Simple preparation method of isoxazoline
A kind of isoxazoline, a simple technology, applied in the field of preparation of isoxazoline, can solve the problems of cumbersome preparation of diazo compounds, unsuitable for the synthesis of drug molecules, expensive and harmful transition metals, etc. good effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034]
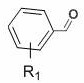
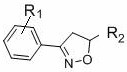
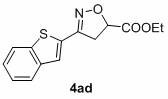
[0035] In air, to a test tube with a magnetic stir bar, p-bromobenzaldehyde (0.65 mmol), p-toluenesulfonylhydrazide (0.7 mmol) and MeOH (1 mL) were added, and the mixture was stirred at 60 °C for 30 min. After the solvent was removed in vacuo, CuCl was sequentially added 2 (0.05 mmol), THF (2.0 mL), ethyl acrylate (0.5 mmol), TMEDA (0.75 mmol), TBN (2.0 mmol) and THF (2.0 mL). Seal the test tube with a parafilm, stir at 65 °C for 24 h, quench with saturated sodium chloride solution, extract with ethyl acetate, use a rotary evaporator to remove the solvent, adsorb on silica gel, and finally use ethyl acetate and petroleum The mixed solvent of ether is subjected to column chromatography to obtain the product isoxazoline 3aa. Yield: 88%; mp: 66-68 o C; 1 H NMR (400MHz, CDCl 3 ) δ 7.51 (s, 4H), 5.15 (dd, J = 10.5, 7.9 Hz, 1H), 4.24 (q, J = 7.1Hz, 2H), 3.592 (d, J = 7.9 Hz, 1H), 3.586 (d, J = 10.5 Hz, 1H), 1.30 (t, J =7.1 Hz, 3H); 13 C NMR (100 MHz, CDCl ...
Embodiment 2
[0037] On the basis of embodiment one, reaction condition is done single factor change:
[0038] The second addition of THF (2.0 mL) was replaced by acetone (2.0 mL), yield: 84%.
[0039] The second addition of THF (2.0 mL) was replaced with acetonitrile (2.0 mL), yield: 54%.
[0040] Tert-butyl nitrite TBN was replaced with isopropyl nitrite (2.0 mmol), yield: 79%.
[0041] Tert-butyl nitrite TBN was replaced by n-butyl nitrite (2.0 mmol), yield: 68%.
[0042] CuCl 2 (0.05 mmol) was replaced by CuCl (0.05 mmol), yield: 77%.
[0043] CuCl 2 (0.05 mmol) was replaced by CuBr (0.05 mmol), yield: 83%.
[0044] CuCl 2 (0.05 mmol) was replaced by CuI (0.05 mmol), yield: 56%.
[0045] CuCl 2 (0.05 mmol) replaced by Cu(OAc) 2 (0.05 mmol), yield: 58%.
[0046] Tetramethylethylenediamine TMEDA was replaced by N,N-dimethylethanolamine DABCO (0.75 mmol), yield: 68%.
[0047] Tetramethylethylenediamine TMEDA was replaced by sodium carbonate (0.75 mmol), yield: 16%.
[0048] Tet...
Embodiment 3
[0051] Embodiment 3 On the basis of Embodiment 1, the copper catalyst is omitted
[0052] In air, to a test tube with a magnetic stir bar, p-bromobenzaldehyde (0.65 mmol), p-toluenesulfonylhydrazide (0.7 mmol) and MeOH (1 mL) were added, and the mixture was stirred at 60 °C for 30 min. After the solvent was removed in vacuo, ethyl acrylate (0.5 mmol), TMEDA (0.75 mmol), TBN (2.0 mmol) and THF (2.0 mL) were added sequentially. Seal the test tube with a parafilm, stir at 65 °C for 24 h, quench with saturated sodium chloride solution, extract with ethyl acetate, use a rotary evaporator to remove the solvent, adsorb on silica gel, and finally use ethyl acetate and petroleum The mixed solvent of ether is subjected to column chromatography to obtain the product isoxazoline 3aa. Yield: 61%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com