or its salts to form amide, removing the above protective group under the action of hydrazine hydrate to obtain crude2-amino-N-(2,2,2-trifluoroethyl)acetamide, carrying out a salt formation reaction on the crude 2-amino-N-(2,2,2-trifluoroethyl)acetamide and an acid to obtain the salt of the 2-amino-N-(2,2,2-trifluoroethyl)acetamide, and adding an alkali to dissociate the 2-amino-N-(2,2,2-trifluoroethyl)acetamide. The method has the advantages of low price of a deprotection reaction reagent used in the invention, simplicity in operation, no use of flammable and explosive hydrogen, realization of the reaction temperature from room temperature to the reflux temperature of a solvent, and mild reaction conditions, and is very suitable for the production of industrial devices.
Owner:荆门医药工业技术研究院
Preparation method of trifluoroethylamine
InactiveCN105906513ALow cost and readily availableEasy to operateAmino compound purification/separationAmino preparation by functional substitutionOrganic solventIodide
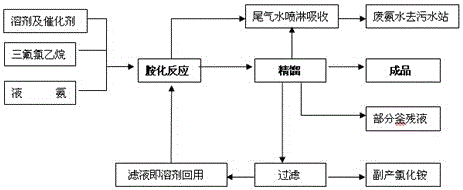
The invention discloses a preparation method of trifluoroethylamine. The preparation method comprises the following steps: amination reaction: taking CHF2CH2X as a raw material, and feeding ammonia gas in the presence of a solvent and a catalyst to ammonify so as to generate trifluoroethylamine; and rectification: rectifying the ammonified product to obtain a finished product, filtering kettle liquid to obtain ammonium chloride as a by-product, and recycling filtrate used as the solvent of amination reaction. The catalyst is inorganic iodide, and the solvent is a frequently used organic solvent DMF or NMP. The purity of the prepared finished product is greater than or equal to 99.5%, and the yield of the finished product is greater than or equal to 90%.
Owner:NANTONG BAOKAI CHEM
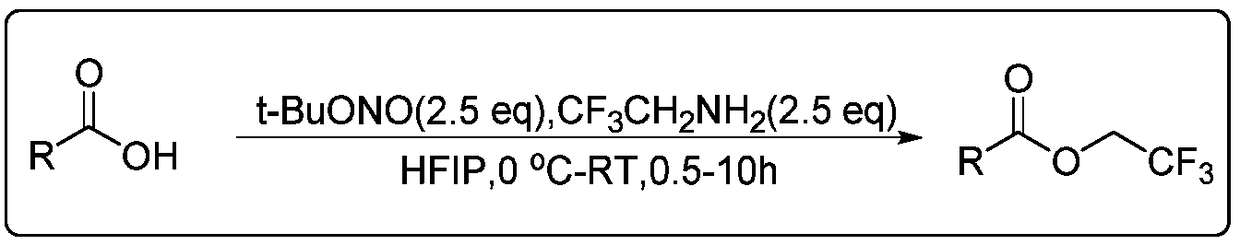
Aromatic carboxylic acid trifluoroethyl ester compound and preparation method thereof
InactiveCN108503549AEasy to operateMild conditionsCarboxylic acid nitrile preparationOrganic compound preparationDrugs synthesisN-butyl nitrite
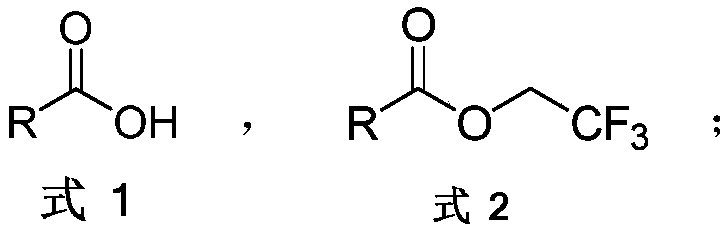
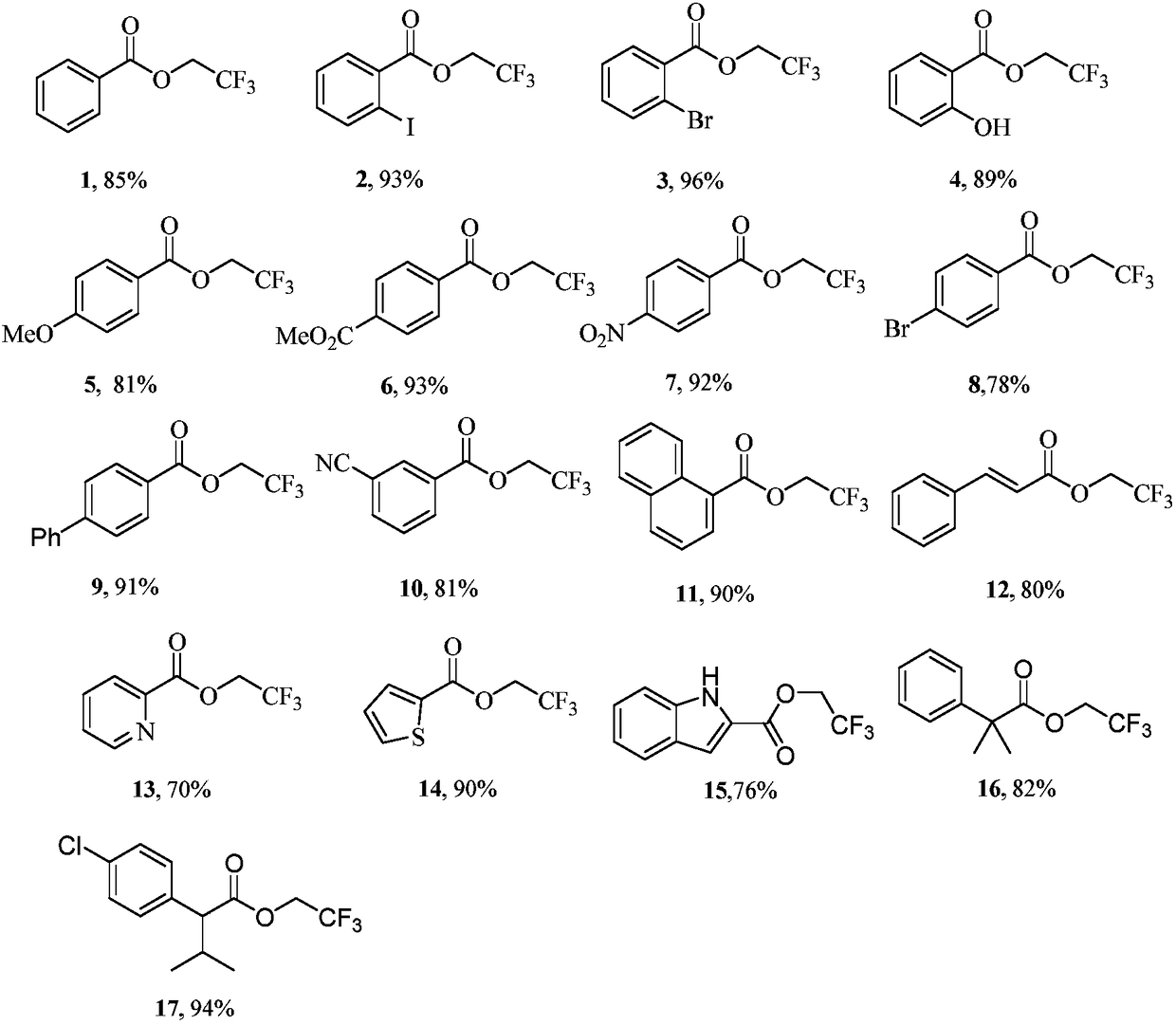
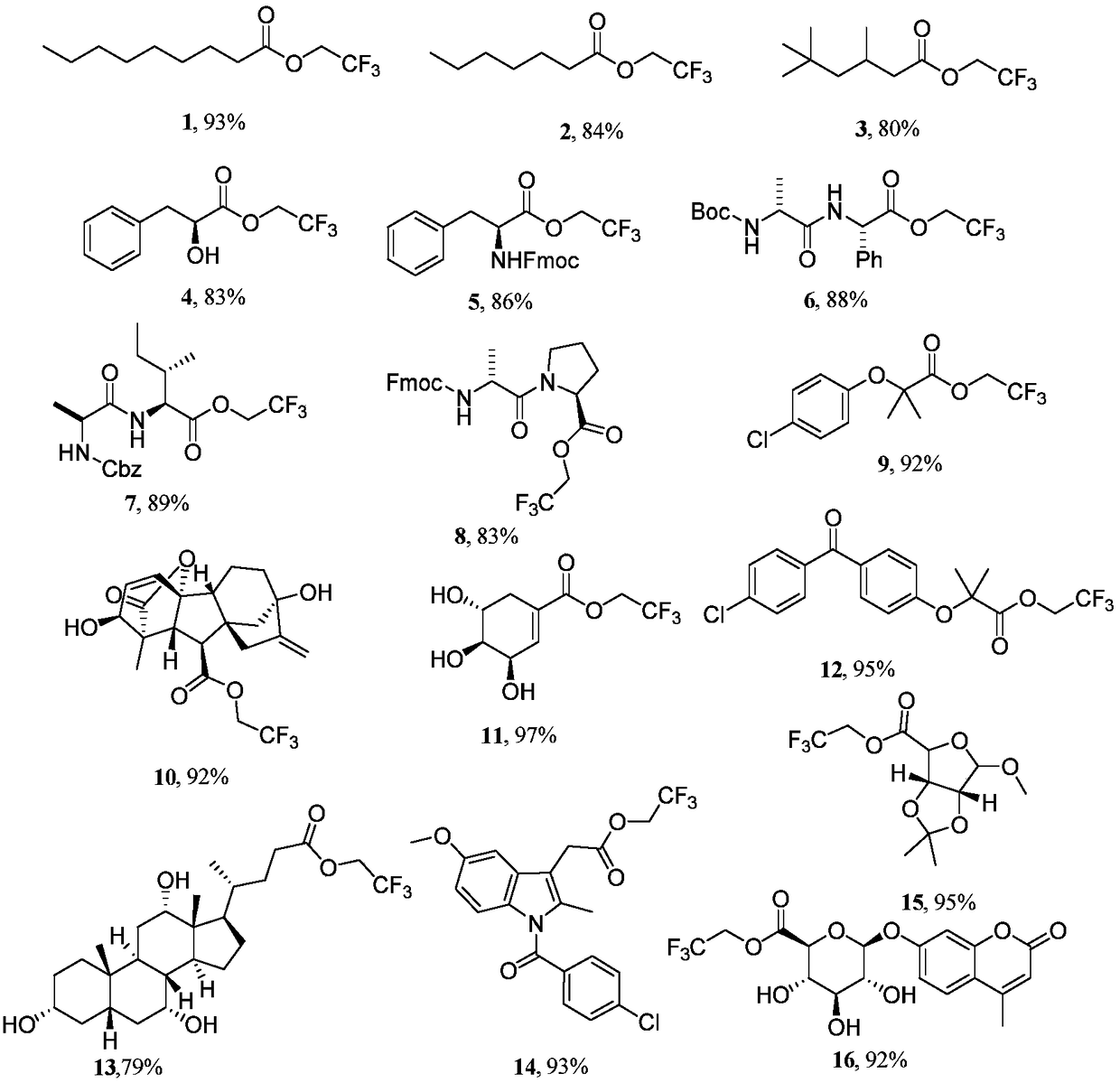
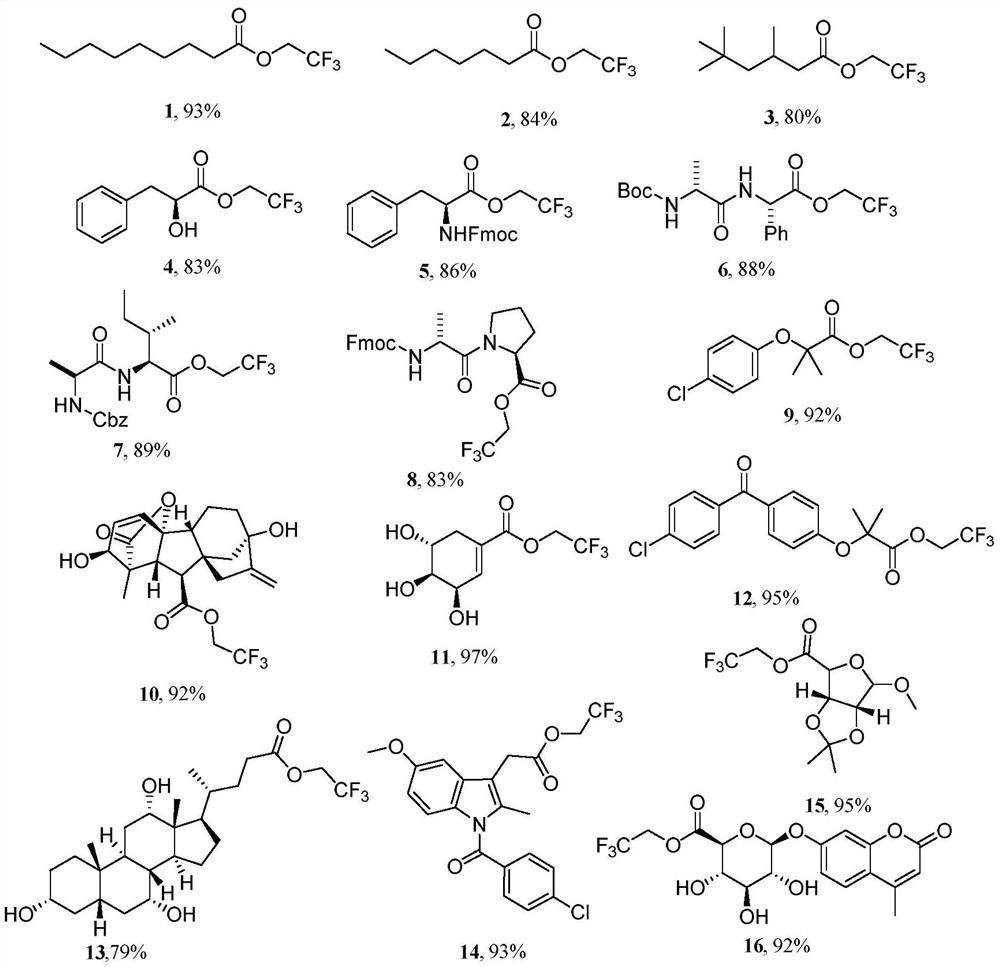
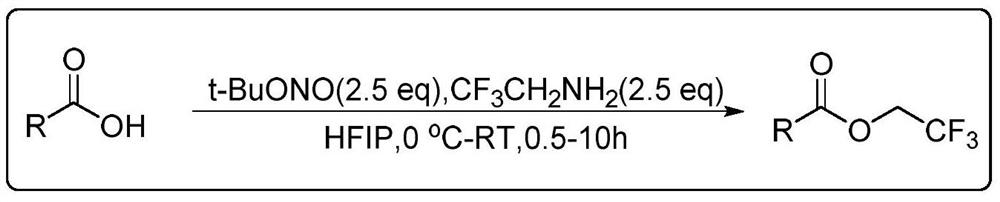
The invention discloses an aromatic carboxylic acid trifluoroethyl ester compound and a preparation method thereof. The invention provides the aromatic carboxylic acid trifluorethylmethyl ester compound and the preparation method thereof. The preparation method comprises the following step of performing reaction on aromatic carboxylic acid, t-butyl nitrite and 2,2,2-trifluoroethylamine with a one-pot method to obtain the carboxylic acid trifluoroethyl ester compound. The method is simple to operate, mild in reaction condition, low in cost, less in byproduct and high in yield, is capable of obtaining the aromatic carboxylic acid trifluoroethyl ester compound without being limited by a substrate so as to establish an aromatic carboxylic acid trifluoroethyl ester compound library and providesa raw material source to drug screening and new drug synthesis.
Owner:JIANGXI NORMAL UNIV
Preparation method for trifluoroethylamine hydrochloride
ActiveCN105801428AHigh purityReduce moistureOrganic compound preparationAmino compound preparationDistillationDesolvation
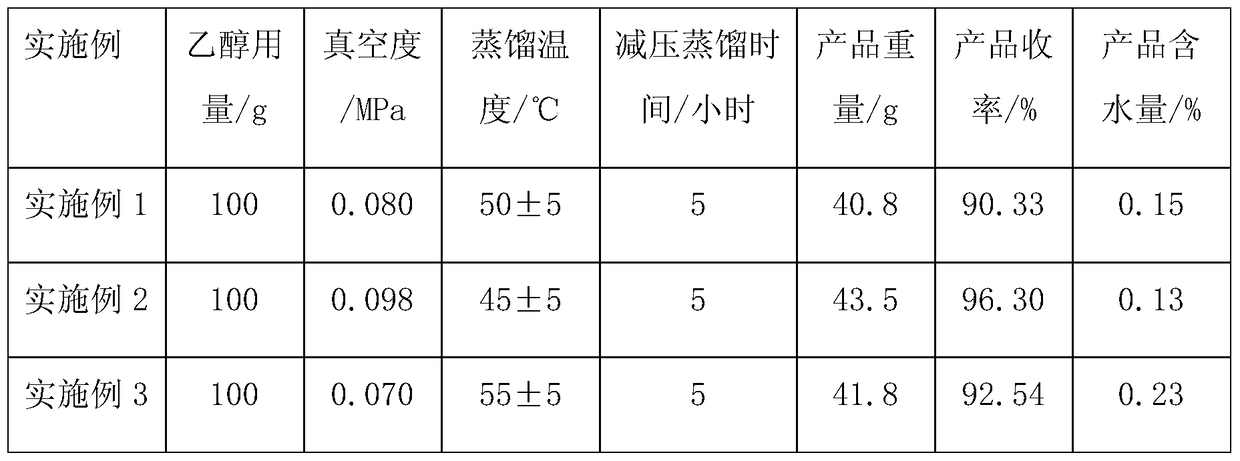
The invention discloses a preparation method for trifluoroethylamine hydrochloride. The preparation method comprises the following steps: subjecting trifluoroethylamine and hydrochloric acid to neutralization reaction in an ethanol-water azeotropic or near-azeotropic system so as to obtain a trifluoroethylamine hydrochloride contained solution after reaction, and removing water and ethanol through reduced pressure distillation and desolvation so as to obtain trifluoroethylamine hydrochloride. The method provided by the invention has safe process, and prepared trifluoroethylamine hydrochloride has high purity and low water content.
Owner:ZHEJIANG LANTIAN ENVIRONMENTAL PROTECTION HI TECH +1
Catalytic preparation method of trifluoroethylamine compound
InactiveCN109096121AImprove conversion rateMild reaction conditionsPreparation by reductive alkylationChemical recyclingChemical industryTrifluoroacetaldehyde hydrate
The invention provides a preparation method of 2,2,2-trifluoroethylamine. Trifluoroformaldehyde or trifluoroacetaldehyde hydrate and ammonia water are taken as raw materials and subjected to reactionin the presence of a mesoporous molecular sieve supported catalyst and a suitable hydrogen donor, a product is subjected to secondary reduction, and trifluoroethylamine is prepared. The process does not need high temperature or high pressure, has mild reaction conditions, high conversion rate, few byproducts and low cost, is clean and environmentally friendly, conforms to the trend of green chemical industry nowadays and has higher industrial production value, and a catalyst is easy to recycle.
Owner:张海英
Fat trifluoro ethyl ester compound and preparation method thereof
ActiveCN108707057AEasy to operateMild conditionsCarbamic acid derivatives preparationSugar derivativesIodo fatty acidNitrite
The invention relates to a fat trifluoro ethyl ester compound and a preparation method thereof. The definition of the fat acid trifluoro ethyl ester compound is same with the definition in the claim.The preparation method comprises the following steps that under the low temperature condition, 2, 2, 2-trifluoroethylamine and tert-butyl nitrite are added into an organic solution with fatty acid dissolved in, and after uniformly stirring, the mixture reacts continuously at the indoor temperature, and a fatty acid trifluoro ethyl ester product can be obtained. The molar ratio among fat acid, 2, 2, 2-trifluoroethylamine and tert-butyl nitrite is 1:(1-2.5):(1-2.5). According to the preparation method, it is not necessary to add catalyst and other additives, and a target product can be obtained,the operation is simple, reaction conditions are mild, the cost is low, the yield is high, the method is environmentally friendly, the trifluoro ethyl ester compound of fat and drug molecule carboxylic acid can be obtained, and the application is wide.
Owner:JIANGXI NORMAL UNIV
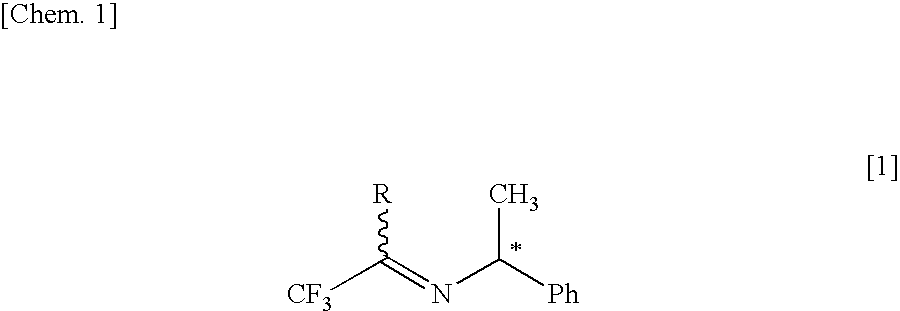
Process for producing optically active 1-alkyl-substituted 2,2,2-trifluoroethylamine
InactiveUS20060281950A1Organic compound preparationAmino compound preparationHydrogen atmosphereMetal catalyst
The present invention relates to a process for producing an optically active 1-alkyl-substituted 2,2,2-trifluoroethylamine, which is an important intermediate of medicines and agricultural chemicals, and which is represented by the formula [3] [in the formula R represents a lower alkyl group of a carbon and * represents an asymmetric carbon], or its salt by subjecting an optically active imine represented by the formula [1] to an asymmetric reduction under hydrogen atmosphere using a metal catalyst of Group VIII to convert it into an optically active secondary amine represented by the formula [2] and then by subjecting the secondary amine or its salt to hydrogenolysis. [Chem. 23]
Owner:CENT GLASS CO LTD
Preparation method of 2,2,2-trifluoroethylamine
ActiveCN101973888BShort reaction timeHigh yieldAmino compound purification/separationAmino preparation by functional substitutionFluid phaseAmmonia
The invention discloses a preparation method of 2,2,2-trifluoroethylamine, which comprises the following steps of: (1) mixing liquid-phase 1,1,1-trifluoro-2-chloroethane dissolved into glycerin with ammonia, wherein the volume ratio of glycerin to 1,1,1-trifluoro-2-chloroethane is 1-3:1, the molar ratio of ammonia to 1,1,1-trifluoro-2-chloroethane is 8-15:1, and the concentration of ammonia ranges from 30wt% to 100wt%; reacting in a pipeline-type reactor for 20min to 30min at 150 DEG C and 200 DEG C under the pressure of 2MPa and 4MPA so as to obtain a mixed solution containing 2,2,2-trifluoroethylamine, wherein the flowing velocity of the reactant in the pipeline-type reactor is 2.0-4.0L / h; and (2) carrying out pressure reduction, flashing and deamination on the mixed solution of 2,2,2-trifluoroethylamine, and adding the liquid subjected to deamination into sodium carbonate for neutralizing , wherein the molar ratio of sodium carbonate to 1,1,1-trifluoro-2-chloroethane is 0.5-2:1; carrying out pressure reduction and rectification on the neutralized solution to obtain 2,2,2-trifluoroethylamine. The method has the advantages of short reaction time and high product yield.
Owner:XIAN MODERN CHEM RES INST
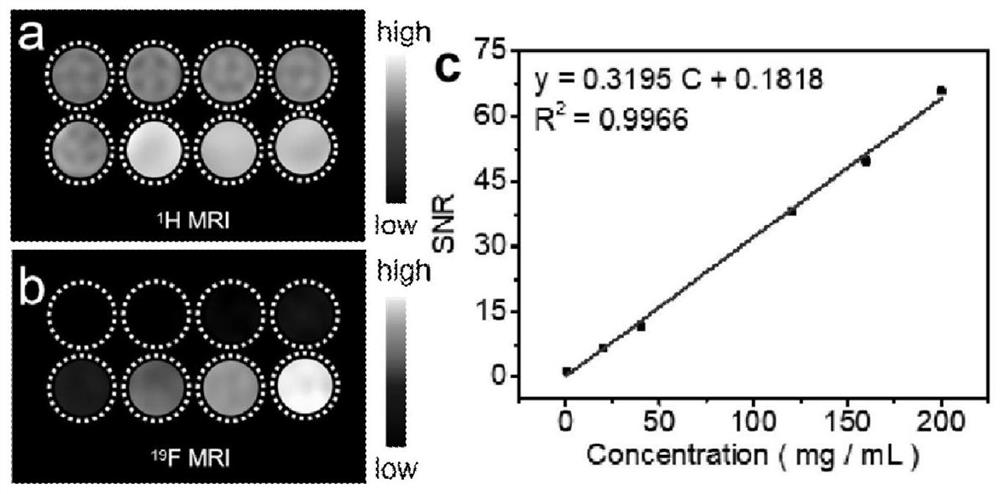
Preparation method of 19F magnetic resonance imaging nanoprobe
InactiveCN111467508AImprove stabilityLong-term storage at room temperatureEmulsion deliveryIn-vivo testing preparationsBiocompatibilityBiomedicine
The invention discloses a preparation method of a 19F magnetic resonance imaging nanoprobe. The method comprises the following steps: in a polar organic solvent, carrying out an imide ring-opening reaction on polysuccinimide and trifluoroethylamine in a hydrothermal kettle with addition or no addition of amino-PEG, carrying out partial high-temperature carbonization, and then carrying out self-assembly to obtain the 19F magnetic resonance imaging nanoprobe. The probe has a good 19F NMR signal and can be directly used for 19F magnetic resonance imaging. The preparation method is simple and rapid and is low in cost, and the obtained nanoprobe is uniform in particle size distribution and good in biocompatibility, does not need hydrophilic modification, is high in stability and can be stored at normal temperature for a long time. The probe can attract wide attention in nano material science and biomedical science, and has important application prospects in biomedical fields such as biosensors, cell imaging, medical diagnosis and the like.
Owner:BEIJING UNIV OF CHEM TECH
Method for synthesizing second-level trifluoromethyl propargyl alcohol
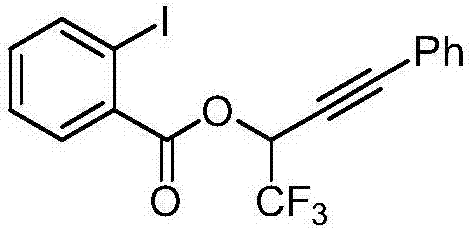
InactiveCN106892936ANothing producedGood chemical stabilitySilicon organic compoundsOxygen-containing compound preparationBenzoic acidKetone
The invention discloses a method for synthesizing second-level trifluoromethyl propargyl alcohol. The method comprises the following steps: enabling 2-iodo benzoic acid and sodium periodate to react so as to generate 1-(hydroxyl)-1,2-iodobenzoyl-3(1H)-ketone (BIOH), generating trimethylsilylacetylene and triisopropylchlorosilane to generate trimethylsilyl (triisopropyl silicon) acetylene, synthesizing 1-[(triisopropyl silicon) acetylene]-1,2-iodobenzoyl-3(1H)-ketone from BIOH and trimethylsilyl (triisopropyl silicon) acetylene, enabling 1-[(triisopropyl silicon) acetylene]-1,2-iodobenzoyl-3(1H)-ketone and trifluoroethylamine hydrochloride and sodium nitrite to synthesize 1-[(triisopropyl silicon) acetylene]-1,2-iodobenzoyl-3(1H)-ketone, and finally performing hydrolysis, thereby synthesizing a target product. The trifluoromethyl propargyl alcohol prepared by using the method is an important fluorine-containing building block molecule, and has very great significances for synthesis of fluorine-containing functional organic molecules and medicinal intermediates.
Owner:HEFEI UNIV OF TECH
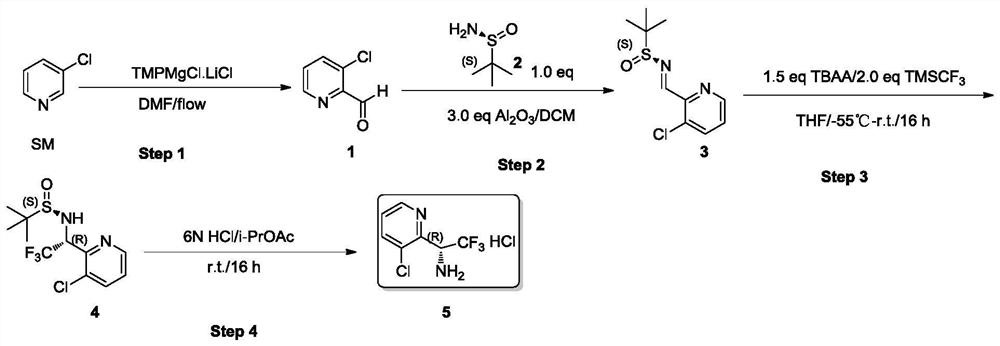
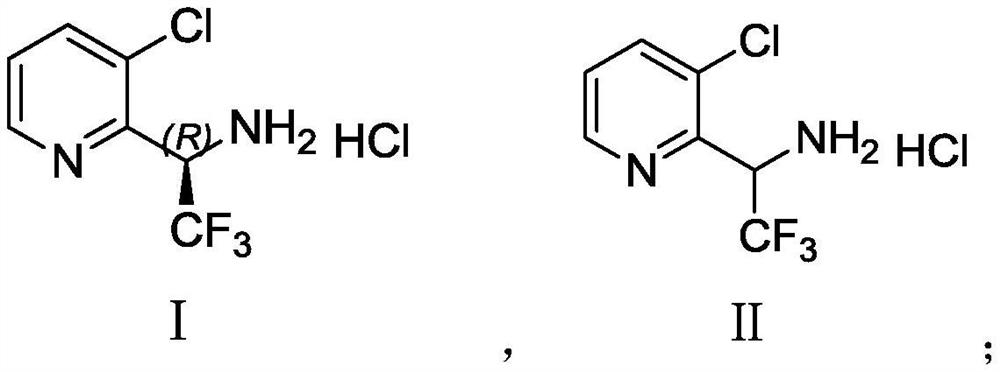
Synthesis method of (R)-3-chloropyridyl-2-trifluoroethylamine hydrochloride
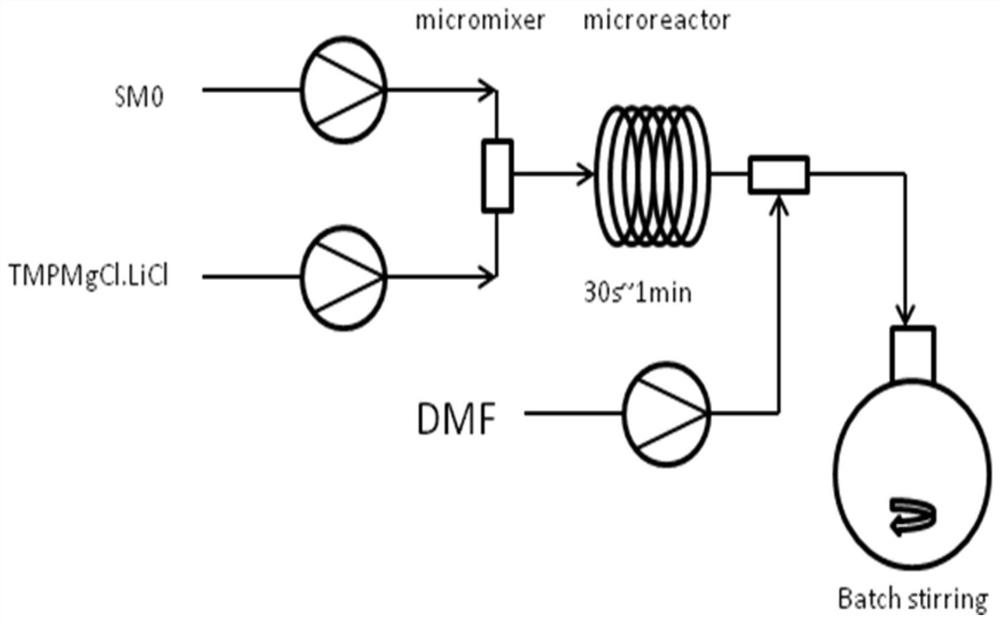
InactiveCN112679421AEfficient mixingHigh chiral purityOrganic chemistryReaction temperatureCombinatorial chemistry
The invention discloses a synthesis method of (R)-3-chloropyridyl-2-trifluoroethylamine hydrochloride, and the synthesis method is characterized in that a Grignard reaction, a chiral induction addition reaction and a deprotection reaction are sequentially carried out to finally prepare a finished product. The invention provides a brand-new synthesis method of the (R)-3-chloropyridyl-2-trifluoroethylamine hydrochloride for the first time. The synthetic method is short in route, raw materials are easy to obtain, operation is easy, a continuous flow microreactor technology is adopted in the first step, reactants are rapidly and effectively mixed, the reaction time and the reaction temperature are accurately controlled, side reactions are avoided, and the conversion rate and safety are effectively improved. The compound 4 with high chiral purity is obtained through chiral induction addition reaction and recrystallization purification, and the de value of the compound 4 is greater than 99.5%. The post-treatment method is simple in post-treatment operation, column chromatography treatment is not needed after each step of reaction, reactants can be purified only through washing and extraction of a solvent, and the method is a novel post-treatment purification method.
Owner:BIRDO (SHANGHAI) PHARMATECH CO LTD
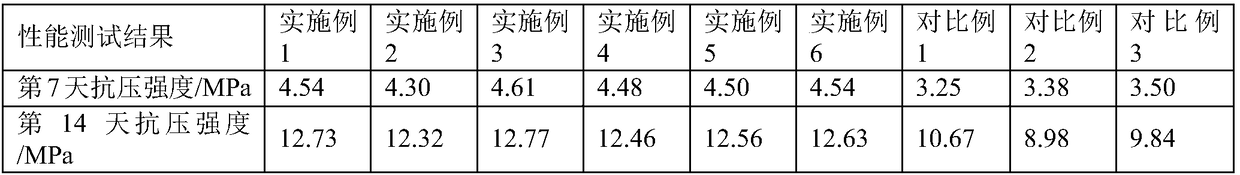
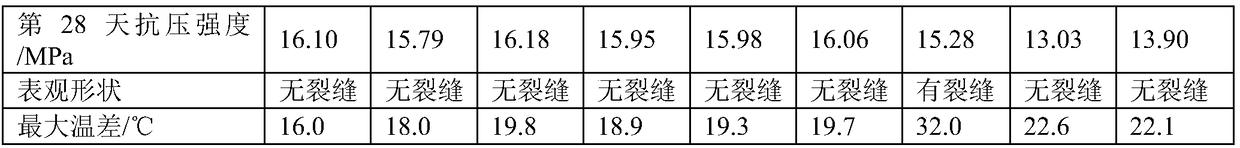
Portland cement and preparation method thereof
The invention relates to Portland cement which is prepared from the following raw materials in parts by weight: 60-70 parts of clinker, 15-19 parts of superfine slag, 5-7 parts of limestone, 5-7 partsof gypsum and 6-9 parts of admixture, wherein the admixture is prepared from silica fume, calcium methacrylate hydrate, 2,2,2-trifluoroethylamine hydrochloride and 2,2-difluoropropylamine hydrochloride. In the early stage of hydration of the Portland cement, the fluorine ions and calcium ions ionized from 2,2,2-trifluoroethylamine hydrochloride and 2,2-difluoropropylamine hydrochloride are utilized to form calcium fluoride which is crystallized more easily than calcium hydroxide, thus a large quantity of calcium ions are consumed, a double-electrode-layer structure is hard to form on the C3Ssurface, the induction period of C3S is shortened and even disappears, the hydration process is quickly accelerated, and the heat release rate is increased in the early stage of hydration. Therefore,the heat release rate of the cement in the hardening and maintaining stage fluctuates between 5% and 10%, the hardened cement has relatively small cracks, and the strength of a cement test piece is improved.
Owner:天津山水水泥有限公司
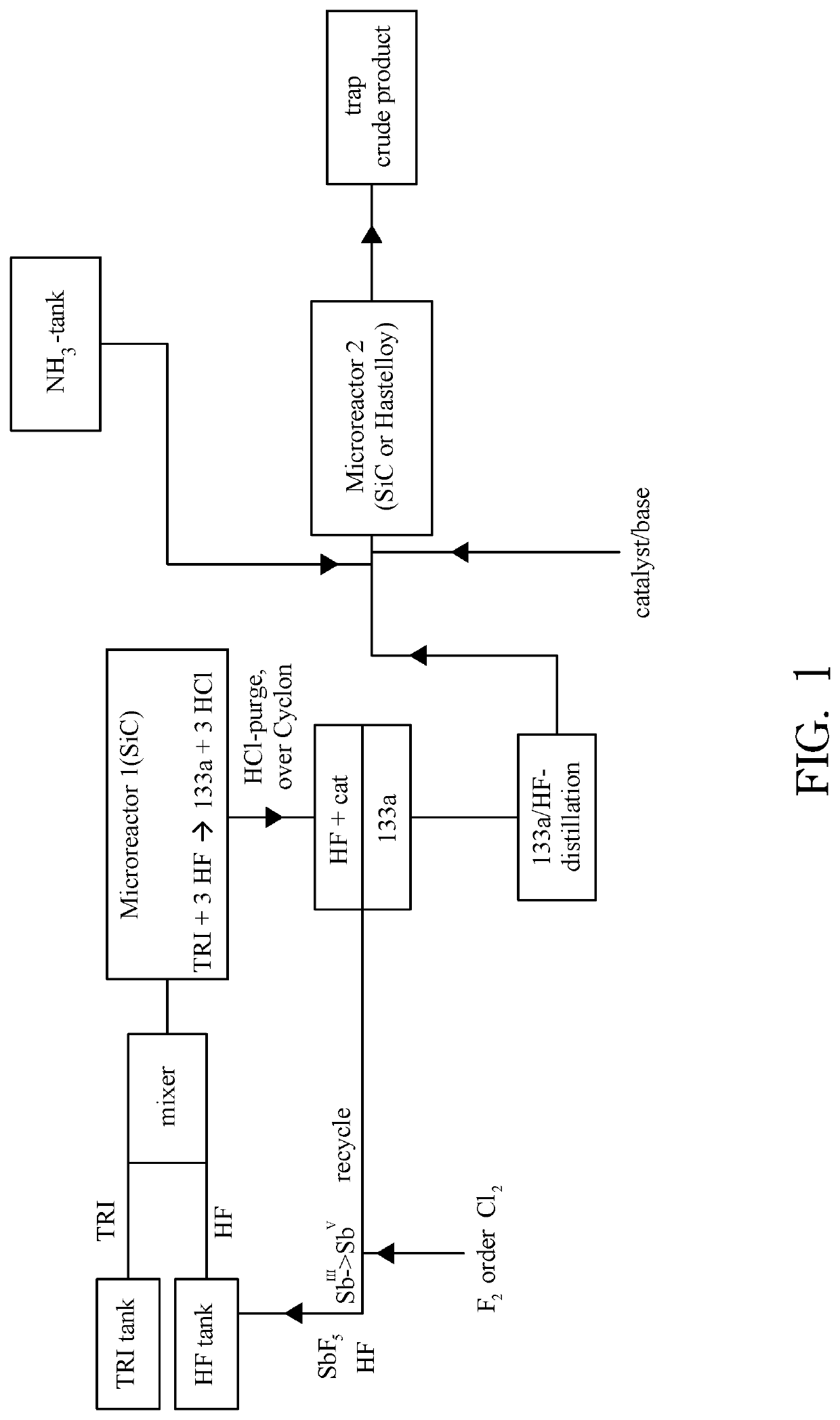
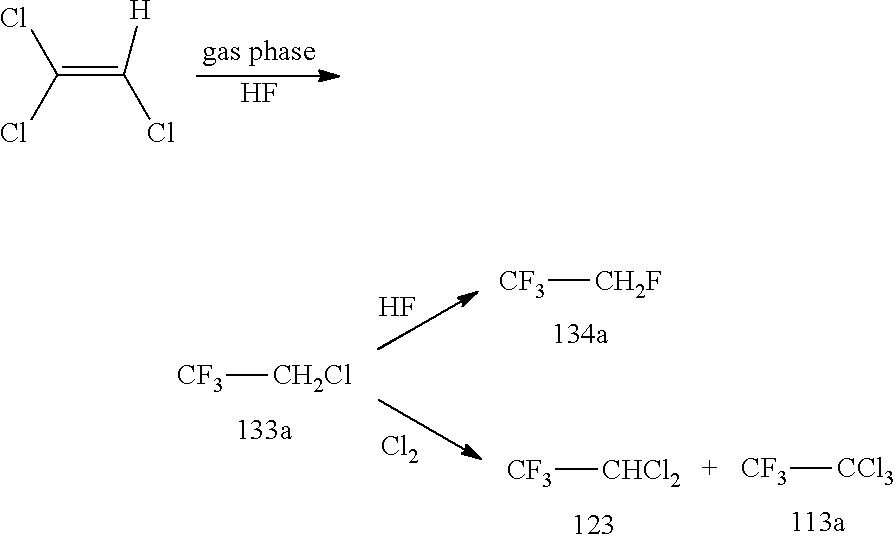
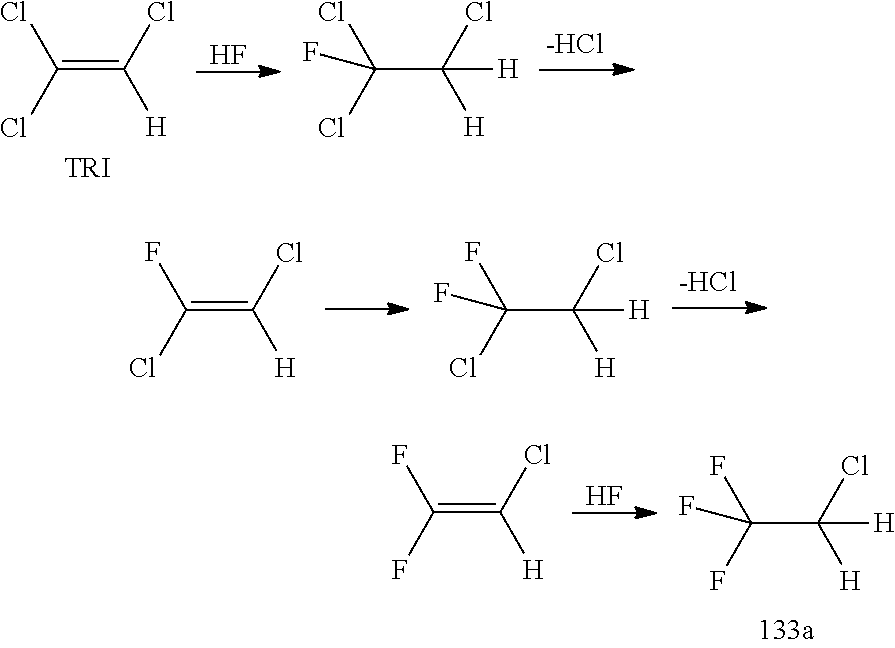
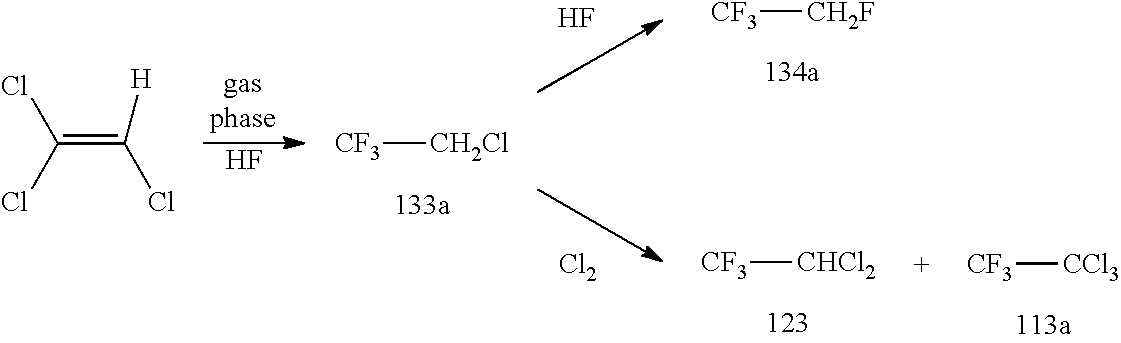
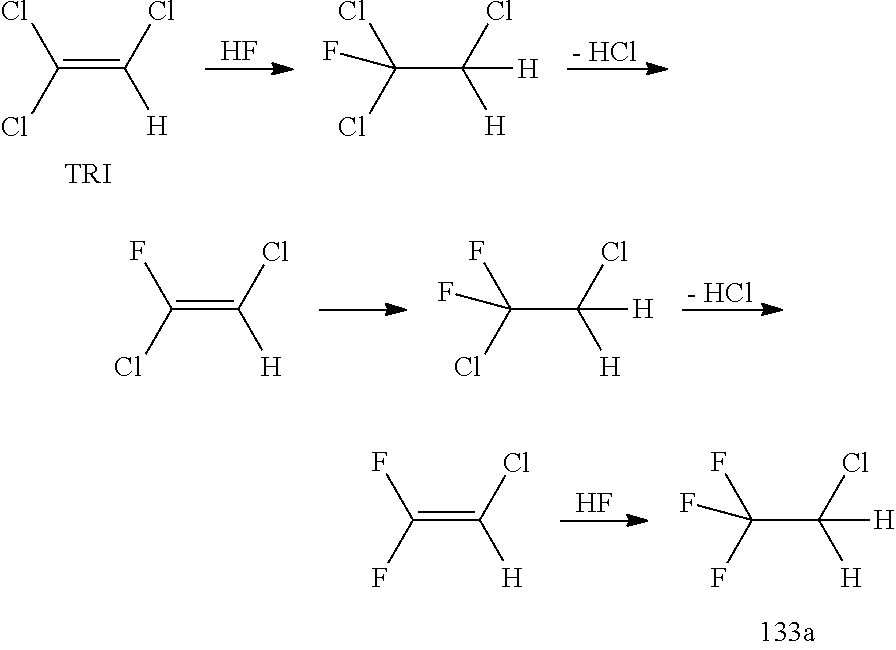
New Process for the Manufacture of 1,1,1-Trifluoro-2-Chloroethaneand/or Trifluoroethylamine
ActiveUS20200148624A1Simplify and reduce numberAvoid a lot of timePreparation by hydrogen halide split-offPhysical/chemical process catalystsHydrogen halideAryl
The invention relates to a new process for the manufacture of fluoroaryl compounds and derivatives thereof, in particular of fluorobenzenes and derivatives thereof, and especially wherein said manufacture relates to an environmentally friendly production of the said compounds. Thus, the present invention overcomes the disadvantages of the prior art processes, and in a surprisingly simple and beneficial manner, and as compared to the prior art processes, in particular, the invention provides a more efficient and energy saving processes, and also provides a more environmentally friendly process, for the manufacture of nuclear fluorinated aromatics, and preferably of nuclear fluorinated fluorobenzenes. Accordingly, in one aspect of the invention, an industrially beneficial process for preparing fluorobenzenes from halobenzene precursors using HF to form hydrogen halide is provided by the present invention. A beneficial and surprisingly simple use of chlorobenzene as an industrially interesting starting material in the manufacture of fluorobenzene is provided.
Owner:FUJIAN YONGJING TECH CO LTD
A method for trifluoroethylation of aromatic secondary amines catalyzed by iron porphyrin
ActiveCN108997145BMild reaction conditionsHigh yieldOrganic-compounds/hydrides/coordination-complexes catalystsCatalytic reactionsPtru catalystPorphyrin
The invention provides a method of catalyzing trifluoro-ethylation of aromatic secondary amine by ferriporphyrin. The method comprises the following steps: adding trifluoroethylamine salt and nitriteto a diazo-reaction first and then adding aromatic primary amine and a ferriporphyrin catalyst for a trifluoro-ethylation reaction to obtain a trifluoro-ethylated aromatic secondary amine compound inan acidic solution system. The reactions of the method are carried out at room temperature, and the reaction condition is mild, and an intermediate product is not separated through a one-pot reaction.The method is few in reaction step, simple to operate, wide in application range of primers, wide in source of raw materials and high in yield of reaction products.
Owner:YUANJIANG HUALONG CATALYST TECH
Method for 2, 5-dimethoxyacyl-1, 4-cyclohexanedione amination by reaction kettle
InactiveCN108440317AStrong fluorescenceThe synthesis method is simpleOrganic compound preparationAmino-carboxyl compound preparationAlcoholFluorescence
The invention discloses a method for 2, 5-dimethoxyacyl-1, 4-cyclohexanedione amination by a reaction kettle. According to the invention, alcohol is adopted as the solvent in a reaction kettle, methylamine, aniline, 2, 2, 2-trifluoroethylamine, 2-methoxyethylamine, ethylamine or isopropylamine is respectively employed for reaction with 2, 5-dimethoxyacyl-1, 4-cyclohexanedione to obtain 1, 4-dialkylamino-2, 5-dimethoxyacyl-1, 4cyclohexanedione derivatives, which have strong fluorescence under both solid and solution states, also a reflux condensation device used in the conventional organic synthesis is avoided, and the cost is saved.
Owner:BEIJING UNIV OF CHEM TECH
Process for the Manufacture of Trifluoroethylamine
ActiveUS20210017118A1Simplify and reduce numberAvoid a lot of timePreparation by hydrogen halide split-offPhysical/chemical process catalystsHydrogen halideAryl
The invention relates to a new process for the manufacture of fluoroaryl compounds and derivatives thereof, in particular of fluorobenzenes and derivatives thereof, and especially wherein said manufacture relates to an environmentally friendly production of the said compounds. Thus, the present invention overcomes the disadvantages of the prior art processes, and in a surprisingly simple and beneficial manner, and as compared to the prior art processes, in particular, the invention provides a more efficient and energy saving processes, and also provides a more environmentally friendly process, for the manufacture of nuclear fluorinated aromatics, and preferably of nuclear fluorinated fluorobenzenes. Accordingly, in one aspect of the invention, an industrially beneficial process for preparing fluorobenzenes from halobenzene precursors using HF to form hydrogen halide is provided by the present invention. A beneficial and surprisingly simple use of chlorobenzene as an industrially interesting starting material in the manufacture of fluorobenzene is provided.
Owner:FUJIAN YONGJING TECH CO LTD
Preparation method of alternating fluorine-containing polymer magnetic resonance imaging probe
ActiveCN113876967AImprove hydrophobicityImprove hydrophilicityNanomedicineNanosensorsEpoxySide chain
The invention discloses a preparation method of an alternating fluorine-containing polymer magnetic resonance imaging probe. According to the preparation method, an epoxy group in an ethylene oxide derivative is used as a modifiable point to connect a fluorine-containing group and a hydrophobic long chain, in the obtained polymer, a fluorine signal is provided by triethylamine, oleylamine increases the hydrophobicity of the polymer, and hydroxyl generated after ring opening of epoxy and amino increases the hydrophilicity of the polymer. The framework structure of the alternating polymer has a polyethylene glycol-like structure, the biocompatibility is good, the polarity is relatively strong, the chemical microenvironment of a side chain containing a fluorinated functional group is consistent, the half-peak width of 19F NMR is narrow, the position of a magnetic resonance peak is single, the dipole-dipole interaction between the fluorine-containing functional groups is weak, and the imaging signal intensity is improved. The alternating polymer is self-assembled to obtain the alternating fluorine-containing polymer magnetic resonance imaging probe, and the probe has a relatively good application prospect in the aspects of diagnosis and treatment of diseases.
Owner:BEIJING UNIV OF CHEM TECH
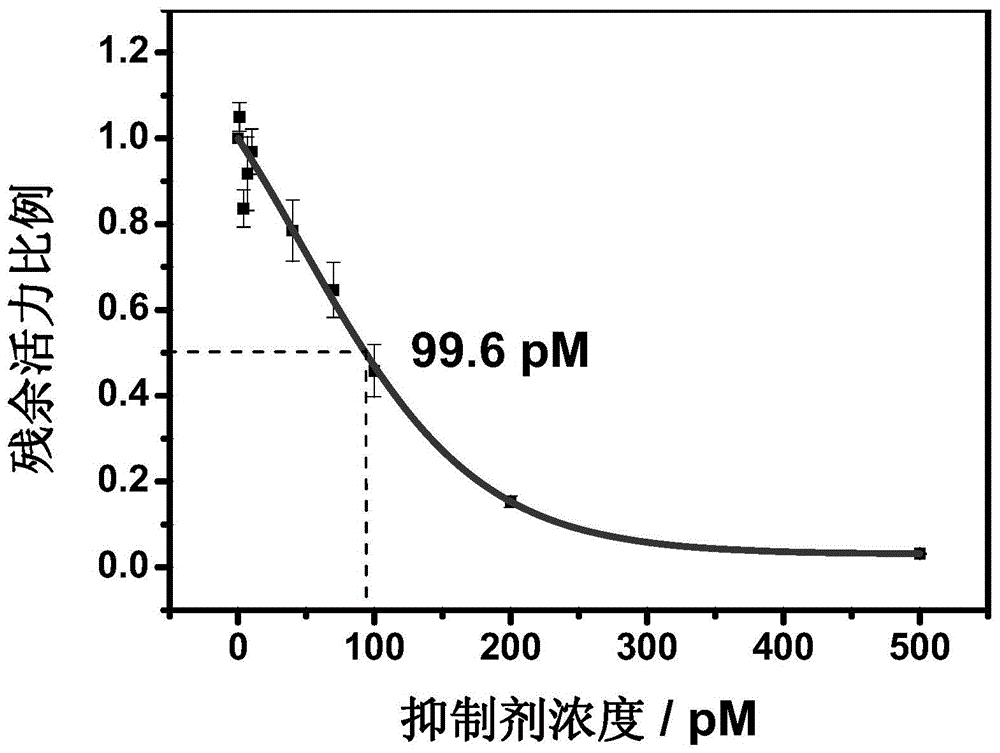
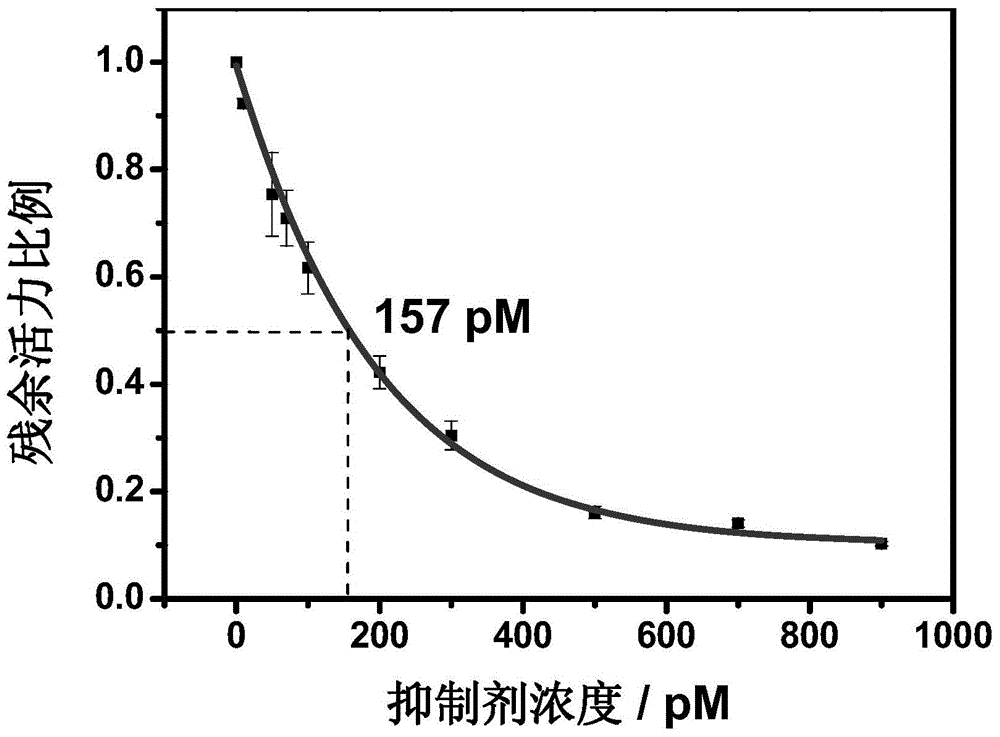
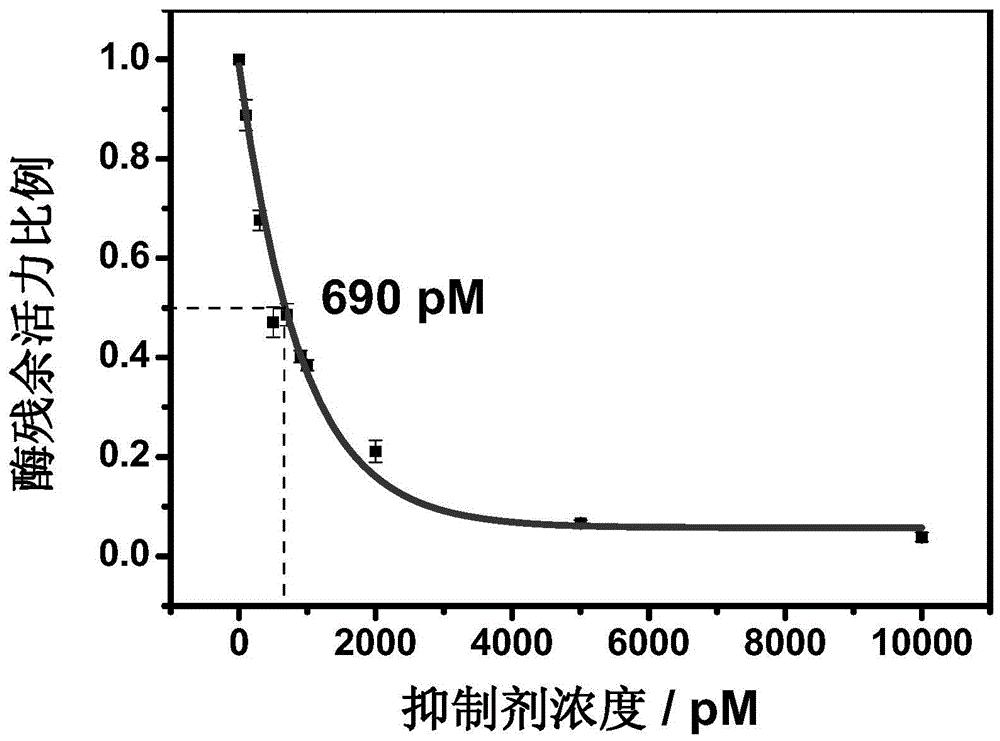
Hydrazonil Cathepsin K Inhibitor Using Trifluoroethylamine as P2-P3 Linking Group and Its Application
The invention belongs to the technical field of cathepsin K inhibitors, and particularly relates to novel nitrile cathepsin K inhibitors using cathepsin K as target protein and trifluoroethylamino groups as P2-P3 linkers and having different P3 position structures. The P2-P3 linkers are trifluoroethylamino groups, P3 positions are aryl groups comprising a bromophenyl group, a p-biphenyl group, a p-terphenyl group and a p-methyl biphenyl group. The nitrile inhibitors have the advantages that the structures are simple relatively, the synthesis is convenient, and the yield is high; inhibition constants (Ki) of the compounds (the inhibitors) to cathepsin K are in the picomole level, so that the effective inhibiting ability to the cathepsin K is reflected; when the P3 position is the P-terphenyl group, the inhibiting ability and the selectivity of the inhibitor to the Cat K (the cathepsin K) are better than those of corresponding amide inhibitors. In vitro cytotoxicity tests show that the inhibitors are free of cytotoxicity, and have the possibility of being applied to clinical trials and being used as potential anti-osteoporosis drug.
Owner:JILIN UNIV
Process for producing fluoro-compounds
InactiveUS20090030228A1Low cost productionLess-costly and readily handleableOrganic compound preparationCarboxylic acid esters preparationArylHydrogen atom
The present invention provides a process for producing highly pure fluoro-compounds by making use of less costly and readily handleable N-(2-chloro-1,1,2-trifluoroethyl)diethylamine. The process produces little or no chlorinated by-products.Specifically the present invention provides a process for producing a fluoro-compound, comprising fluorinating an alcohol derivative represented by the following general formula (1):R1R2R3COH (1)(wherein R1 represents a hydrogen atom, a substituted or unsubstituted alkyl group, aryl group, alkylcarbonyl group, alkoxycarbonyl group, arylcarbonyl group or aryloxycarbonyl group; and R2 and R3are each independently a substituted or unsubstituted alkyl group, aryl group, alkylcarbonyl group, alkoxycarbonyl group, arylcarbonyl group or aryloxycarbonyl group, wherein at least two of R1, R2 and R3 may together form part of a ring structure, either with or without a heteroatom) with N,N-diethyl-2-chloro-1,1,2-trifluoroethylamine to form a fluoro-compound represented by the following general formula (2):R1R2R3CF (2)(wherein R1, R2 and R3 are as defined above), the process being characterized in that an alcohol derivative represented by the following general formula (3):R4OH (3)(wherein R4 represents a substituted or unsubstituted alkyl group or aryl group) is added to the reaction system.
Owner:TOSOH F TECH INC
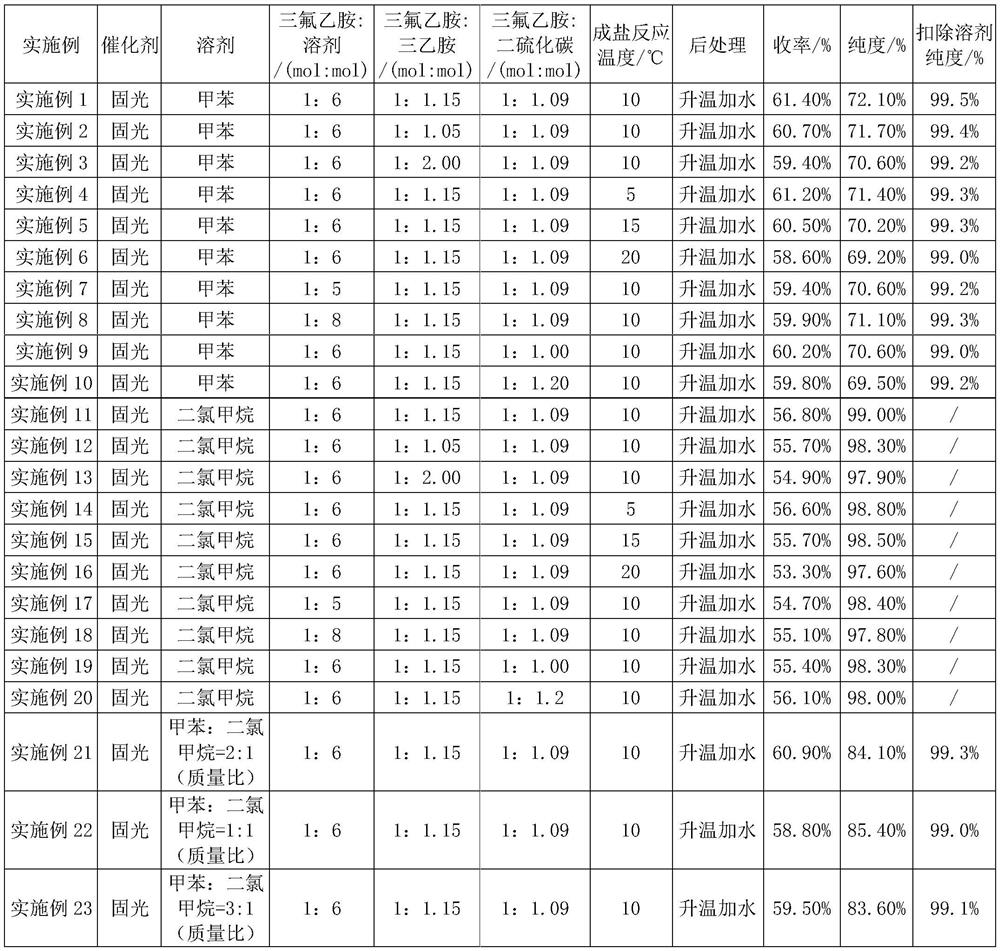
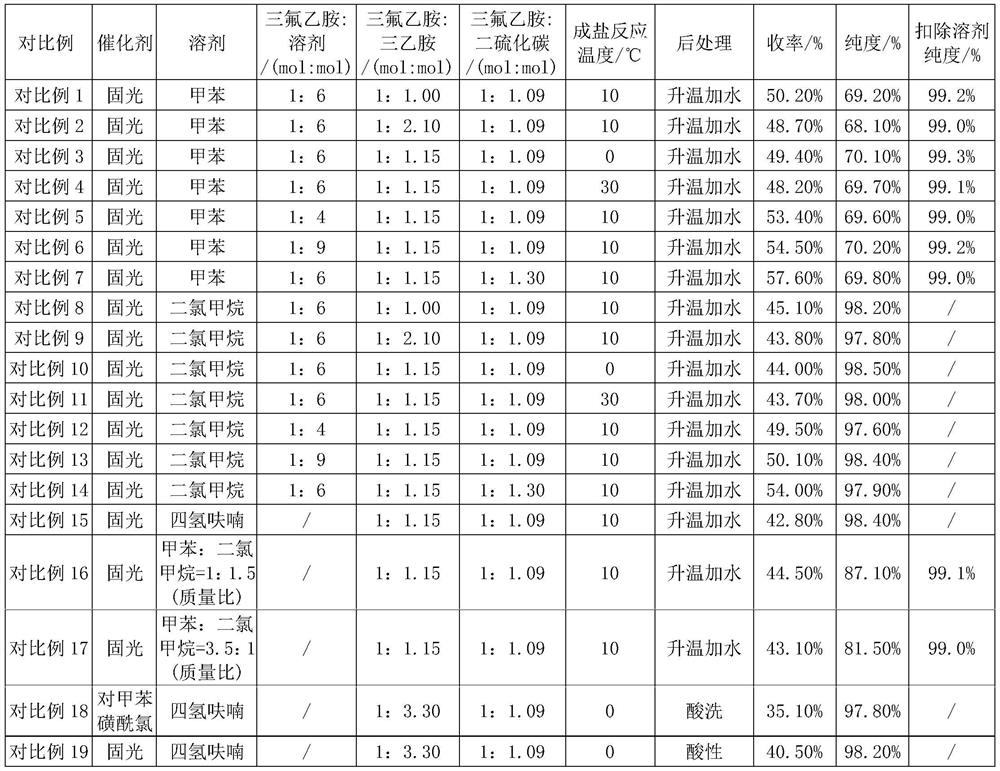
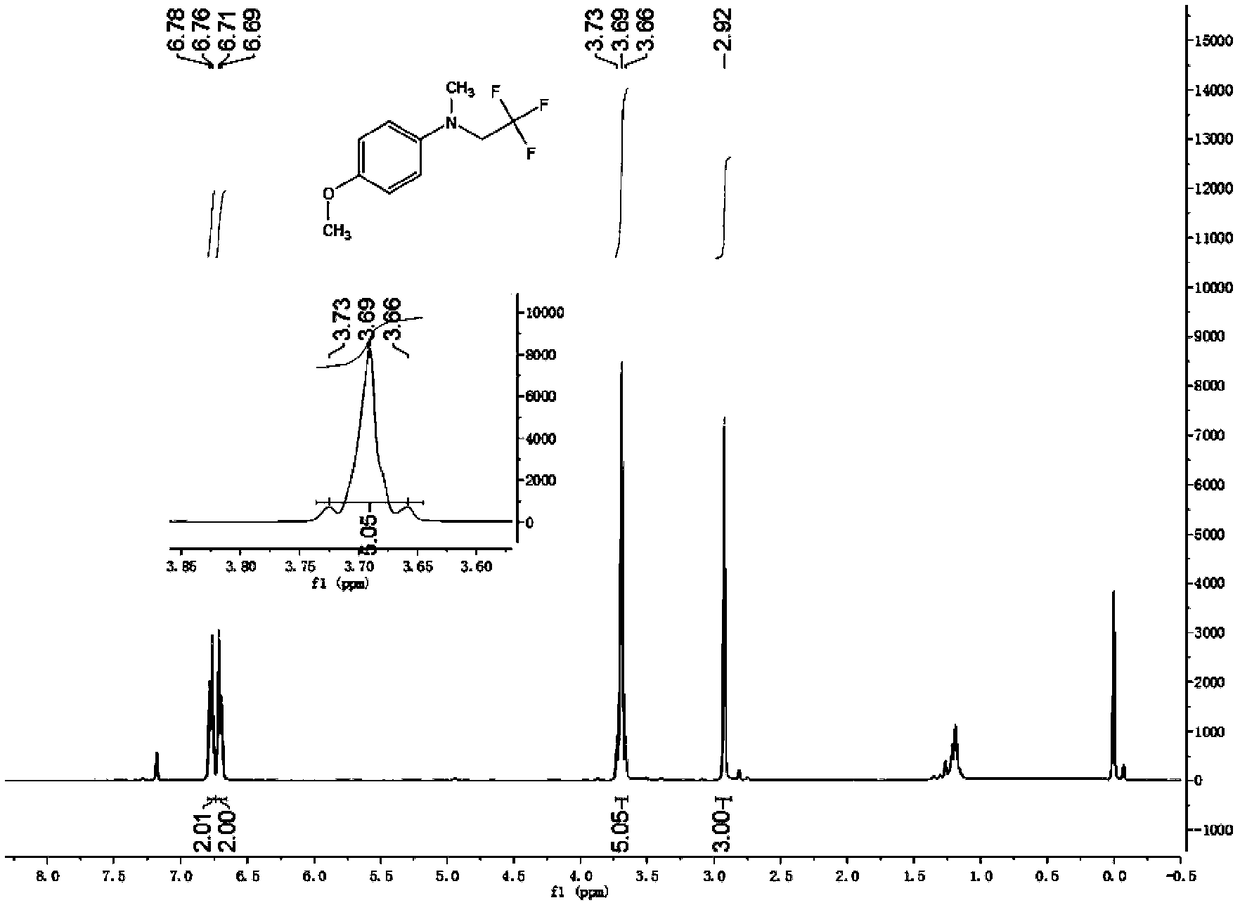
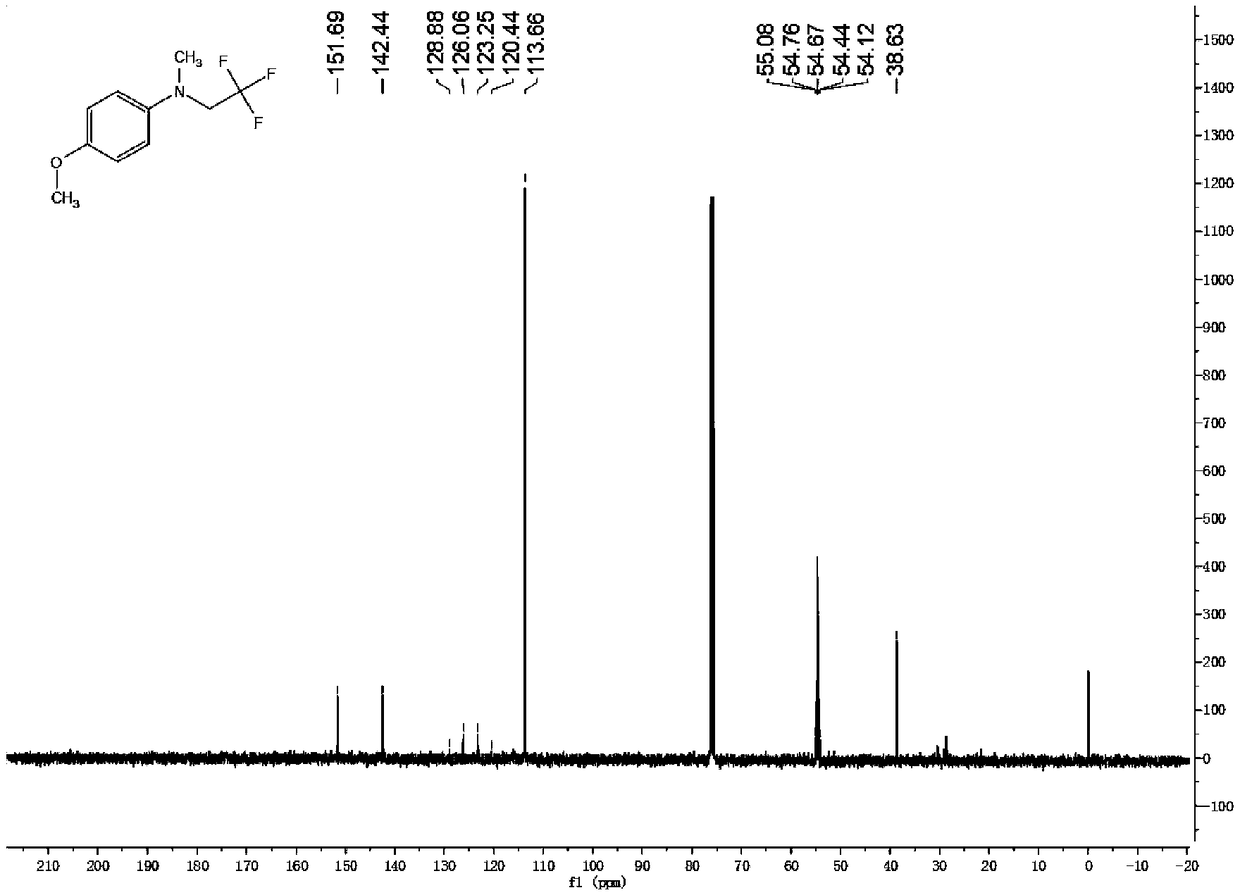
Preparation method of trifluoroisothiocyanate ethane
The invention relates to a preparation method of trifluoroisothiocyanate ethane. The preparation method comprises the steps of 1, mixing trifluoroethylamine, triethylamine and a solvent to obtain a first reaction mixture; controlling the temperature to be 10-12 DEG C, adding carbon disulfide, and reacting at the temperature of 5-20 DEG C to obtain a second reaction mixture containing trifluoroethylamino thioformate, wherein the molar ratio of the trifluoroethylamine to the triethylamine is 1: (1.05-2), the molar ratio of trifluoroethylamine to carbon disulfide is 1: (1-1.2), the molar ratio of trifluoroethylamine to the solvent is 1: (5-8), and the solvent is toluene, xylene or dichloromethane; 2, cooling the second reaction mixture obtained in the step 1 to 5-10 DEG C, adding solid light, stirring at room temperature, heating to the temperature of 35-45 DEG C, and reacting to obtain a trifluoroisothiocyanate ethane mixture; and then cooling, adding water and rectifying to obtain trifluoroisothiocyanate ethane. Compared with the prior art, the method has the advantages of high product yield and less three wastes by adopting solid light as a catalyst. According to the method, toluene / dichloromethane is adopted as the solvent, the problems that tetrahydrofuran and water are mixed and dissolved, part of products are brought into a water phase, the reaction yield is too low, and the solvent is difficult to recover are solved, the solvent can be recovered and reused after being evaporated, the recovery rate reaches 88% or above, the reaction cost is reduced, and emission of three wastes is reduced.
Owner:ZHEJIANG HISUN CHEM
Method of catalyzing trifluoro-ethylation of aromatic secondary amine by ferriporphyrin
ActiveCN108997145AMild reaction conditionsHigh yieldOrganic-compounds/hydrides/coordination-complexes catalystsCatalytic reactionsReaction stepDiazo
The invention provides a method of catalyzing trifluoro-ethylation of aromatic secondary amine by ferriporphyrin. The method comprises the following steps: adding trifluoroethylamine salt and nitriteto a diazo-reaction first and then adding aromatic primary amine and a ferriporphyrin catalyst for a trifluoro-ethylation reaction to obtain a trifluoro-ethylated aromatic secondary amine compound inan acidic solution system. The reactions of the method are carried out at room temperature, and the reaction condition is mild, and an intermediate product is not separated through a one-pot reaction.The method is few in reaction step, simple to operate, wide in application range of primers, wide in source of raw materials and high in yield of reaction products.
Owner:YUANJIANG HUALONG CATALYST TECH
Fatty trifluoroethyl ester compound and its preparation method
ActiveCN108707057BEasy to operateMild conditionsCarbamic acid derivatives preparationSugar derivativesN-butyl nitritePharmaceutical drug
Owner:JIANGXI NORMAL UNIV
A kind of preparation method of trifluoroethylamine hydrochloride
ActiveCN105801428BHigh purityReduce moistureOrganic compound preparationAmino compound preparationDistillationMoisture
Owner:ZHEJIANG LANTIAN ENVIRONMENTAL PROTECTION HI TECH +1
Process for producing fluoro-compounds
InactiveUS7615669B2Low cost productionLess costlyOrganic compound preparationCarboxylic acid esters preparationArylHydrogen atom
Owner:TOSOH F TECH INC
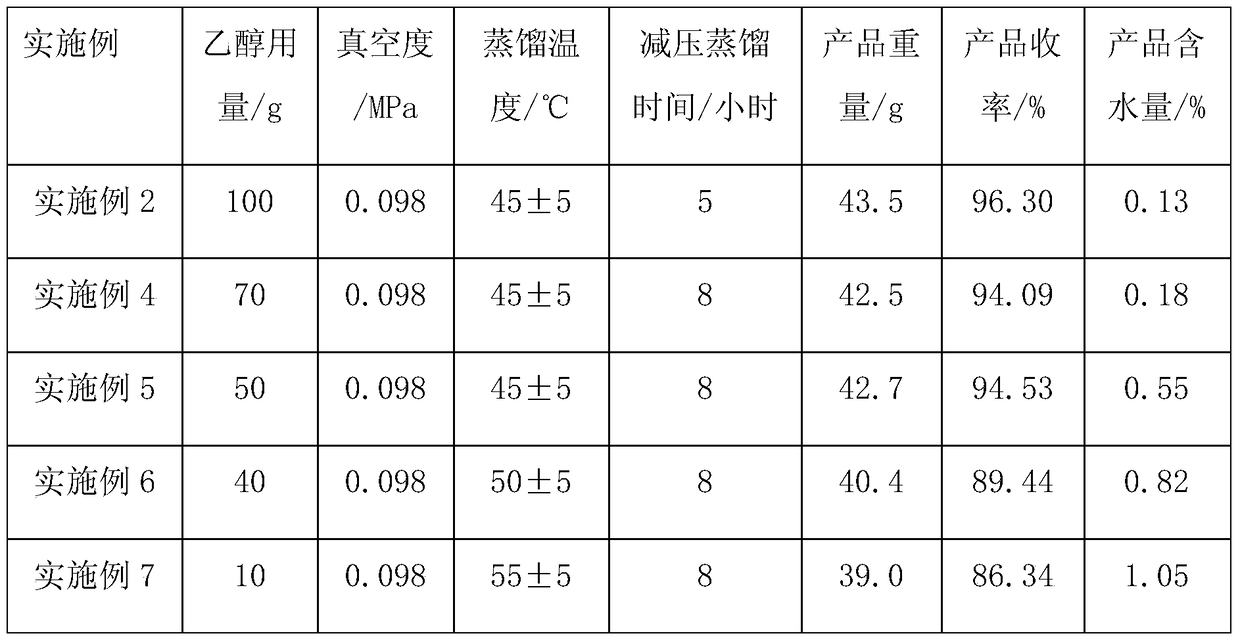
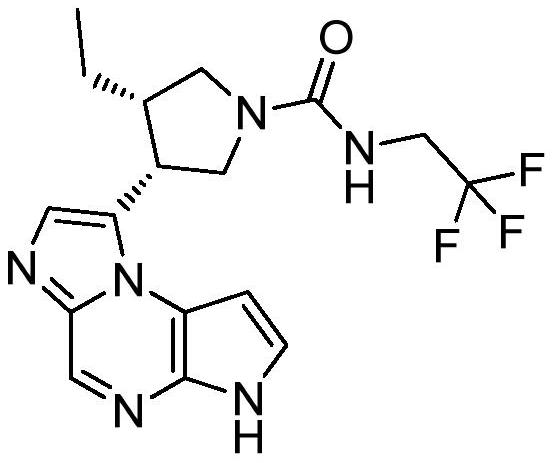
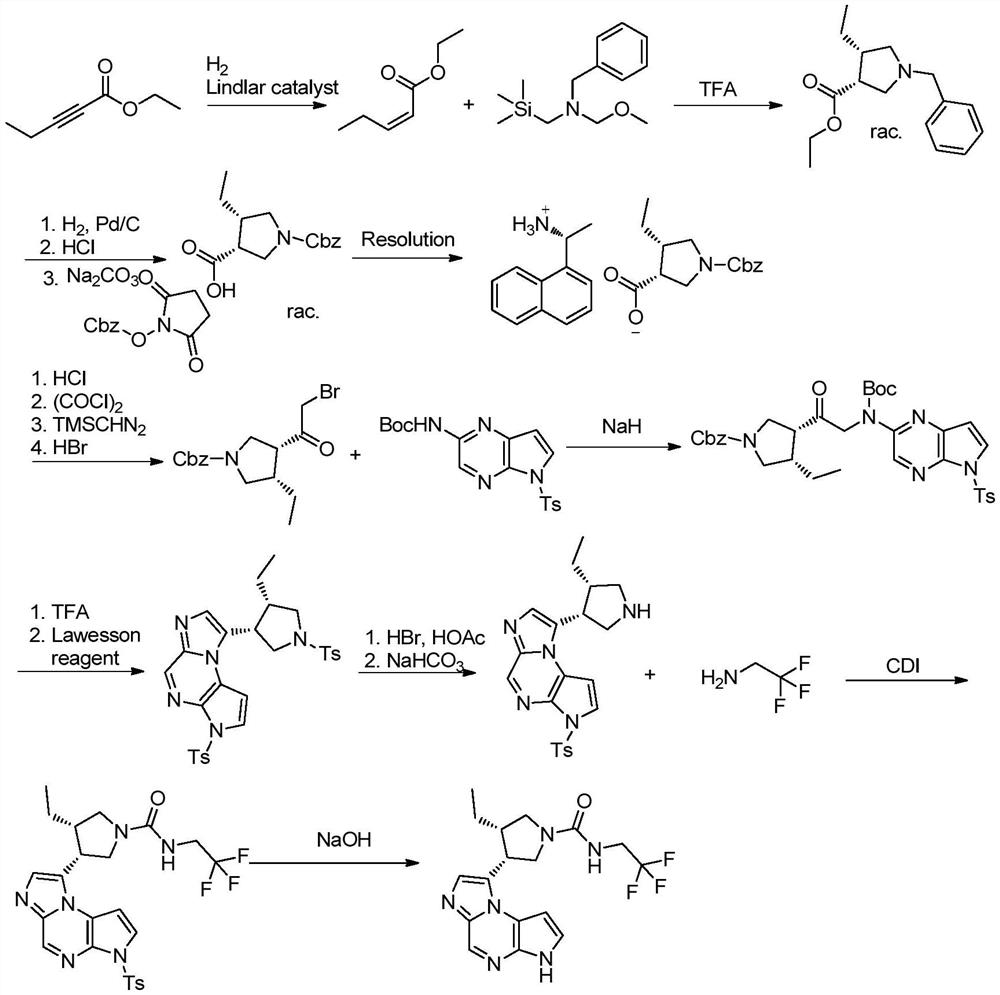
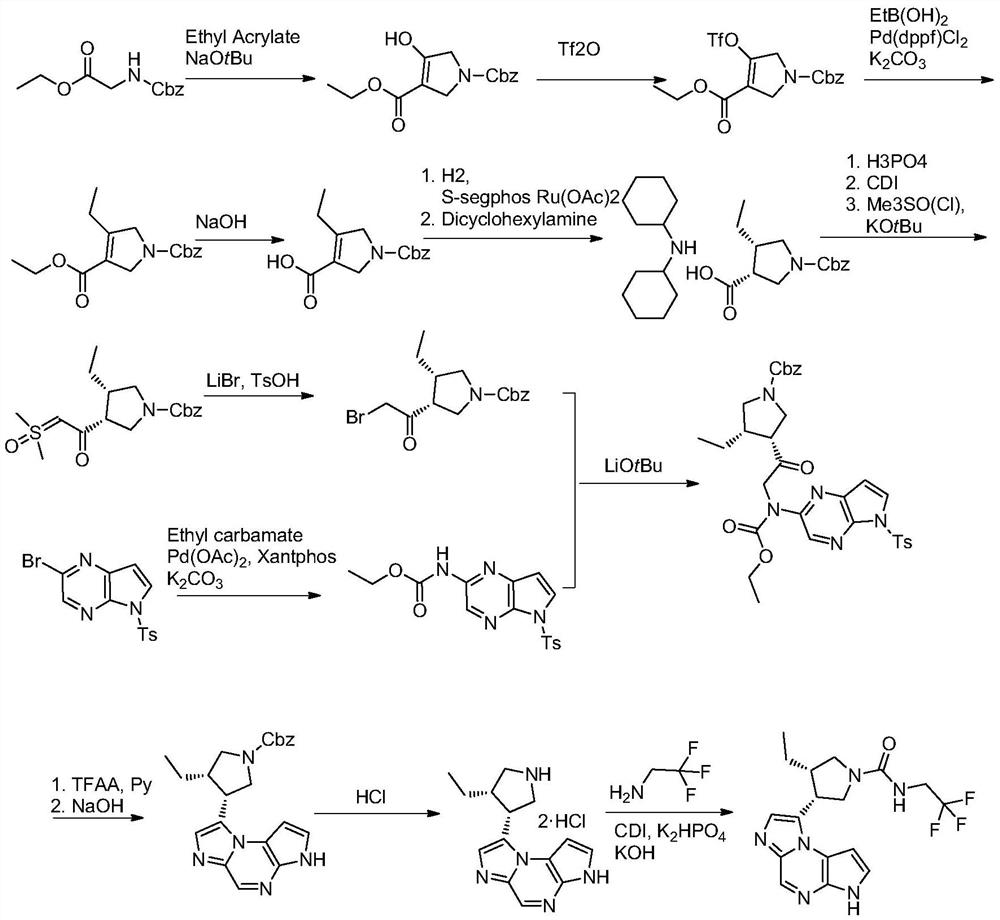
The synthetic method of upadatinib
The present invention provides a synthetic method for upatinib, which uses compound 1 as a starting material to obtain compound formula 4 through chlorination, coupling, hydrolysis, and hydrogenation into a salt, and then derivatizes and halogenates with McBurney's acid to obtain intermediate Body compound 6. Next, we use 2-bromo-5-p-toluenesulfonyl-5H-pyrrolo[2,3-b]pyrazine compound 7 as raw material to obtain intermediate compound 9 through ammonolysis reaction and amino protection. The docking reaction of compound 6 and compound 9 obtained chemical formula 10, and then the key nucleus 11 of upadatinib was obtained by cyclization with trifluoroacetic anhydride. Finally, the docking reaction of trifluoroethylamine was optimized, and upadatinib was obtained by both methods. These improvements have greatly improved the route efficiency, reduced the process steps of using noble metal catalysts, reduced the process cost, and greatly reduced the generation of by-chiral products, which is conducive to improving the purity of the final product. The route is:
Owner:HANGZHOU CHEMINSPIRE TECH CO LTD
Method of catalyzing trifluoro-ethylation of aromatic primary amine by ferriporphyrin
ActiveCN108997144AMild reaction conditionsHigh yieldOrganic-compounds/hydrides/coordination-complexes catalystsCatalytic reactionsRoom temperatureOne pot reaction
The invention provides a method of catalyzing trifluoro-ethylation of aromatic primary amine by ferriporphyrin. The method comprises the following steps: adding trifluoroethylamine salt and nitrite toa diazo-reaction first and then adding aromatic primary amine and a ferriporphyrin catalyst for a trifluoro-ethylation reaction to obtain a trifluoro-ethylated aromatic primary amine compound in an acidic solution system. The reactions of the method are carried out at room temperature, the reaction condition is mild, and an intermediate product is not separated through a one-pot reaction. The method is few in reaction step, simple to operate, wide in application range of primers, wide in source of raw materials and high in yield of reaction products.
Owner:YUANJIANG HUALONG CATALYST TECH
Method for analyzing related impurities of isoxazoline veterinary drug intermediate ammonium salt
ActiveCN114414678AEfficient separationHigh sensitivityComponent separationClimate change adaptationGlycineGas liquid chromatographic
The invention discloses a method for analyzing related impurities of an isoxazoline veterinary drug intermediate ammonium salt, the intermediate is 2-amino-N-(2, 2, 2-trifluoroethyl) acetamide, the related impurities are trifluoroethylamine hydrochloride and glycine, and the method comprises the following steps: dissolving the intermediate ammonium salt and a reference substance by using a diluent to obtain a sample solution and a reference substance solution; a reversed-phase high performance liquid chromatograph is used for detecting glycine, and gas chromatography is used for detecting trifluoroethylamine hydrochloride. The method provided by the invention can effectively separate impurities of 2-amino-N-(2, 2, 2-trifluoroethyl) acetamide, and has the advantages of high efficiency, simplicity, convenience, high sensitivity and good separation effect.
Owner:LIVZON NEW NORTH RIVER PHARMA
Process for producing optically active 1-alkyl-substituted 2,2,2-trifluoroethylamine
InactiveUS7393979B2Organic compound preparationAmino compound preparationHydrogen atmosphereMetal catalyst
The present invention relates to a process for producing an optically active 1-alkyl-substituted 2,2,2-trifluoroethylamine, which is an important intermediate of medicines and agricultural chemicals, and which is represented by the formula [3] [in the formula R represents a lower alkyl group of a carbon and * represents an asymmetric carbon], or its salt by subjecting an optically active imine represented by the formula [1] to an asymmetric reduction under hydrogen atmosphere using a metal catalyst of Group VIII to convert it into an optically active secondary amine represented by the formula [2] and then by subjecting the secondary amine or its salt to hydrogenolysis.[Chem. 23]
Owner:CENT GLASS CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com