Method of catalyzing trifluoro-ethylation of aromatic primary amine by ferriporphyrin
A technology of iron porphyrin catalyzing aromatic primary amine trifluoroethyl, aromatic primary amine, applied in chemical instruments and methods, catalytic reaction, physical/chemical process catalyst, etc., can solve the separation of trifluorodiazonium salt, high reaction temperature , poor tolerance of substrate functional groups, etc., to achieve the effect of low cost, simple operation and few reaction steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
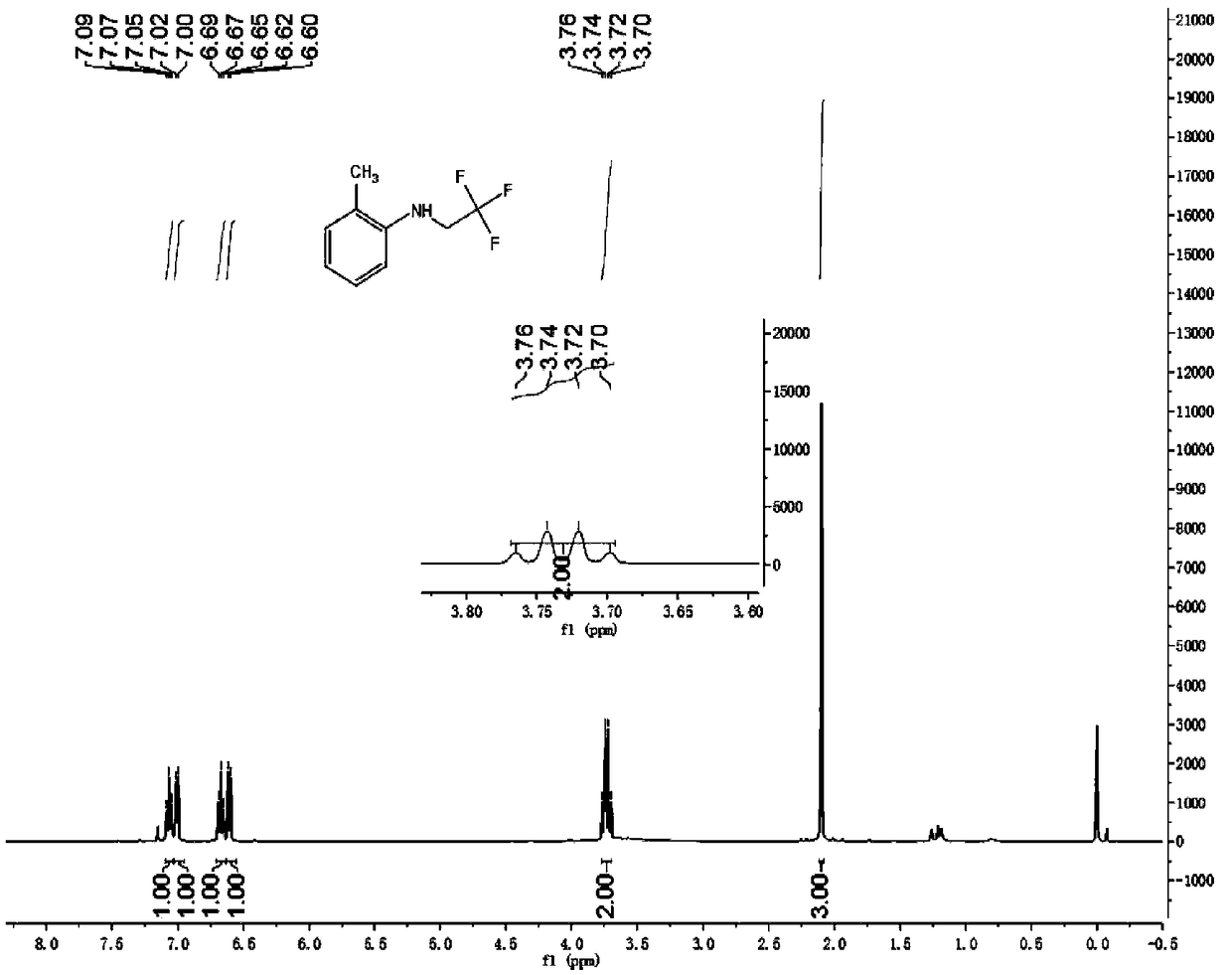
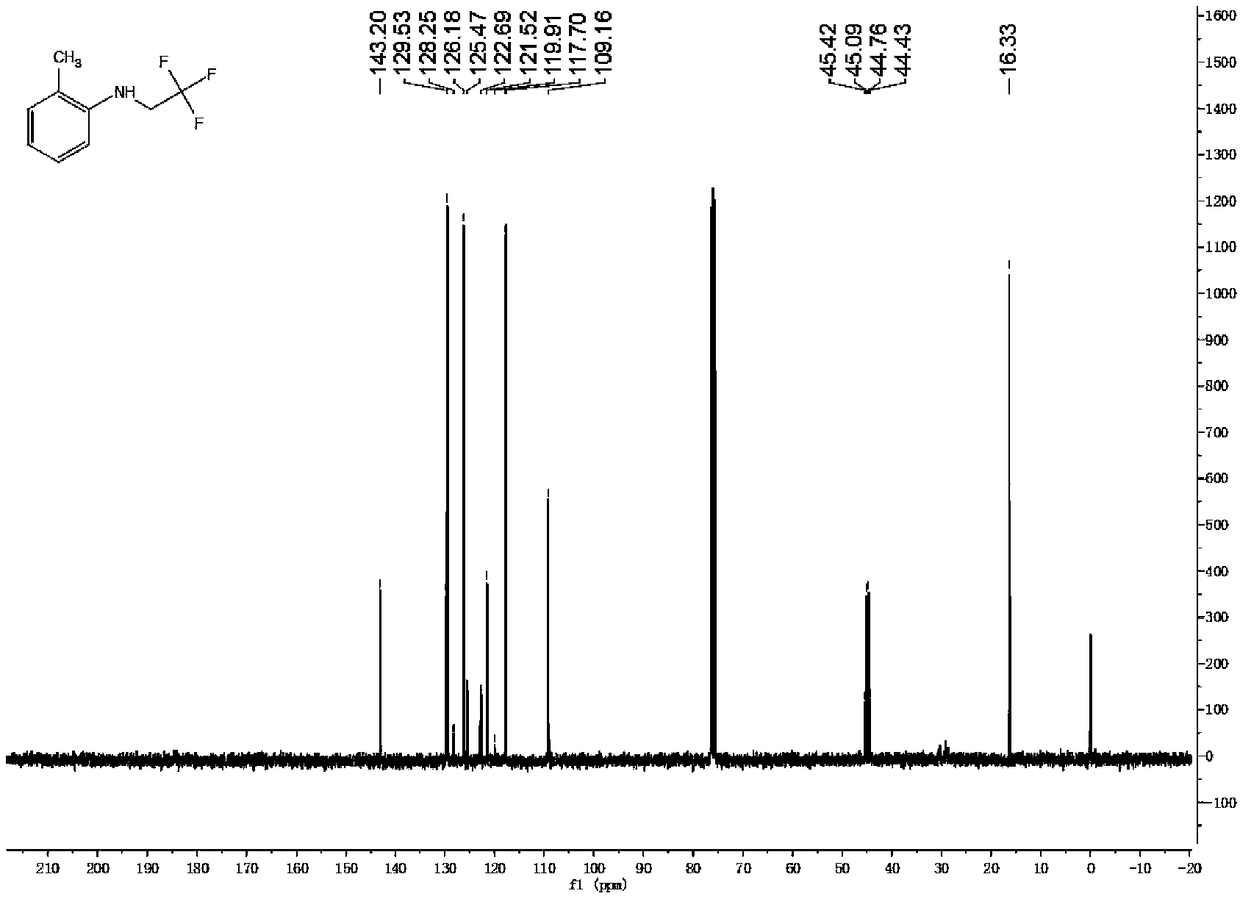
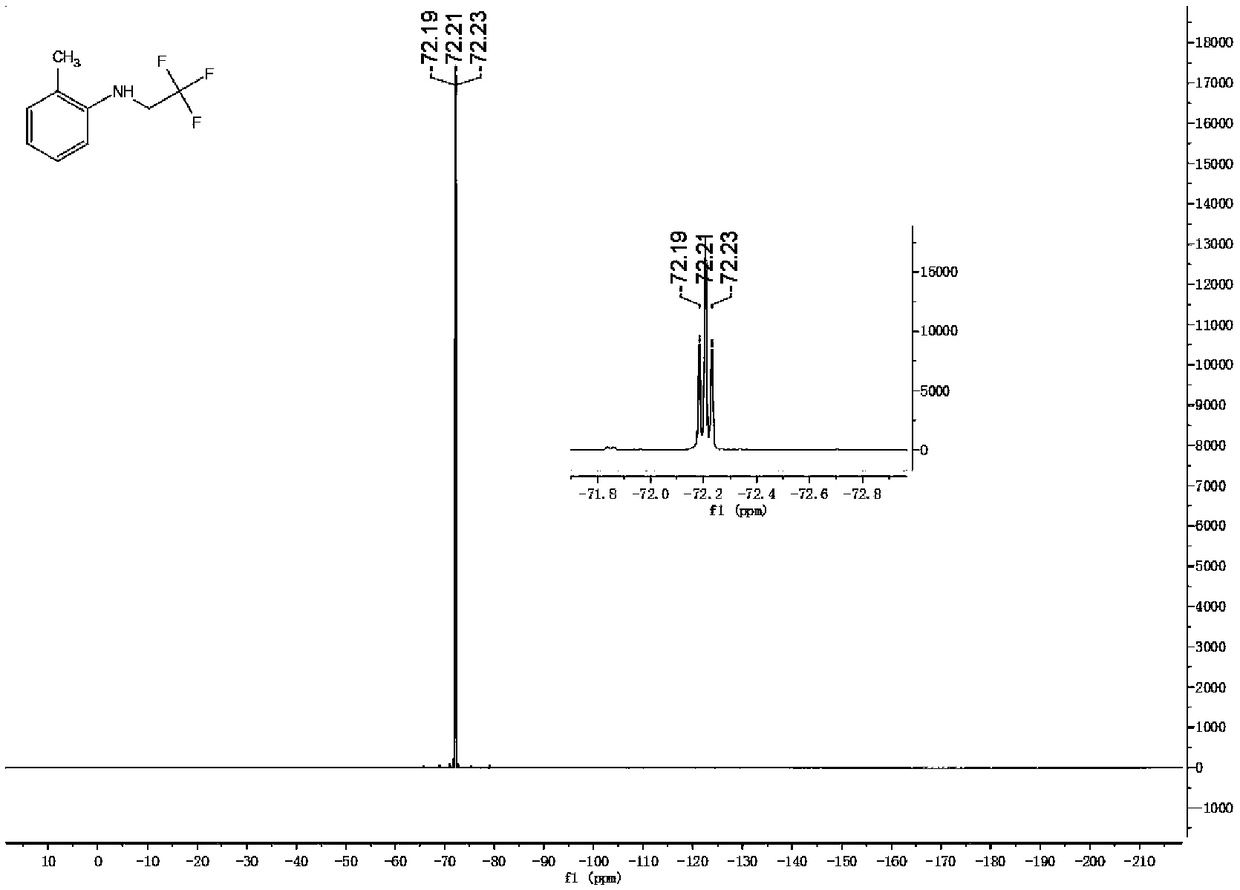
[0045] Synthesis of 2-methyl-N-(2,2,2-trifluoroethyl)aniline
[0046]Add 2mmol trifluoroethylamine hydrochloride, 1mL water, 34uL acetic acid, 1mL dichloromethane into the reaction tube, cover with a rubber stopper, and fix it on the stirrer. Take 42mg of sodium nitrite in a 1.5mL sample tube, add 1mL of water to the sample tube, shake the sample tube to dissolve the sodium nitrite. Add the dissolved sodium nitrite solution dropwise into the reaction tube with a syringe, and keep stirring for half an hour at room temperature. Dissolve the iron porphyrin (R 2 = R 3 = H, R 1 =Cl, L=Cl) (catalytic amount, 9 / 1000 of the molar amount of primary amine), take 0.24 mmol of 2-methylaniline in a sample tube. After half an hour, the mixed solution in the sample tube was added dropwise into the reaction tube, stirred while adding, and reacted for twelve hours. The reaction solution was cooled to room temperature, filtered to remove some impurities, concentrated and purified by column...
Embodiment 2
[0083] Synthesis of 3-methyl-N-(2,2,2-trifluoroethyl)aniline
[0084] Add 2mmol trifluoroethylamine hydrochloride, 1mL water, 34uL acetic acid, 1mL dichloromethane into the reaction tube, cover with a rubber stopper, and fix it on the stirrer. Take 42mg of sodium nitrite in a 1.5mL sample tube, add 1mL of water to the sample tube, shake the sample tube to dissolve the sodium nitrite. Add the dissolved sodium nitrite solution dropwise into the reaction tube with a syringe, and keep stirring for half an hour at room temperature. Dissolve the iron porphyrin (R 2 = R 3 = H, R 1 =Cl, L=Cl) (catalytic amount, 9 / 1000 of the molar amount of primary amine), take 0.24 mmol of 3-methylaniline in a sample tube. After half an hour, the mixed solution in the sample tube was added dropwise into the reaction tube, stirred while adding, and reacted for twelve hours. The reaction solution was cooled to room temperature, filtered to remove some impurities, concentrated and purified by colum...
Embodiment 3
[0089] Synthesis of 4-methyl-N-(2,2,2-trifluoroethyl)aniline
[0090] Add 2mmol trifluoroethylamine hydrochloride, 1mL water, 34uL acetic acid, 1mL dichloromethane into the reaction tube, cover with a rubber stopper, and fix it on the stirrer. Take 42mg of sodium nitrite in a 1.5mL sample tube, add 1mL of water to the sample tube, shake the sample tube to dissolve the sodium nitrite. Add the dissolved sodium nitrite solution dropwise into the reaction tube with a syringe, and keep stirring for half an hour at room temperature. Dissolve the iron porphyrin (R 2 =R 3 =CH 3 , R 1 =Cl, L=Cl) (catalytic amount, 9 / 1000 of the molar amount of primary amine), take 0.24 mmol of 3-methylaniline in a sample tube. After half an hour, the mixed solution in the sample tube was added dropwise into the reaction tube, stirred while adding, and reacted for twelve hours. The reaction solution was cooled to room temperature, filtered to remove some impurities, concentrated and purified by co...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com