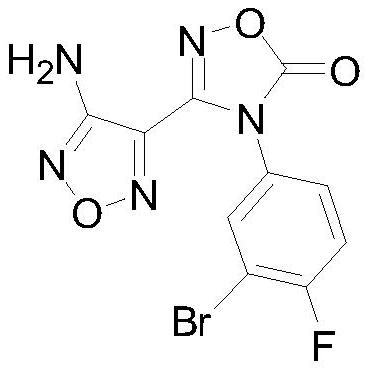
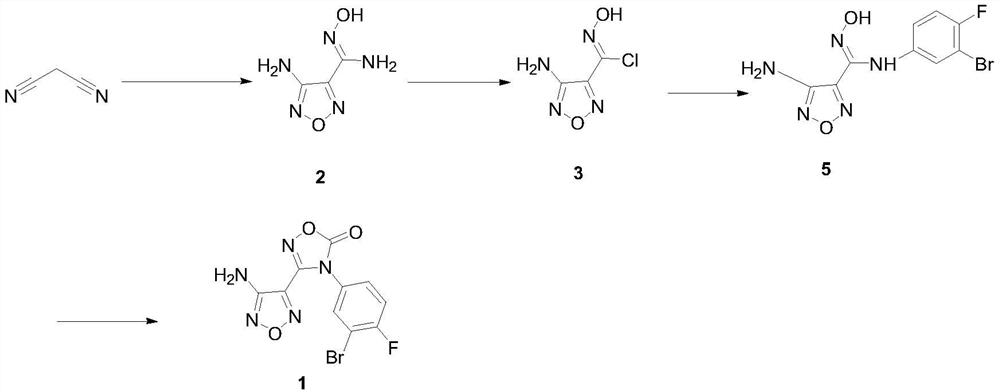
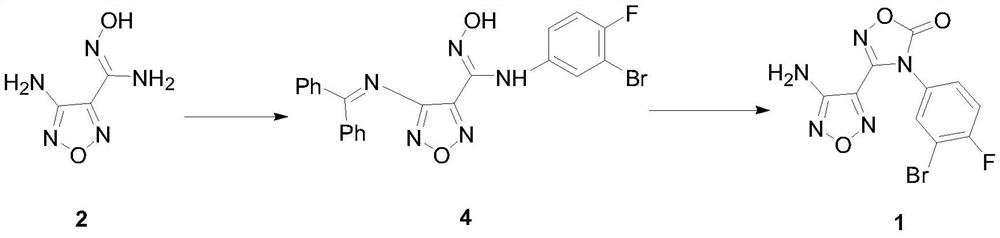
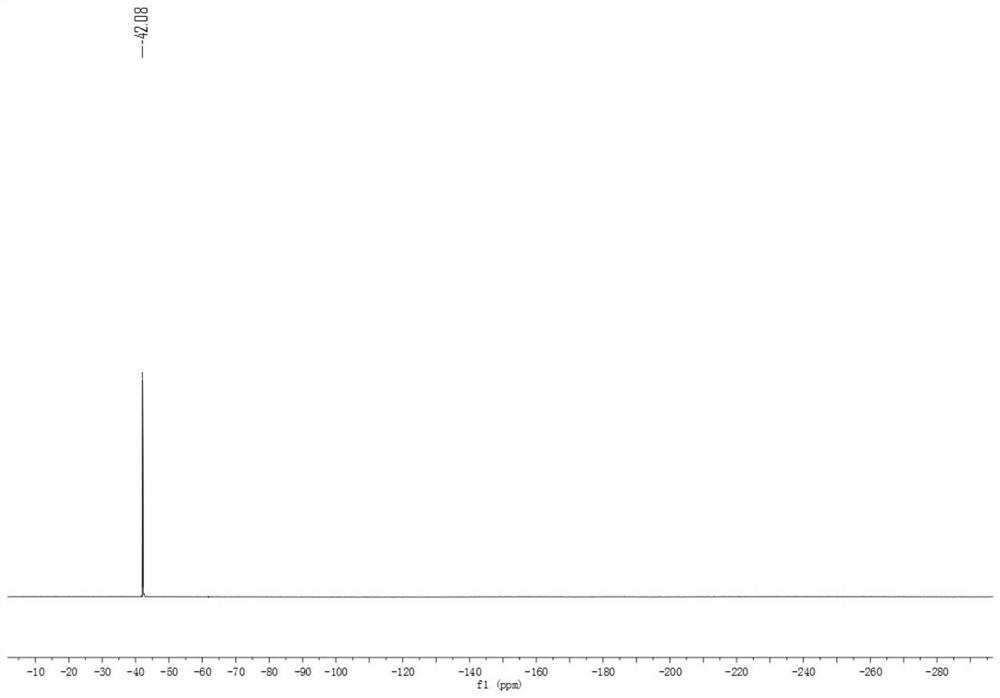
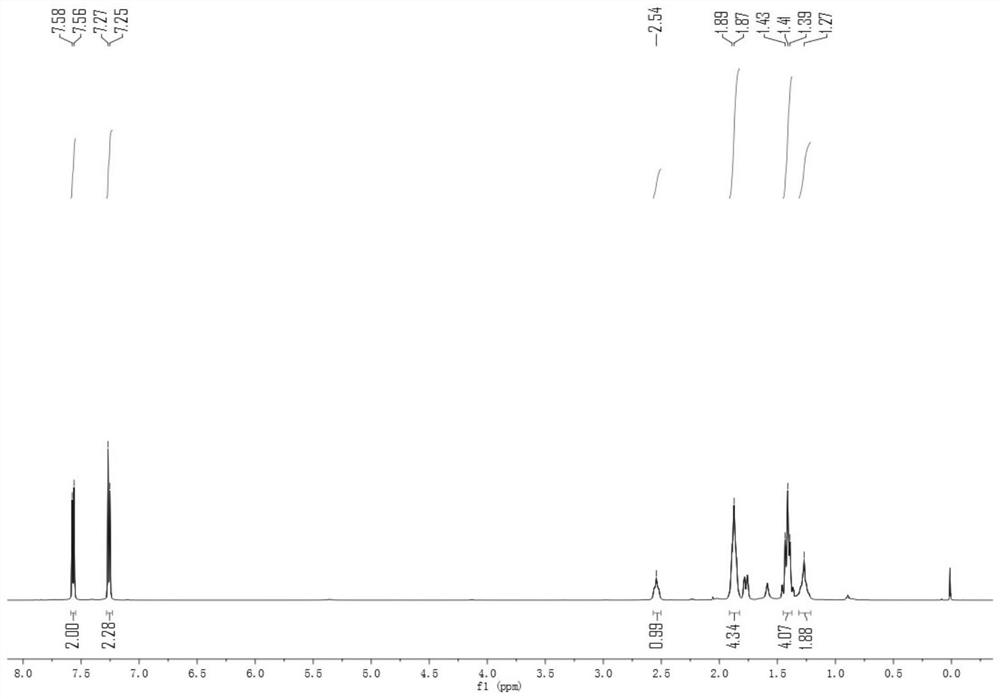
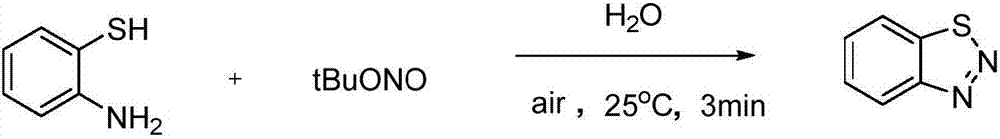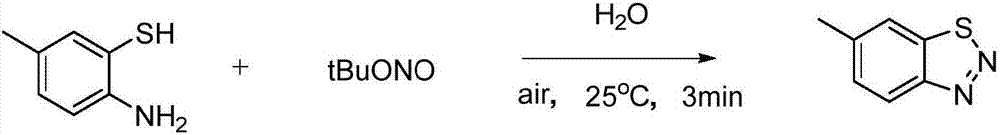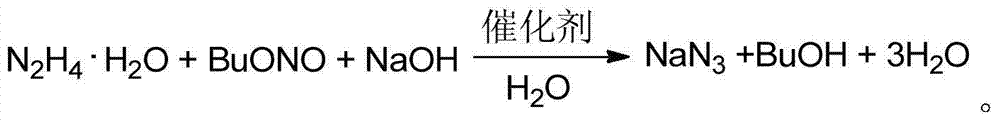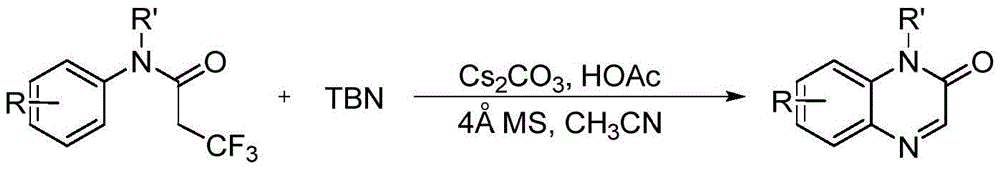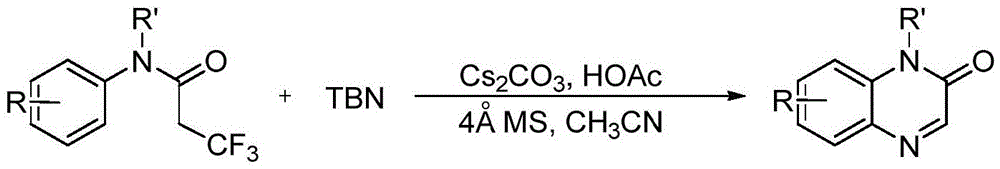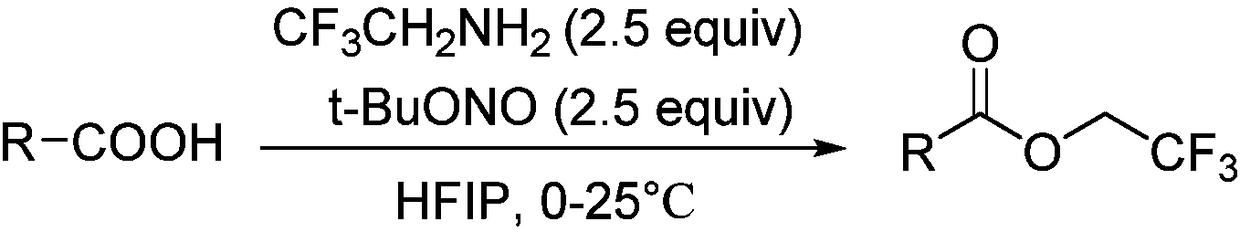Patents
Literature
75 results about "N-butyl nitrite" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Butyl nitrite is an alkyl nitrite made from n-butanol. Butyl nitrite is used recreationally as poppers. Synonyms include 1-butyl nitrite and nitrous acid, butyl ester. It can be prepared by reacting nitrous acid (generated in situ by reacting a metal nitrite with a mineral acid) with n-butanol.
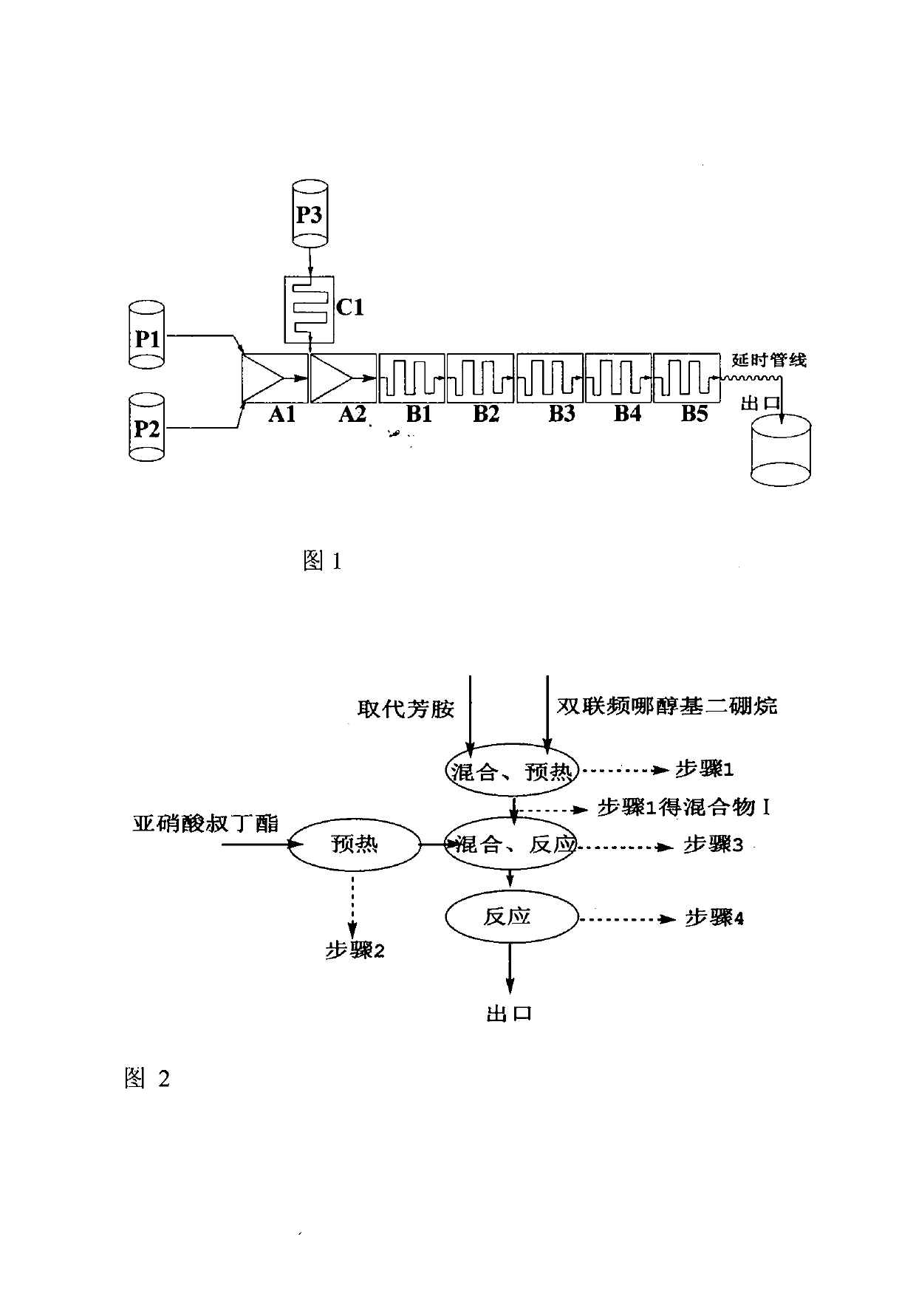
Method for continuously synthesizing arylboronic acid ester by utilizing microreactor
InactiveCN103275112ADegree of reductionShort reaction timeGroup 3/13 element organic compoundsOrganic synthesisN-butyl nitrite
The invention relates to a method for continuously synthesizing arylboronic acid ester by utilizing a microreactor, which belongs to the technical field of green organic synthesis application. The method comprises the following steps of: preheating substituted arylamine, tert-butyl nitrite, bisdiborane in a continuous-flow micro-channel reactor system by using substituted arylamine, acetonitrile, tert-butyl nitrite and bisdiborane as starting materials; and mixing the substituted arylamine with the bisdiborane and then reacting the obtained mixture with the tert-butyl nitrite, wherein in the reaction, the molar ratio of the substituted arylamine to the bisdiborane is (1:0.5)-(1:1.25), the molar ratio of the substituted arylamine to isoamyl nitrite is (1:1.1)-(1:1.5), the reaction temperature is 60 DEG C-120 DEG C, the reaction time is 50 seconds-3600 seconds, and the effective conversion ratio of the substituted arylamine is 50%-90%. The continuous-flow microreactor, which is capable of strengthening the mixing effect, the mass transfer effect and the heat transfer effect, is especially suitable for carrying out homogeneous reaction of the method. Moreover, the method has the characteristics of stable temperature control, safe process and less waste material.
Owner:JINAN SHAOYUAN MEDICAL TECH
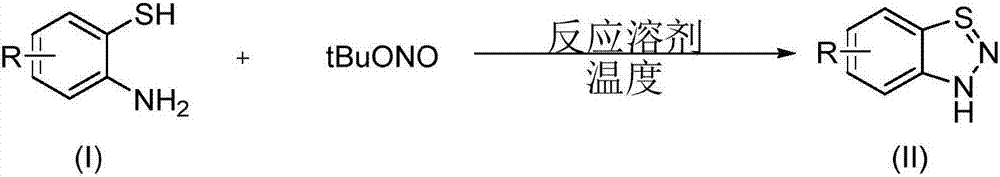
Synthesis method of 1,2,3-diazosulfide compound
InactiveCN107311960APost-processing is simpleSimple and fast operationOrganic chemistrySynthesis methodsTert butyl
The invention discloses a synthesis method of a 1,2,3-diazosulfide compound. A o-aminothiophenol compound with a structure shown as a formula (I) and tert-butyl nitrite are taken as raw materials, and are subjected to intramolecular diazo-reaction to obtain the 1,2,3-diazosulfide compound with a structure shown as a formula (II); a reaction equation is as follows (shown in the description), wherein R=hydrogen, fluorine, chlorine, ester group, nitro or methyl. The synthesis method has the beneficial effects that (1) the operation is simple and convenient in a preparation process, an obtained product is easy for after-treatment, and large-scale industrialized production is suitable; (2) reaction conditions are mild; (3) the synthesis cost is lowered; (4) a reaction substrate is high in functional group tolerance, is wide in range and easy to prepare; (5) the reaction is efficient, the yield is high, and the reaction efficiency is higher after reaction amplification; (6) environment pollution is avoided, and environment friendliness is achieved.
Owner:WENZHOU UNIVERSITY
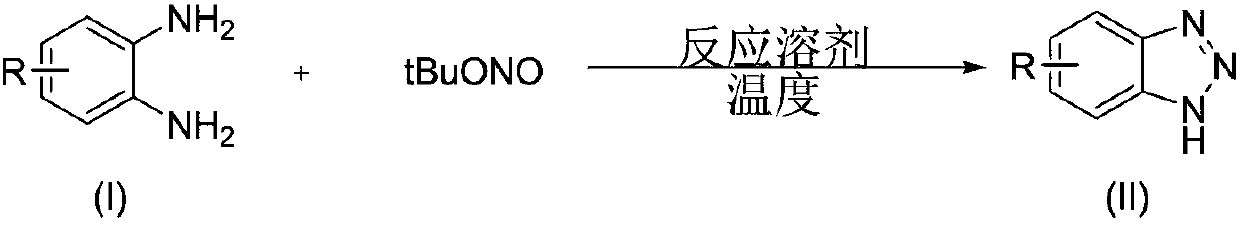
Benzotriazole compounds and a preparing method thereof
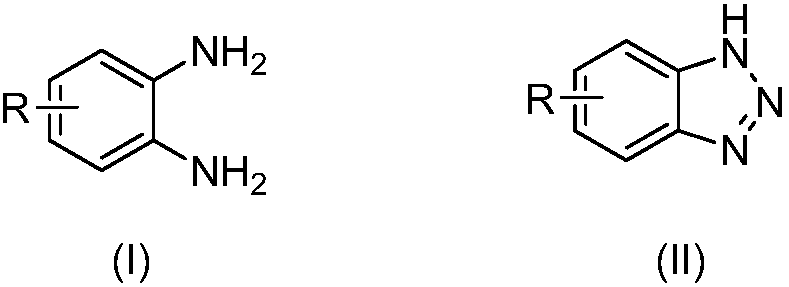
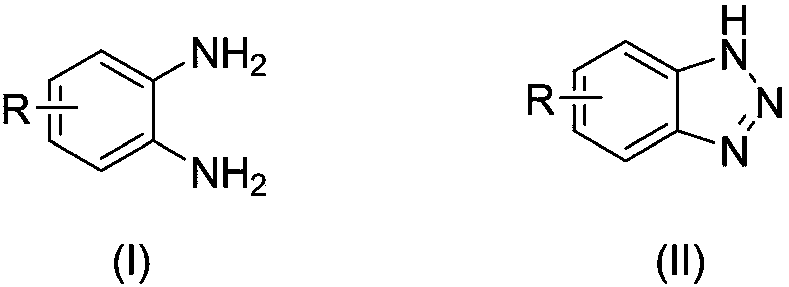
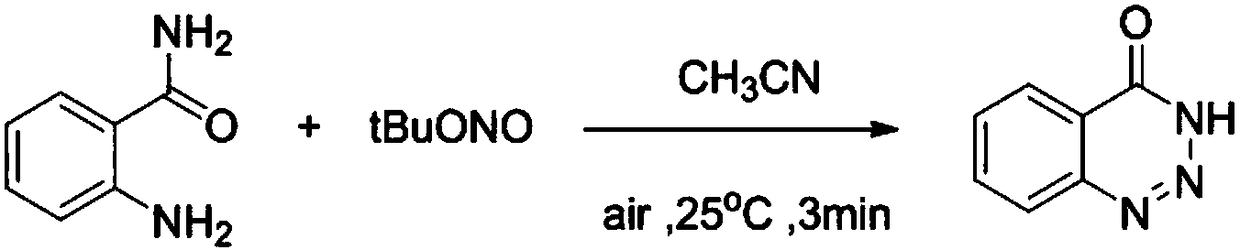
The invention relates to benzotriazole compounds and a preparing method thereof. o-phenylenediamine or an o-phenylenediamine compound n-butyl nitrite are adopted as reaction raw materials and are subjected to an intramolecular diazo-reaction at room temperature in a reaction solvent to obtain the corresponding benzotriazole compound. According to the method, the range of substrates is wide, reaction conditions are mild, after-treatment is simple, and a product yield and quality are high. A novel synthesis route and method for the benzotriazole compounds are developed. The benzotriazole compounds have good application potentials and research value.
Owner:WENZHOU UNIVERSITY
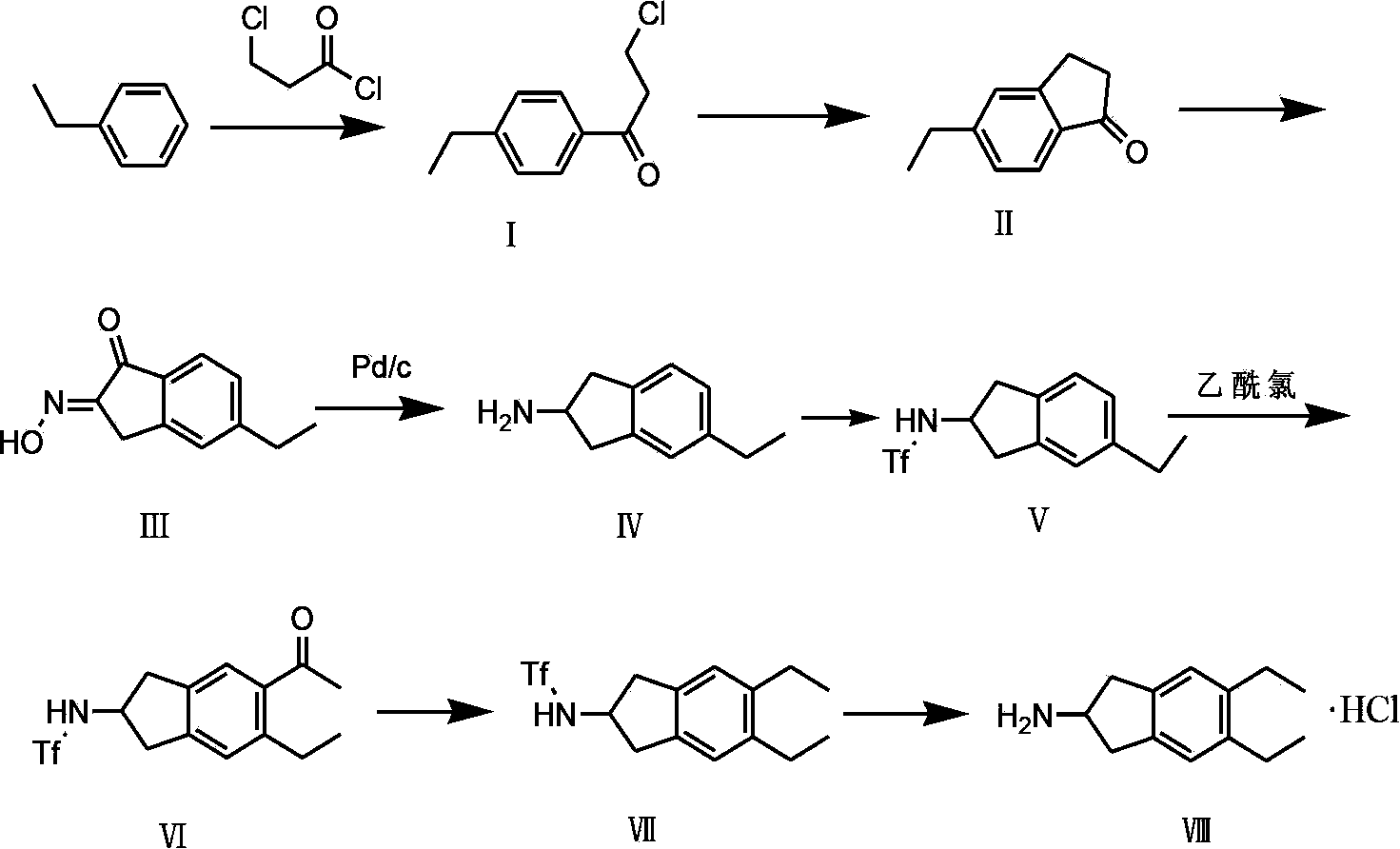
Preparation method of 5,6-diethyl-2,3-dihydro-1H-indene-2-amine hydrochloride
InactiveCN103539677AEase of industrial implementationOrganic compound preparationAmino compound preparationIndacaterolN-butyl nitrite
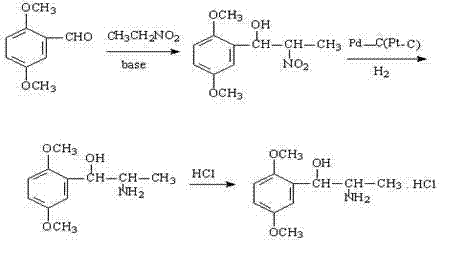
The invention belongs to the technical field of synthesis of a medical immediate, and particularly relates to a synthesis method of a key intermediate 5,6-diethyl-2,3-dihydro-1H-indene-2-amine hydrochloride of indacaterol. The method comprises the following steps: by taking ethylbenzene as a raw material, preparing a compound I by propionyl chloride; preparing a compound II from the compound I by cyclization reaction; preparing a compound III by reaction of the compound II and butyl nitrite; preparing a compound IV from the compound III by palladium hydrogen reduction; finally preparing a compound V from the compound IV under protection of trifluoroacetyl; preparing a compound VI from the compound V by amino acetylation reaction; preparing a compound VII from the compound VI by reduction; carrying out deprotection, hydrolysis and acidification on the compound VII, so as to obtain the final product compound VIII, namely the 5,6-diethyl-2,3-dihydro-1H-indene-2-amine hydrochloride. The method disclosed by the invention is simple and convenient to operate, reasonable in reaction flow, low in cost, good in product quality, free of pollution to environment, and applicable to industrial production; the content is greater than 99%.
Owner:湖北万知化工医药股份有限公司
Diesel light oil composition
InactiveUS20090151232A1Improve ignition performanceReduce the amount requiredLiquid carbonaceous fuelsFuel additivesButyl nitrateN-butyl nitrite
To provide a diesel light oil composition having an excellent ignitability while containing butanol. The diesel light oil composition comprises a diesel light oil base material, butanol having a volume percentage in a range of 9% to 20% and butyl nitrate or butyl nitrite having a volume percentage in a range of 0.8% to 4%. It is preferable that the butanol is 20 v / v %, and the butyl nitrate or butyl nitrite is 4 v / v %. The butyl nitrite or butyl nitrate is derived from butanol as a material.
Owner:HONDA MOTOR CO LTD
Aqueous-phase synthetic method of sodium azide with n-butyl alcohol circularly recycled
ActiveCN104760939AEasy to recycleReduce pollutionHydrazoic-acids/azides/halogen-azidesNitrous acid preparation ester preparationButyl nitrateHydrazine compound
The invention discloses an aqueous-phase synthetic method of sodium azide with n-butyl alcohol being circularly recycled, wherein the method includes following steps: (1) mixing butyl nitrite, sodium hydroxide, hydrazine hydrate, a catalyst and water to form a reaction system; (2) after the reaction finished, recycling the n-butyl alcohol and water; and (3) filtering a reaction solution to obtain a sodium azide (white solid) and circularly recycling the recycled n-butyl alcohol. In the method, by means of the butyl nitrite for synthesizing the sodium azide, so that compared with a synthesis route from ethyl nitrite, the synthetic method is free of high-pressure operations and the risk of leakage of gas. Meanwhile, the reaction can be carried out in the aqueous phase and just needs to added with the catalyst in a micro amount (especially, herein the catalyst may be composited of tributylamine and methanol), so that the synthetic method is environmental-protective. The method is high in yield of product, is low in the content of free alkali and is high in product purity. Meanwhile, the reaction achieves the recycle of a by-product n-butyl alcohol for synthesizing the butyl nitrite, by means of the technical scheme, the environmental pollution is reduced while the economic benefit is increased.
Owner:ZHEJIANG HAILAN CHEM GRP
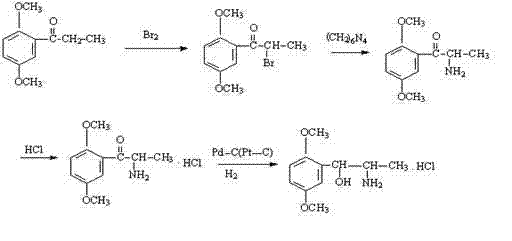
Preparation method for erythro-structure methoxamine hydrochloride
ActiveCN103755578AAvoid the disadvantages of low boiling point, easy to volatilize, difficult to measure, etc.Increased hydrogenation rateOrganic compound preparationAmino-hyroxy compound preparationN-butyl nitriteSolvent
A disclosed preparation method for erythro-structure methoxamine hydrochloride comprises the following steps: (1) dissolving an initial raw material 2,5-dimethylpropiophenone in an organic solvent, under the condition of introducing dry hydrogen chloride gas, dropwise adding a 1-butyl nitrite solution to perform an oximation reaction, so as to obtain an intermediate I; (2) under the acidic condition, taking a methanol solution as a solvent, take palladium on activated carbon as a catalyst, employing hydrogen to reduce the oximido group in the intermediate I, so as to obtain an intermediate II; and (3) performing hydrogenation reduction reaction on the intermediate II to obtain the erythro-structure methoxamine hydrochloride. The method provided by the invention is simple in operation, economic, environment-friendly, mild in reaction conditions, excellent in product quality and high in yield, and can help to obtain the single erythro-structure methoxamine hydrochloride.
Owner:GUANGDONG JIABO PHARM CO LTD
Hydrogen sulfide fluorescent probe as well as preparation method and application thereof
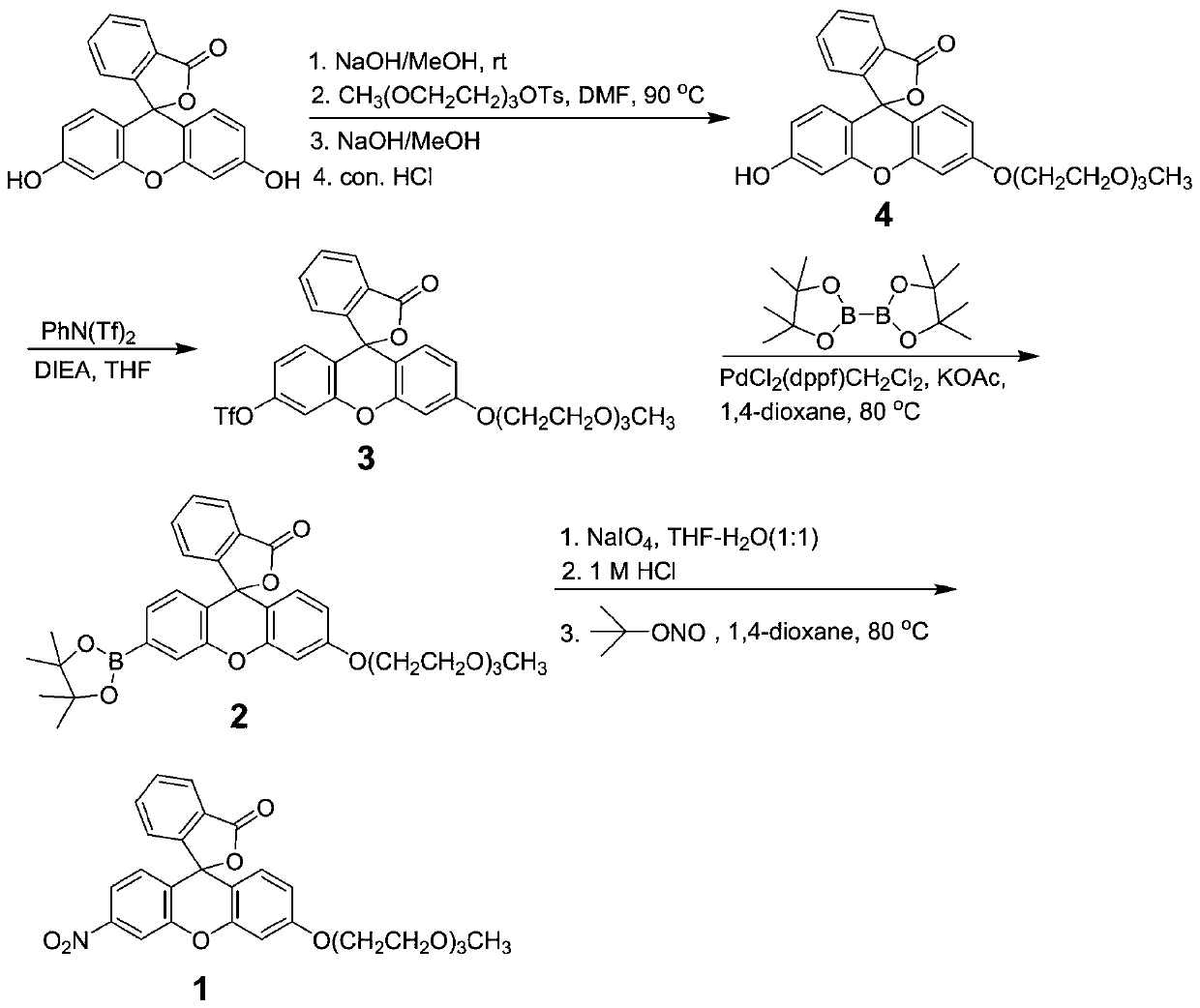
InactiveCN110734450AHigh selectivityOrganic chemistryFluorescence/phosphorescenceFluoProbesTosylic acid
The invention relates to a hydrogen sulfide fluorescent probe as well as preparation and application thereof. The molecular formula of the fluorescent probe is C27H25NO9; the preparation method of thefluorescent probe comprises the following steps: reacting a fluorescein with sodium hydroxide; reacting an obtained product with 2-(2-(2-methoxyethoxy)ethoxy)ethyl 4-methylbenzenesulfonate to obtaina compound 4, reacting the compound 4 with N-Phenylbis(trifluoromethanesulphonimide) to obtain a compound 3, reacting the compound 3 with bis (pinacolato) diboron to obtain a compound 2, hydrolyzing the compound 2, and reacting an obtained product with tert-butyl nitrite to obtain the hydrogen sulfide fluorescent probe. The probe shows relatively high selectivity on detection of hydrogen sulfide in a pure water solution.
Owner:HEZHOU UNIV
Method for preparing chiral 3-nitroindole compound through nickel-catalyzed asymmetric nitration reaction
PendingCN113666862ALow costHigh reaction stereoselectivityOrganic-compounds/hydrides/coordination-complexes catalystsCatalytic reactionsNickel catalystPtru catalyst
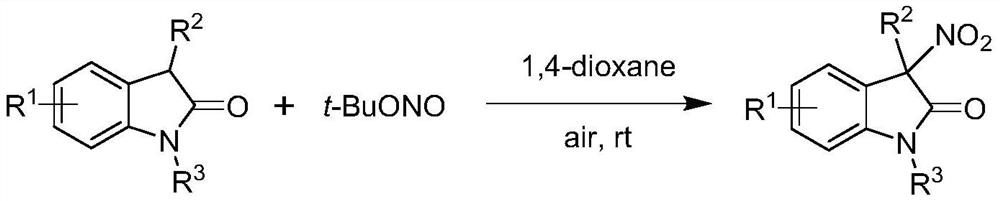
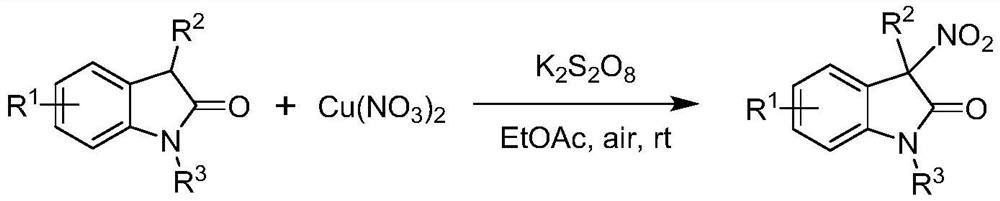
The invention provides a method for preparing a chiral 3-nitroindole compound through a nickel-catalyzed asymmetric nitration reaction. The method comprises the following steps: in a solvent, under the action of an additive, a nickel catalyst and a ligand, carrying out an asymmetric nitration reaction on an indole-2-one compound I and tert-butyl nitrite II to obtain the chiral 3-nitroindole compound III. According to the invention, chiral 3-nitroindole is constructed through a nickel-catalyzed asymmetric nitration reaction, and the method has the advantages of low catalyst cost, convenience in operation, wide substrate application range, cheap and easily available reaction raw materials and the like.
Owner:SHANDONG UNIV
Synthesis method of 1,2,3-phentriazine-4(3H)-one compound
InactiveCN108084104APost-processing is simpleEasy to operateOrganic chemistryNitriteSynthesis methods
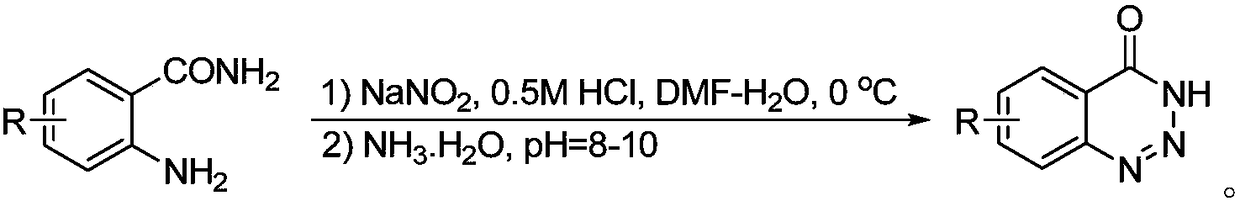
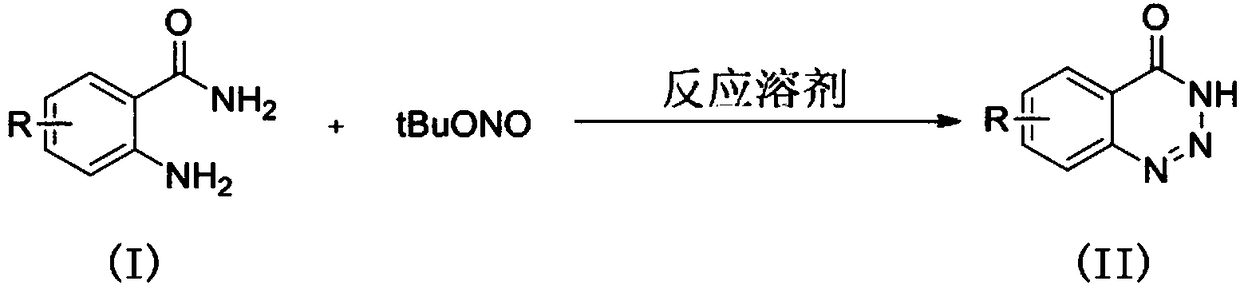
The invention discloses a synthesis method of a 1,2,3-phentriazine-4(3H)-one compound. 2-aminobenzamide and are used as raw materials and tert-butyl nitrite stirred at the room temperature in a reaction solvent, and the 1,2,3-phentriazine-4(3H)-one compound is obtained through an intramolecular diazotization reaction. The reaction equation is shown in the description, wherein R is H, fluorine, chlorine, bromine, trifluoromethyl, nitryl or methyl. The synthesis method has the benefits as follows: (1) the synthesis method is convenient in experiment operation, easy in posttreatment, mild in reaction condition and suitable of large-scale industrial production; (2) reaction substrate functional groups have high tolerance, and the substrate is wide in range and easy to obtain; (3) the reactionefficiency, yield and purity are higher.
Owner:WENZHOU UNIVERSITY
Preparation method of quinoxalin-2-one derivative and product purification method
The invention discloses a preparation method of a quinoxalin-2-one derivative and a product purification method. The preparation method comprises the following steps: using N-methyl-N-phenyltrifluoropropionamide or its derivative as a substrate, adding tert-butyl nitrite into the substrate, using an acetonitrile solution as a solvent, and heating to carry out a reaction in the environment of a buffer solution prepared from a 4 Angstrom molecular sieve, cesium carbonate and acetic acid so as to prepare a crude product. Then, the crude product undergoes purification. The purification method comprises the following steps: filtering the crude product and removing acid to obtain residue; carrying out silica-gel column chromatography on the residue, leaching with an eluate and collecting an eluant; merging product-containing eluants; condensing the merged eluant to remove the solvent; and finally carrying out vacuum drying to obtain a target product. The invention has advantages of simple technological process, low cost and high yield.
Owner:WENZHOU UNIVERSITY
Method for synthesizing Crizotinib intermediate
InactiveCN102898449AEasy to synthesizeReduce manufacturing costGroup 3/13 element organic compoundsPtru catalystBenzoyl peroxide
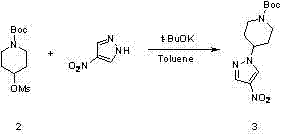
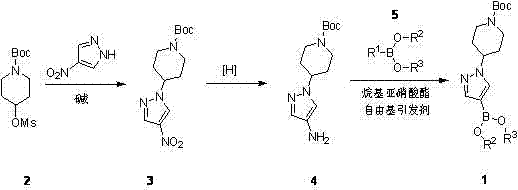
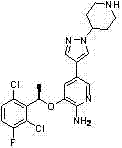
The invention belongs to the technical field of medicine synthesis, and in particular relates to a method for synthesizing a Crizotinib intermediate. The method comprises steps of: a) reacting a raw material 4-mesylate piperidine-1-formic acid tert-butyl ester (2) with 4-nitro pyrazole to prepare a compound 3; b) reducing nitro by using hydrazine hydrate to obtain an amino compound 4; and c) diazotizing the compound 4 by using tert-butyl nitrite, and reacting the compound 4 with a boric acid ester compound 5 in the presence of a free radical initiator benzoyl peroxide, so as to prepare the Crizotinib intermediate (1). Compared with an existing synthesis method, the method provided by the invention has the following advantages: a diazotization method is used to synthesize the boric acid ester product; and compared with an existing Miyaura boronation method catalyzed by Pd, the method avoids the usage of expensive palladium catalyst and ligand, and has the merits of mild reaction condition, high yield, simple operation, cheap and easily available raw materials and short reaction period, and is quite easy for industrialized mass production.
Owner:TONGJI UNIV
Aromatic carboxylic acid trifluoroethyl ester compound and preparation method thereof
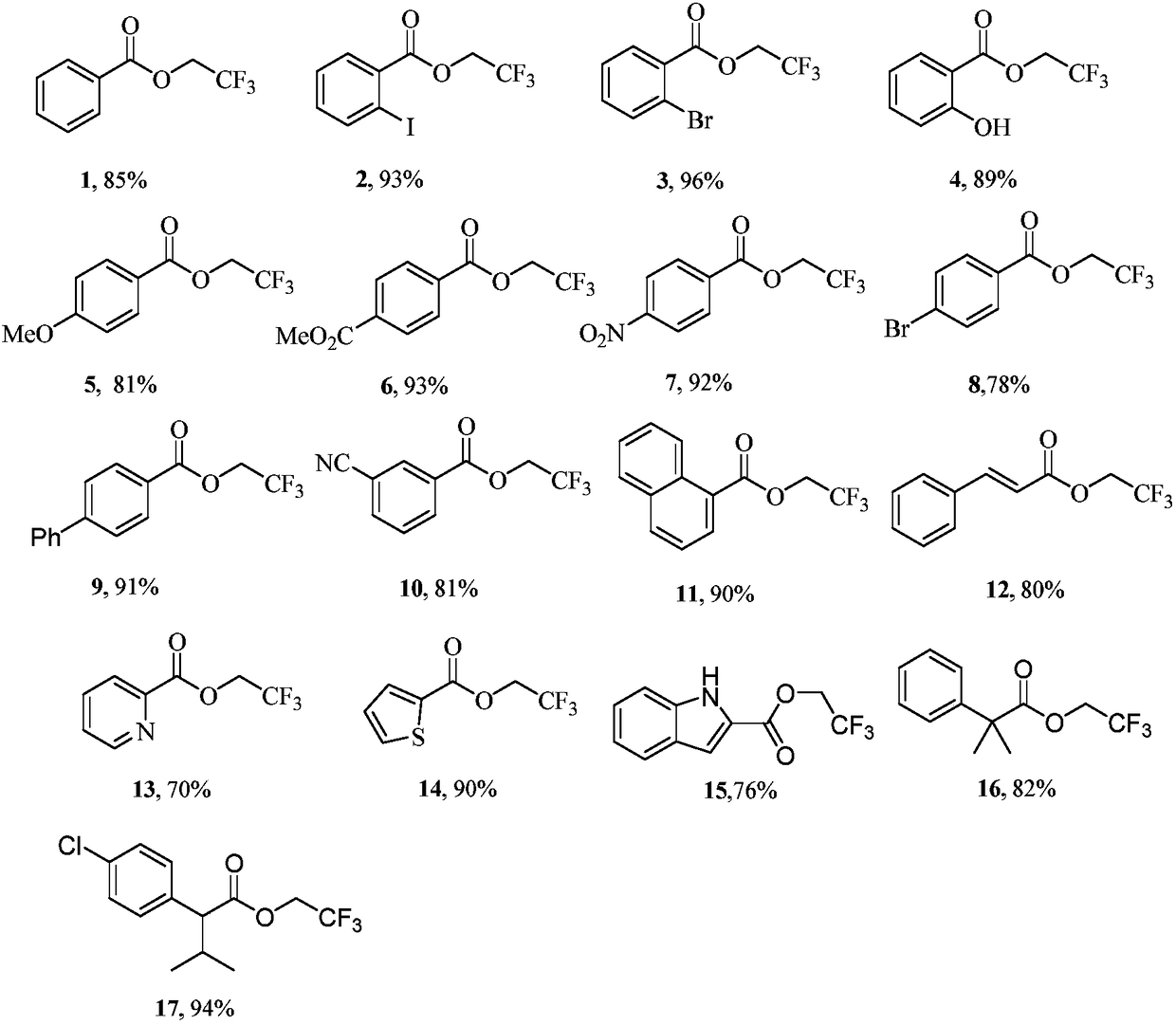
InactiveCN108503549AEasy to operateMild conditionsCarboxylic acid nitrile preparationOrganic compound preparationDrugs synthesisN-butyl nitrite
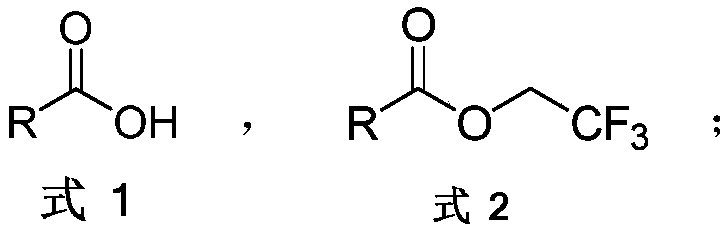
The invention discloses an aromatic carboxylic acid trifluoroethyl ester compound and a preparation method thereof. The invention provides the aromatic carboxylic acid trifluorethylmethyl ester compound and the preparation method thereof. The preparation method comprises the following step of performing reaction on aromatic carboxylic acid, t-butyl nitrite and 2,2,2-trifluoroethylamine with a one-pot method to obtain the carboxylic acid trifluoroethyl ester compound. The method is simple to operate, mild in reaction condition, low in cost, less in byproduct and high in yield, is capable of obtaining the aromatic carboxylic acid trifluoroethyl ester compound without being limited by a substrate so as to establish an aromatic carboxylic acid trifluoroethyl ester compound library and providesa raw material source to drug screening and new drug synthesis.
Owner:JIANGXI NORMAL UNIV
Method for preparing aromatic hydrocarbon alpha ketone carbonyl compound through continuous visible light catalytic molecular oxygen oxidation in micro-channel reactor
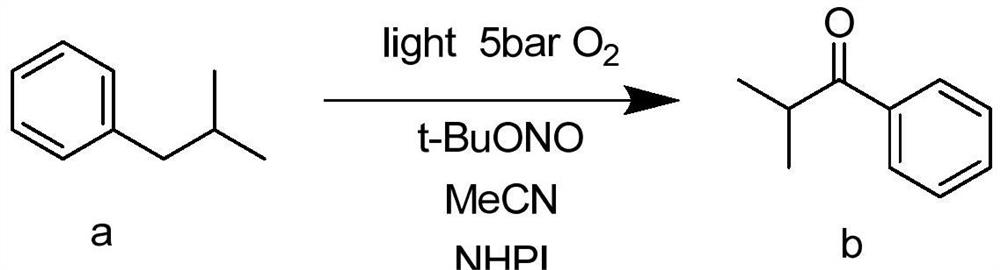
InactiveCN111635299ALow costPhotosensitizing activityOrganic compound preparationChemical/physical/physico-chemical microreactorsPtru catalystN-butyl nitrite
The invention provides a method for preparing aromatic hydrocarbon alpha ketone carbonyl compounds through continuous visible light catalytic molecular oxygen oxidation in a micro-channel reactor, andbelongs to the technical field of continuous flow photocatalytic synthesis. According to the method, molecular oxygen is used as an oxidizing agent, N-hydroxyphthalimide and tert-butyl nitrite are used as catalysts, and isobutylbenzene is used for preparing isobutyryl benzene under the condition of visible light. The product isobutyryl benzene has photosensitive activity, the catalytic reaction is carried out under visible light, and the highest yield of the obtained isobutyryl benzene can reach 86.3%. The reaction conditions are mild, the isobutyryl benzene can be efficiently prepared without additional metal, alkali and the like, and the operation is simple. With application of the micro-channel reactor, the reaction time is greatly shortened, the mass and heat transfer efficiency is obviously improved, the energy consumption is reduced, and three-waste emission is reduced.
Owner:DALIAN UNIV OF TECH
Photocatalytic oxidation synthesis method of benzocoumarin compound
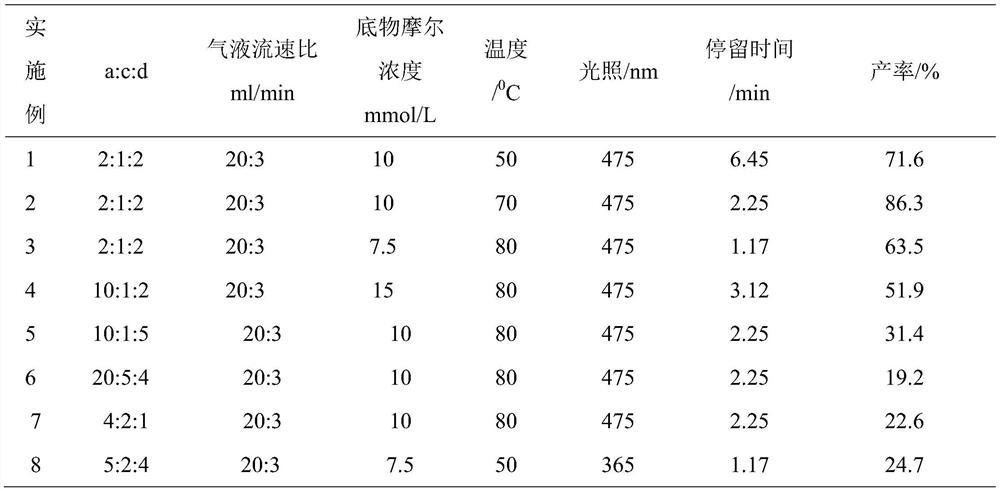
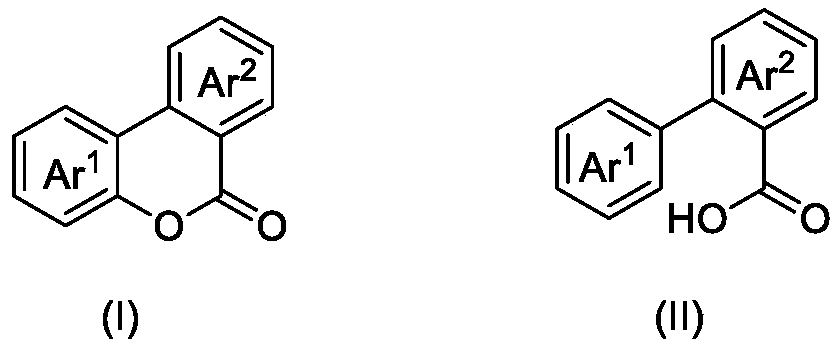
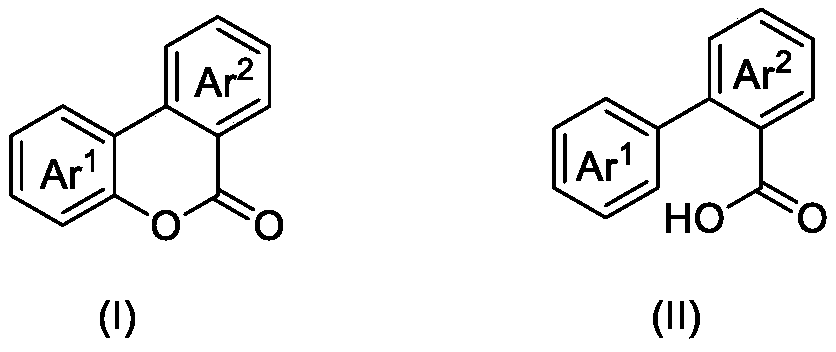
ActiveCN110862368ASave energyOvercome the problem of metal residueOrganic chemistryPtru catalystN-butyl nitrite
The invention discloses a photocatalytic oxidation synthesis method of a benzocoumarin compound. A 2-aryl-aryl formic acid compound is used as a reaction raw material; 2,3-dichloro-5,6-dinitrile-1,4-benzoquinone (DDQ) and tert-butyl nitrite (TBN) are used as catalysts, oxygen is used as an oxidant, a reaction substrate is subjected to a reaction in an organic solvent under the conditions of normaltemperature, normal pressure and blue light irradiation, and after the reaction is finished, separation treatment is performed to obtain the benzocoumarin compound. Compared with a traditional heating reaction, the synthesis method provided by the invention has the advantages that the illumination reaction can save energy; oxygen is used as a terminal oxidant, so that the environmental cost is reduced; a transition metal catalyst is not used, so that the problem of metal residues in the product can be solved.
Owner:ZHEJIANG UNIV OF TECH
Method for preparing nitrate ester compound by adopting micro-flow field reaction technology
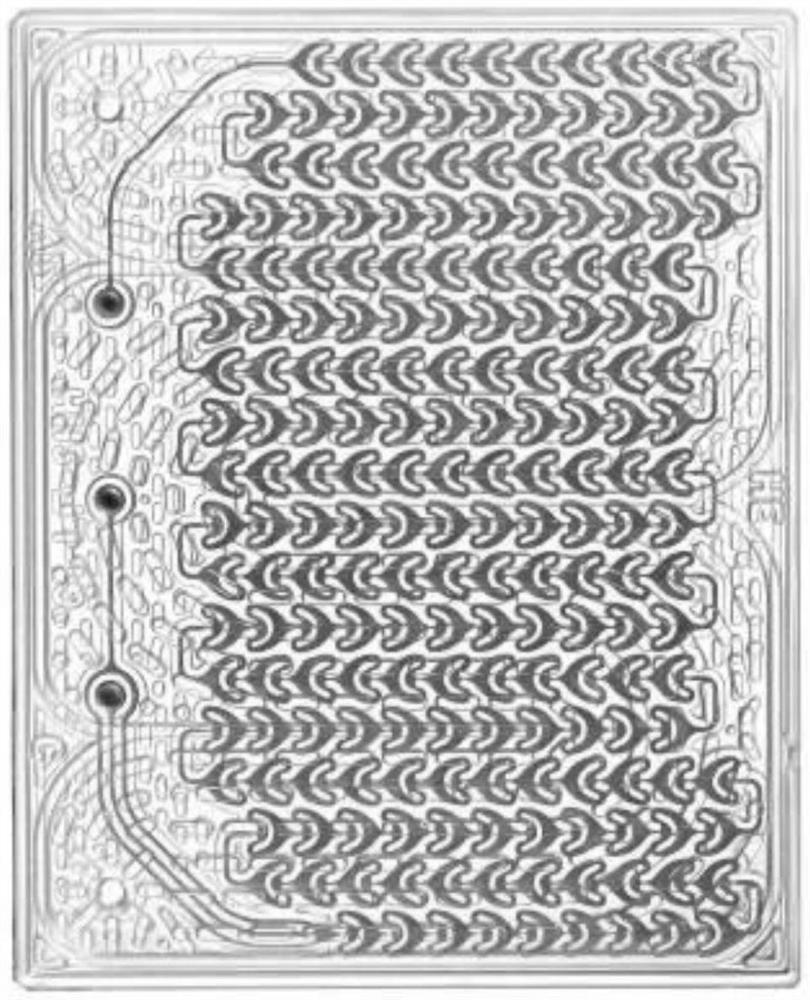
ActiveCN107721874AHigh yieldReduce contentOrganic compound preparationChemical/physical/physico-chemical microreactorsMicro structureOrganic solvent
The invention discloses a method for preparing a nitrate ester compound by adopting a micro-flow field reaction technology. The method comprises the following steps: dissolving an oxazole compound andtert-butyl nitrite into an organic solvent to obtain a mixed solution; pumping the obtained mixed solution into a micro-channel modular reaction device for reaction to prepare the nitrate ester compound. The micro-channel modular reaction device comprises a micro-structure mixer, a micro-structure reactor and a product collector which are sequentially connected with each other through pipelines,and temperature in the micro-structure reactor is controlled by a micro-heat exchanger. According to the method provided by the invention, a conventional nitrification method for preparing the nitrateester compound is changed, and the use of concentrated sulfuric acid and concentrated nitric acid is avoided; moreover, the method has the advantages of simple production device, easiness for processcontrol, easiness for wastewater treatment and the like.
Owner:NANJING UNIV OF TECH
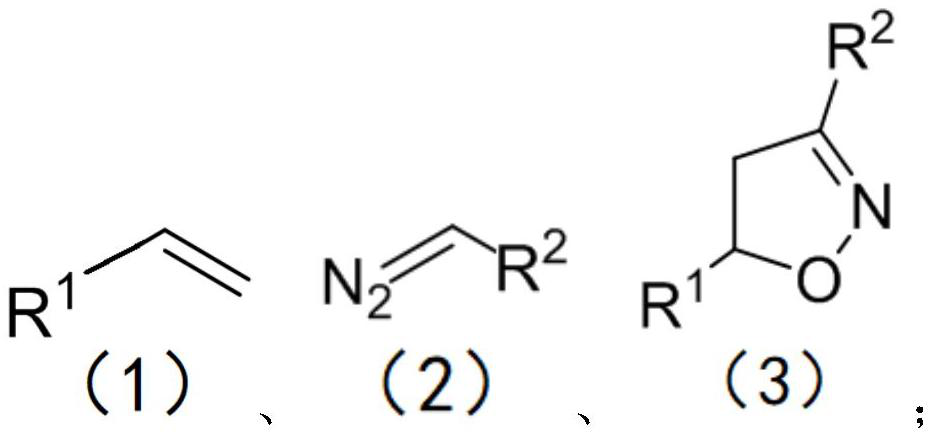
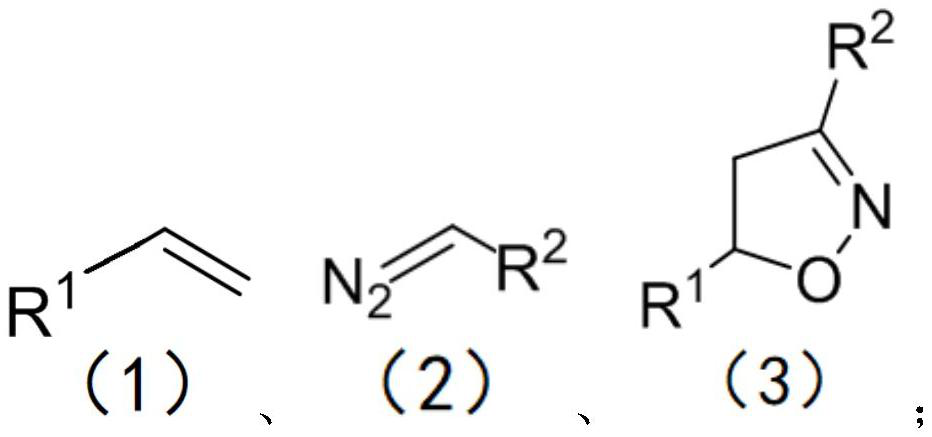
Simple preparation method of isoxazoline
The invention discloses a simple preparation method of isoxazoline. The simple preparation method is characterized in that a series of isoxazoline compounds are efficiently synthesized by using aldehyde, p-toluenesulfonhydrazide, olefin and tert-butyl nitrite as substrates, copper chloride as a catalyst and tetramethylethylenediamine (TMEDA) as alkali through the one-pot two-step method. The method has the advantages that the catalyst is cheap, reaction is economical, good substrate universality is achieved, the raw materials are easy to obtain, later-stage functionalization is more convenient, reaction conditions are mild, and gram-level scale reaction is good; and especially, the compounds with medium yield can be obtained without the catalyst, post-treatment is simple and convenient, the application of the compoumds in drug molecule synthesis and large-scale industrialization is facilitated, and the requirements and trends of modern green chemistry and medicinal chemistry are met.
Owner:SUZHOU UNIV
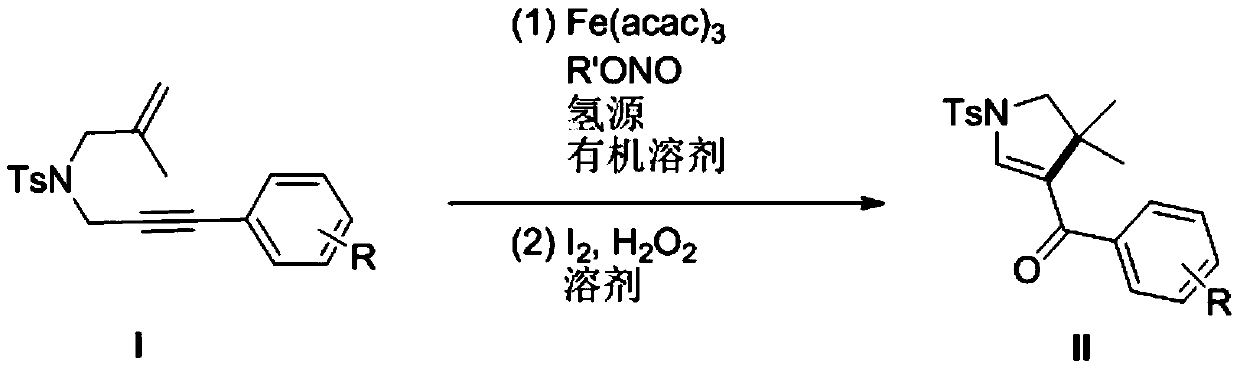
Preparation method of polysubstituted pyrroline compound
The invention discloses a preparation method of a polysubstituted pyrroline compound, and belongs to the technical field of fine chemical engineering. According to the method, N-2-methylallyl-N-3-arylpropargyl p-toluenesulfonamide is used as a raw material, a specific ferric salt is used as a catalyst, tert-butyl nitrite is used as an oxidizing agent, polymethylsiloxane (PMHS) is used as a hydrogen source, and a reaction is carried out in an inert atmosphere; and then a reaction is performed in an I2 / H2O2 system to synthesize a target product, namely the polysubstituted pyrroline compound. The method has the advantages of cheap catalyst, mild reaction condition and the like, and has a good application prospect.
Owner:JIANGNAN UNIV
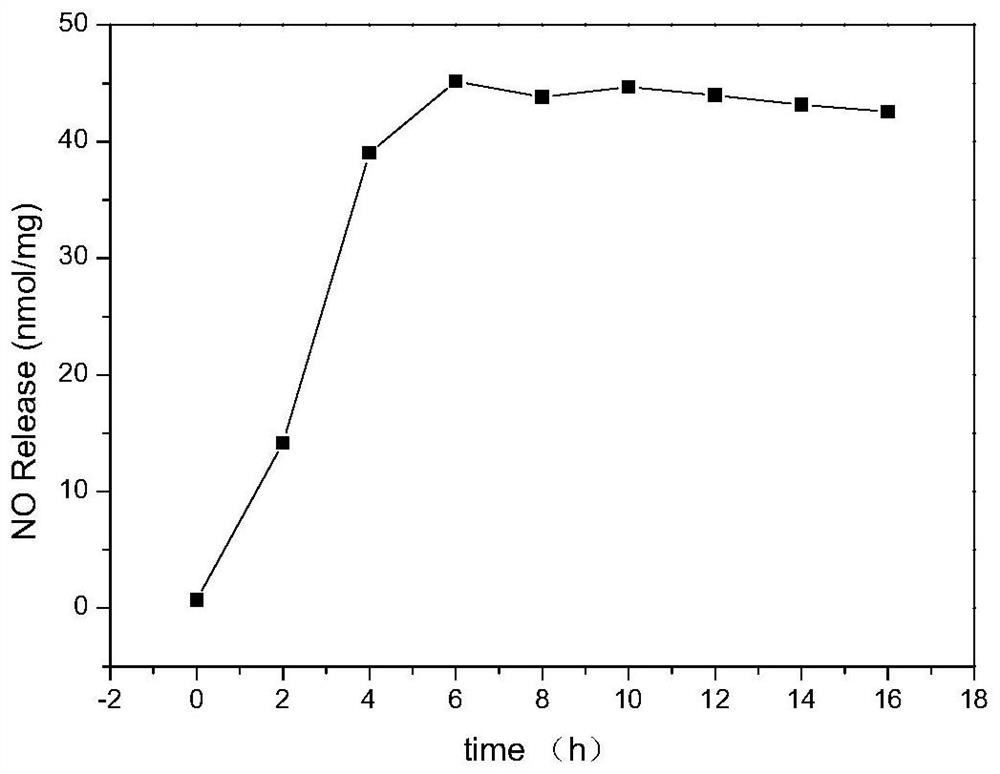
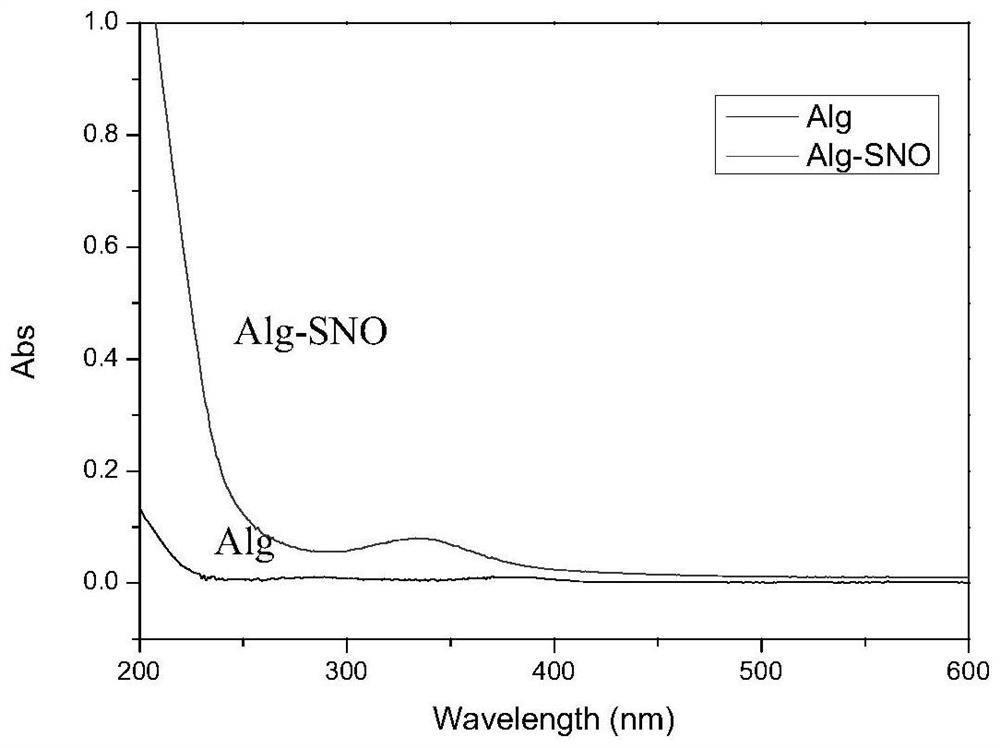
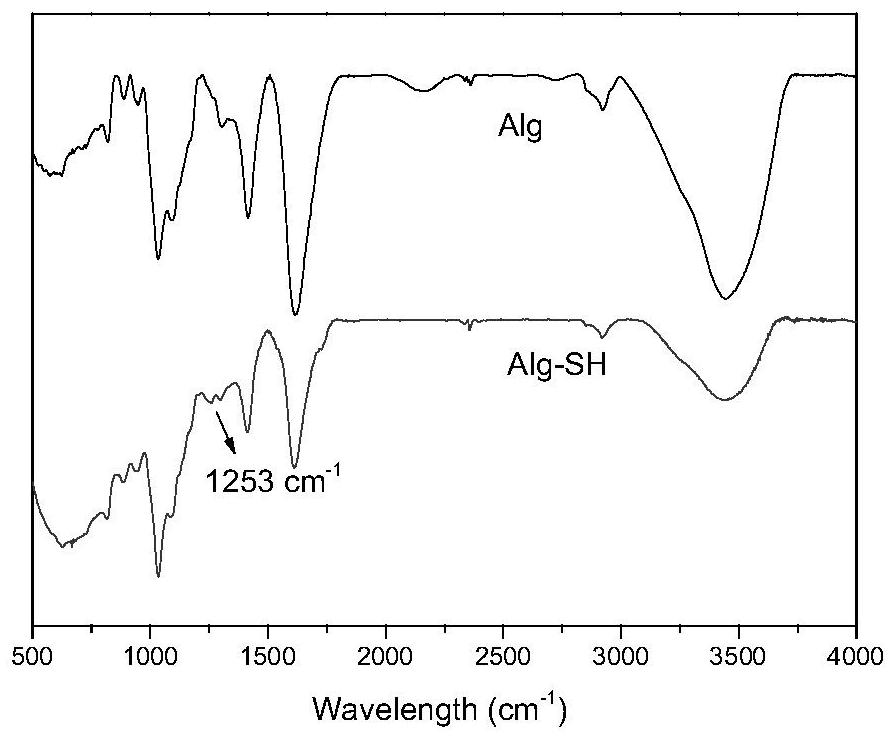
Nitric oxide donor based on sodium alginate as well as synthesis method and application of nitric oxide donor
PendingCN114652743AGood water solubilityGood donor stabilityOrganic active ingredientsAerosol deliveryNitrosoN-butyl nitrite
The invention discloses a sodium alginate-based nitric oxide donor as well as a synthesis method and application thereof. The synthesis method comprises the following steps: dissolving sodium alginate in ultrapure water, adding an activating agent for reaction, adding L-cysteine hydrochloride or cystamine dihydrochloride for continuous reaction; and dialyzing the reaction solution, adding tert-butyl nitrite, continuously reacting, dialyzing the obtained solution, and freeze-drying to obtain the sodium alginate nitric oxide donor. The sodium alginate nitric oxide donor synthesized by the method is S-nitrosylated sodium alginate, sulfydryl is grafted on sodium alginate, SNO groups are formed through nitrosation, the synthesis method is simple and easy to implement, non-toxic and low in cost, and the prepared nitric oxide donor is long in release time, can exist stably, and can be used for preparing the sodium alginate nitric oxide donor. And the material and other polymer materials are expected to be applied to preparation of multifunctional nitric oxide release composite materials.
Owner:NANJING NORMAL UNIVERSITY
Rubber containing modified graphene
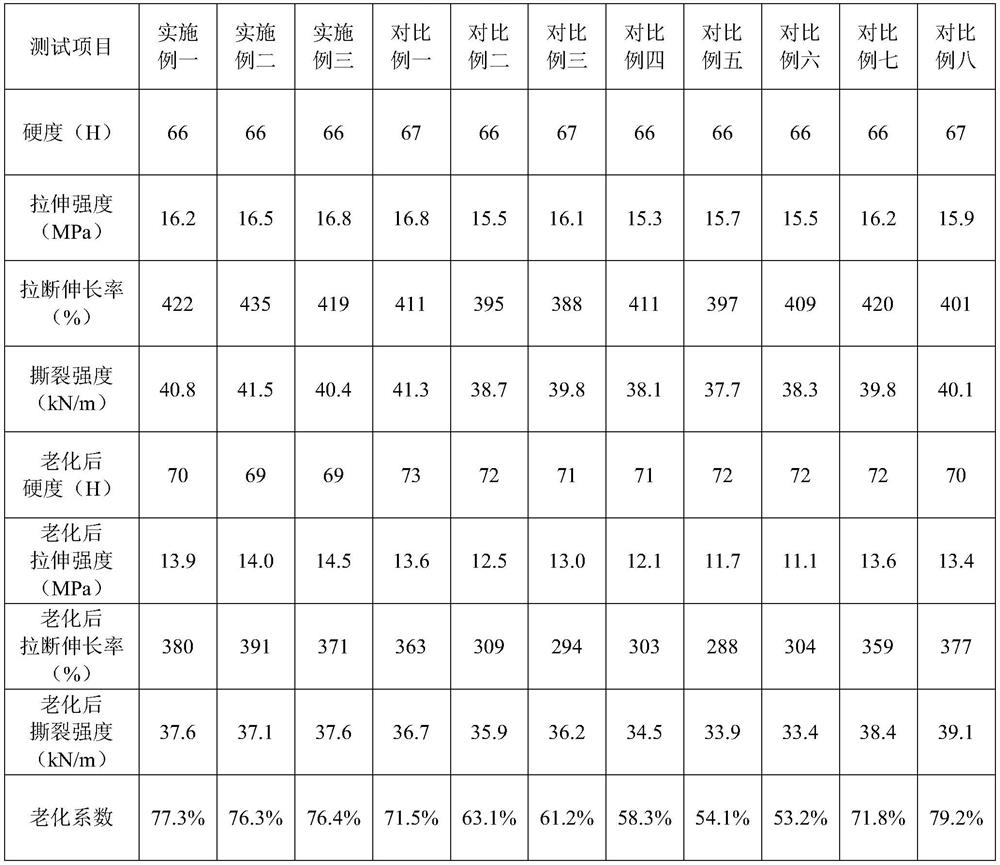
The invention relates to the technical field of synthetic rubber, and discloses rubber containing modified graphene, which comprises the following components: rubber, carbon black, zinc oxide, stearic acid, sulfur, an accelerant, a scorch retarder and modified graphene. The modified graphene is obtained by fully mixing and ball-milling graphite and a modifier, then adding tert-butyl nitrite and a solvent, mixing and ball-milling, fully mixing, washing and drying; and the modifying agent is one or more of amino propionyl aniline, 4-aminobenzoyl aniline, 2-amino-N-cyclohexyl-N-methyl benzene methylamine, 4-(4-isopropyl piperazine-1-yl) aniline, (4-aminobenzene)-4-(4-methoxyphenyl) piperazine, (4-(4-aminobenzyl) piperazine-1-yl) ethanone and (4-aminobenzene)-4-(4-methoxyphenyl) piperazine. Rubber formed by mixing and refining the modified graphene and other components of rubber has a better anti-aging effect.
Owner:QUANGANG PETROCHEM RES INST OF FUJIAN NORMAL UNIV
A kind of synthetic method of novel chiral nitrobinaphthol and its derivatives
ActiveCN108658774BLow priceImprove compatibilityOrganic chemistry methodsNitro compound preparationOrganic synthesisN-butyl nitrite
The invention relates to a synthesizing method of novel chiral nitro binaphthol and derivatives thereof, and belongs to the field of organic synthesis. A novel green free radical nitrifying method isprovided, and comprises the following steps of using chiral binaphthol as the raw material, and generating free radical nitrifying reaction with a green nitrifying reagent (TBN (tert-butyl nitrite)) at room temperature by using tetrahydrofuran as a reaction solvent, so as to obtain the chiral nitro binaphthol. The synthesizing method has the advantages that the metal is not used in reaction, and the TBN is directly used in the nitrifying reaction; only the product and tertiary butanol are generated in reaction, and the pollution to environment is reduced; the important application prospect isrealized in the synthesizing field of the chiral nitro binaphthol, the green nitrifying is truly realized, and a novel concept is provided for the large-scale industrialized production of the nitro binaphthol.
Owner:QINGDAO INST OF BIOENERGY & BIOPROCESS TECH CHINESE ACADEMY OF SCI
Imidazo heterocyclic azo derivative and preparation method and application thereof
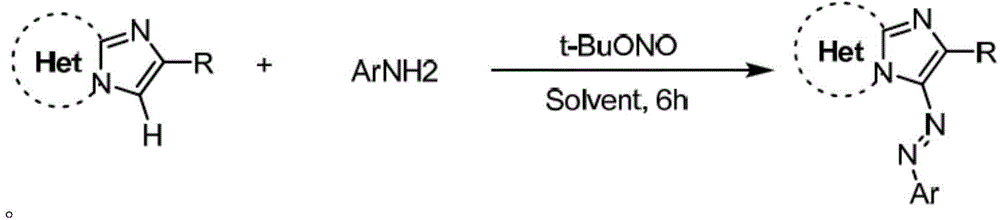
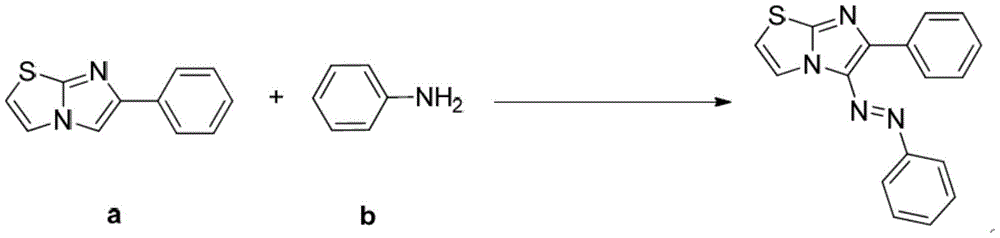
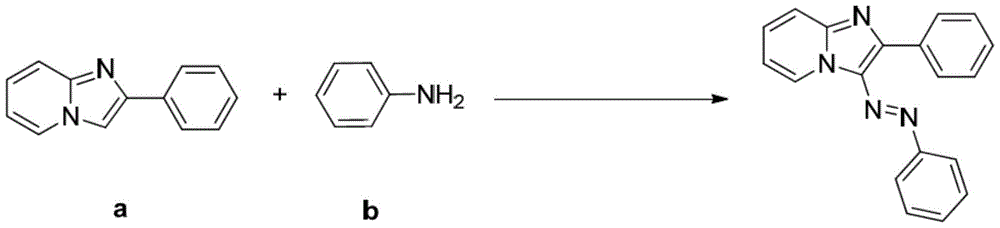
ActiveCN105713015AEconomical green synthesis methodSimple green synthesis methodMonoazo dyesOrganic chemistryOrganic synthesisStructural formula
The invention belongs to the field of organic synthesis and azo dyes and particularly discloses an imidazo heterocyclic azo derivative and a preparation method and application thereof. The imidazo heterocyclic azo derivative has a structural formula shown as in formula (1), wherein heterocyclic Het is one of pyridine, thiazole and pyrimidine; R is C1-C6 alkyl groups, or aryl groups containing F, Cl, Br, MeO, CN and NO2 or aryl groups that are acetyl-substituted; Ar is aryl groups containing F, Cl, Br, MeO, CN and NO2 or aryl groups that are acetyl-substituted. The preparation method of the invention is an economical, simple and environment-friendly green synthetic method, neither the addition of mass hydrochloric acid or sulfuric acid and nitrites nor the pre-preparation of diazonium salts is required in the method, and imidazo heterocyclic azo compounds are directly produced under the action of tert-butyl nitrites. The imidazo heterocyclic azo derivative prepared herein has good fluorescent properties and is useful in the fields such as fluorescent dyes, luminescent materials and bactericides.
Owner:SOUTH CHINA AGRI UNIV
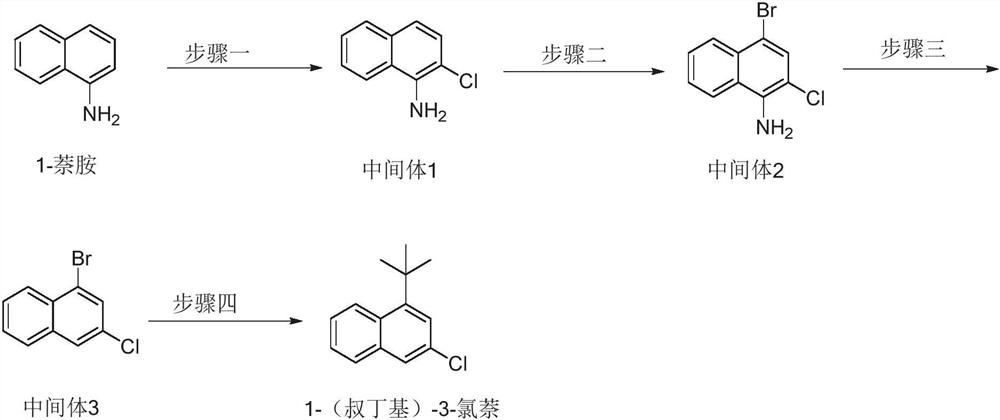
Preparation method of 1-(tert-butyl)-3-chloronaphthalene
ActiveCN114736099ASuitable for industrial productionHigh yieldOrganic compound preparationChemical recyclingPtru catalystN-butyl nitrite
The invention discloses a preparation method of 1-(tert-butyl)-3-chloronaphthalene. The preparation method comprises the following steps: reacting 1-naphthylamine with N-chlorosuccinimide in a solvent to generate a first intermediate; reacting the first intermediate with N-bromosuccinimide in a solvent to generate a second intermediate; reacting a first reaction system containing the second intermediate, lewis acid, tert-butyl nitrite and a solvent, and then adding hypophosphorous acid and cuprous oxide into the first reaction system for reaction to generate a third intermediate; and reacting a second reaction system containing a catalyst ligand, nickel acetylacetonate, lithium tert-butoxide, isopropyl magnesium chloride and a solvent in a protective atmosphere, and then adding the third intermediate into the second reaction system for reaction to generate 1-(tert-butyl)-3-chloronaphthalene. The preparation method disclosed by the invention is short in process route, simple to operate, mild in condition, environment-friendly, low in cost, high in target product yield, few in by-products and beneficial to industrial production.
Owner:JIANGSU NATA OPTO ELECTRONICS MATERIAL
Method for preparing isoxazoline
ActiveCN112028848AReaction economyBroad substrate versatilityOrganic chemistryPtru catalystOrganic solvent
The invention relates to a method for preparing isoxazoline, which comprises the steps of reacting olefin, a diazo compound and tert-butyl nitrite in an organic solvent at the temperature of 25-50 DEGC under the action of a Lewis acid catalyst to obtain isoxazoline after complete reaction. Lewis acid is used as a catalyst for synthesizing isoxazoline, reaction conditions are mild and can be carried out under the condition that the temperature is as low as the room temperature, use of transition metal is avoided, and the product yield is high.
Owner:SUZHOU UNIV
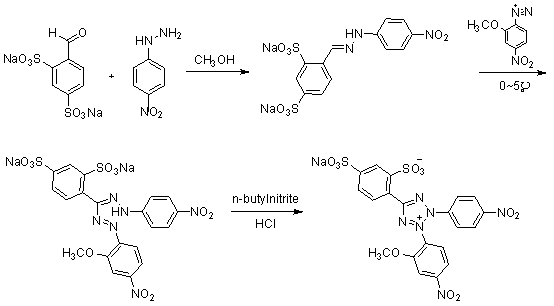
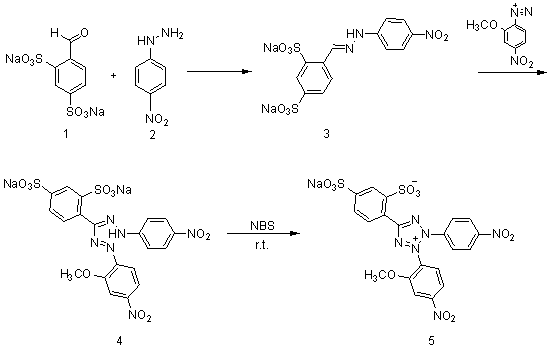
Synthesis method of 2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazole monosodium salt
The invention discloses a synthesis method of 2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazole monosodium salt (WST-8), belonging to the technical field of chemical synthesis. According to the synthesis method, the raw materials benzaldehyde-2,4-sodium disulfonate and p-nitrophenylhydrazine are subjected to four-step reaction by using N-bromosuccinimide (NBS) as an oxidizer, thereby obtaining the WST-8. The invention is safe and simple to operate and easy for after-treatment, and avoids using chlorine gas, n-butyl nitrite and other high-toxicity high-price compounds; and the total yield of the four-step reaction is up to 34.4%, thereby being beneficial to industrial production.
Owner:ZHENGZHOU UNIV
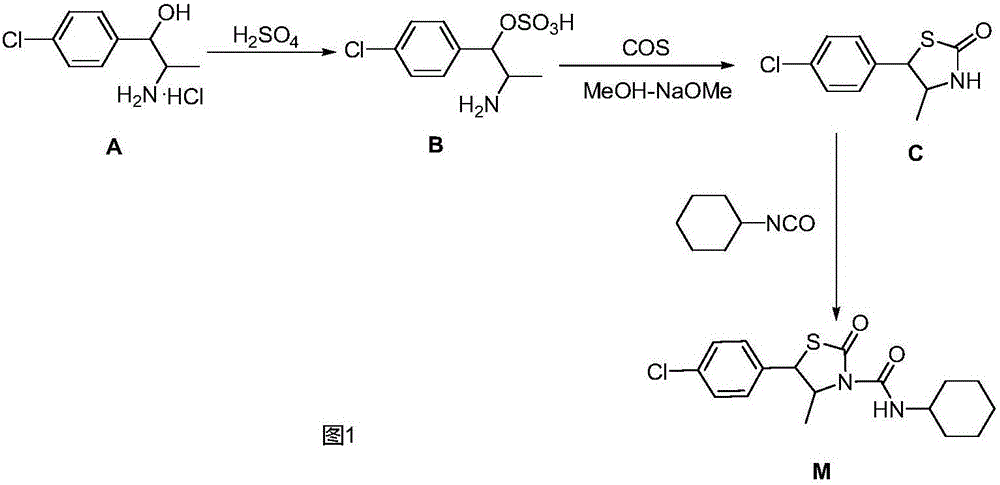
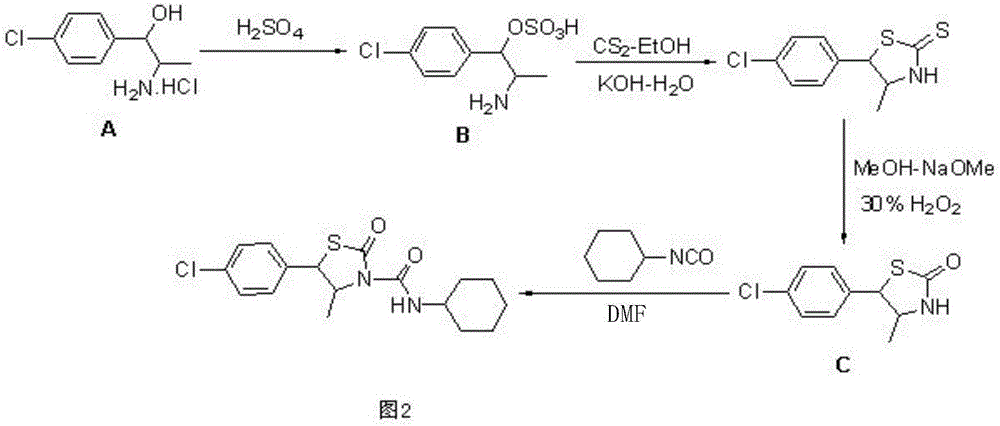
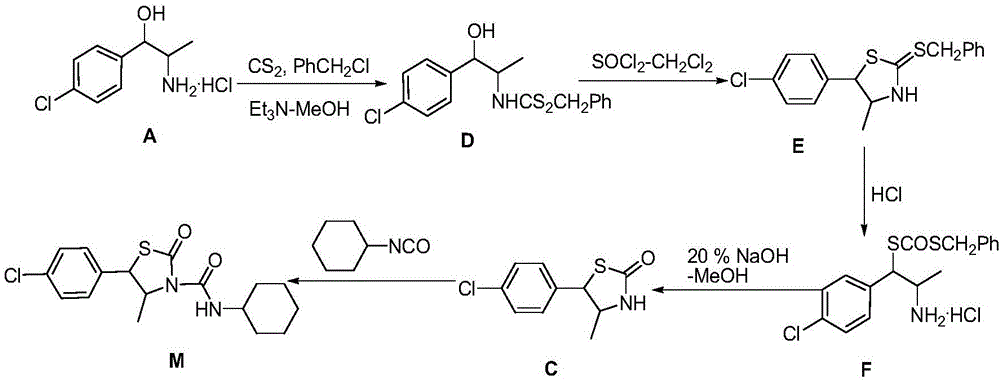
Hexythiazox and preparation method thereof
The invention discloses hexythiazox and a preparation method thereof. The method includes: taking chloropropiophenone as a starting material to react with n-butyl nitrite in a mass ratio of 70:47 to generate ketoxime; performing hydrogenation reduction to obtain 2-amino-1-p-chlorophenyl propanol hydrochloride; directly subjecting 2-amino-1-p-chlorophenyl propanol hydrochloride to reaction with carbon disulfide to synthesize trans-5-(4-chlorphenyl)-4-methyl-2-sulfo-thiazolidone which is then oxidized through hydrogen peroxide to generate 5-(4-chlorphenyl)-4-methyl-2-oxo-thiazolidone; subjecting 5-(4-chlorphenyl)-4-methyl-2-oxo-thiazolidone to additive reaction with cyclohexyl isocyanate to obtain hexythiazox which is a final product. The preparation method is suitable for industrial production, a technical process is simplified, reaction yield is increased, synthesis difficulty is lowered, by-products are reduced, the problem of environment pollution in a preparation process is reduced, and production cost is reduced as well.
Owner:WENZHOU UNIVERSITY +1
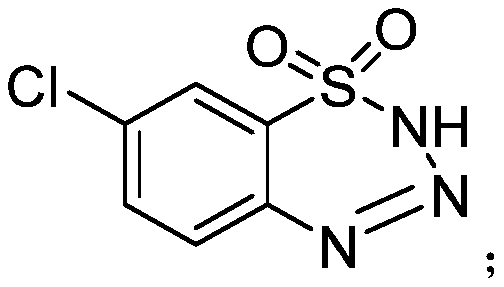
Chromium-containing waste leather scrap treatment method
The invention discloses a chromium-containing waste leather scrap treatment method. The method comprises the following steps: carrying out hydrolysis treatment on chromium-containing waste leather scraps serving as a raw material, and carrying out enzyme hydrolysis on the chromium-containing waste leather scraps by using protease; carrying out further hydrolysis on the chromium-containing waste leather scraps by using an acid solution to hydrolyze the chromium-containing waste scraps into collagen polypeptide molecules, wherein the acid solution is a mixture of an aqueous sulfuric acid solution and an aqueous lactic acid solution; then modifying the collagen polypeptide molecules with a modifier, wherein the modifier is consisting mixture of of 2,3-pyridinedioic anhydride, 7-chloro-2H-benzo[e][1,2,3,4]thiatriazine-1,1-dioxide and 4,4'-diamino-3,3'-biphenyl disulfonic acid, wherein the 7-chloro-2H-benzo[e][1,2,3,4]thiatriazine-1,1-dioxide is obtained by reacting 2-amino-5-chlorobenzenesulfonamide with tert-butyl nitrite; and performing treatment to process the chromium-containing waste leather scraps into a retanning filler. The retanning filler has a good tanning and retanning filling effect, and tanned leather is full, soft, good in elasticity and clear and fine in grain.
Owner:WENZHOU UNIVERSITY
The preparation method of iodosulfuron sodium salt intermediate
The invention discloses a preparation method of an intermediate of iodosulfuron-methyl sodium. The intermediate is obtained by performing a reaction on 6-aminosaccharin and potassium iodide in the presence of alkyl nitrite and acetic acid in one step, wherein a molar ratio of the potassium iodide to the 6-aminosaccharin is 1:1 to 1.2:1; a molar ratio of the alkyl nitrite to the 6-aminosaccharin is0.95:1 to 1:1; the alkyl nitrite is ethyl nitrite, isopropyl nitrite, n-butyl nitrite or isoamyl nitrite; and a molar ratio of the acetic acid to the 6-aminosaccharin is 1:1 to 1.2:1. According to the method provided by the invention, the alkyl nitrite is adopted for diazotization, the potassium iodide is added at the same time, so that decomposition and hydrolysis of a diazonium salt can be avoided, and the reaction yield is greatly improved; and the method does not require a large amount of water as a solvent and only adopts a small amount of water to wash an organic layer in the post-treatment, and therefore wastewater produced by adopting the method provided by the invention does not exceed 2 times the weight of the target product.
Owner:江苏省农用激素工程技术研究中心有限公司 +1
A kind of preparation method of intermediate of heterocyclic urea indoleamine-2,3-dioxygenase inhibitor
The invention discloses a preparing method of a heterocyclic urea indoleamine-2-3-dioxygenase enzyme inhibitor intermediate. Malononitrile and hydroxylamine hydrochloride serve as raw materials, and 3-amino-4-aminoxime furza is obtained through a sodium nitrite reaction; afterwards, benzophenone is adopted for amino protection, and then 3-amino-4-aminoxime furza is reacted with 3-bromine-4-fluoroaniline after being diazotized with tert-butyl nitrite to obtain 4-diphenyl agag amino-N-(3-bromine-4-fluorophenyl)-N'-hydroxy-1,2,5-oxadizole-3-formamidine; after protection of carbonic ester ring closure and benzophenone, the heterocyclic urea indole amines-2-3-dioxygenase enzyme inhibitor intermediate, namely 3-(4-amino-1,2,5-oxadiazole-3-radical)-4-(3-bromine-4-fluorophenyl)-1,2,4-oxidazole-5(4H)-ketone is generated. The preparing method is high in reaction yield, convenient to operate, low in cost and suitable for industrialized production.
Owner:宁夏蓝博思化学技术有限公司
Method for synthesizing aromatic trifluoromethylthio compound
The invention discloses a method for synthesizing an aromatic trifluoromethylthio compound. The method comprises the following steps: reacting sodium trifluoromethanesulfinate with Chlorodiphenyl phosphine at room temperature; after reacting for a period of time, sequentially adding an aniline compound and tert-butyl nitrite, fully reacting at 70 + / -5 DEG C by taking CuSO4 as a catalyst and acetonitrile as a solvent, cooling to room temperature after the reaction is finished, extracting, carrying out reduced pressure distillation, and carrying out column chromatography separation and purification on the reaction mixture to obtain the aromatic trifluoromethylthio compound. The method is simple and safe in process operation and high in reaction conversion rate, the used raw materials are economical and practical, few three wastes are generated, and no extra organic solvent needs to be treated.
Owner:NANJING UNIV OF SCI & TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com